Abstract
Cardiovascular diseases are the major causes of preventable health loss from disease in the world and lead to functional disturbances including hematological parameters. The inflammatory and hypoxemic nature of cardiovascular diseases causes a stimulus in the bone marrow and, depending on the intensity of this stimulus, there is a release of immature cells or increase of other cells in the bloodstream. Therefore, their presence in the circulation is an important variable used to diagnose, stratify and predict diseases.
In the last five decades, with the advent of automated counting of immature cells in the peripheral blood, the hemogram was transformed into a clinical tool of great importance in hospital surveillance for demonstrating this daily variability in the hematopoietic response according to the
existing injury in the patient. Studies have shown that the presence of nucleated red blood cells and increases in mean platelet volume, immature granulocytes and neutrophil to lymphocyte ratio in the systemic circulation are independent prognostic biomarkers.
This review article has as main objective to demonstrate the association of these hematological parameters to cardiovascular diseases, emphasizing their importance in clinical decision making.
Keywords: Nucleated red blood cells, Neutrophil to lymphocyte ratio, Mean platelet volume, Immature granulocytes, Biomarkers, Cardiovascular diseases
1. INTRODUCTION
The cardiovascular diseases (CVD) are among the major causes of preventable health loss from disease in the world, regardless of a country’s income socio-demographic status [1]. These diseases are influenced by population aging, genetic predisposition, eating habits, stress, dyslipidemia, diabetes mellitus, systemic arterial hypertension, sedentary lifestyle and risk behaviors such as smoking and alcohol abuse [2]. Studies have demonstrated a strong association between hematological parameters and the risk of adverse outcomes in patients with CDV due to the inflammation and systemic hypoxemia implicated in the pathophysiology mechanisms, and depending on the extent of the injury, causing circulatory failure and, consequently, organic dysfunction [3-5].
The technological advancement of the last five decades and the development of modern automated equipment in the evaluation of blood cells have a great impact on the research of these blood biomarkers, mainly in the prognostic evaluation in cardiovascular diseases (Table 1). In healthy adults, we did not find immature blood cells in the systemic circulation. Apart from hematological and oncological diseases, the inflammatory and hypoxemic processes are the main stimuli for the greater production by the bone marrow of the constituents of the blood. The presence of these immature young cells in the peripheral circulation is directly associated with the intensity of systemic inflammation and hypoxemia and, consequently, with a poorer prognosis [3, 4].
Table 1. Complete blood count parameters (adapted by Ralph and Sebastian W-Hogiu [18]).
| Methodology | Discoverer / Year |
|---|---|
| Microscope | Vierordt / 1852 |
| Light scattering and absorption measured by eye | Oliver / 1896 |
| Light scattering and absorption measured with a photodetector | Marcandier et al. /1928 |
| Impedance measurement | Coulter / 1953 |
| Impedance measurement and Electrostatic cell sorting | Fulwyler / 1965 |
| Fluorescence-based flow cytometry |
Dittrich and Göehde/ 1968 |
| Fluoresce-activated cell sorting | Julius et al. / 1972 |
| Light scattering in flow cytometry |
George and Groner / 1973 |
| Parameters | Reporting Units |
| RBC count | (usually x 1012)/L |
| MCV | fL |
| MCH | pg or fmol |
| MCHC | g/dL, g/L, mmol/L |
| RDW | %CV or fL |
| Hematocrit | % or L/L |
| Hb concentration | g/dL, g/L, mmol/L |
| Reticulocyte count | (usually x 109)/L |
| NRBC | (usually x 109)/L |
| WBC count | (usually x 109)/L |
| Lymphocytes | (usually x 109)/L |
| Monocytes | (usually x 109)/L |
| Neutrophil granulocytes |
(usually x 109)/L |
| Eosinophil granulocytes |
(usually x 109)/L |
| Basophil granulocytes |
(usually x 109)/L |
| Platelet count | (usually x 109)/L |
| MPV | fL |
Abbreviations: RBC, red blood cell; MCH, mean cell Hb; MCHC, mean cell Hb Concentration; RDW, red cell distribution width; MPV, Mean platelet volume; %CV, % coefficient of variation; fL: femtoliter (x 10-15L); fmol: femtomol (x 10-15mol); pg: picogram (x 10-12g).
Previous studies have demonstrated the prognostic value of these hematological parameters in stroke [5, 6], coronary artery disease [7, 8], heart failure [9, 10], systemic arterial hypertension [11, 12], atrial fibrillation [13] and cardiovascular disease associated with septicemia [3, 14]. Therefore, it is important to review these blood cells count as potential clinical analyzers (Table 2) [15].
