Highlights
-
•
C1/C2 hypomobility is an important finding in women with migraine and is related to migraine chronicity.
-
•
Mobility of the C1/C2 segment is influenced by neck pain related-disability.
-
•
Chronic migraine patients have a reduced global cervical range of motion.
Keywords: Headache, Migraine disorders, Joint range of motion, Neck
Abstract
Objective
To compare flexion rotation test and global active cervical mobility in women with chronic migraine, episodic migraine, and headache-free controls. The influence of neck pain-related disability on the flexion rotation test was also analyzed.
Methods
Women with chronic migraine (n = 25), episodic migraine (n = 30), and those who were headache-free (n = 30) were evaluated. Upper cervical mobility was measured using the flexion rotation test and global active mobility was assessed using the cervical range of motion device. Neck pain related-disability was assessed using the Neck Disability Index. Statistical analyses were performed using a MANOVA test, prevalence ratios, and linear regression.
Results
Chronic (right, MD: −15°; 95%CI: −21° to −11°; left, MD: −13°; 95%CI: −20° to −12°) and episodic (right, MD: −8°; 95%CI: −13° to −4°; left, MD: −8°; 95%CI: −12° to −5°) migraine groups achieved lower flexion rotation test mobility bilaterally than headache-free women. Only chronic migraine was associated with a lower global cervical range of motion compared to that of headache-free women during flexion, (MD: −8°; 95%CI: −15° to −1°), extension (MD: −13°; 95%CI: −20° to −4°), right lateral flexion (MD: −4°; 95%CI: −9° to −0.2°), left lateral flexion (MD: −6°; 95%CI: −10° to −2°), right rotation (MD: −9°; 95%CI: −15° to −4°), and left rotation (MD: −8°; 95%CI: −13° to −2°). Migraine was associated with a 2.85-fold increase in the risk of a positive flexion rotation test. Flexion Rotation Test was influenced by disability-related neck pain (R2 = 19.1; p = 0.001).
Conclusion
Women with migraine have a lower upper cervical range of motion than headache-free women. Women with chronic migraine demonstrated reduced global cervical range of motion when compared to headache-free women. Migraine was associated with in increased likelihood of a positive Flexion Rotation Test. Reduction in mobility was influenced by migraine frequency and disability-related neck pain.
Introduction
Migraine is a primary headache disorder and is considered the second most important cause of disability in the world.1 This condition is associated with increased excitability of the central nervous system and dysregulation of central pain control and is characterized by activation of trigeminovascular pain pathways.2, 3 Although the pathophysiology of migraine is not directly related to the presence of musculoskeletal disorders, patients often report neck pain.4, 5, 6 The association between migraine and neck pain is believed to be a consequence of sensitization of the trigeminocervical nucleus (TCN), since this nucleus is sensitized in migraine pathology and receives inputs from the upper cervical nerve roots.2
It is possible that, once the TCN is sensitized, dysfunction of the upper cervical segment of the nerve could induce pain at the dermatomes.7 The presence of neck pain is an important factor related to moderate-to-severe migraine phenotypes, which predict worse migraine-related disability.8 In addition, individuals with chronic migraine (CM) are more likely to exhibit cervical impairment and joint hypomobility leading to a significant risk of neck disability than individuals with episodic migraine (EM).5, 8, 9, 10, 11
Although previous studies have investigated the global cervical range of motion (ROM) in individuals with migraine,9, 12, 13 few of them have addressed dysfunction, pain, or mobility of the upper cervical segments (C1/C2). Furthermore, the results of these studies are contradictory.13, 14, 15, 16, 17, 18 Biomechanically, the C1/C2 segment is responsible for nearly 40–60% of cervical mobility, in particular movements involving rotation.19 The flexion rotation test (FRT)15, 20, 21, 22 is used to identify rotational impairment of the C1/C2 segment and previous studies have demonstrated that this test has diagnostic validity for clinical differentiation of cervicogenic headaches.15, 18, 20 However, impairment of the C1/C2 segment is not considered as a diagnostic criterion for cervicogenic headache according to the International Headache Classification (3rd edition).23 Only one study reported an increased prevalence of positive FRT mobility in individuals with migraine compared to that in controls,17 while three did not report any difference in prevalence.15, 18, 24
Identifying the characteristics of neck dysfunction in individuals with migraine and any association with worse clinical presentation would be helpful, highlighting issues that physical therapists may encounter in the management of these individuals.
