Highlights
-
•
Explaining pain to patients who survived cancer should be individually tailored.
-
•
Take the patient's pain beliefs, cognitions, pain memories, social factors and dominant pain mechanism into account.
-
•
Explaining pain implies teaching patients about the underlying biopsychosocial mechanisms of pain.
-
•
Pain neuroscience education is a potential solution to improve pain outcome in cancer survivors, but should never be a stand-alone treatment.
-
•
Pain neuroscience education should precede interventions such as graded activity, exercise therapy, stress management, sleep management and dietary advice.
Keywords: Oncology, Education, Communication, Rehabilitation, Breast cancer, Prostate cancer
Abstract
Background
Pain is one of the most prevalent and debilitating symptom following cancer treatment.
Objectives
This paper entails a practical guide for clinicians willing to apply pain neuroscience education (PNE) in this specific population, or clinical researchers willing to examine the effects of PNE in patients suffering from pain following cancer.
Results
Patient-specific information (i.e. beliefs, cognitions, pain memories, social factors) as well as identification of the dominant pain mechanism are needed to tailor the education to the specific needs and beliefs of the patient. Therapists require an in-depth understanding of pain mechanisms, the skills to explain to their patients various pain mechanisms, specific communication skills (e.g. Socratic-style dialogof education) and experience with current evidence-based biopsychosocially-driven pain management strategies for successful implementation of PNE in the clinic. Rather than purely focusing on the biomedical characteristics of pain following cancer (e.g., tissue damage due to past cancer treatment), PNE implies teaching patients about the underlying biopsychosocial mechanisms of pain. Its application is backed-up by mounting evidence supporting the effectiveness of PNE in non-cancer pain populations, and a pilot study in patients having pain following cancer.
Conclusion
PNE is a potential solution to improve pain outcome in cancer survivors. Further research using sufficiently powered and well-designed randomized clinical trials should be conducted to examine the potential of PNE in patients having pain following cancer.
Background
Life expectancy for people following cancer treatment has increased due to the early detection and advances in cancer treatment.1, 2 Unfortunately, cancer survivors often face long-term symptoms that arise or persist beyond the completion of treatment, making it an increasingly important population for the field of physical therapy and rehabilitation. Together with fatigue, pain is the most frequent persistent symptom following cancer and cancer treatment,3 accounting for poor quality of life and day-to-day functioning.4, 5, 6, 7, 8 The high prevalence of pain in the growing cancer survivor population is of considerable concern due to the fact that pain is associated with reduced quality of life and day-to-day functioning.4, 5, 6, 7, 8
In the treatment phase of cancer, pain may be considered to be part of the suffering, but after this process it is difficult for patients to understand that they are ‘cured’ or in remission yet continue to suffer from pain. Most patients experience dull pain and fail to identify its origin; which tends to be frightening and cause worrisome when one has had cancer. The happiness of having survived and successfully ‘beaten’ cancer, however with a toll: he/she now has to deal with persistent and debilitating pain every single day. In general, cancer patients indicate that they do not have enough knowledge regarding pain during or after cancer, what the possibilities of pain relief are and how they can access support when needed.9, 10 Hence, the education of patients about pain should be the first part of the management of pain following cancer.
A meta-analysis of 21 trials concluded that patient-based educational interventions are effective for improving pain severity, knowledge and self-efficacy for treating cancer related pain.11 Yet education about pain is underused in the field of oncology.11 The content of the used education is variable and poorly established,11 with most of the educational interventions for cancer patients restricted to more biomedical pain management instructions (e.g., use of analgesics).11 The latter is problematic as the biomedical model falls short in explaining (persistent) pain following cancer, therefore current patient-based educational interventions emphasize a biopsychosocial framework.12 When providing a pain education program to patients suffering from cancer related pain, implementation of contemporary pain neuroscience into this program seems warranted.13 This includes explaining to patients that the pain is the sum of many processes within the nervous system, which may or may not include nociception, and thus frequently poorly reflects current tissue damage or illness status. In non-cancer pain populations, such pain neuroscience education (PNE) is welcomed very positively,14, 15 and has proven to be effective in changing pain beliefs and improving health status as well as pain coping strategies.14, 15, 16, 17, 18, 19, 20, 21, 22 An observational, uncontrolled study showed decreased pain, catastrophizing (including pain helplessness and rumination about pain) and improved pain related quality of life (medium effect sizes) following a single session of PNE in 30 patients suffering from pain following cancer.23
This article will introduce PNE in the field of oncology, specifically applied to patients suffering from pain following cancer. It is explained how PNE can be applied in patients suffering from pain following cancer. This paper entails a practical guide for clinicians willing to apply PNE in this specific population, or clinical researchers willing to examine the effects of PNE in patients suffering from pain following cancer.
Information needed to allow individually-tailored pain neuroscience education
In order to allow the clinician to adopt the PNE to the specific features, needs and perceptions of patients suffering from pain following cancer, a thorough assessment within a biopsychosocial framework is needed. This includes, but is certainly not limited to, the items listed in Table 1. A comprehensive overview of all variables required to obtain a comprehensive biopsychosocial profile of the patients having pain following cancer is beyond the scope of this paper.
Table 1.
Potential items of importance to question/assess before initiating pain neuroscience education for pain following cancer (and information needed to individually tailor the education).
