Abstract
Background and aim
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of liver diseases with simple steatosis on one end and hepatocellular carcinoma on the other. Although obesity is a known risk factor for NAFLD, individuals with normal body mass index (BMI) also have hepatic fatty infiltration, now termed “lean-NAFLD”. It represents a distinct entity with a strong underlying genetic component. The present study aimed to sequence the complete exonic regions of individuals with lean-NAFLD to identify germline causative variants associated with disrupted hepatic fatty acid metabolism, thereby conferring susceptibility to NAFLD.
Methods
Whole blood was collected from patients with lean-NAFLD (n = 6; BMI < 23.0 kg/m2) and matched lean controls (n = 2; discovery set). Liver fat was assessed using acoustic radiation force impulse (ARFI) imaging. Patients with ultrasound-detected NAFLD (n = 191) and controls (n = 105) were part of validation set. DNA was isolated, and whole-exome sequencing (WES) was performed in the discovery cohort (Ion Proton™; Ion AmpliSeq™ Exome RDY Kit). Data were analyzed (Ion Reporter software; Life Technologies), and variants identified. Validation of variants was carried out (Taqman probes; Real time-PCR). Student's t test and Fisher's exact test were used to analyze the statistical significance.
Results
Although WES identified ∼74,000 variants in individual samples, using various pipelines. variants in genes namely phosphatidylethanolamine N-methyltransferase (PEMT) and oxysterol-binding protein-related protein10 (OSBPL10) that have roles in dietary choline intake and regulation of cholesterol homeostasis, respectively, were identified (discovery set). Furthermore, significant differences were noted in BMI (p = 0.006), waist/hip circumference (p > 0.001), waist/hip ratio (p > 0.001), aspartate aminotransferase (p > 0.001), alanine aminotransferase (p > 0.001), and triglycerides (p = 0.002) between patients and controls. Validation of variants (rs7946-PEMT and rs2290532-OSBPL10) revealed that variant in PEMT but not OSBPL10 gene was associated (p = 0.04) with threefold increased risk of NAFLD in lean individuals.
Conclusion
Our results demonstrate the association of rs7946 with lean-NAFLD. WES may be an effective strategy to identify causative variants underlying lean-NAFLD.
Keywords: lean-NAFLD, nonalcoholic fatty liver disease, OSBPL10, PEMT, whole-exome sequencing
Abbreviations: PEMT, Phosphatidylethanolamine N-methyltransferase; NAFLD, Nonalcoholic fatty Liver disease; BMI, Body mass index; ARFI, Acoustic Radiation Force Impulse; DNA, Deoxyribonucleic acid; WES, Whole-Exome Sequencing; OSBPL10, Oxysterol-binding protein-related protein10; AST, aspartate aminotransferase; ALT, alanine aminotransferase; NASH, nonalcoholic steatohepatitis; FFAs, free fatty acids; SNPs, Single-nucleotide polymorphisms; GWAS, Genome-wide association studies; SNVs, Single-nucleotide variants; gDNA, genomic Deoxyribonucleic acid; pM, pico mole; indel, insertion deletion; SIFT, Sorting Intoleratnt from Tolerant; PCR, Polymerase chain reaction; ng, nano gram; HDL, high-density lipoproteins; CI, confidence interval; WC, Waist circumference; HCC, Hepatocellular carcinoma; PHRED, Phil's Read Editor; PE, phosphatidylethanolamine; PC, phosphatidylcholine
Nonalcoholic fatty liver disease (NAFLD) is a condition that is characterized by hepatic fat infiltration (≥5%) in the absence of alcohol consumption (≤20 mg/day in females and ≤30 mg/day in males). NAFLD is a spectrum of liver diseases majorly contributing to development of hepatocellular carcinoma (HCC)1 and has high morbidity and mortality with few known effective treatments.2 Thus, the emphasis is on early detection for effective management. The prevalence of NAFLD is 20–30% in Western countries3 and ∼25–30% in India4 in the general population. The hallmark of NAFLD is hepatic neutral lipid accumulation. Although obesity, diabetes, age, hypertension, and hypertriglyceridemia are known risk factors for NAFLD, it is now known that in 26–35% of patients, genetic factors may contribute to the development of the disease.5, 6
Obesity is a known risk factor for NAFLD; however, fatty infiltration is encountered in individuals with normal body mass index (BMI < 23 kg/m2) that is now termed “lean-NAFLD” and may have strong underlying genetic susceptibility. The prevalence of lean-NAFLD is ∼20% in India,7, 8 15.2% in Japan,9 15% in China,10 12% in Greece,11 and 12.6% in South Korea12 as well as USA.13 Lean-NAFLD may represent a distinct entity and is the most frequent cause of cryptogenic liver disease;14 moreover, it suggests a phenotypic distinctiveness that raises the possibility of a different pathophysiology. Previously, it was reported that 50% of lean-NAFLD had biopsy-proven nonalcoholic steatohepatitis (NASH), which is much higher than what has been reported in general population studies.15 The biology of lean-NASH is much similar to that of obese NASH; however, the absence of significant adiposity suggests a phenotypic uniqueness with shared biology strongly raising the possibility of genetic risk.