The aims of the current study were: (1) to investigate the differences in FRT and global active cervical mobility between women with chronic migraine (CM) or EM and headache-free individuals and (2) to analyze the influence of neck pain-related disability on the FRT mobility. Our hypothesis was that individuals with migraine would present with a reduced cervical ROM and lower C1/C2 mobility, which could be verified using the FRT. We believed that this reduction would be more pronounced in individuals with CM.
Methods
Patient characteristics
From September 2014 to March 2015, volunteers from a university-based headache center were screened according to specific eligibility criteria. Migraine disorder was diagnosed by an experienced neurologist using the diagnostic criteria set out in the 3rd edition of the International Classification of Headache Disorders (ICHD-III).23 In order to be included, individuals had to experience recurrent headaches lasting 4–72 h, with unilateral pulsating pain of moderate to severe intensity (pain classified as >4 in the numerical pain rating scale [NPRS]), aggravated by routine physical activity and associated with nausea and/or photophobia and phonophobia. The ICHD-III criteria were used to classify migraines as episodic or chronic. The presence of a headache for more than 15 days in a month during the last 3 months, with at least 8 migraineurs episodes was defined as CM. The presence of a migraines headache for less than 15 days per month was defined as EM.23
Individuals were excluded according to any of the following criteria: (1) diagnosis of any primary and/or secondary headache other than migraine, including medication overuse headache; (2) a report of migraine during the assessment; (3) a history of neck/head trauma (e.g. whiplash); (4) pregnancy; (5) a history of herniated cervical disk or cervical osteoarthritis; (6) any systemic disease, such as rheumatoid arthritis or lupus erythematosus; (7) an associated diagnosis of fibromyalgia syndrome; or (8) an anesthetic block performed in the last 3 months. A careful clinical examination of each individual was conducted to determine eligibility according to the inclusion and exclusion criteria.
Headache-free individuals were recruited from the general community and we included women with no history of headaches in the past 6 months and those with an occasional headache who did not experience recurrence or require treatment. The exclusion criteria for the headache-free group were the same as those for the migraine group. This study was approved by the Committee of Research Ethics of the Hospital das Clínicas of the Ribeirão Preto Medical School (process number 12521/2014), Ribeirão Preto, São Paulo, Brazil, and all participants provided informed consent prior to inclusion in the study.
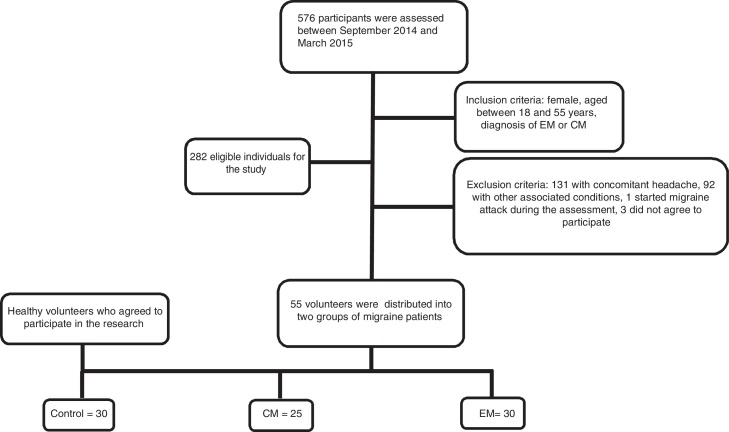
The final sample consisted of 25 women with CM, 30 women with EM, and 30 healthy women in the control group. Fig. 1 shows a flow diagram of the patient recruitment process.
Figure 1.
Sample screening flowchart. EM, episodic migraine; CM, chronic migraine.
Data collection
Clinical history was obtained for each individual prior to physical evaluation. Data collected included age, body mass index, and medical comorbidities. The clinical characteristics of the frequency of migraines, age at onset (years), whether a headache was present during data collection and the intensity of pain (NPRS ranging from 0 [no pain] to 10 [maximum pain]) were also recorded.