| Item | Mode of assessment |
|---|---|
| Past cancer & pain treatment | Questioning + medical record screening |
| Current cancer & pain treatment | Questioning + medical record screening |
| Premorbid pain | Questioning + medical record screening |
| Pain evolution | Questioning |
| Pain location | Pain drawing88, 89 |
| Pain history, including possible pain memories | Questioning + medical record screening |
| Scar tissues | Clinical examination including inspection, palpation and possible movement investigation |
| Musculoskeletal system | Clinical examination including inspection, palpation and possible movement investigation |
| Pain beliefs/perceptions: – Interpretation of pain by the patient – Personal beliefs about provoking factors – Personal beliefs about etiology of pain – Beliefs about how long the pain will last/anticipated course – Beliefs about the consequences of the pain on life – Beliefs about the extent of which the patient thinks he can control the (course of the) pain – The extent to which one understands the pain – Influence of the pain on once mood |
Questioning and/or (brief) Illness Perceptions Questionnaire90, 91, 92 |
| Pain coping strategy | Questioning and/or a pain coping questionnaire (e.g. Pain-Coping Inventory,93 the Vanderbilt Pain Management Inventory94) |
| Pain catastrophizing | Pain Catastrophizing Scale24, 25 |
| Pain-related fear | Fear of pain questionnaire95 |
| Pain-related fear of movement | Tampa Scale Kinesiophobia96 |
| Avoidance of pain and cognitive fusion with pain | Psychology Inflexibility in Pain Scale97 |
| Anxiety | State and Trait Anxiety Inventory98 |
| Depression | Beck Depression Inventory99 or Hospital Anxiety and Depression Scale100 |
| Pain acceptance, i.e. the extent to which they accept their pain | History taking or chronic pain acceptance questionnaire101 |
| Pain (hyper)vigilance | Pain vigilance and awareness questionnaire102 |
| Perceived injustice | Injustice experience questionnaire37 |
| Social support | Questioning |
Beliefs, cognitions and pain memories
During history taking, pain perceptions (e.g. “You have successfully beaten cancer, yet you continue to suffer from pain. What do you think is causing your pain?”) and cognitions (e.g. pain catastrophizing assessed through the Pain Catastrophizing Scale24, 25) of the patient should be thoroughly assessed. Most important are perceptions about the physical and mental causes of pain as well as the consequences (Table 1). Furthermore, the following factors should be questioned; the expectations for care (anticipated outcome as well as the content of the treatment), expectations regarding the prognosis of their pain problem, coherence (the extent to which one understands the pain) and emotional representation of the pain.26
In some patients, pain is one of the reasons why they initially consult a physician. In such instances, pain will be strongly linked to the cancer itself, will be connected in the brain with receiving the cancer diagnosis, and hence may become an uncertain threat of disease recurrence.27 Indeed, receiving the cancer diagnosis is a very stressful event,28 leading to the release of cortisol and adrenaline which facilitate long-term potentiation of excitatory synapses within parts of the central nervous system.29 The mechanism of long-term potentiation is crucial for (re)learning and developing new (pain-related) memories,30, 31 and hence for altering pain memories in the brain.32 Stress burns those memories (connections) in the brain, and they will not go away easily and therefore can be a source of chronic pain following cancer. In addition to these stress mechanisms, other factors such as fear and memory, fear of pain and diagnosis, neuroinflammation, the influence of drugs on the brain, depression and anxiety may contribute to such pain memories. It is of importance to question whether such (or other) pain memories are relevant to the patient (e.g. “Was pain one of the reasons why you consulted a physician and how you found out you had cancer?”), as they can be addressed (i.e. explained) during pain neuroscience education.
Maladaptive cognitive patterns, such as catastrophizing, perceived injustice or perceived harm are equally important to recognize (Table 1 provides the mode assessment of each of these constructs). Pain catastrophizing comprises of rumination, magnification and helplessness, and has been identified as an important treatment mediator of mindfulness-based cognitive therapy on pain intensity in women treated for primary breast cancer.33 Women with persistent pain following breast cancer surgery can show high levels of pain catastrophizing that mediate alterations in central pain modulatory systems.34 Indeed, when comparing post-mastectomy patients with and without post-surgical pain, they did not differ in treatment or disease-related variables, but differed in terms of psychological factors (more anxiety, catastrophizing, depression and somatization in the patients having pain).35 Perceived injustice or perceived harm is less-well studied in the post-cancer pain population, but is an established prognostic factor in the non-cancer pain population.36, 37 Anecdotally, patients who have lived a healthy life might perceive not only the cancer itself, but especially the post-cancer pain as highly unfair, generating negative emotions that contribute to the pain experience.
Determining motivation and readiness to change is vital for further treatment. The perceptions about the cause of pain and the treatment expectations are crucial to know in order to target and modify them during the treatment,38 which can be done using PNE.
Social factors
Social factors should also be assessed (through questioning/history taking) and can be divided into housing, social environment, work, relationship with the partner (including being able to be sexually active and have an intimate relationship), prior/other treatments, socioeconomic and environmental influences (especially in low-income countries, where access to hospitals and treatment can be an issue). It is important to find out whether certain social factors are helpful and supportive, stressful or unconsciously unhelpful. Other important social factors are prior/simultaneous treatments and attitudes of the healthcare professionals that delivered these treatments. These prior/simultaneous treatments, advices and explanations will influence the perceptions and current coping strategy of patients having pain following cancer. It is important to know them and to discuss them with the patient during PNE, also to prevent contrasting views from various health care practitioners.
Low social support contributes to symptoms and poor quality of life following cancer,39, 40 but tends to be an underestimated factor in clinical practice. PNE provides the opportunity to facilitate social support in patients following cancer. By simply stimulating to share the PNE content with a significant other (partner, parent, child, close friend), social support can be enhanced. Involvement is possible through various ways, depending on the patient preferences (shared decision-making): inviting the significant other to join the patient to a face-to-face session of pain neuroscience education, the patient asking the significant other to read the information leaflet or to watch an online education video about explaining pain.
Predominant pain mechanism(s)
In order to allow tailoring PNE to the underlying pain mechanisms it is important to differentiate between the 3 major pain types (nociceptive, neuropathic and central sensitization pain). Without such pain mechanism-based tailoring, PNE may include in part explanations that do not match the patient's clinical picture and personal pain experience. For example, explaining the mechanism of central sensitization to someone without spreading of the pain or hypersensitivity symptoms, might lead to confusion rather than recognizing their pain experience in the provided information, which in turn might jeopardize the therapeutic alliance.
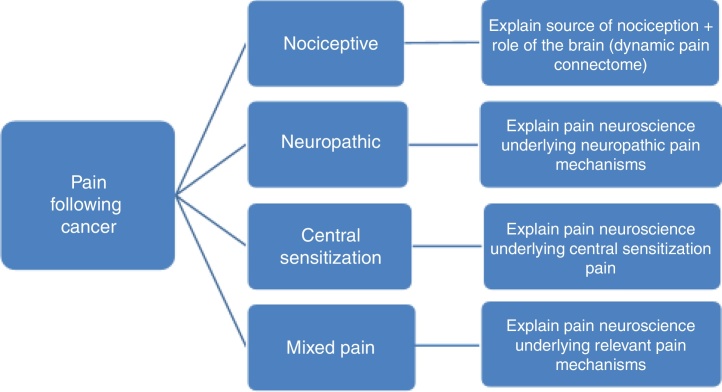
To differentiate between the various pain mechanisms among pain patients following cancer, identification of red flags and excluding the possibility of cancer recurrence or any other serious pathology is often the first step.41, 42 Next, neuropathic pain, defined as pain caused by a primary lesion or disease of the somatosensory nervous system,43 can be diagnosed by applying international guidelines.44, 45 Within the cancer survivor population, neuropathic pain is most prevalent, but mixed type of pain is also common, reflecting the complexity of the pain experience in those who completed cancer treatment.46 Examples of neuropathic pain following cancer include neuropathy from direct insult during surgery and lymph node dissection, e.g. the intercostobrachial nerve during axillary lymph node dissection47 or postsurgical adhesions and inflammation in the area of the nerve.48 In case of neuropathic pain, PNE should include the underlying (sensitizing) mechanisms explaining pain and other symptoms (e.g. sensory hypersensitivity) typically seen in neuropathic pain (Fig. 1). The PNE should also aim at decatastrophizing about the ‘neuropathic pain’ diagnosis, which can sound frightening to patients.