Genetic factors play a significant role in the causation of lean-NAFLD.16, 17 The hepatic lipid imbalance between availability and disposal eventually triggers ectopic fat infiltration, leading to hepatocyte damage, a process defined as ‘lipotoxicity’. The molecular factors of lipotoxicity need to be identified more precisely, unraveling the role of different free fatty acids (FFAs) in this context.18 Some of the lead single-nucleotide polymorphisms (SNPs) identified through case–control and genome-wide association studies (GWAS) are found in or near genes known to cause dyslipidemias, thus facilitating the identification of the functional process that governs their relationship with the lipoprotein trait. GWAS-based identification of single-nucleotide variants (SNVs) is an unbiased approach for discovery of genes involved in complex genetic traits. Efforts to identify the molecular basis of the disease using whole-exome sequencing (WES) revealed several candidate variants and appear to be responsible for the phenotype.6 In the present study, we performed WES in a discovery cohort of individuals with lean-NAFLD to identify germline variants that are associated with hepatic fatty acid metabolism, and the identified variants were validated in an independent cohort with NAFLD.
Methods
Study population
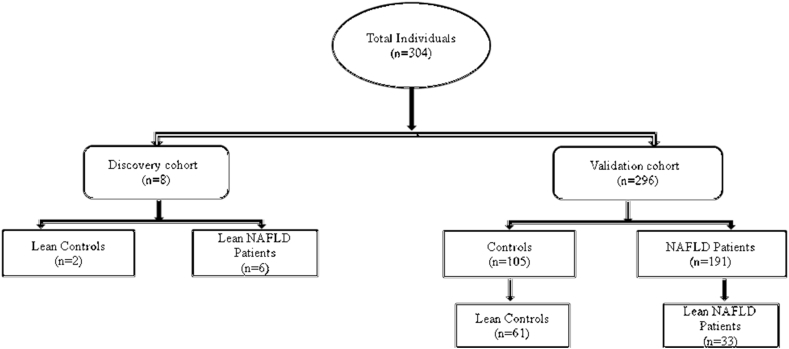
In this case–control study, a total of 304 individuals were included and grouped into discovery and validation cohorts. The discovery cohort included patients with (n = 6) and without (n = 2) lean-NAFLD (BMI <23 kg/m2). Liver fat/fibrosis examination was carried out in the discovery cohort by acoustic radiation force impulse (ARFI) imaging, an ultrasound-based quantitative elastography technology. A total of 191 patients with ultrasound-confirmed NAFLD (Hepatology clinic, Asian Institute of Gastroenterology, Hyderabad) and 105 controls (without fatty infiltration) were included in the validation set (Figure 1). Anthropometric data and blood samples were collected from all the individuals, and WES and biochemical analysis were performed. Written informed consent was obtained from individuals, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review (Scientific) Board.
Figure 1.
Flowchart depicting sample recruitment.
DNA extraction and WES (discovery set)
DNA was isolated from whole blood using a commercial kit (Bioserve Biotechnologies, Hyderabad) following the manufacturer's protocol. Whole-exome target regions were amplified using 100 ng of genomic DNA (gDNA) (Ion AmpliSeq™ Exome RDY kit). Individual samples were then ligated to specific Ion Xpress™ Barcode Adapters. Libraries were amplified and purified using AMPure™ XP reagent to remove unamplified libraries. The amplified libraries were quantified on Qubit™ 2.0 fluorometer instrument and diluted to ∼100 pM final concentration. Two barcoded exome libraries were combined to prepare templates and were loaded on a single Ion PI™ chip v2 (using Ion Chef), and the sequencing run was carried out on Ion Proton™ system.