Volunteers were asked about neck pain and instructed to fill out the Neck Disability Index (NDI). This self-reported questionnaire consists of 10 items designed to assess influence of neck pain on daily activities. The NDI score ranges from 0 to 50 points, with the level of neck disability classified as none (0–4), mild (5–14), moderate (15–24), severe (25–34), or completely disabling (≥35).25, 26
Global active cervical ROM was assessed using a cervical range of motion (CROM®) instrument.27, 28 Volunteers were instructed to remain seated in a relaxed position with the back supported on a chair, both feet on the ground, with ankles, knees, and hips at 90°, resting the upper extremities on the thighs.9 The CROM® instrument was positioned on the individual's head and a familiarization trial was performed. The CROM® measurement instrument has demonstrated excellent intra-rater reliability (ICC > 0.79) and moderate to excellent inter-rater (ICC > 0.68) reliability in individuals with migraine.9
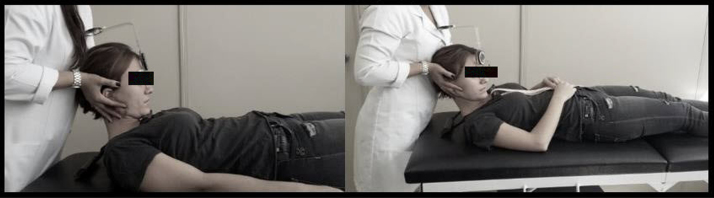
For the FRT, participants assumed a supine position with the CROM® instrument placed on the head (Fig. 2). The evaluator performed maximum flexion of the cervical spine followed by a rotation to each side,15, 20, 21 based on the criteria described by Hall et al.20 The limit of rotation was determined when the evaluator perceived resistance or when the patient reported pain in the upper cervical area during the movement. A test was reported as positive when individuals demonstrated a reduction of movement of 10° compared to the normal range of motion (<34°).20
Figure 2.
The flexion rotation test performed using the CROM® device.
Global active cervical motion during flexion, extension, lateral flexion, rotation, and right and left FRT were randomly assessed. Each measurement was repeated 3 times at 30 s intervals. The mean value obtained from the 3 trials was used for data analysis. An experienced examiner who was blinded to the individual's conditions assessed all outcomes.
Statistical analysis
The sample size was calculated based on criteria provided by Singer,29 with power set at 90% and α = 0.05, based on a pilot study including 30 women (10 in each group). The average of FRT in the EM group was 27.38° (SD: 6.87°) to the right and 34.18° (SD: 6.9°) to the left; in the CM group it was 34.36° (SD: 8.14°) to the right and 27.47° (SD: 8.23°) to the left; and in the headache-free women it was 46.76° (SD: 8.32°) to the right and 44° (SD: 5.17°) to the left. The sample size calculation was based on a difference of 8° in the FRT between the three groups and resulted in a minimum of 12 participants for each group.
Statistical analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Sample characteristics were compared using one-way analysis of variance, independent Student's t-test and the chi-square test. Between-group comparisons of global active cervical ROM and FRT were conducted using multi-analysis of variance (MANOVA), with Bonferroni post hoc correction. The prevalence ratio was calculated based on the frequency of positive FRT (with a cut-off of <34°) in the CM, EM, and headache-free groups. Additionally, linear regression models were applied to identify the influence of neck pain-related disability (>4 points on the NDI) on the FRT score, based on the results from the entire sample (n = 85).
Clinical relevance was determined for statistically significant results according to the minimal important difference (MID) and the effect size (ES).30, 31 A clinically relevant difference was identified when between-group differences were greater than the MID and the ES was >0.4.
Results
Table 1 summarizes the characteristics of the three groups. Women with CM exhibited an increased frequency (p < 0.05) and longer duration of symptoms (p = 0.05), but experienced similar migraine onset as compared to those with EM. Both EM and CM groups reported a higher prevalence (χ2 = 34.6, p < 0.05) and longer history of neck pain symptoms than the controls (p < 0.05). In addition, the intensity of neck pain and neck pain-related disability was greater in the CM group than in the EM (p = 0.05) and control (p < 0.05) groups.
Table 1.
Characteristics of participants.
| Chronic migraine (n = 25) | Episodic migraine (n = 30) | Headache-free (n = 30) | |
|---|---|---|---|
| Mean (95%CI) | Mean (95%CI) | Mean (95%CI) | |
| Age | 35.1 (31.8; 38.9) | 35.4 (30.7; 39.4) | 32 (27.8; 36.2) |
| Body mass index (kg/cm2) | 23.8 (22.4; 25.2) | 24.2 (22.4; 26.0) | 23.8 (22.3; 25.3) |
| Frequency (days/month)* | 22.8 (20.2; 25.4) | 7.9 (6.5; 9.2) | – |
| Duration of attack (h)* | 16.8 (13.4; 20.2) | 11.7 (8.4; 15.0) | – |
| History of migraine (years) | 19.4 (15.1; 23.8) | 15.1 (10.4; 19.8) | – |
| Headache intensity (NPRS) | 8.2 (7.6; 8.9) | 8.3 (7.7; 8.8) | – |
| Neck Pain (n, %)# | 23 (92%) | 21 (70%) | 5 (16%) |
| Neck pain intensity (NPRS)#,* | 6.1 (5.0; 7.2) | 4.3 (3.1; 5.6) | 0.7 (0.2; 1.3) |
| History of neck pain (years)# | 5.8 (2.7; 8.9) | 5.2 (1.5; 8.8) | 0.3 (−0.1; 0.7) |
| Neck Disability Index (NDI)#,* | 14.2 (11.1; 17.2) | 8.5 (5.6; 10.5) | 1.97 (0.7; 3.2) |
Abbreviations: 95%CI, 95% confidence interval; NDI, neck disability index; NPRS, numerical pain rating scale.