Figure 1.
Pain neuroscience education for patients having pain following cancer: tailoring based on mechanism-based pain classification.
For clinical purposes, the term nociceptive pain can be used when pain is proportional to nociceptive input, while central sensitization is a common pathophysiological mechanism to explain related symptoms (including pain) for which no specific organic cause can be found. Pain in patients where central nervous system sensitization dominates the clinical picture is labeled now as ‘nociplastic pain’ by the International Association for the Study of Pain,49 who defines nociplastic pain as pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain. In order to diagnose central sensitization pain, the first and either the second or third (or both) of the following three questions should be answered positively46:
-
1.
Is the pain severity disproportionate to the nature and extent of the injury or pathology (i.e., tissue damage or structural impairments which might cause nociceptive pain following cancer)? Frequent sources of nociception following cancer include bone fractures, connective tissue fibrosis, lymphatic cording, skin rashes, ulcers and scars, and inflammation.46
-
2.
Does the pain pattern presents a neuroanatomical distribution, i.e. one that is neuroanatomically plausible for the presumed source(s) of nociception?
-
3.
Does the patient scores 26 or higher on part A of the Central Sensitization Inventory?50 The Central Sensitization Inventory assesses symptoms common to central sensitization, with the total score ranging from 0 to 100.51, 52 The clinimetric properties of the Central Sensitization Inventory in the cancer survivor population have been examined and were found satisfactory, and the receiver operating characteristic curve (ROC) analysis yielded a cutoff point of the Central Sensitization Inventory score of 26 out of 100.53
In case of predominant central sensitization pain, the PNE content should focus on explaining the mechanisms of central sensitization (Fig. 1), including the increased sensitivity of the dorsal horn neurons, poor functioning of top-down inhibitory control and overactivity in the dynamic pain connectome.54
Pain neuroscience education: content and protocol when applied to post-cancer pain
Therapists’ prerequisites
The therapist can be a psychotherapist, physical therapist, nurse or any other healthcare professional as long as they have certain prerequisites for providing PNE to patients having pain following cancer. First, therapists require an in-depth understanding of pain mechanisms55 and the dysfunctional central nociceptive processing in those with chronic pain.56, 57 This includes a thorough understanding of biopsychosocial factors in the development and sustainment of chronic pain.58 Second, therapists require the skills to explain to their patients various pain mechanisms, including central sensitization and neuropathic pain as evidence-based explanations for pain following cancer.59, 60 Third, specific communication skills are required. For instance, a Socratic-style dialog of education61 is preferred over ‘lecturing’ to the patient, and therapists are advised to use shared decision making to optimize the therapeutic alliance. Finally, PNE is not a standard intervention or standalone treatment, but rather a treatment concept that should be individually tailored to the specific features, needs and perceptions of patients suffering from pain following cancer. Indeed, PNE typically clears the path for more active approaches to pain management, therapists should preferentially be familiar (or experienced) with current evidence-based biopsychosocially-driven pain management strategies including graded activity,62 graded exposure in vivo,63 stress-management and acceptance-based interventions (e.g. acceptance and commitment therapy).64, 65
Clinicians are advised to take into account that pain following cancer is contextualized by the serious condition that has been a precursor for post-cancer pain. In many cases, the pain experienced by these patients has often, at least initially, been associated with real damage and danger and therefore, has potentially a higher threat value compared to other (chronic) pain populations. Secondly, in this population, fear for recurrence might be present,66 making it harder to reconceptualise the pain as an unreliable and harmless signal. Therefore, it is very important for both patient and therapist to be confident/reassured about that issue. Finally, it is possible that cancer survivors are less admissive about their pain, due to the enormous psychological burden caused by the disease.
Learning objectives
The learning objectives of PNE comprise decreasing the threat value of pain, increasing the patients’ knowledge of pain, and reconceptualising pain. In order to achieve this, the patient needs to understand that all pain is produced, constructed and modulated by the brain (e.g. pain triggered or worsened by pain anticipation, similar to anticipatory nausea and vomiting), that their pain symptoms can partly relate to hypersensitivity of the central nervous system rather and not only (ongoing) tissue damage. Still, it is equally important to acknowledge the tissue damage that contributes to the pain experience, but at the same time broaden the patient's view and understanding regarding their pain. Finally, systematic evaluation of the effects of exercise on a multitude of cancer-related concerns, e.g., fatigue, sleep and mood reveals it is effective for these impairments, but also shows some efficacy for decreasing pain in this population.67 Pain beliefs such as pain catastrophizing,33, 68 fear of movement69 and fear of cancer (recurrence) can prevent patients following cancer of returning to normal physical activity levels or limit effect sizes of exercise interventions. Therefore, at the end of the PNE, the patient should be able to put their post-cancer pain into the right perspective and feel less threatened by the pain, leading to the willingness to perform physical activity with progression toward feared or avoided movements.
Format
The format can vary from online learning to group sessions, one-on-one sessions to blended learning formats, but in other conditions online interventions only show little positive effects.70 Preferentially (at least part of) the PNE is provided individually, allowing to adopt the PNE (content) to the individual perceptions/beliefs. For example, patients can participate in three interactive and individual sessions of PNE that will last about 30 min each, or the PNE can comprise of one group session (of approximately 5–6 patients per group) followed by an online session and one individual session.71 Given the social context of pain, at least one significant other is encouraged to participate.
To facilitate (deep) learning, the information should be presented verbally (explanation by the therapist) and visually (summaries, pictures, metaphors and diagrams on computer, whiteboard and/or paper). To assess the degree of learning and to identify possible learning gaps, patients are asked to complete the (revised) Neurophysiology of Pain Test.72 This can be done immediately after the first or second educational session, depending on the therapists’ preference.
At the end of the first session, patients receive an information leaflet about the neurophysiology of pain73 and are asked to read it carefully at home. Alternatively, patients can be asked to watch online lessons of PNE (e.g., retrainpain.org, brainman,74 etc.). Research has informed us that PNE delivered using an information leaflet only is not effective.70 Other learning formats in addition to an information leaflet are essential to achieve positive effects.75 A more detailed description of how PNE can be provided is presented elsewhere.15
Content
The content and pictures of the educational sessions can be based on the book ‘Explain Pain’55 and the “Why you hurt” therapeutic PNE system.76 PNE focuses on the nervous system and covers the physiology of nociception and pain, all presented in layman's speech, including the following topics: the physiology of:
-
1.
the neuron (receptor, axon, terminal);
-
2.
the synapse (action potential, neurotransmitters, postsynaptic membrane potential, chemically driven ion channel);
-
3.
descending nociceptive inhibition and facilitation (the influence of stress, emotions, thoughts, physical activity, etc.);
-
4.
peripheral sensitization;
-
5.
differentiation between types of pain especially acute and chronic pain;
-
6.
contributing (personal) factors in the pain experience;
-
7.