WES data analysis
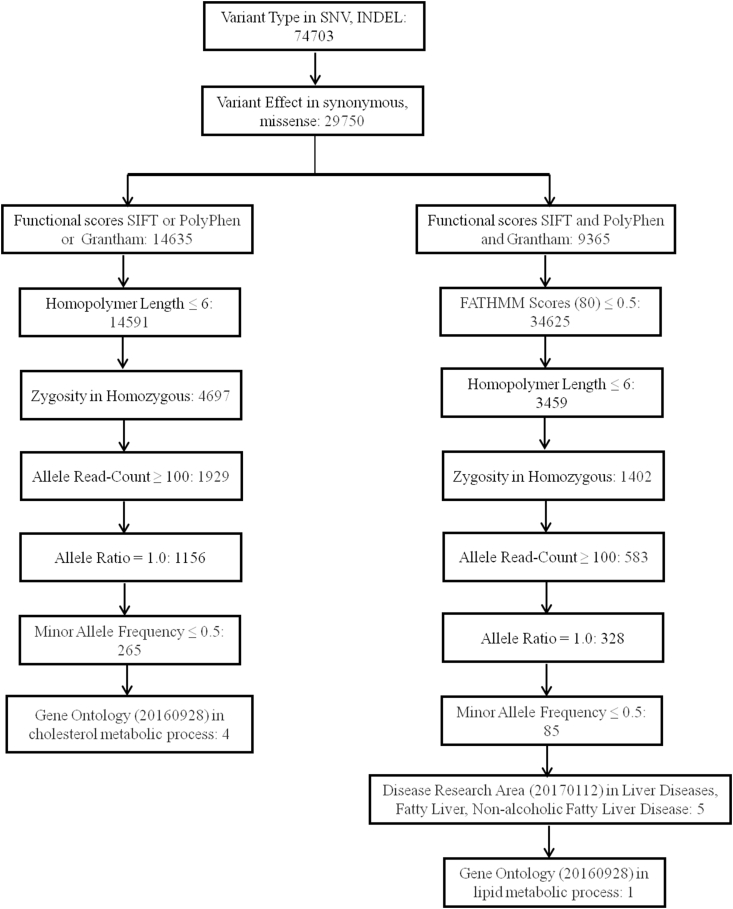
The generated AmpliSeq Exome files were aligned with human reference genome hg19, and coverage analysis was conducted using Ion torrent™ software. The AmpliSeq Exome files (.BAM files) were uploaded from Ion torrent server to cloud-based Ion Reporter™ software. The raw data were analyzed using Ion Reporter™ software (version 5.6) with AmpliSeq exome paired (control Vs patient) workflow. After the alignment of raw sequence data, the variant analysis annotation was carried out applying a series of pipelines; Variant Type filter-SNV, indel, variant effect-synonymous, missense, Functional scores-SIFT and/or PolyPhen and/or Grantham, Homopolymer Length ≤6, Zygosity-Homozygous, Allele Read-Count ≥ 100, Allele Ratio = 1.0, Minor Allele Frequency ≤0.5, Gene Ontology (20160928) - lipid metabolic process, cholesterol metabolic process filters were used and causal variants were identified.
Genotyping (validation set)
All the samples from the validation set were genotyped by Taqman single-nucleotide genotyping assay (Life Technologies, USA) on the Real-Time polymerase chain reaction (PCR) platform (Step one; Life technologies). PCR for genotyping consisted of 5 μl of 2X Taqman genotyping master mix, 0.5 μl of 1X assay mix (rs7946-PEMT, assay ID C_26319323_10; rs2290532-OSBPL10, assay ID C_9245965_10), and 4.5 μl consisting of 8–10 ng of DNA in a final volume of 10 μl. PCR was performed on Step One Real-Time PCR (Life technologies, USA) with the following cycling conditions: 95 °C for 10 min, 95 °C for 15 s, and 60 °C for 1 min with fluorescence read after each cycle for a total of 40 cycles. Genotyping calls were made using the allelic discrimination software (Life Technologies, USA), and only auto calls made by the software were considered for further analysis. A known heterozygous and homozygous variant sample was replicated across all the plates, and these known genotypes were verified manually during analysis in all the plates.
Definitions
An alcohol intake of ≤20 mg/day in females and ≤30 mg/day in males was considered as nonalcoholic as per the European Association for the Study of the Liver (EASL)19 and the American Association for the Study of the Liver (AASLD).20 According to Asian standards, individuals with BMI21 ≤22.9 kg/m2 were defined as normal and less than 23 kg/m2 were defined as lean,7, 21 and a cutoff of 1.2 m/s for ARFI22, 23 considered normal. Waist circumference (WC) cutoff of ≤ 90 cm in men and ≤ 80 cm in women24, 25; cutoff for liver enzymes26 (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) of ≤30 IU/L; and a value of <150 mg/dL for triglycerides, ≥ 40 mg/dL in males and 50 mg/dL in females for high-density lipoprotein (HDL), were considered normal.
Statistical analysis
Demographic data were collected and entered into MS Excel sheet for further analysis. Continuous variables were presented as mean ± standard deviation, and categorical variables were presented as percentages. Goodness of fit was used to confirm the agreement of the observed genotype frequencies with those of expected (Hardy–Weinberg equilibrium), χ2 test was used to analyze the statistical significance. Student's t test was performed between the two groups, and Chi-square and Fisher's exact tests were used to investigate the SNP associations between patients and controls. The relative risk and association of the studied SNPs with disease were expressed as odds ratio (95% confidence interval [CI]). In this study, a P value of ≤0.05 was considered statistically significant. Data were analyzed using GraphPad Prism software and Med cal C package.