Differences between chronic and episodic migraine (p < 0.05);
Differences between both migraine groups and headache-free (p < 0.05).
MANOVA revealed that women with CM exhibited a decreased ROM in the FRT compared to those with EM and these two groups exhibited bilaterally reduced FRT ROM compared to those of headache-free individuals (p < 0.05) (Table 2).
Table 2.
Scores (degrees) obtained for the flexion rotation test (FRT) and the global active cervical range of motion for individuals with chronic migraine, episodic migraine, and headache-free women.
| Chronic Migraine (n = 25) | Episodic Migraine (n = 30) | Headache-free (n = 30) | Mean differences (95%CI). Effects Sizes |
|||
|---|---|---|---|---|---|---|
| Mean (95%CI) | Mean (95%CI) | Mean (95%CI) | CM × EM | CM × HF | EM × HF | |
| Right FRT | 26 (23; 28)*,a,#,b | 33 (31; 36)*,a | 41 (39; 45) | −7° (−13°; −3°) 1.13 | −15° (−21°; −11°) 2.08 | −8° (−13°; −4°) 1.09 |
| Left FRT | 27 (24; 30)*,a,#,b | 32 (30; 34)*,a | 40 (38; 43) | −5° (−10°; −1.5°) 0.87 | −13° (−20°; −12°) 1.89 | −8° (−12; −5°) 1.32 |
| Flexion | 50 (45; 55)*,a | 54 (50; 57) | 58 (55; 62) | −4° (−9°; 4°) 0.31 | −8° (−15°; −1°) 0.79 | −4° (−13°; 5°) 0.50 |
| Extension | 59 (54; 64)*,a | 65 (60; 70) | 72 (68; 7) | −6° (−14°; 2°) 0.45 | −13° (−20°; −4°) 1.15 | −7° (−15°; −3°) 0.54 |
| Right Lateral flexion | 33 (30; 36)*,a | 35 (33; 37) | 37 (34; 39) | −2° (−6°; 2°) 0.42 | −4° (−9°; −0.2°) 0.62 | −2° (−6°; 4°) 0.30 |
| Left Lateral flexion | 33(30; 36)*,a | 36 (34; 38) | 39 (36; 41) | −3° (−7; 1°) 0.57 | −6° (−10°; −2°) 0.86 | −3° (−4°; 6°) 0.38 |
| Right Rotation | 58 (54; 61)*,a | 61 (58; 64)*,a | 67 (64; 70) | −3° (−7°; 3°) 0.37 | −9° (−15°; −4°) 1.15 | −5° (−9°; −6°) 0.86 |
| Left Rotation | 54 (50; 59)*,a | 58 (55; 62) | 62 (58; 65) | −4° (−9°; 3°) 0.41 | −8° (−13°; −2°) 0.72 | −6° (−9°; 5°) 0.35 |
Abbreviations: 95%CI, confidence interval of 95%; CM, chronic migraine group; EM, episodic migraine group; HF, headache-free group.
p < 0.05 compared to headache-free group;
p < 0.05 compared to episodic migraine.
Clinically relevant factors determined using the headache-free group as a reference. Chronic migraine MIDs: FRT = 6.9°, flexion = 5.2°; extension = 5.4°; lateral-flexion = 6.5°, rotation = 8.6°. Episodic migraine MIDs: FRT = 6.01°, flexion = 4.8°, extension = 6.1°, lateral-flexion = 6.6°, rotation = 7.6°.
Clinically relevant factors when comparing chronic and episodic migraine. MIDs: FRT = 5.4°, flexion = 5.6°; extension = 6.5°; lateral-flexion = 5.9°, rotation = 8.5°.
A total of 23 individuals in the CM and 24 from the EM group had positive FRT, while only 9 individuals had a positive FRT in the headache-free group. Among these, only 3 volunteers from the CM group, 4 from the EM group and 3 from the headache-free group presented unilateral restriction in the FRT. Among the individuals with a positive FRT, the prevalence of pain as a reason for interruption of the test was higher in the CM group (n = 17, 68%) and in the EM group (n = 18, 60%) compared to the headache-free group (n = 2, 6.7%) (χ2: 30.91; p < 0.001).