Table 2. Potential clinical measures of hematological parameters analyzers (adapted by Thomas Pierre and Michael Bernimoulin [15]).
| Blood Cells | Parameters | Clinical Measures |
|---|---|---|
| Red blood cells | ||
| % hypochromic red blood cells | Assessment of iron-restricted erythropoiesis | |
| Low hemoglobin density | Assessment of iron-restricted erythropoiesis | |
| Red cell distribution width | Anisocytosis | |
| Immature reticulocyte fraction | % Immature erythrocytes | |
| Reticulocyte hemoglobin content | Iron deficiency anemia | |
| Nucleated red blood cells | Bleeding disorders, hypoxemia | |
| Schistocytes | Hemolytic anemia | |
| White blood cells | ||
| Immature granulocyte | Infections | |
| White cell volume | Infections | |
| High fluorescent lymphocytes |
Atypical lymphocytes | |
| Platelets | ||
| Immature platelet fraction |
% immature platelet and thrombocytopenia | |
| Platelet distribution width | thrombocytopenia | |
| Mean platelet volume | Platelet activation and thrombocytopenia | |
| Large cell ratio | Bleeding disorders and thrombocytopenia | |
| Platelet dry mass | Histogram of platelet | |
| Plateletcrit | Platelet activation |
2. PATHOPHYSIOLOGICAL MECHANISMS
The organ responsible to produce blood cells (red blood cells, leucocytes and platelets) is the bone marrow, a process called hematopoiesis. These cells are originated from a single progenitor cell called the stem cell. These pluripotent stem cells can reproduce when necessary and lead to differentiation processes in distinct hematological cell lines [16] (Fig. 1). There are proteins responsible for the induction of cell multiplication and other proteins are responsible for cellular differentiation, called differentiation inducers, which are controlled by factors external to the bone marrow. For example, blood exposure to low oxygen concentrations over a long period results in the induction of growth, differentiation and production of an increased number of red blood cells. This stimulus to the bone marrow is produced by erythropoietin, a glycoprotein produced in 90% by the kidneys, the rest being formed, for the most part, in the liver, in the presence of hypoxemia [16].
Fig. (1).
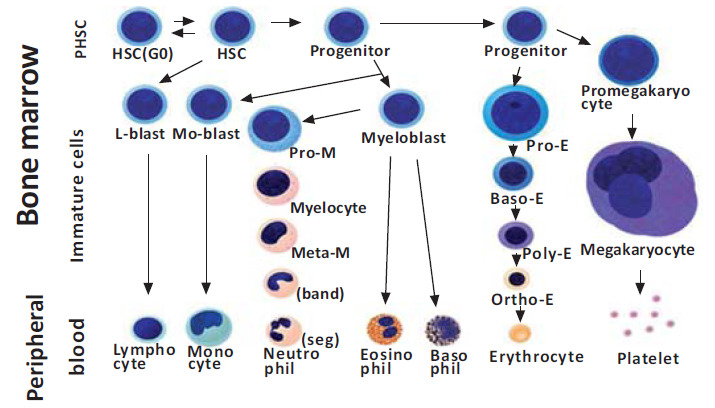
Formation of blood cell differences from pluripotent hematopoietic stem cell (PHSC) in bone marrow.
Infectious diseases cause growth, differentiation and final formation of specific types of leukocytes for each pathogen. Therefore, in the inflammatory process caused by bacteria, trauma, chemical agents, heat or any other phenomenon, there is a specific cellular differentiation at the spinal level. In relation to platelets, which are cells formed in the bone marrow from megakaryocytes, there are small fragments of anucleated cells that have a characteristic discoidal form and vary from 1 to 3 μm in diameter, which have an important role in hemostasis, inflammation and innate immunity [16, 17].
3. HEMOGRAM: NEW PARAMETERS
The development of microscopy over more than 300 years ago by Van Leeuwenhoek was a milestone in laboratory medicine. Consequently, experimental research was boosted due to the observation that the blood is formed by small red blood cells and their sizes were eventually determined. In 1870, due to the improvement of optical microscopy with objective corrective lenses by Paul Ehrlich, it was possible to distinguish between different types of white blood cells. However, despite this differentiation in morphology, it was observed that the number of cells changed in many diseases; therefore, the need for the quantitative measurement of these cells. A laborious manual measurement was introduced as it was counted cell-by-cell, attempting to quantify and describe the volume and geometry of cells [18].
Another method of automated measurement based on electrical impedance has been developed since the work of Wallace Coulter in 1940. Therefore, with technological advances in optics, electronics and microfluidics, new improvements are being directed towards the development of new agents and contrast methods, which will allow greater accuracy and precision in the automated analysis [18].
3.1. Nucleated Red Blood Cells
Nucleated red blood cells (NRBCs), also known as erythroblasts, are immature erythrocyte cells present in the bone marrow in the process of hematopoiesis. It is normal to find NRBCs in the peripheral blood of newborns for a few days. However, its permanence in the systemic circulation of children and adults is associated with serious diseases, mainly hematological and oncological. Prior studies have shown that severe hypoxemia and/or infection are responsible for the presence of NRBCs in peripheral blood, when hematological diseases, cancer, congestive heart failure, acute and chronic anemias are excluded [19-21]. It has also been demonstrated that patients with serum NRBCs (NRBCs present in the peripheral circulation) have high concentrations of erythropoietin, interleukin-3 and interleukin-6 suggestive of an important role of tissue oxygenation and/or inflammation, caused by local or systemic disorders, and presenting a poorer prognosis [19].