Results
Discovery cohort characteristics
The discovery cohort consisted of lean controls and patients with a mean age of 46 ±1.4 and 45.7 ±6.5 years (P = 0.95) and mean BMI of 21.3 ±2.4 and 21.9 ±0.6 kg/m2 (P = 0.5), respectively. There were significant differences in the mean ARFI [0.86 ±0.04 Vs 1.47 ±0.12 (P = 0.005)], ALT [18.5 ±2.1 Vs 107 ±53 (P = 0.06)], and AST levels [21.5 ±7.8 Vs 99.5 ±39.8 (P = 0.04)] between the control and lean patient groups. The individual characteristics of the discovery cohort are as given in Table 1.
Table 1.
Demographic Data of the Discovery Cohort.
| S.No. | Subjects | Age (Yr) | Gender | BMI (kg/m2) | ARFI (m/s) | ALT (IU/L) | AST (IU/L) |
|---|---|---|---|---|---|---|---|
| 1 | Control-1 | 47 | Male | 22.9 | 0.88 | 20 | 27 |
| 2 | Control-2 | 45 | Male | 19.6 | 0.83 | 17 | 16 |
| 3 | Patients-1 | 41 | Male | 21.6 | 1.6 | 88 | 65 |
| 4 | Patients-2 | 44 | Male | 22.3 | 1.3 | 215 | 178 |
| 5 | Patients-3 | 55 | Male | 22.9 | 1.6 | 86 | 87 |
| 6 | Patients-4 | 38 | Female | 21.6 | 1.4 | 84 | 88 |
| 7 | Patients-5 | 52 | Male | 21.5 | 1.5 | 80 | 84 |
| 8 | Patients-6 | 44 | Male | 21.4 | 1.4 | 89 | 95 |
BMI: body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ARFI: acoustic radiation force impulse.
WES data
Average mapped read coverage in targeted regions in the six patients and two controls was 23–51 million, with 93–95% reads on target. The coverage mean depth was 55–136 with 77–78% uniformity, and amplicons coverage in targeted regions with at least 1x and 20x was 97–98.9% and 72–85%, respectively. Whole-exome variant analysis identified ∼74,000 variants in each of lean-NAFLD individuals and controls. Although the alignment of raw sequences to the reference genome hg19 revealed 74,703 variants, using a series of pipelines, we narrowed down these variants to 85–265 specific SNVs and InDels (Figure 2). Based on gene ontology (lipid metabolic process and cholesterol metabolic process), variants, namely rs7946-PEMT and rs2290532-OSBPL10, were selected for validation. The complete details for the variants are as given in Table 2.
Figure 2.
Pipelines used during analysis of whole-exome sequencing data.
Table 2.
Details of Variants Identified in the Whole-Exome Sequencing Analysis.
| Genes | PEMT | OSBPL10 |
|---|---|---|
| Locus | chr17:17409560 | chr3:31789582 |
| Genotype | T/T | C/C |
| Ref | C | T |
| Allelic coverage | C = 0, T = 139 | T = 0, C = 398 |
| Allelic ratio | C = 0.0, T = 1.0 | T = 0.0, C = 1.0 |
| MAF | 0.461 (ref) | 0.459 (ref) |
| Type | SNV | SNV |
| Strand | – | – |
| Exon | 6 | 5 |
| Transcript | NM_148172.2 | NM_017784.4 |
| Coding | c.634G > A | c.760A > G |
| Amino acid change | p.Val212Met | p.Asn254Asp |
| Variant effect | Missense | Missense |
| PHYLOP | 0.38 | 0.97 |
| SIFT | 0.06 | 0.79 |
| GRANTHAM | 21 | 23 |
| POLYPHEN | 0.945 | 0.011 |
| FATHMM | 0.05 | 0.89 |
| dbSNP | rs7946 | rs2290532 |
OSBPL10, oxysterol-binding protein-related protein10; PEMT, phosphatidylethanolamine N-methyltransferase; MAF, minor allele frequency; PHYLOP, phylogenetic p-value; SIFT, sorting intolerant from tolerant; GRANTHAM, Grantham; POLYPHEN, polymorphism phenotyping; FATHMM, functional analysis through hidden Markov models.
Replication of discovery data in the validation cohort
The variants identified in the discovery cohort were genotyped in a validation cohort comprising 296 individuals (controls, n = 105 and patients with NAFLD, n = 191). The mean age of the control and patients was 35.1 ±11.6 and 35.5 ±8.2 years, respectively, with predominantly males. There were significant differences in the clinical characteristics between patients and controls, as shown in Table 3. There was no significant difference in the genotype frequencies of the studied variants between total patients and controls, as shown in Table 4.
Table 3.