Reduced global active cervical ROM was observed in the CM group for all directions when compared to headache-free controls (p < 0.05), except during right lateral flexion. The differences were considered clinically relevant (ES > 0.62) (Table 2).
A post hoc analysis also revealed that women with EM had reduced right cervical rotation (p < 0.05), but no reduction in other cervical movements compared to headache-free individuals. These differences were clinically relevant (ES > 0.86). The global active cervical ROM was similar between the CM and EM groups (Table 2).
Positive FRT were more prevalent in both migraine groups than in the headache-free group (χ2 = 31.58; p < 0.001) and were more prevalent in the CM group than in the EM group (χ2 = 25.55; p < 0.001). The presence of migraine, independent of the frequency of attacks, was associated with a prevalence ratio (PR) of 2.85 (95%CI: 1.63–4.98) for risk of hypomobility on the FRT, with the headache-free group used as a reference (p < 0.05). Women with CM had a PR of 3.07 (95%CI: 1.75–5.36), whereas those with EM had a PR of 2.67 (95%CI: 1.50–4.74). When the EM group was used as a reference, individuals with CM showed a higher, but not significant, association with the presence of hypomobility (PR: 1.15, 95%CI: 0.93–1.42) (Table 3).
Table 3.
Prevalence ratio (PR) of the flexion rotation test (FRT) between women with chronic or episodic migraine and headache-free women.
| Headache-free (n = 30) | Chronic Migraine (n = 25) | Episodic migraine (n = 30) | Migraine (n = 55) | |
|---|---|---|---|---|
| FRT positive (%) | 30% | 92%*,# | 80%# | 85.5% |
| PR (95%CI) | Ref. | 3.1 (1.7; 5.4) | 2.7 (1.5; 4.7) | 2.8 (1.6; 5) |
| PR (95%CI) | – | 1.1 (0.9; 1.4) | Ref. | – |
Abbreviations: Ref, reference group used for prevalence ratio analysis.
p < 0.05 in relation to the episodic migraine group.
p < 0.05 in relation to the headache-free group.
Finally, linear regression revealed that the presence of neck pain disability could explain 19% of the FRT (B: −15.32, 95%CI: −21.98 to −8.65; β: −0.45, p = 0.001), independent of the diagnosis of migraine.
Discussion
Our results showed that women with migraine demonstrated a greater prevalence of positive FRTs and the frequency of migraine attacks seemed to be associated with decreased and/or more painful C1-C2 mobility, since individuals with CM were more likely to have a positive FRT and lower ROM compared to individuals with EM. Individuals with CM had lower global active cervical ROM than headache-free individuals for all movements assessed, whereas EM individuals only differed in right cervical rotation. Women with migraine had an approximately 3-fold greater association with the risk of positive FRT than headache-free individuals and an increase in the frequency of attacks increased this association. Furthermore, neck pain-related disability is able to explain 19% of the reduction in FRT, independent of the diagnosis of migraine.
The reduction of global active cervical ROM in migraine patients has been already reported.9, 32 In our study, this general reduction only occurred when comparing individuals with CM to headache-free individuals. This suggests that diagnosis of migraine is not a determinant factor for the reduction in global cervical ROM. However, global cervical ROM was more closely related to the frequency of attacks than to migraine itself. Furthermore, the dominant side of headaches does not influence the interpretation of the FRT for both primary and secondary headaches.33, 34
The FRT based observation of impaired mobility in individuals with migraine was consistent with results of previous studies.13, 17 However, our results differ to those of other studies.15, 18, 24 The main reason for these discrepancies may be the eligibility criteria applied, since our sample was obtained from a specialized headache clinic. We included both individuals with migraine and headache-free individuals, not taking reports of neck pain into account, assuming that this would yield a pragmatic sample. Previous investigations evaluated migraine using questionnaires and excluded individuals who experienced neck pain.15, 18, 24
The differences reported in FRT are related not only to the migraine itself, but also to the frequency of attacks. The association of lower C1/C2 mobility with the frequency of headache has already been reported in individuals with Cervicogenic headache (CGH)15, 18, 35 and in individuals with migraine during manual passive assessment.13, 14 The prevalence of positive FRT is similar to that of 78% described for CGH.15, 18, 35 However, only the range measured in CM is similar to that reported for CGH (27°),35 despite a prevalence of 70% of self-reported neck pain in the EM group. Although these factors confirm the hypothesis that the frequency of migraine attacks plays a role in the FRT, there is also the possibility that another comorbidity or factor related to migraine, rather than headache frequency alone, could also have influenced the decreased ROM reported here. Therefore, future studies should address factors other than frequency of attack that could potentially influence ROM.