Therefore, the presence of serum NRBCs in hospitalized patients with cardiovascular diseases is associated with increased mortality and the need for these patients to stay in the intensive care unit even with clinical improvement [3]. In a study published by our research group [3], we observed that the inclusion of the NRBCs in the model including the APACHE II score would result in an increase in the area under the ROC curve from 0.7668 to 0.7929, p = 0.011 (Fig. 2).
Fig. (2).
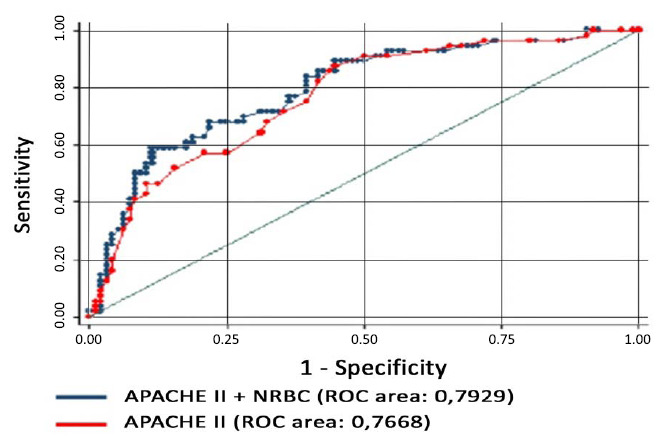
comparison of the ROC curves, p = 0.01.
Another important parameter in clinical practice is the measurement of erythrocyte size variability in the systemic circulation, the RDW (Red Blood cell distribution range), which, in healthy adults, is about 4.0 to 6.0 x 106 / mm3 but high levels, according to some studies, are associated with poorer prognosis in heart failure and coronary disease [22, 23]. Another variable also well studied is the aggregation of erythrocytes, which is associated with cardiovascular diseases [24]. Several studies conditions increased erythrocyte’s aggregation with myocardial ischemia and infarction [25], cerebral ischemia and infarction [26] and peripheral vascular diseases [27].
3.2. Neutrophil to Lymphocyte Ratio
In the investigation of atherosclerosis, we have focused on inflammation, which participates articulately in all stages, from the beginning of the lesion to the progression and destabilization of the plaque, which determined studies evaluating certain inflammatory factors in the prognosis of cardiovascular risk [28]. C-reactive protein (CRP) has been the most studied and most applicable in clinical practice. However, its predictive value was recently questioned and the need for new complementary markers is evident [29].
The blood count is performed routinely on admission and carries important prognostic information. The neutrophil to lymphocyte ratio (NLR), a combination of two independent markers of inflammation, is considered a simple and non-specific marker of inflammation [30]. White blood cells, particularly lymphocytes, cause an important modulation in the inflammatory response. Lymphopenia has been associa-ted with progressive atherosclerosis in clinical and experimental studies [31].
Studies have shown that the NLR is a strong predictor of short and long-term mortality in stable and unstable coronary insufficiency. Patients with non-ST-segment elevation myocardial infarction with NLR > 4.7 have a mortality rate of 29.8% compared to those with NLR < 3, with a mortality rate of 8.4 (p <0.001) [32]. In another study, using a 2.54 cut-off point, NLR was a predictor of severe atherosclerosis with a sensitivity of 74% and specificity of 53% (ROC curve 0.627; 95% CI: 0.545-0.704, p = 0.004) [33]. There are also studies demonstrating the association between NLR and the extent and severity of coronary artery disease [34, 35].
3.3. Plaquetogram
The multiple quantitative and qualitative platelet parameters, evaluated by the electronic counters, justify applying the term plaquetogram, in analogy to the other subdivisions of the hemogram. Parameters evaluated included platelet count (PLT), mean platelet volume (MPV), platelet distribution width (PDW), cross-linked platelets (RT) and platelet-cell ratio (P-LCR) [36, 37].
Platelets are found in the systemic circulation with a concentration between 150,000 and 450,000 mm3, 70% of which are present in the circulation for approximately 10 days and 30% in the spleen [38]. Platelet counts represent a dynamic equilibrium of many simultaneous processes, including altered capillary permeability and inflammatory cascades, as well as the coagulation process [39]. An abnormal fall in the number of platelets in the bloodstream, known as thrombocytopenia (<150 x 109/L), may result in increased bleeding. Studies have associated this decline in platelet count with unfavorable clinical outcomes [39-41]. In a recent publication of our research group [42], we categorized patients for serum platelet count as mild (100-149 x 109/L), moderate (50- 99 x 109/L) and severe (<50 x 109/L) associated with mortality during hospitalization period (Fig. 3).
Fig. (3).
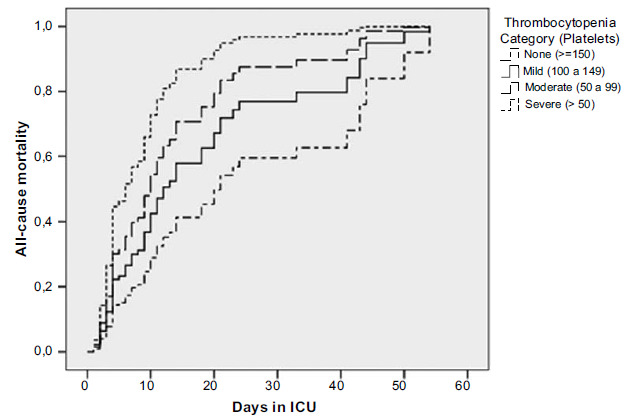
Kaplan-Meier curve for mortality (death). Patients categorized by platelet count during the period of hospital stay (days).