(in case of predominant neuropathic or central sensitization pain) central sensitization (receptor field growth, potentiation of the postsynaptic membrane, changes at cortical and subcortical level, etc.).
The content is provided in a standard format, but always individually tailored to the patient's (medical) background, pain perceptions, pain mechanisms (Fig. 1) and other possible relevant factors identified during the intake/baseline assessment (Table 1). Sample PNE educational material are freely available online in 6 languages.77
Contrary to PNE provided to nonspecific non-cancer pain, the contribution of (healing or past) tissue (including nerve) damage and ongoing nociception is emphasized and explained as ‘normal’ consequences of the life-saving cancer treatment. This, together with explaining the neurophysiology of nociception, is used to build the therapeutic alliance and prevents the patient from suspecting that the therapist beliefs ‘it's all in my head’. This is important as many patients are not prone for a psychological explanation in the early stage of treatment. In addition, viewing their pain as a side effects of life-saving treatment may aid patients in accepting their pain. Once the therapeutic alliance is build, other (modifiable) contributing factors, including psychological and behavioral factors and their underlying (neuro)biology, can be explained to the patient.
Initially many patients following cancer do not relate nerve damage to pain. On the contrary, anecdotally many patients wonder how they can feel pain ‘when the nerves are cut?!?’. Hence, the first step is explaining that the damaged nerves can become more sensitive and signal the brain about ongoing damage.
PNE plays a major role in explaining the role of the brain in the sensation of pain. Patients need to be made aware that despite ongoing tissue damage, the brain will channel incoming stimuli and sort which gets to generate pain. In order to achieve this, one can include the following conversation when explaining (chemotherapy-induced) neuropathic pain:
Therapist (T): ‘Now you understand that the chemotherapy has slightly damaged your small nerve fibers in your fingers and toes, let's have a look at this picture of a kind lady who had her left breast removed surgically. Breasts are full of nerve (endings), similar to the ones that are damaged in your fingers and toes. Do you think that surgical removal of her breast resulted in persisting breast pain (Here we focus on phantom breast pain rather than the more prevalent post-mastectomy shoulder/neck pain.)?’
Patient (P): ‘It must have!’
T: ‘That would seem reasonable, doesn’t it? Yet only a small minority of people receiving such breast surgery (i.e. between 9 and 25%)47, 78 will experience persisting (phantom) breast pain. Can you think of a reason why the great majority of people having had one of their breasts (partly) removed do not experience persisting (phantom breast) pain?’
P: ‘They reconstructed the nerves?!?’
T: ‘Nice reasoning! Unfortunately those nerves are too small to reconstruct surgically. Any other idea?’
P: ‘I have no idea.’
T: ‘Do you agree that the brain will receive similar signals from the damaged nerves in her (pointing to the picture of the patient who had her breast removed) situation as the signals arising from the damaged nerves in your fingers and toes?’
P: ‘I don’t know … are people having similar damage in their finger nerves as I have, who don’t have pain?’
T: ‘I really like the way you are reasoning right now. This is exactly what we need to get at. Not only the nerve damage will determine the pain you will or will not experience, many factors contribute to the pain. Remember that last time (during the intake) you mentioned several factors that aggravate your pain, and you also mentioned things that can relief your pain. For instance, you mentioned that you experience less pain when you are having fun with your friends. In such a situation, the nerve damage is still the same isn’t it? How can you explain that?’
….
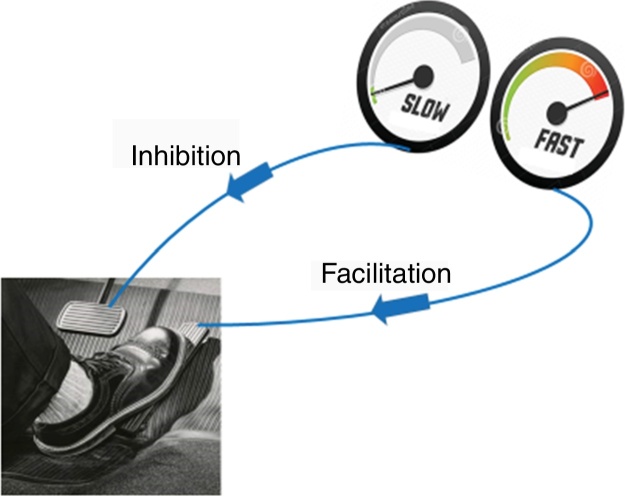
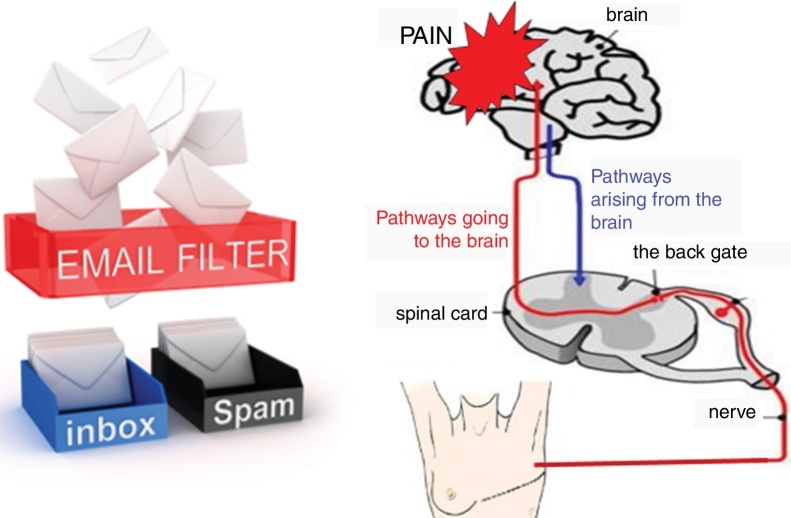
The aggravating factors can then be explained as the ones pushing on the accelerator (Fig. 2), while the pain relieving factors activate brain-orchestrated descending nociceptive inhibition,79 which can be explained easily to patients using the brake (Fig. 2) or spam filter metaphor (Fig. 3).
Figure 2.
Pain neuroscience education slide illustrating how the brain controls two top-down systems that can either inhibit (the brake) or facilitate (the accelerator) pain.
Figure 3.
Pain neuroscience education slide illustrating nociceptive pathways and using the spam filter metaphor to illustrate descending nociceptive inhibition.
For explaining the spam filter metaphor, the following conversation can be included:
T: ‘Do you use email?’
P: ‘Yes I do.’
T: ‘Do all emails send from anywhere in the world to your email account end up in your inbox?’
P: ‘I hope not, the spam filter should keep out inappropriate messages including publicity.’