This study revealed that neck-related disability influences approximately 19% of the variability in FRT mobility. The presence of neck pain-related disability is considered to be a predisposing factor for reduced cervical ROM in individuals with migraine.10 This reinforces the musculoskeletal impact associated with the close relationship between neck pain and migraine that has been extensively explored in the literature.5, 6, 32, 34, 36, 37, 38
These results suggest that, in our sample, two factors were associated with impaired mobility and higher prevalence of positive FRT: migraine frequency and neck-related disability. The results of this study suggest that both migraine and neck pain may play an important role in the mobility of the cervical spine. As neck pain is very common in individuals with migraine, it may be considered a part of the migraine phenotype or it could be a separate, associated condition. Therefore, we cannot determine which factor is responsible for the results of this study. The results emphasize that the combination of neck pain and frequent migraine attacks, is related to worse physical findings, which is in line with a worse prognosis and increased disability previously reported.8, 10, 11, 39
It is important to emphasize that the co-occurrence of two or more types of headache are common in individuals who experience intermittent headaches.35 For this reason, care was taken by the neurologists performing the diagnosis of the participants in this study, in order to exclude any other headache than migraine. If the International Headache Society (IHS) diagnostic criteria for CM assume that at least 7 days of headache may not present migraine-like features, there may be additional types of headache associated with CM. However, this hypothesis is not in accordance with the IHS,23 since it explains that these episodes may assume tension-type-like characteristics, as characteristics of CM may change day to day and can even change within the same day. Due to the frequency and continuity of migraine, it is impossible to distinguish episodes separately. Additionally, if there is some evidence of secondary headaches, for example, CGH, the patient should be diagnosed with both CM and CGH.23
The novelty of the current study is the finding of impairment of the C1/C2 segment in women with both EM and CM. These results indicate the presence of musculoskeletal dysfunction in the upper cervical spine in this population. Although EM demonstrated a greater associated risk of a positive FRT, the mean differences compared to the headache-free group were lower than those observed for CM and global deficits were not as obvious as only right rotation was reduced in the global ROM. This information could help physical therapists to perform better evaluations and to develop a more precise kinetic diagnosis, focused on the presence of pain and hypomobility of the upper cervical segment.
This study has several limitations. First, this is a cross-sectional study; therefore a causal relationship between migraine and cervical mobility impairment cannot be determined. Second, the absence of a group with a diagnosis of CGH prevented us from performing a direct comparison of the magnitude of C1/C2 impairment between these groups. Third, the psychosocial factors were not investigated in these individuals. Analysis of these factors would have been helpful, as the majority of participants presented with chronic pain. Fourth, only women were included, since they are more often affected than men.40 Consequently, it is unclear whether these results would be replicated in a sample of men with migraine. Finally, the participants of this study were recruited from a tertiary outpatient hospital and may represent a subgroup of the general population with migraine.
Conclusions
Women who experience migraine exhibit a reduced range of motion on FRT, compared to headache-free women. However, only individuals with CM had a reduced global active cervical range of motion compared to headache-free women. Migraine was associated with a higher likelihood of a positive FRT. A reduction in C1/C2 mobility was aggravated by higher migraine frequency and influenced by neck pain-related disability.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors would like to acknowledge the Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil for funding this study.