Large platelets are metabolically and enzymatically more active than small platelets and they are reflected by elevation in mean platelet volume (MPV). Therefore, platelets are heterogeneous in relation to platelet volume and density, biological variables that determine platelet function and activity, with an important role in the development of intravascular thrombus [38]. In the study by Uysal et al. [33], the value of the MPV above 10.4 is a predictor of severe atherosclerosis with a sensitivity of 39% and specificity of 90% (ROC curve: 0.631, 95% CI: 0.549-0.708, p = 0.003), and can be used as a predictor and cardiac risk identifier in patients with coronary artery disease. Therefore, MPV has been identified as an independent risk factor for acute myocardial infarction (AMI) in patients with coronary artery disease [43, 44].
Recent studies have demonstrated that immature platelets, also known as reticulated platelets, because they are analogous to reticulocytes, immature red blood cells, contain measurable amounts of cytosolic messenger ribonucleic acid (mRNA) that, unlike normal mature platelets, continue in protein synthesis and aggravate their prothrombotic potential in acute coronary syndrome (ACS) [45]. In addition, the response of these immature platelets to the ticlopidine clopidogrel and prasugrel is significantly decreased and obviously related to the serum level [46]. Therefore, these findings indicate that there is a justification for the evaluation of these platelets, cross-linking for risk stratification in patients with ACS who receive dual antiplatelet therapy with aspirin and thienopyridines [46, 47].
3.4. Immature Granulocytes
Immature granulocytes (IG), which comprise the sum of metamyelocytes, myelocytes and promyelocytes, are cells released into the systemic circulation resulting from bone marrow stimuli, such as bacterial infection, acute inflammatory diseases, cancer (particularly spinal metastasis), tissue necrosis, acute transplant rejection, surgical trauma, orthopedic trauma, myeloproliferative disease, steroid use and pregnancy (especially in the third trimester). However, in the elderly, neonates or immunosuppressed individuals, IG > 2% may be indicative of acute infection, even if the patient does not have neutrophilia or neutropenia [48].
Therefore, the counting of immature cells by automation is faster, more accurate, and determined while the other blood count parameters are released. This means agility in the knowledge of diagnostic hypotheses and patient care. Ansari-Lari et al. [49] observed that the IG > 3% is a specific predictor of sepsis. According to the Surviving Sepsis Campaign: the International Guidelines for the Management of Severe Sepsis and Septic Shock (2012), the inflammatory variables below are part of the diagnostic criteria of sepsis: 1 - Leukocytosis (leukocytes > 12 x 103 / μL), 2 - Leukopenia (leukocytes < 4.0 x 103 / μL) and 3 - Normal leukocyte count and IG > 10% [50].
4. CLINICAL IMPLICATIONS
The appearance of nucleated red blood cells and increases in mean platelet volume, neutrophil to lymphocyte ratio and immature granulocytes in peripheral blood are directly associated with the intensity of systemic inflammation and hypoxemia. These two mechanisms are directly implicated in the pathophysiology of organic dysfunction, according to clinical studies (Table 3).
Table 3. Hematological parameters in clinical studies.
| Clinical studies (First Author / Year) | Hematological Parameters (Results) | |
|---|---|---|
| ISCHEMIC STROKE | ||
| Lou Fan / 2017 Martin Söderholm / 2015 |
Eosinophil: OR 0.74 (95% CI: 0.67-0.82) Neutrophil: OR 0.76 (95% CI: 0.67-0.84) WBC: OR 0.72 (95% CI: 0.64-0.81) RDW: OR 0.65 (95% CI: 0.56-0.73) NLR: OR 0.76 (95% CI: 0.68-0.84) Monocyte: OR 0.67 (95% CI: 0.59-0.76) Lymphocyte: OR 0.75 (95% CI: 0.67-0.83) RDW: Total stroke: HR 1.31 (95% CI: 1.11-1.54) Cerebral infarction: HR 1.32 (95% CI: 1.10-1.58) Intracerebral hemorrhage: Adjusted HR 1.44 (95% CI: 0.90-2.30) Subarachnoid hemorrhage: Adjusted HR 0.94 (95% CI: 0.43-2.07) |
|
| ATHEROSCLEROSIS (CORONARY DISEASE) | ||
| Jan Budziannowski / 2017 José Gildo / 2018 |
Review article: WBC >10.000; NLR >4.9; RDW >14.0%; PDW >17%; MPV > 7.1% provide risk stratification, pathophysiology and optimal management. NRBC: HR 2.42 (95% CI: 1.35-4.36) NLR: HR 5.02 (95% CI: 1.68-15.0) MPV: HR 2.97 (95% CI: 1.15-7.67) |
|
| HEART FAILURE | ||
| Guoxing Wan / 2018 Sem Liu / 2016 |
NLR ≥3.96 Gene SLC22A4: OR 5.219 (95% CI: 1.762-15.458) Gene ILIR2: OR 5.228 (95% CI: 1.560-17.515) Gene VNN3: OR 3.478 (95% CI: 1.322-9.195) RDW: OR 2.531 (95% CI: 1.371-4.671) |
|
| SYSTEMIC ARTERIAL HYPERTENSION | ||
| Marzieh Emamian / 2017 Bamlaku Enawgaw / 2017 |
Multivariate OR: WBC: 1.01 (95% CI: 0.94-1.09) RBC: 0.92 (95% CI: 0.66-1.28) HCT: 1.02 (95% CI: 1.003-1.04) MCV: 0.98 (95% CI: 0.96-1.002) RDW: 0.98 (95% CI: 0.94-1.02) Median (IQR) value with p <0.005: WBC, RBC, Hgb, HCT, MCV Mean (SD) vakue with p <0.005: MCHC, RDW, MPV, PDW Median (IQR) value p=0.262: PLT |