T: ‘That's exactly how your nervous system works. It has a powerful spam filter in the back gate of the spinal cord (Fig. 3). What part of your body is in control of your spam filter?’
P: ‘Perhaps the brain is?’
T: ‘Exactly! During the treatment program, we will teach you how you can control the spam filter in your spinal cord.’
The conversation regarding the spam filter continues when explaining the pain matrix (or the dynamic pain connectome54):
T: ‘When you open your email inbox and you see all the messages that have passed the spam filter, do you read them all?’
P: ‘No, the first thing I do is deleting all messages that disinterest me.’
T: ‘I do the same! And so does the brain! The brain is a fantastic computer, but it simply has not enough capacity to process all input it constantly receives. Therefore, it has the spam filter in the spinal cord, but it will also be highly selective to the messages passing the spam filter and entering the brain. So even when a message arising from your painful fingers or toes will enter your brain, processing of those messages will not per se imply that you will feel pain.’
In case (older) patients are unfamiliar with using email, one need to use an alternative metaphor to explain the descending nociceptive inhibition, like a volume nod from a radio than one can turn up or down. After the patient understands the idea of descending inhibition and facilitation, (modifiable) psychological and behavioral factors can be slowly introduced and related to the patient's situation and experiences, with emphasis on how they will be addressed during subsequent phases of the comprehensive treatment program (e.g. stress as a pain amplifier will be addressed during stress management, poor sleep by sleep management; exercise therapy or graded activity will be introduced to active descending inhibition, etc.).80
For explaining the role of pain memories, the “pain library” metaphor can be introduced when explaining the pain matrix. The hippocampus serves as the memory center of the brain, and may be considered the “brain's library” which may contain some pain books/memories. Yet the patient sitting in front of you has experienced a lot of pain over the past couple of months, implying that many more pain memories were added, new books were written and the patient ended up with an entire pain library in the brain. Nearly everything the patient does on a regular day has been previously connected with a pain experience, and all those memories are written down somewhere in that library. This makes it very easy for the brain to connect everyday situations with previous pain experiences, even when the situation is not threatening at all. The brain is trying to warn the patient of being in (suspected) danger, even though there is currently nothing to be afraid of. This is similar to anticipatory nausea and vomiting in the setting of chemotherapy induced nausea and vomiting. If patients’ nausea and vomiting was not well controlled, and patients experience it, they may anticipate its recurrence prior to subsequent chemotherapy or with a trigger that was present in the environment when they experienced nausea/vomiting such as the smell of a perfume.
Further questions to be asked during the PNE session can be related to the patient's understanding and opinion regarding the PNE content (e.g. ‘Do you understand that pain and damage are not synonymous terms?’, ‘Do you think your body was recently damaged, in a way that requires pain as a warning signal for you?’, ‘Do you still think that the pain might reflect that the cancer is returning?’, ‘Do you think that your nervous system has become more sensitive?’, etc.). The therapist and patient discuss these answers by relating them to the PNE content.
The PNE will typically address past or current (cancer) treatments, including radiotherapy, surgery or chemotherapy and its (long-term) consequences. For instance, many patients who survived cancer use adjuvant endocrine therapy like aromatase inhibitors. Unfortunately, aromatase inhibitors often result in debilitating side effects like pain: approximately 50% of breast cancer survivors on aromatase inhibitor therapy report musculoskeletal pain.81 Such musculoskeletal pain remains even after stopping the adjuvant endocrine therapy. The reason for this common side effect of aromatase inhibitors is still an active area of research, and advances have been made. Animal work suggests that aromatase inhibitors selectively target the transient receptor potential ankyrin 1 (TRPA1) channel, a major pathway in pain transmission and neurogenic inflammation.82 Aromatase inhibitors activate TRPA1 channels in nociceptors, resulting in the release of sensory neuropeptides (i.e. neuro-inflammation) and mechanical hypersensitivity, together corresponding to a picture of generalized peripheral sensitization. Indeed, TRPA1 is not only expressed on the distal, but also on the central endings of the primary afferent nociceptive fibers, located within the spinal dorsal horn.83 When explaining this to patient, it often suffices to detail that:
-
-
the adjuvant endocrine therapy sensitizes the nerve endings in the musculoskeletal system rather than damaging any tissues;
-
-
the increased nerve sensitivity rather than any ongoing tissue damage explains the pain;
-
-
the adjuvant endocrine therapy increases disease free survival time,84, 85 and should therefore be continued.
Conclusion
PNE has been suggested repeatedly as an important add to current management strategies for patients having pain following cancer.13, 80 The present paper explained how PNE can be applied in patients suffering from pain following cancer, and includes a practical guide for clinicians willing to apply PNE in this specific population, or clinical researchers willing to examine the effects of PNE in patients suffering from pain following cancer. Patient-specific information (i.e. beliefs, cognitions, pain memories, social factors) as well as identification of the dominant pain mechanism are needed to tailor the education to the specific needs and beliefs of the patient. Therapists require an in-depth understanding of pain mechanisms, the skills to explain to their patients various pain mechanisms, specific communication skills (e.g. Socratic-style dialog of education) and experience with current evidence-based biopsychosocially-driven pain management strategies for successful implementation of PNE in the clinic. PNE is a potential solution to improve pain outcome in cancer survivors. Anecdotal evidence and uncontrolled study data86 illustrate the potential of PNE for patients following cancer, yet further research using sufficiently powered and well-designed randomized clinical trials should be conducted to examine the potential of PNE in patients having pain following cancer. According to the meta-analysis mentioned previously, education for cancer related pain did not result in positive effects on interference with daily activities,11 indicating that education can be a first and important step but requires follow-up with more active interventions including stress management as well as exercise and functional activity interventions.87
Funding
This work is partially funded by the Berekuyl Academy Chair, funded by the European College for Decongestive Lymphatic Therapy, the Netherlands, and awarded to Jo Nijs, Vrije Universiteit Brussel, Belgium.
Conflicts of interest
JN has co-authored a Dutch book for clinicians on pain neuroscience education, but the royalties for that book are collected by the Vrije Universiteit Brussel and not him personally. PvW co-authored a Dutch book for clinicians on PNE and receives royalties. Besides that, the authors have no conflict of interest to disclose.
Acknowledgements
This work is partially funded by the Berekuyl Academy Chair, funded by the European College for Decongestive Lymphatic Therapy, the Netherlands, and awarded to Jo Nijs, Vrije Universiteit Brussel, Belgium.