Demographic and Clinical Data of the Complete Patient and Control Group.
| NAFLD characteristics | Controls (mean ± SD) (n = 105) | Patients (mean ± SD) (n = 191) |
|---|---|---|
| Age (yr) | 35.1 ± 11.6 | 35.5 ± 8.2 |
| Gender, male/female (n) | 98/7 | 182/9 |
| BMI (kg/m2) | 21.9 ± 3.5 | 26.3 ± 4.2** |
| Waist circumference (cm) | 81.5 ± 9.4 | 94.3 ± 10.3** |
| Hip circumference (cm) | 89.8 ± 6.5 | 96.8 ± 9.1** |
| Waist/hip ratio | 0.9 ± 0.06 | 0.98 ± 0.13** |
| AST (IU/L) | 22.8 ± 5.4 | 70.6 ± 37.3** |
| ALT (IU/L) | 21.8 ± 8.8 | 117.2 ± 64.3** |
| Triglycerides (mg/dL) | 123.8 ± 55.2 | 182.7 ± 94.1* |
| HDL (mg/dL) | 35.1 ± 6.8 | 39 ± 12.6 |
BMI: body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TG: triglycerides; HDL: high-density lipoprotein; SD: standard deviation.
**P < 0.001; *P = 0.002.
Table 4.
Association Analysis for the Variants in NAFLD Group.
| PEMT (rs7946) | Wild type (CC) | Heterozygous (CT) | Mutant (TT) | χ2 | *P-Value |
|---|---|---|---|---|---|
| Controls (n) | 32 (30.5%) | 49 (46.7%) | 24 (22.8%) | 0.21 | 0.89 |
| Patients (n) | 55 (28.8%) | 88 (46.0%) | 48 (25.2%) | ||
| OSBPL (rs2290532) | Wild type (TT) | Heterozygous (CT) | Mutant (CC) | ||
| Controls (n) | 24 (26.7%) | 37 (41.1%) | 29 (32.2%) | 1.36 | 0.5 |
| Patients (n) | 44 (25.0%) | 85 (48.3%) | 47 (26.7%) |
*χ2 test was used.
NAFLD, nonalcoholic fatty liver disease; PEMT, phosphatidylethanolamine N-methyltransferase.
Association of a variant in PEMT with lean-NAFLD
The mean age of lean controls (n = 61) and patients with lean-NAFLD (n = 33) was 32.4 ±10.9 and 35.4 ±8.0 years, respectively, with predominantly males. There were significant differences in the clinical characteristics between the groups, as shown in Table 5. Although there was no significant difference in the genotype frequency of the variant in OSBPL10 gene between lean patients and controls, there was a significant difference (p = 0.04) in the genotype frequency of the variant in PEMT gene, conferring a three-fold higher risk of the disease (95% CI, 0.9–9.9), as shown in Table 6.
Table 5.
Demographic Data of Lean-NAFLD and Lean Control Groups.
| Lean-NAFLD characteristics | Controls (mean ± SD) (n = 61) |
Patients (mean ± SD) (n = 33) |
|---|---|---|
| Age (yr) | 32.4 ± 10.9 | 35.4 ± 8.0 |
| Gender, male/female (n) | 60/1 | 35/0 |
| BMI (kg/m2) | 19.7 ± 2.2 | 21.2 ± 1.5** |
| Waist circumference (cm) | 77.2 ± 7.2 | 91.2 ± 8.7** |
| Hip circumference (cm) | 88.2 ± 5.5 | 88.8 ± 5.2 |
| Waist/hip ratio | 0.88 ± 0.06 | 1.03 ± 0.1** |
| AST (IU/L) | 22.6 ± 4.8 | 85.2 ± 52.5** |
| ALT (IU/L) | 20.3 ± 7.5 | 142.8 ± 71.1** |
| Triglycerides (mg/dL) | 119.5 ± 61.2 | 142.8 ± 81.4 |
| HDL (mg/dL) | 34.2 ± 6.8 | 37.5 ± 6.5 |
BMI: body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TG: triglycerides; HDL: high-density lipoprotein; SD: standard deviation.
**P < 0.001.
Table 6.
Association Analysis for the Variants in Lean-NAFLD Group.
| PEMT (rs7946) | Wild type (CC) | Heterozygous (CT) | Mutant (TT) | aP-Value | Odds ratio | 95% CI |
|---|---|---|---|---|---|---|
| Controls (n) | 18 (29.5%) | 27 (44.3%) | 16 (26.2%) | 0.04 | 3.03 | 0.9–9.9 |
| Patients (n) | 4 (12.1%) | 16 (48.5%) | 13 (39.4%) | |||
| OSBPL (rs2290532) | Wild type (TT) | Heterozygous (CT) | Mutant (CC) | 0.12 | 1.46 | 0.4–5.0 |
| Controls (n) | 14 (24.6%) | 25 (43.9%) | 18 (31.6%) | |||
| Patients (n) | 4 (12.5%) | 16 (50.0%) | 12 (37.5%) |
NAFLD, nonalcoholic fatty liver disease; PEMT, phosphatidylethanolamine N-methyltransferase; CI, confidence interval.