|
| ATRIAL FIBRILLATION | ||
| Alexander Weymann / 2017 | Systematic review with meta-analysis: 22 studies were analyzed including a total of 6098 patients. Weighted mean difference (preoperative): PC: -7.07 x 109/L and p <0.001 MPV: 0.53 FL and p <0.001 WBC: 0.130 x 109/L and p <0.001 NLR: 0.33 and p <0.001 RDW: 0.36% p <0.001 Weighted mean difference (postoperative): WBC: 1.36 x 109/L and p <0.001 NLR: 0.74 and p <0.001 |
|
| SEPTICEMIA | ||
| José Gildo / 2015 | NRBC: Univariate model: OR 3.37 (95% CI: 1.64-6.89) Multivariate model: OR 1.81 (95% CI: 0.79-4.12) |
|
Abbreviations: OR, Odds ratio; HR, Hazard ratio; WBC, white blood cell; RBC, red blood cell; PDW, platelet distribution width; HTC, hematocrit; Hgb, hemoglobin; MCV, mean corpuscular volume; MCH, mean cell Hb; MCHC, mean cell Hb Concentration; RDW, red cell distribution width; NRBC, nucleated red blood cell; MPV, Mean platelet volume; NLR, neutrophil to lymphocyte ratio; PDW, platelet distribution width; PLT, platelet count.
Ischemic stroke (IS) is one of the leading causes of disability and death around the world. Fan et al. [5] hypothesized that the prognostic value of these routine hematological parameters can be explained by inflammation and stress. This study indicated that routine hematological parameters, including NLR, eosinophil and RDW are useful
and independent prognostic factors for IS. Another study by Martin Söderholm et al. [6] demonstrated the association between high RWD with an increased incidence of total stroke and cerebral infarction, but there was no significant association between RDW and incidence of intracerebral or subarachnoid hemorrhage.
Atherosclerosis is currently considered as a chronic inflammatory disease, leading to an endothelial dysfunction associated with the activation of the immune system. Coronary disease is most commonly caused by atherosclerosis. In the review article by Jan Budzianowski et al. [7], the authors shall describe the main hematological parameters and their prognostic and diagnostic values in patients with the acute coronary syndrome. For examples, NRL > 4.9 enabled a clinician to predict stent thrombosis and the high mortality rate among patients with acute myocardial infarction (70% accuracy and 65% specificity in predicting in-hospital mortality) and MPV was a strong and independent predictor of impaired reperfusion and 6-month mortality in patients with acute myocardial infarction who underwent primary percutaneous coronary intervention. They conclude the article referring to the importance of these hematological parameters in the identification of high cardiovascular risk in secondary prevention and tailor the therapy to their needs. Monteiro Junior et al. [8] associated increased NRBC, MPV and NRL in peripheral blood with higher in-hospital mortality in patients with acute myocardial infarction. Therefore, they considered these variables an important in-hospital tool of clinical surveillance.
Heart failure, commonly the end-stage of most cardiovascular diseases, is a complex inflammatory neuroendocrine syndrome not merely an exclusive problem of low cardiac output [9]. Wan et al. [9] described the association between NLR and three genes SLC22A4, IL1R2 and VNN3 with heart failure. NLR is considered a useful prognostic index for many cardiovascular diseases. The current study is the first to investigate the potential decisive factors associated with elevated NLR in heart failure by microarray assay of genes. The researcher Liu et al. [10] demonstrated in patients with heart failure, the elevated RDW with an independent risk factor for mortality.
Atrial fibrillation is one of the most critical common complications after cardiovascular surgery such as thromboembolism, cerebrovascular events, prolonged hospital stay and readmissions to the intensive care unit (ICU) and hospital, organ failure, as well as an increase in health care costs and mortality [13]. In this study, the authors concluded that hematological parameters may predict the risk of postoperative atrial fibrillation before surgery and these exams should be evaluated in all patients submitted to myocardial revascularization and valvular surgical or combined procedures.
In septicemia, the immature granulocyte is associated with the bacterial infectious process. Therefore, it helps the clinician in differentiating whether the inflammation is due to an infectious process or not. In addition, it is a prognostic marker of severity in patients with infectious disease. Musher et al. [14] recently published a review article associating acute infection and myocardial infarction. This study has confirmed that acute bacterial and viral infections are associated with an increased risk of myocardial infarction in the short, intermediate and long term. However, we will need to discuss the mechanisms that might explain this association.