References
- 1.Ghoncheh M., Mirzaei M., Salehiniya H. Incidence and mortality of breast cancer and their relationship with the Human Development Index (HDI) in the World in 2012. Asian Pac J Cancer Prev. 2015;16(18):8439–8443. doi: 10.7314/apjcp.2015.16.18.8439. [DOI] [PubMed] [Google Scholar]
- 2.Krok-Schoen J.L., Fisher J.L., Baltic R.D., Paskett E.D. White-Black differences in cancer incidence stage at diagnosis, and survival among adults aged 85 years and older in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(11):1517–1523. doi: 10.1158/1055-9965.EPI-16-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington S., Gilchrist L., Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- 4.Cleeland C.S., Gonin R., Hatfield A.K. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330(9):592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 5.Gureje O., Von Korff M., Simon G.E., Gater R. Persistent pain and well-being: a World Health Organization Study in Primary Care. JAMA. 1998;280(2):147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 6.Avis N.E., Crawford S., Manuel J. Quality of life among younger women with breast cancer. J Clin Oncol. 2005;23(15):3322–3330. doi: 10.1200/JCO.2005.05.130. [DOI] [PubMed] [Google Scholar]
- 7.Arnow B.A., Hunkeler E.M., Blasey C.M. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262–268. doi: 10.1097/01.psy.0000204851.15499.fc. [DOI] [PubMed] [Google Scholar]
- 8.Rudy T.E., Kerns R.D., Turk D.C. Chronic pain and depression: toward a cognitive-behavioral mediation model. Pain. 1988;35(2):129–140. doi: 10.1016/0304-3959(88)90220-5. [DOI] [PubMed] [Google Scholar]
- 9.Binkley J.M., Harris S.R., Levangie P.K. Patient perspectives on breast cancer treatment side effects and the prospective surveillance model for physical rehabilitation for women with breast cancer. Cancer. 2012;118(8 Suppl.):2207–2216. doi: 10.1002/cncr.27469. [DOI] [PubMed] [Google Scholar]
- 10.McGuire D.B. Occurrence of cancer pain. J Natl Cancer Inst Monogr. 2004;(32):51–56. doi: 10.1093/jncimonographs/lgh015. [DOI] [PubMed] [Google Scholar]
- 11.Bennett M.I., Bagnall A.M., Jose Closs S. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta-analysis. Pain. 2009;143(3):192–199. doi: 10.1016/j.pain.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Moseley G.L., Butler D.S. Fifteen years of explaining pain: the past, present, and future. J Pain. 2015;16(9):807–813. doi: 10.1016/j.jpain.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Malfliet A., Leysen L., Pas R. Modern pain neuroscience in clinical practice: applied to post-cancer, paediatric and sports-related pain. Braz J Phys Ther. 2017;21(5):378–387. doi: 10.1016/j.bjpt.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louw A., Diener I., Butler D.S., Puentedura E.J. The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Arch Phys Med Rehabil. 2011;92(12):2041–2056. doi: 10.1016/j.apmr.2011.07.198. [DOI] [PubMed] [Google Scholar]
- 15.Nijs J., Paul van Wilgen C., Van Oosterwijck J., van Ittersum M., Meeus M. How to explain central sensitization to patients with ‘unexplained’ chronic musculoskeletal pain: practice guidelines. Man Ther. 2011;16(5):413–418. doi: 10.1016/j.math.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Van Oosterwijck J., Meeus M., Paul L. Pain physiology education improves health status and endogenous pain inhibition in fibromyalgia: a double-blind randomized controlled trial. Clin J Pain. 2013;29(10):873–882. doi: 10.1097/AJP.0b013e31827c7a7d. [DOI] [PubMed] [Google Scholar]
- 17.Van Oosterwijck J., Nijs J., Meeus M. Pain neurophysiology education improves cognitions, pain thresholds, and movement performance in people with chronic whiplash: a pilot study. J Rehabil Res Dev. 2011;48(1):43–58. doi: 10.1682/jrrd.2009.12.0206. [DOI] [PubMed] [Google Scholar]
- 18.Moseley G.L. Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. Eur J Pain. 2004;8(1):39–45. doi: 10.1016/S1090-3801(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 19.Moseley G.L. Widespread brain activity during an abdominal task markedly reduced after pain physiology education: fMRI evaluation of a single patient with chronic low back pain. Aust J Physiother. 2005;51(1):49–52. doi: 10.1016/s0004-9514(05)70053-2. [DOI] [PubMed] [Google Scholar]
- 20.Moseley G.L. Joining forces – combining cognition-targeted motor control training with group or individual pain physiology education: a successful treatment for chronic low back pain. J Manual Manipulative Ther. 2003;11:88–94. [Google Scholar]
- 21.Moseley G.L., Nicholas M.K., Hodges P.W. A randomized controlled trial of intensive neurophysiology education in chronic low back pain. Clin J Pain. 2004;20(5):324–330. doi: 10.1097/00002508-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Moseley G.L. Combined physiotherapy and education is efficacious for chronic low back pain. Aust J Physiother. 2002;48(4):297–302. doi: 10.1016/s0004-9514(14)60169-0. [DOI] [PubMed] [Google Scholar]
- 23.Pas R, Leysen L, de Goeij W, Vossebeld L, De Groef A, van Wilgen CP. Is pain neurophysiology education useful in cancer survivors with persistent pain? A pilot study. Submitted, 2018. [DOI] [PubMed]
- 24.Crombez G, Vlaeyen JW. The pain catastrophizing scale. 1996. Unpublished Dutch/Flemish translation.