References
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators* Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. 2018;17(2):174–182. doi: 10.1016/S1474-4422(17)30435-0. [DOI] [PubMed] [Google Scholar]
- 3.Cutrer F.M. Pathophysiology of migraine. Semin Neurol. 2010;30(2):120–130. doi: 10.1055/s-0030-1249222. [DOI] [PubMed] [Google Scholar]
- 4.Borg-Stein J. Cervical myofascial pain and headache. Curr Pain Headache Rep. 2002;6(4):324–330. doi: 10.1007/s11916-002-0055-0. [DOI] [PubMed] [Google Scholar]
- 5.Calhoun A.H., Ford S., Millen C., Finkel A.G., Truong Y., Nie Y. The prevalence of neck pain in migraine. Headache. 2010;50(8):1273–1277. doi: 10.1111/j.1526-4610.2009.01608.x. [DOI] [PubMed] [Google Scholar]
- 6.Ashina S., Bendtsen L., Lyngberg A.C., Lipton R.B., Hajiyeva N., Jensen R. Prevalence of neck pain in migraine and tension-type headache: a population study. Cephalalgia. 2015;35(3):211–219. doi: 10.1177/0333102414535110. [DOI] [PubMed] [Google Scholar]
- 7.Robertson B., Morris M. The role of cervical dysfunction in migraine: a systematic review. Cephalalgia. 2008;28(5):474–483. doi: 10.1111/j.1468-2982.2008.01545.x. [DOI] [PubMed] [Google Scholar]
- 8.Ford S., Calhoun A., Kahn K., Mann J., Finkel A. Predictors of disability in migraineurs referred to a tertiary clinic: neck pain, headache characteristics, and coping behaviors. Headache. 2008;48(4):523–528. doi: 10.1111/j.1526-4610.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 9.Bevilaqua-Grossi D., Pegoretti K.S., Goncalves M.C., Speciali J.G., Bordini C.A., Bigal M.E. Cervical mobility in women with migraine. Headache. 2009;49(5):726–731. doi: 10.1111/j.1526-4610.2008.01233.x. [DOI] [PubMed] [Google Scholar]
- 10.Florencio L.L., Chaves T.C., Carvalho G.F. Neck pain disability is related to the frequency of migraine attacks: a cross-sectional study. Headache. 2014;54(7):1203–1210. doi: 10.1111/head.12393. [DOI] [PubMed] [Google Scholar]
- 11.Calhoun A.H., Ford S., Pruitt A.P. Presence of neck pain may delay migraine treatment. Postgrad Med J. 2011;123(2):163–168. doi: 10.3810/pgm.2011.03.2274. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-de-las-Peñas C., Cuadrado M.L., Pareja J.A. Myofascial trigger points, neck mobility and forward head posture in unilateral migraine. Cephalalgia. 2006;26(9):1061–1070. doi: 10.1111/j.1468-2982.2006.01162.x. [DOI] [PubMed] [Google Scholar]
- 13.Tali D., Menahem I., Vered E., Kalichman L. Upper cervical mobility, posture and myofascial trigger points in subjects with episodic migraine: case–control study. J Bodyw Mov Ther. 2014;18(4):569–575. doi: 10.1016/j.jbmt.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Vernon H., Steiman I., Hagino C. Cervicogenic dysfunction in muscle contraction headache and migraine: a descriptive study. J Manip Physiol Ther. 1992;15(7):418–429. [PubMed] [Google Scholar]
- 15.Hall T.M., Briffa K., Hopper D., Robinson K. Comparative analysis and diagnostic accuracy of the cervical flexion-rotation test. J Headache Pain. 2010;11(5):391–397. doi: 10.1007/s10194-010-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson D.H., Drummond P.D. Head pain referral during examination of the neck in migraine and tension-type headache. Headache. 2012;52(8):1226–1235. doi: 10.1111/j.1526-4610.2012.02169.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferracini G.N., Florencio L.L., Dach F. Musculoskeletal disorders of the upper cervical spine in women with episodic or chronic migraine. Eur J Phys Rehabil Med. 2017;53(3):342–350. doi: 10.23736/S1973-9087.17.04393-3. [DOI] [PubMed] [Google Scholar]
- 18.Ogince M., Hall T., Robinson K., Blackmore A.M. The diagnostic validity of the cervical flexion-rotation test in C1/2-related cervicogenic headache. Man Ther. 2007;12(3):256–262. doi: 10.1016/j.math.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Anderst W.J., Donaldson W.F., 3rd, Lee J.Y., Kang J.D. Cervical motion segment contributions to head motion during flexion\extension, lateral bending, and axial rotation. Spine J. 2015;15(12):2538–2543. doi: 10.1016/j.spinee.2015.08.042. [DOI] [PubMed] [Google Scholar]
- 20.Hall T., Briffa K., Hopper D., Robinson K. Long-term stability and minimal detectable change of the cervical flexion-rotation test. J Orthop Sports Phys Ther. 2010;40(4):225–229. doi: 10.2519/jospt.2010.3100. [DOI] [PubMed] [Google Scholar]