Therefore, the presence of nucleated red blood cells and increases in neutrophil to lymphocyte ratio, mean platelet volume and immature granulocyte in peripheral blood are an important tool of daily monitoring of the clinical response after treatment. Persistence or worsening of these variants in the bloodstream is associated with poorer prognosis.
CONCLUSION
These new hematological parameters derived from the hemogram, obtained in an automated way, simple and low cost, need to be valued as a daily tool of prognostic surveillance of hospitalized patients. As we have seen, there is much evidence in the medical literature that corroborates that nucleated red blood cells, neutrophil to lymphocyte ratio, mean platelet volume and immature granulocyte are independent biomarkers of cardiovascular death risk.
However, more research on these biomarkers is necessary to establish these variables in daily practice, helping the clinician in the diagnosis and prognostic evaluation of cardiovascular diseases.
ACKNOWLEDGEMENTS
Conceived and research design: Monteiro Junior JGM, Torres DOC, Sobral Filho DC; Writing of the manuscript and critical revision of the manuscript regarding important intellectual content: Monteiro Junior JGM, Torres DOC, Sobral Filho DC.
LIST OF ABBREVIATIONS
- ACS
Acute Coronary Syndrome
- AMI
Acute Myocardial Infarction
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- CVD
Cardiovascular Diseases
- CRP
C-Reactive Protein
- IG
Immature Granulocytes
- ICU
Intensive Care Unit
- MPV
Mean Platelet Volume
- NLR
Neutrophil to Lymphocyte Ratio
- NRBCs
Nucleated Red Blood Cells
- PDW
Platelet Distribution Width
- P-LCR
Platelet-Cell Ratio
- PLT
Platelet Count
- PROCAPE
Pernambuco Cardiac Emergency Hospital
- RDW
Red Blood Cell Distribution Range
- ROC
Receiver Operating Characteristic
- RT
Cross-linked Platelets
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Thomas H., Diamond J., Vieco A., et al. Global atlas of cardiovascular disease 2000-2016: The path to prevention and control. Glob. Heart. 2018;13(3):143–163. doi: 10.1016/j.gheart.2018.09.511. [DOI] [PubMed] [Google Scholar]
- 2.Li L., Xie W., Zheng X., Yin W., Tang C. A novel peptide adropin in cardiovascular diseases. Clin. Chim. Acta. 2016;453:107–113. doi: 10.1016/j.cca.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Monteiro Júnior J.G.M., Torres D.O.C., Silva M.C.F.C., et al. Nucleated red blood cells as predictors of all-cause mortality in cardiac intensive care unit patients: A prospective cohort study. PLoS One. 2015;10(12):e0144259. doi: 10.1371/journal.pone.0144259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verbrugge S.E., Huisman A. Verification and standardization of blood cell counters routine clinical laboratory tests. Clin. Lab. Med. 2015;(35):183–196. doi: 10.1016/j.cll.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Fan L., Gui L., Chai E.Q., Wei C.J. Routine hematological parameters are associated with short- and long-term prognosis of patients with ischemic stroke. J. Clin. Lab. Anal. 2018 doi: 10.1002/jcla.22244. Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Söderholm M., Borné Y., Hedblad B., Persson M., Engström G. Red cell distribution width in relation to incidence of stroke and carotid atherosclerosis: A population-based cohort study. PLoS One. 2015;10(5):e0124957. doi: 10.1371/journal.pone.0124957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budziannowski J., Pieszko K., Burchardt P., Rzezniczak J., Hiczkiewicz J. The role of hematological indices in patients with acute coronary syndrome. Dis. Markers. 2017;2017:3041565. doi: 10.1155/2017/3041565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteiro Júnior J.G.M., Torres D.O.C., da Silva M.C.F.C., et al. Prognostic value of hematological parameters in patients with acute myocardial infarction: Intrahospital outcomes. PLoS One. 2018;13(4):e0194897. doi: 10.1371/journal.pone.0194897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan G., Ji L., Xia W., Cheng L., Zhang Y. Screening genes associated with elevated neutrophil-to-lymphocyte ratio in chronic heart failure. Mol. Med. Rep. 2018;18(2):1415–1422. doi: 10.3892/mmr.2018.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S., Wang P., Shen P.P., Zhou J.H. Predictive values of red blood cell distribution width in assessing severity of chronic heart failure. Med. Sci. Monit. 2016;22:2119–2125. doi: 10.12659/MSM.898103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emamian M., Hasanian S.M., Tayefi M., et al. Association of hematocrit with blood pressure and hypertension. J. Clin. Lab. Anal. 2017;31(6) doi: 10.1002/jcla.22124. Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enawgaw B., Adane N., Terefe B., Asrie F., Melku M. A comparative cross-sectional study of some hematological parameters of hypertensive and normotensive individuals at the university of Gondar hospital, Northwest Ethiopia. BMC Hematol. 2017;17:21. doi: 10.1186/s12878-017-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weymann A., Ali-Hasan-Al-Saegh S., Popov A.F., et al. Hematological índices as predictors of atrial fibrillation following isolated coronary artery by-pass grafting, valvular surgery or combined procedures: A systematic review with meta-analysis. Kardiol. Pol. 2018;76(1):107–118. doi: 10.5603/KP.a2017.0179. [DOI] [PubMed] [Google Scholar]