Fisher's exact test.
Discussion
Lean-NAFLD represents a distinct subset of NAFLD, with an evidence of hepatic steatosis on imaging but with normal BMI. It has a unique phenotype and may carry a poor prognosis21, 27 compared with obese NAFLD, and therefore, individuals at risk need to be identified aggressively. Genetic variants might play a key role in conferring susceptibility to lean-NAFLD,28, 29 and therefore, an unbiased identification of causal variant(s) is warranted. We sequenced the complete exonic regions of patients with fatty infiltration with a normal BMI (lean-NAFLD) and subsequently identified and validated two variants (rs7946-PEMT and rs2290532-OSBPL10) related to cholesterol handling in both patients with lean-NAFLD and general NAFLD. We report the association of the variant in PEMT, but not that of OSBPL10 gene, with lean-NAFLD.
As is expected, there were significant differences in BMI; WC; waist–hip ratio (W/H); and triglyceride, ALT, and AST levels between patients with NAFLD and controls, with higher values in the patient group. Interestingly, although the BMI was within the normal range in both patients with lean-NAFLD and controls, significantly higher WC and W/H were seen in patients with lean-NAFLD than in lean controls. Furthermore, significantly elevated ALT and AST levels were also noted. This reiterates data from an earlier study in which the risk in lean-NAFLD was attributed primarily to alterations in body fat distribution as measured by WC or W/H.30, 25, 9 It was further suggested that the accumulation of visceral adipose tissue triggered ectopic fat infiltration, leading to hepatocyte damage, and was closely related to the progression of NASH31, 32 cirrhosis and finally to HCC. Furthermore, this unique phenotype (lean-NAFLD) may be associated with causal variants, and therefore, WES in this cohort is justified; to the best of our knowledge, this is the first study that performed WES in NAFLD in Indian population.
WES is known to generate abundant volumes of data, and in the process, it presents the challenge of determining variants of importance and those that need subsequent validation.33 Although there are no standard and accepted ways to analyze the data,34 variants with low-quality calls and coverage score are generally excluded from further analysis. Variants with good-quality Phil's Read Editor scores and read depth have a positive effect on the overall performance of variant calling pipelines35 and therefore are generally included for better overall success of identifying causative variants. In the present study, acceptable base coverage, mean depth, and uniformity were maintained. It is a herculean task in narrowing the variants from an initial large number, and the usual accepted approach is to sort the variants based on variant type, effect, and coverage.36, 37
By using pipelines as suggested previously to sort variants generated in the discovery set, we identified two variants, namely rs7946-PEMT and rs2290532-OSBPL10. Both these variants in patients with lean-NAFLD were found to be homozygous. Furthermore, the variants were classified as missense and deleterious and were known to be involved in lipid and cholesterol metabolic processes. Based on the importance of these variants in the context of NAFLD including lean, they were selected for further validation. Patients with NAFLD (with higher BMI) were also included to assess the association of these variants with NAFLD. Replication of these variants in the validation cohort established the association of the variant in PEMT, but not OSBPL10, with lean-NAFLD. Although the lack of association of the variant in PEMT gene with obese NAFLD is intriguing, the possibility of other gene–gene and gene–environmental interactions in precipitating the phenotype need to be explored.
PEMT is an enzyme that catalyzes de novo synthesis of choline via the triple methylation of phosphatidylethanolamine (PE) to form phosphatidylcholine (PC)38; choline governs the secretion of hepatic triglycerides in the form of very-low-density lipoprotein and has been implicated in NAFLD.39 The PEMT rs7946 was found to confer an enhanced risk (1.27- and 3.76-fold, respectively) of NAFLD and NASH. Decrease in the activity of PEMT reduces the availability of PC, leading to the accumulation of fat droplets in the cytosol of hepatocytes.40 Although its activity is comparatively lower in the adipose tissue, the conversion of PE to PC appears to be important for the stabilization of lipid droplets and normal fat distribution.41 In the present study, we found that the variant rs7946 in PEMT gene was associated with conferring a ∼threefold higher risk of lean-NAFLD; however, there was no enhanced risk for NAFLD. An earlier study suggested that the variant rs7946-PEMT results in a V175M substitution in the encoded protein that occurs more frequently in patients with NAFLD42 and lean-NASH.43 Although it is unclear on the role of the variant only in lean-NAFLD but not NAFLD, further studies are warranted to elucidate its precise contribution.