- 21.Hall T.M., Robinson K.W., Fujinawa O., Akasaka K., Pyne E.A. Intertester reliability and diagnostic validity of the cervical flexion-rotation test. J Manip Physiol Ther. 2008;31(4):293–300. doi: 10.1016/j.jmpt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Smith K., Hall T., Robinson K. The influence of age, gender, lifestyle factors and sub-clinical neck pain on the cervical flexion-rotation test and cervical range of motion. Man Ther. 2008;13(6):552–559. doi: 10.1016/j.math.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Headache Classification Committee of the International Headache S. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 24.Dugailly P.M., Decuyper A., Salem W., De Boe A., Espí-López G.V., Lepers Y. Analysis of the upper cervical spine stiffness during axial rotation: a comparative study among patients with tension-type headache or migraine and asymptomatic subjects. Clin Biomech. 2017 Feb;42:128–133. doi: 10.1016/j.clinbiomech.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Ackelman B.H., Lindgren U. Validity and reliability of a modified version of the neck disability index. J Rehabil Med Suppl. 2002;34(6):284–287. doi: 10.1080/165019702760390383. [DOI] [PubMed] [Google Scholar]
- 26.Cook C., Richardson J.K., Braga L. Cross-cultural adaptation and validation of the Brazilian Portuguese version of the Neck Disability Index and Neck Pain and Disability Scale. Spine (Phila Pa 1976) 2006;31(14):1621–1627. doi: 10.1097/01.brs.0000221989.53069.16. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher J.P., Bandy W.D. Intrarater reliability of CROM measurement of cervical spine active range of motion in persons with and without neck pain. J Orthop Sports Phys Ther. 2008;38(10):640–645. doi: 10.2519/jospt.2008.2680. [DOI] [PubMed] [Google Scholar]
- 28.Audette I., Dumas J.P., Cote J.N., De Serres S.J. Validity and between-day reliability of the cervical range of motion (CROM) device. J Orthop Sports Phys Ther. 2010;40(5):318–323. doi: 10.2519/jospt.2010.3180. [DOI] [PubMed] [Google Scholar]
- 29.Singer J. A simple procedure to compute the sample size needed to compare two independent groups when the population variances are unequal. Stat Med. 2001;20(7):1089–1095. doi: 10.1002/sim.722. [DOI] [PubMed] [Google Scholar]
- 30.Armijo-Olivo S., Warren S., Fuentes J., Magee D.J. Clinical relevance vs. statistical significance: using neck outcomes in patients with temporomandibular disorders as an example. Man Ther. 2011;16(6):563–572. doi: 10.1016/j.math.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Armijo-Olivo S. The importance of determining the clinical significance of research results in physical therapy clinical research. Braz J Phys Ther. 2018;22(3):175–176. doi: 10.1016/j.bjpt.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvalho G.F., Chaves T.C., Goncalves M.C. Comparison between neck pain disability and cervical range of motion in patients with episodic and chronic migraine: a cross-sectional study. J Manip Physiol Ther. 2014;37(9):641–646. doi: 10.1016/j.jmpt.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-de-las-Peñas C., Arendt-Nielsen L., Cuadrado M.L., Pareia J.A. Generalized mechanical pain sensitivity over nerve tissues in patients with strictly unilateral migraine. Clin J Pain. 2009;25(5):401–406. doi: 10.1097/AJP.0b013e31819655b3. [DOI] [PubMed] [Google Scholar]
- 34.Landgraf M.N., von Kries R., Heinen F., Langhagen T., Straube A., Albers L. Self-reported neck and shoulder pain in adolescents is associated with episodic and chronic migraine. Cephalalgia. 2015;36(8):807–811. doi: 10.1177/0333102415610875. [DOI] [PubMed] [Google Scholar]
- 35.Hall T.M., Briffa K., Hopper D., Robinson K.W. The relationship between cervicogenic headache and impairment determined by the flexion-rotation test. J Manip Physiol Ther. 2010;33(9):666–671. doi: 10.1016/j.jmpt.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Amiri M., Jull G., Bullock-Saxton J., Darnell R., Lander C. Cervical musculoskeletal impairment in frequent intermittent headache. Part 2: subjects with concurrent headache types. Cephalalgia. 2007;27:891–898. doi: 10.1111/j.1468-2982.2007.01346.x. [DOI] [PubMed] [Google Scholar]
- 37.Fernández-de-las-Peñas C., Hernandez-Barrera V., Carrasco-Garrido P. Population-based study of migraine in Spanish adults: relation to socio-demographic factors, lifestyle and co-morbidity with other conditions. J Headache Pain. 2010;11(2):97–104. doi: 10.1007/s10194-009-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaschek A., Decke S., Albers L. Self-reported neck pain is associated with migraine but not with tension-type headache in adolescents. Cephalalgia. 2014;34(11):895–903. doi: 10.1177/0333102414523338. [DOI] [PubMed] [Google Scholar]
- 39.Lipton R.B., Fanning K.M., Buse D.C. Identifying natural subgroups of migraine based on comorbidity and concomitant condition profiles: results of the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2018;58(7):933–947. doi: 10.1111/head.13342. [DOI] [PubMed] [Google Scholar]
- 40.Queiroz L.P., Peres M.F., Kowacs F. Chronic daily headache in Brazil: a nationwide population-based study. Cephalalgia. 2008;28(12):1264–1269. doi: 10.1111/j.1468-2982.2008.01670.x. [DOI] [PubMed] [Google Scholar]