- 14.Musher D.M., Abers M.S., Corrales-Medina V.F. Acute infection and myocardial infarction. N. Engl. J. Med. 2019;380(2):171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 15.Lecompte T.P., Bernimoulin M.P. Novel parameters in blood cell counters. Clin. Lab. Med. 2015;(35):209–224. doi: 10.1016/j.cll.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Guyton A.C., Hall J.E. Textbook of Medical Physiology 11th edition, editing in Portuguese by Elsevier 2006. ISBN 978-85-352-1641-7; 32: 419-28; 33: 429-38; 36: 457-68. 2006 [Google Scholar]
- 17.Machlus K.R., Italiano J.E., Jr The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 2013;201(6):785–796. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green R., Wachsmann-Hogiu S. Development, history, and future of automated cell counters. Clin. Lab. Med. 2015;35(1):1–10. doi: 10.1016/j.cll.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Stachon A., Sebbers E., Holland-Letz T., Kempf R., Herinf S., Krieg M. Nucleated red blood cells in the blood of medical intensive care patients indicate increased mortality risk: A prospective cohort study. Crit. Care. 2007;11:R62. doi: 10.1186/cc5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai S., Jones S.L., Turner K.L., Hall J., Moore L.J. Nucleated red blood cells are associated with a higher mortality rate in patients with surgical sepsis. Surg. Infect. (Larchmt.) 2012;13(6):360–365. doi: 10.1089/sur.2011.089. [DOI] [PubMed] [Google Scholar]
- 21.Kuert S., Holland-Letz T., Friese J. stachon A. association of nucleated red blood cells in blood and arterial oxygen partial tension. Clin. Chem. Lab. Med. 2011;49(2):257–263. doi: 10.1515/CCLM.2011.041. [DOI] [PubMed] [Google Scholar]
- 22.Felker G.M., Allen L.A., Pocock S.J., et al. Red distribution width as a novel prognostic marker in heart failure. J. Am. Coll. Cardiol. 2007;50(1):40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 23.Tonelli M., Sacks F., Arnold M., Moye L., Davis B., Pfeffer M. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117(2):163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 24.Baskurt O.K., Meiselman H.J. Erythrocyte aggregation: Basic aspects and clinical importance. Clin. Hemorheol. Microcirc. 2013;53(1-2):23–37. doi: 10.3233/CH-2012-1573. [DOI] [PubMed] [Google Scholar]
- 25.Ami R.B., Barshtein G., Zeltser D., et al. Parameters of red blood cell aggregation as correlates of the inflammatory state. Am. J. Physiol. Heart Circ. Physiol. 2001;280(5):H1982–H1988. doi: 10.1152/ajpheart.2001.280.5.H1982. [DOI] [PubMed] [Google Scholar]
- 26.Tikhomirova I.A., Oslyakova A.O., Mikhailova S.G. Microcirculation and blood rheology in patients with cerebrovascular disorders. Clin. Hemorheol. Microcirc. 2011;49:295–395. doi: 10.3233/CH-2011-1480. [DOI] [PubMed] [Google Scholar]
- 27.Koscielny J., Jung E.M., Mrowietz C., Kiesewetter H., Latza R. Blood fluidity, fibrinogen, and cardiovascular risk factors of occlusive arterial disease: Results of the Aachen study. Clin. Hemorheol. Microcirc. 2004;31(3):185–195. [PubMed] [Google Scholar]
- 28.Packard R.R.S., Libby P. Inflamation in atherosclerosis: From vascular biology to biomarker discovery and risk prediction. Clin. Chem. 2008;54(1):24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 29.Zazula A.D., Précoma-Neto D., Gomes A.M., et al. Avaliação da relação neutrófilos/linfócitos em pacientes com suspeita de síndrome coronariana aguda. Arq. Bras. Cardiol. 2008;90(1):31–36. [Google Scholar]
- 30.Association between the Neutrophil / Lymphocyte Ratio and the Degree of Electrocardiographic Ischemia in the STEMI. Arq. Bras. Cardiol. 2015;104(2):112–119. doi: 10.5935/abc.20140179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Núñez J., Núñez E., Bodí V., et al. Low lymphocyte count in acute phase of ST-segment elevation myocardial infarction predicts long-term recurrent myovardial infarction. Coron. Artery Dis. 2010;21:1–7. doi: 10.1097/mca.0b013e328332ee15. [DOI] [PubMed] [Google Scholar]