OSBPL10 plays a role in regulation of cholesterol homeostasis, and Kandutsch et al.45 proposed that the oxysterol mediates feedback regulation of cholesterol biosynthesis rather than cholesterol regulating itself.44, 45 In the present study, we could not replicate the association of the variant rs2290532 in OSBPL10 genes with either of the disease phenotypes (lean and NAFLD).
In conclusion, WES is an effective strategy to identify potentially causative missense variants underlying lean and NAFLD. Identifying causative variants in lean-NAFLD particularly related to the cholesterol mechanism may be important to identify not only at-risk individuals for lean-NAFLD but also NAFLD in the general population.
Conflicts of interest
The authors have none to declare.
Acknowledgments
The authors acknowledge all the participants for their consent to participate in the study. The authors acknowledge the technical help rendered by Ms. Urmila Steffie Avanthi and Mr. E. Suthagar in generating whole-exome sequencing data.
References
- 1.Farrell G.C., Larter C.Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:99–112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 2.Kleiner D.E., Brunt E.M., Van Natta M. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 4.Duseja A. Nonalcoholic fatty liver disease in India: a lot done, yet more required! Indian J Gastroenterol. 2010;29:217–225. doi: 10.1007/s12664-010-0069-1. [DOI] [PubMed] [Google Scholar]
- 5.Schwimmer J.B., Celedon M.A., Lavine J.E. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinstein S.E., Rein M., Abdelmalek M.F. Whole-exome sequencing study of extreme phenotypes of NAFLD. Hepatology Communications. 2018;2:1021–1029. doi: 10.1002/hep4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhat G., Baba C.S., Pandey A., Kumari N., Choudhuri G. Insulin resistance and metabolic syndrome in nonobese Indian patients with non-alcoholic fatty liver disease. Trop Gastroenterol. 2013;34:18–24. doi: 10.7869/tg.2012.86. [DOI] [PubMed] [Google Scholar]
- 8.Kanth V.V., Sasikala M., Padaki Nagaraja Rao U.S., Rao K.R., Reddy D.N. Pooled genetic analysis in ultrasound measured non-alcoholic fatty liver disease in Indian subjects: a pilot study. World J Hepatol. 2014;6:435. doi: 10.4254/wjh.v6.i6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishioji K., Sumida Y., Kamaguchi M. Prevalence of and risk factors for non-alcoholic fatty liver disease in a non-obese Japanese population, 2011–2012. J Gastroenterol. 2015;50:95–108. doi: 10.1007/s00535-014-0948-9. [DOI] [PubMed] [Google Scholar]
- 10.Feng R.N., Du S.S., Wang C. Lean-nonalcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World J Gastroenterol. 2014;20:17932–17940. doi: 10.3748/wjg.v20.i47.17932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margariti E., Deutsch M., Manolakopoulos S., Papatheodoridis G.V. Non-alcoholic fatty liver disease may develop in individuals with normal body mass index. Ann Gastroenterol. 2012;25:45–51. [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H.J., Kim H.J., Lee K.E. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169–2175. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 13.Younossi Z.M., Stepanova M., Negro F. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltim) 2012;91:319–327. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 14.Vos B., Moreno C., Nagy N. Lean non-alcoholic fatty liver disease (Lean-NAFLD): a major cause of cryptogenic liver disease. Acta Gastroenterol Belg. 2011;74:389–394. [PubMed] [Google Scholar]
- 15.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 16.Argo C.K., Henry Z.H. “Lean” NAFLD: metabolic obesity with normal BMI… Is it in the genes? Am J Gastroenterol. 2017;112:111. doi: 10.1038/ajg.2016.527. [DOI] [PubMed] [Google Scholar]
- 17.Palmer N.D., Musani S.K., Yerges-Armstrong L.M. Characterization of European ancestry nonalcoholic fatty liver disease-associated variants in individuals of African and Hispanic descent. Hepatology. 2013;58:966–975. doi: 10.1002/hep.26440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marra F., Gastaldelli A., Baroni G.S., Tell G., Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;29:72–81. doi: 10.1016/j.molmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 19.European Association for the Study of Liver EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 20.O'Shea R.S., Dasarathy S., McCullough A.J. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 21.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 22.Sporea I., Bota S., Peck-Radosavljevic M. Acoustic Radiation Force Impulse elastography for fibrosis evaluation in patients with chronic hepatitis C: an international multicenter study. Eur J Radiol. 2012;81:4112–4118. doi: 10.1016/j.ejrad.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Raghuwanshi B., Jain N., Jain M. Normal values in healthy liver in central India by acoustic radiation force impulse imaging. J Clin Diagn Res. 2013;7:2498. doi: 10.7860/JCDR/2013/7479.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waist Circumference and Waist-Hip Ratio. Report of a WHO Expert Consultation; Geneva: 2008. pp. 8–11. [Google Scholar]