- 25.Lame I.E., Peters M.L., Kessels A.G., Van Kleef M., Patijn J. Test–retest stability of the Pain Catastrophizing Scale and the Tampa Scale for Kinesiophobia in chronic pain over a longer period of time. J Health Psychol. 2008;13(6):820–826. doi: 10.1177/1359105308093866. [DOI] [PubMed] [Google Scholar]
- 26.Wijma A.J., van Wilgen C.P., Meeus M., Nijs J. Clinical biopsychosocial physiotherapy assessment of patients with chronic pain: the first step in pain neuroscience education. Physiother Theory Pract. 2016;32(5):368–384. doi: 10.1080/09593985.2016.1194651. [DOI] [PubMed] [Google Scholar]
- 27.Heathcote L.C., Eccleston C. Pain and cancer survival: a cognitive-affective model of symptom appraisal and the uncertain threat of disease recurrence. Pain. 2017;158(7):1187–1191. doi: 10.1097/j.pain.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 28.Groarke A., Curtis R., Kerin M. Global stress predicts both positive and negative emotional adjustment at diagnosis and post-surgery in women with breast cancer. Psychooncology. 2013;22(1):177–185. doi: 10.1002/pon.2071. [DOI] [PubMed] [Google Scholar]
- 29.Timmermans W., Xiong H., Hoogenraad C.C., Krugers H.J. Stress and excitatory synapses: from health to disease. Neuroscience. 2013;248:626–636. doi: 10.1016/j.neuroscience.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 30.Ji R.R., Kohno T., Moore K.A., Woolf C.J. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26(12):696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Zhuo M. A synaptic model for pain: long-term potentiation in the anterior cingulate cortex. Mol Cells. 2007;23(3):259–271. [PubMed] [Google Scholar]
- 32.Nijs J., Lluch Girbes E., Lundberg M., Malfliet A., Sterling M. Exercise therapy for chronic musculoskeletal pain: innovation by altering pain memories. Man Ther. 2015;20(1):216–220. doi: 10.1016/j.math.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Johannsen M., O’Connor M., O’Toole M.S., Jensen A.B., Zachariae R. Mindfulness-based cognitive therapy and persistent pain in women treated for primary breast cancer: exploring possible statistical mediators – results from a randomized controlled trial. Clin J Pain. 2018;34(1):59–67. doi: 10.1097/AJP.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 34.Edwards R.R., Mensing G., Cahalan C. Alteration in pain modulation in women with persistent pain after lumpectomy: influence of catastrophizing. J Pain Symptom Manage. 2013;46(1):30–42. doi: 10.1016/j.jpainsymman.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreiber K.L., Martel M.O., Shnol H. Persistent pain in postmastectomy patients: comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. Pain. 2013;154(5):660–668. doi: 10.1016/j.pain.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrari R. A prospective study of perceived injustice in whiplash victims and its relationship to recovery. Clin Rheumatol. 2015;34(5):975–979. doi: 10.1007/s10067-014-2693-0. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan M.J., Adams H., Horan S., Maher D., Boland D., Gross R. The role of perceived injustice in the experience of chronic pain and disability: scale development and validation. J Occup Rehabil. 2008;18(3):249–261. doi: 10.1007/s10926-008-9140-5. [DOI] [PubMed] [Google Scholar]
- 38.Turk D.C., Okifuji A. Psychological factors in chronic pain: evolution and revolution. J Consult Clin Psychol. 2002;70(3):678–690. doi: 10.1037//0022-006x.70.3.678. [DOI] [PubMed] [Google Scholar]
- 39.Faller H., Schilling S., Otteni M., Lang H. Social support and social stress in tumor patients and their partners. Z Psychosom Med Psychoanal. 1995;41(2):141–157. [PubMed] [Google Scholar]
- 40.Servaes P., Verhagen S., Bleijenberg G. Determinants of chronic fatigue in disease-free breast cancer patients: a cross-sectional study. Ann Oncol. 2002;13(4):589–598. doi: 10.1093/annonc/mdf082. [DOI] [PubMed] [Google Scholar]
- 41.Waddell G. Churchill Livingstone; Edinburgh: 1998. The Back Pain Revolution. [Google Scholar]
- 42.Koes B.W., van Tulder M.W., Ostelo R., Kim Burton A., Waddell G. Clinical guidelines for the management of low back pain in primary care: an international comparison. Spine (Phila Pa 1976) 2001;26(22):2504–2513. doi: 10.1097/00007632-200111150-00022. [discussion 13-4] [DOI] [PubMed] [Google Scholar]
- 43.Merskey H., NBatITFoT . Part III: Pain terms, a current list with definitions and notes on usage. In: Merskey H., NBatITFoT, editors. Classification of Chronic Pain. second edition. IASP Press; Seattle, USA: 1994. pp. 209–214. [Google Scholar]
- 44.Treede R.D., Jensen T.S., Campbell J.N. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 45.Haanpää M.T.R. Diagnosis and classification of neuropathic pain. Pain Clin Updates. 2010;XVII:7. [Google Scholar]
- 46.Nijs J., Leysen L., Adriaenssens N. Pain following cancer treatment: guidelines for the clinical classification of predominant neuropathic, nociceptive and central sensitization pain. Acta Oncol. 2016;55(6):659–663. doi: 10.3109/0284186X.2016.1167958. [DOI] [PubMed] [Google Scholar]
- 47.Peuckmann V., Ekholm O., Rasmussen N.K. Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain. 2009;13(5):478–485. doi: 10.1016/j.ejpain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Kim J.H., Dougherty P.M., Abdi S. Basic science and clinical management of painful and non-painful chemotherapy-related neuropathy. Gynecol Oncol. 2015;136(3):453–459. doi: 10.1016/j.ygyno.2015.01.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kosek E., Cohen M., Baron R. Do we need a third mechanistic descriptor for chronic pain states? Pain. 2016;157(7):1382–1386. doi: 10.1097/j.pain.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 50.Mayer T.G., Neblett R., Cohen H. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012;12(4):276–285. doi: 10.1111/j.1533-2500.2011.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neblett R., Cohen H., Choi Y. The Central Sensitization Inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain. 2013;14(5):438–445. doi: 10.1016/j.jpain.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neblett R., Hartzell M.M., Cohen H. Ability of the central sensitization inventory to identify central sensitivity syndromes in an outpatient chronic pain sample. Clin J Pain. 2014;31(4) doi: 10.1097/AJP.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 53.Scheepbouwer P.N.J. Vrije Universiteit Brussel; Brussels: 2017. Assessment of Central Sensitization in Cancer Survivors: Psychometric Properties of the Dutch Central Sensitization Inventory. [Google Scholar]
- 54.Kucyi A., Davis K.D. The dynamic pain connectome. Trends Neurosci. 2015;38(2):86–95. doi: 10.1016/j.tins.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Butler D., Moseley G.L. NOI Group Publishing; Adelaide: 2003. Explain Pain. [Google Scholar]
- 56.Woolf C.J. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl.):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woolf C.J., Salter M.W. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 58.Vlaeyen J.W., Crombez G. Fear of movement/(re)injury, avoidance and pain disability in chronic low back pain patients. Man Ther. 1999;4(4):187–195. doi: 10.1054/math.1999.0199. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez-Lao C., Cantarero-Villanueva I., Fernandez-de-las-Penas C., Del-Moral-Avila R., Menjon-Beltran S., Arroyo-Morales M. Widespread mechanical pain hypersensitivity as a sign of central sensitization after breast cancer surgery: comparison between mastectomy and lumpectomy. Pain Med. 2011;12(1):72–78. doi: 10.1111/j.1526-4637.2010.01027.x. [DOI] [PubMed] [Google Scholar]