- 32.Basem A., Zaher M., Weiserbs K.F., et al. Usefulness of neutrophil to lymphocyte ratio in predicting short and long-term mortality after non-ST elevation myocardial infarction. Am. J. Cardiol. 2010;106(4):470–476. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 33.Uysal H.B., Dagli B., Akgullu C., et al. Blood count parameteres can predict the severity of coronary artery disease. Korean J. Intern. Med. (Korean. Assoc. Intern. Med.) 2016;31:1093–1100. doi: 10.3904/kjim.2015.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verdoia M., Barbieri L., Di Giovine G., et al. Neutrophil to lymphocyte ratio and the extent of coronary artery disease: Results from a large cohort study. Angiology. 2016;67(1):75–82. doi: 10.1177/0003319715577529. [DOI] [PubMed] [Google Scholar]
- 35.Kaya H., Ertas F., Islamoglu Y., et al. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin. Appl. Thromb. Hemost. 2014;20(1):50–54. doi: 10.1177/1076029612452116. [DOI] [PubMed] [Google Scholar]
- 36.Larsen S.B., Grove E.L., Hvas A.M., Kristensen S.D. Platelet turnover in stable coronary artery disease – influence of thrombopoietin and low-grade inflamation. PLoS One. 2014;9(1):e85566. doi: 10.1371/journal.pone.0085566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grove E.L., Hvas A.M., Mortensen S.B., Larsen S.B., Kristensen S.D. Effect of platelet turnover on whole blood platelet aggregation in patients with coronary artery disease. J. Thromb. Haemost. 2011;9:185–191. doi: 10.1111/j.1538-7836.2010.04115.x. [DOI] [PubMed] [Google Scholar]
- 38.Wendland A.E., Farias M.G., Manfroi W.C. Volume plaquetário médio e doença cardiovascular. J Brasileiro Patologia Medicina Laboratorial. 2009;45(5):371–378. [Google Scholar]
- 39.Puertas M., Zayas-Castro J.L., Fabri P.J. Statiscal and prognostic analysis of dynamic changes of platelet count in ICU patients. Physiol. Meas. 2015;36:939–953. doi: 10.1088/0967-3334/36/5/939. [DOI] [PubMed] [Google Scholar]
- 40.Greinacher A., Selleng K. Thrombocytopenia in the intensive care unit patient. Hematology. 2010;2010:135–143. doi: 10.1182/asheducation-2010.1.135. [DOI] [PubMed] [Google Scholar]
- 41.Labriolle A.D., Bonello L., Lemesle G., et al. Decline in platelet count in patients treated by percutaneous coronary intervencion: Defition, incidence, prognostic importance, and predictive factors. Eur. Heart J. 2010;31(9):1079–1087. doi: 10.1093/eurheartj/ehp594. [DOI] [PubMed] [Google Scholar]
- 42.Monteiro Júnior J.G.M., Torres D.O.C., Silva M.C.F.C., et al. Evaluation of platelet parameters as prognostic analysis in cardiac intensive care units patients. Atherosclerosis. 2017;2:1. [Google Scholar]
- 43.Murat S.N., Duran M., Kalay N., et al. Relation between mean platelet volume and severity of atherosclerosis in patients with acute coronary syndromes. Angiology. 2013;64(2):131–136. doi: 10.1177/0003319711436247. [DOI] [PubMed] [Google Scholar]
- 44.Klovaite J., Benn M., Yazdanyar S., Nordestgaard B.G. High platelet volume and increased risk of myocardial infarction: 39,531 participants from the general population. J. Thromb. Haemost. 2011;9(1):49–56. doi: 10.1111/j.1538-7836.2010.04110.x. [DOI] [PubMed] [Google Scholar]
- 45.Ibrahim H., Schutt R.C., Hannawi B., DeLao T., Barker C.M., Kleiman N.S. Association of immature platelets with adverse cardiovascular outcomes. J. Am. Coll. Cardiol. 2014;2014:2122–2129. doi: 10.1016/j.jacc.2014.06.1210. [DOI] [PubMed] [Google Scholar]
- 46.Bernlochner I., Goedel A., Plischke C., et al. Impact of immature platelets on platelet response to ticagrelor and prasugrel in patients with acute coronary syndrome. Eur. Heart J. 2015;36:3202–3210. doi: 10.1093/eurheartj/ehv326. [DOI] [PubMed] [Google Scholar]
- 47.Grove E.L., Hvas A.M., Mortensen S.B., Larsen S.B., Kristensen S.D. Effect of platelet turnover on whole blood platelet aggregation in patients with coronary artery disease. J. Thromb. Haemost. 2011;9:185–191. doi: 10.1111/j.1538-7836.2010.04115.x. [DOI] [PubMed] [Google Scholar]
- 48.Buttarello M., Plebani M. Automated blood cell counts: State of the art. Am. J. Clin. Pathol. 2008;130(1):104–116. doi: 10.1309/EK3C7CTDKNVPXVTN. [DOI] [PubMed] [Google Scholar]
- 49.Ansari-Lari M.A., Kickler T.S., Borowitz M.J. Immature granulocyte measurement using the Sysmex XE-2100. Relationship to infection and sepsis. Am. J. Clin. Pathol. 2003;120(5):795–799. doi: 10.1309/LT30-BV9U-JJV9-CFHQ. [DOI] [PubMed] [Google Scholar]
- 50.Dellinger R.P., Levy M.M., Rhodes A., et al. Surviving sepsis campaign: Internacional guidelines for management of severe sepsis and septic shock 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