- 25.Misra A., Vikram N.K., Gupta R., Pandey R.M., Wasir J.S., Gupta V.P. Waist circumference cutoff points and action levels for Asian Indians for identification of abdominal obesity. Int J Obes. 2006;30:106. doi: 10.1038/sj.ijo.0803111. [DOI] [PubMed] [Google Scholar]
- 26.Kang H.S., Um S.H., Seo Y.S. Healthy range for serum ALT and the clinical significance of “unhealthy” normal ALT levels in the Korean population. J Gastroenterol Hepatol. 2011;26:292–299. doi: 10.1111/j.1440-1746.2010.06481.x. [DOI] [PubMed] [Google Scholar]
- 27.Feldman A., Eder S.K., Felder T.K. Clinical and metabolic characterization of lean Caucasian subjects with non-alcoholic fatty liver. Am J Gastroenterol. 2017;112:102. doi: 10.1038/ajg.2016.318. [DOI] [PubMed] [Google Scholar]
- 28.Kumar R., Rastogi A., Sharma M.K. Clinicopathological characteristics and metabolic profiles of non-alcoholic fatty liver disease in Indian patients with normal body mass index: do they differ from obese or overweight non-alcoholic fatty liver disease? Indian journal of endocrinology and metabolism. 2013;17:665. doi: 10.4103/2230-8210.113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wattacheril J., Sanyal A.J. Lean NAFLD: an underrecognized outlier. Current hepatology reports. 2016;15:134–139. doi: 10.1007/s11901-016-0302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C., Yu C., Ma H., Xu L., Miao M., Li Y. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai Study. Am J Gastroenterol. 2013;108:1299–1304. doi: 10.1038/ajg.2013.104. [DOI] [PubMed] [Google Scholar]
- 31.Eguchi Y., Mizuta T., Sumida Y. The pathological role of visceral fat accumulation in steatosis, inflammation, and progression of nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:70–78. doi: 10.1007/s00535-010-0340-3. [DOI] [PubMed] [Google Scholar]
- 32.Petta S., Amato M.C., Di Marco V. Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;35:238–247. doi: 10.1111/j.1365-2036.2011.04929.x. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen R., Paul J.S., Albrechtsen A., Song Y.S. Genotype and SNP calling from next-generation sequencing data. Nat Rev Genet. 2011;12:443–451. doi: 10.1038/nrg2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nekrutenko A., Taylor J. Next-generation sequencing data interpretation: enhancing reproducibility and accessibility. Nat Rev Genet. 2012;13:667–672. doi: 10.1038/nrg3305. [DOI] [PubMed] [Google Scholar]
- 35.Girard S.L., Gauthier J., Noreau A. Increased exonic de novo mutation rate in individuals with schizophrenia. Nat Genet. 2011;43:860–863. doi: 10.1038/ng.886. [DOI] [PubMed] [Google Scholar]
- 36.Xu B., Roos J.L., Dexheimer P. Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nat Genet. 2011;43:864. doi: 10.1038/ng.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Roak B.J., Vives L., Fu W. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridgway N.D., Vance D.E. Kinetic mechanism of phosphatidylethanolamine N-methyltransferase. J Biol Chem. 1988;263:16864–16871. [PubMed] [Google Scholar]
- 39.Musso G., Gambino R., Cassader M. Recent insights into hepatic lipidmetabolism in non-alcoholic fatty liver disease (NAFLD) Prog Lipid Res. 2009;48:1–26. doi: 10.1016/j.plipres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Watkins S.M., Zhu X., Zeisel S.H. Phosphatidylethanolamine-Nmethyltransferase activity and dietary choline regulate liver-plasma lipid flux and essential fatty acid metabolism in mice. J Nutr. 2003;133:3386–3391. doi: 10.1093/jn/133.11.3386. [DOI] [PubMed] [Google Scholar]
- 41.Hörl G., Wagner A., Cole L.K. Sequential synthesis and methylation of phosphatidylethanolamine promote lipid droplet biosynthesis and stability in tissue culture and in vivo. J Biol Chem. 2011;286:17338–17350. doi: 10.1074/jbc.M111.234534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song J., Da Costa K.A., Fischer L.M. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD) FASEB J. 2005;19:1266–1271. doi: 10.1096/fj.04-3580com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakatsuka A., Matsuyama M., Yamaguchi S. Insufficiency of phosphatidylethanolamine N-methyltransferase is risk for lean non-alcoholic steatohepatitis. Sci Rep. 2015;6:21721. doi: 10.1038/srep21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gill S., Chow R., Brown A.J. Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog Lipid Res. 2008;47:391–404. doi: 10.1016/j.plipres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Kandutsch A.A., Chen H.W., Heiniger H.J. Biological activity of some oxygenated sterols. Science. 1978;201:498–501. doi: 10.1126/science.663671. [DOI] [PubMed] [Google Scholar]