- 60.Liu S., Liu Y.P., Yue D.M., Liu G.J. Protease-activated receptor 2 in dorsal root ganglion contributes to peripheral sensitization of bone cancer pain. Eur J Pain. 2013;18(3) doi: 10.1002/j.1532-2149.2013.00372.x. [DOI] [PubMed] [Google Scholar]
- 61.Siemonsma P.C., Schroder C.D., Dekker J.H., Lettinga A.T. The benefits of theory for clinical practice: cognitive treatment for chronic low back pain patients as an illustrative example. Disabil Rehabil. 2008;30(17):1309–1317. doi: 10.1080/09638280701610254. [DOI] [PubMed] [Google Scholar]
- 62.Macedo L.G., Smeets R.J., Maher C.G., Latimer J., McAuley J.H. Graded activity and graded exposure for persistent nonspecific low back pain: a systematic review. Phys Ther. 2010;90(6):860–879. doi: 10.2522/ptj.20090303. [DOI] [PubMed] [Google Scholar]
- 63.de Jong J.R., Vlaeyen J.W., Onghena P., Cuypers C., den Hollander M., Ruijgrok J. Reduction of pain-related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. Pain. 2005;116(3):264–275. doi: 10.1016/j.pain.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 64.Wicksell R.K., Ahlqvist J., Bring A., Melin L., Olsson G.L. Can exposure and acceptance strategies improve functioning and life satisfaction in people with chronic pain and whiplash-associated disorders (WAD)?. A randomized controlled trial. Cogn Behav Ther. 2008;37(3):169–182. doi: 10.1080/16506070802078970. [DOI] [PubMed] [Google Scholar]
- 65.Wicksell R.K., Olsson G.L., Hayes S.C. Psychological flexibility as a mediator of improvement in Acceptance and Commitment Therapy for patients with chronic pain following whiplash. Eur J Pain. 2010;14(10) doi: 10.1016/j.ejpain.2010.05.001. 1059 e1–e11. [DOI] [PubMed] [Google Scholar]
- 66.Heins M.J., Korevaar J.C., Rijken P.M., Schellevis F.G. For which health problems do cancer survivors visit their general practitioner? Eur J Cancer. 2012;49(1) doi: 10.1016/j.ejca.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 67.Mishra S.I., Scherer R.W., Geigle P.M. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8:Cd007566. doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terkawi A.S., Tsang S., Alshehri A.S., Mulafikh D.S., Alghulikah A.A., AlDhahri S.F. The burden of chronic pain after major head and neck tumor therapy. Saudi J Anaesth. 2017;11(Suppl. 1) doi: 10.4103/sja.SJA_162_17. S71–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Velthuis M.J., Peeters P.H., Gijsen B.C. Role of fear of movement in cancer survivors participating in a rehabilitation program: a longitudinal cohort study. Arch Phys Med Rehabil. 2012;93(2):332–338. doi: 10.1016/j.apmr.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 70.van Ittersum M.W., van Wilgen C.P., van der Schans C.P., Lambrecht L., Groothoff J.W., Nijs J. Written pain neuroscience education in fibromyalgia: a multicenter randomized controlled trial. Pain Pract. 2014;14(8):689–700. doi: 10.1111/papr.12137. [DOI] [PubMed] [Google Scholar]
- 71.Malfliet A., Kregel J., Meeus M. Blended-learning pain neuroscience education for people with chronic spinal pain: randomized controlled multicenter trial. Phys Ther. 2018;98(5):357–368. doi: 10.1093/ptj/pzx092. [DOI] [PubMed] [Google Scholar]
- 72.Moseley L. Unraveling the barriers to reconceptualization of the problem in chronic pain: the actual and perceived ability of patients and health professionals to understand the neurophysiology. J Pain. 2003;4(4):184–189. doi: 10.1016/s1526-5900(03)00488-7. [DOI] [PubMed] [Google Scholar]
- 73.van Wilgen C.P., Nijs J. Bohn Stafleu van Loghum; 2010. Pijneducatie: een praktische handleiding voor (para) medici. [Google Scholar]
- 74.Brainman Youtube channel. 2015.
- 75.Louw A., Zimney K., Puentedura E.J., Diener I. The efficacy of pain neuroscience education on musculoskeletal pain: a systematic review of the literature. Physiother Theory Pract. 2016;32(5):332–355. doi: 10.1080/09593985.2016.1194646. [DOI] [PubMed] [Google Scholar]
- 76.Louw A. International Spine & Pain Institute; USA: 2014. Why You Hurt. Therapeutic Neuroscience Education System. [Google Scholar]
- 77.Group PiMIR . 2018. Pain Neuroscience Education Material Available in 6 Languages. http://www.paininmotion.be/education/tools-for-clinical-practice (accessed 04-12-2018) [Google Scholar]
- 78.Beyaz S.G., Ergonenc J.S., Ergonenc T., Sonmez O.U., Erkorkmaz U., Altintoprak F. Postmastectomy pain: a cross-sectional study of prevalence pain characteristics, and effects on quality of life. Chin Med J (Engl) 2016;129(1):66–71. doi: 10.4103/0366-6999.172589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Millan M.J. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 80.Nijs J., Leysen L., Pas R. Treatment of pain following cancer: applying neuro-immunology in rehabilitation practice. Disabil Rehabil. 2016:1–8. doi: 10.1080/09638288.2016.1261418. [DOI] [PubMed] [Google Scholar]
- 81.Peppone L.J., Janelsins M.C., Kamen C. The effect of YOCAS(c)((R)) yoga for musculoskeletal symptoms among breast cancer survivors on hormonal therapy. Breast Cancer Res Treat. 2015;150(3):597–604. doi: 10.1007/s10549-015-3351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fusi C., Materazzi S., Benemei S. Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nat Commun. 2014;5:5736. doi: 10.1038/ncomms6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pertovaara A., Koivisto A. TRPA1 ion channel in the spinal dorsal horn as a therapeutic target in central pain hypersensitivity and cutaneous neurogenic inflammation. Eur J Pharmacol. 2011;666(1–3):1–4. doi: 10.1016/j.ejphar.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 84.Hind D., Ward S., De Nigris E., Simpson E., Carroll C., Wyld L. Hormonal therapies for early breast cancer: systematic review and economic evaluation. Health Technol Assess. 2007;11(26) doi: 10.3310/hta11260. iii–iv, ix–xi, 1–134. [DOI] [PubMed] [Google Scholar]
- 85.John-Baptiste A.A., Wu W., Rochon P., Anderson G.M., Bell C.M. A systematic review and methodological evaluation of published cost-effectiveness analyses of aromatase inhibitors versus tamoxifen in early stage breast cancer. PLoS One. 2013;8(5):e62614. doi: 10.1371/journal.pone.0062614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Goeij W., Vossebeld L. Vrije Universiteit Brussel; Brussels: 2017. Development and Pilot Testing of a Pain Neuroscience Education Program For Cancer Survivors. [Google Scholar]
- 87.Nijs J., Leysen L., Pas R. Treatment of pain following cancer: applying neuro-immunology in rehabilitation practice. Disabil Rehabil. 2018;40(6):714–721. doi: 10.1080/09638288.2016.1261418. [DOI] [PubMed] [Google Scholar]
- 88.Margolis R.B., Tait R.C., Krause S.J. A rating system for use with patient pain drawings. Pain. 1986;24(1):57–65. doi: 10.1016/0304-3959(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 89.Margolis R.B., Chibnall J.T., Tait R.C. Test–retest reliability of the pain drawing instrument. Pain. 1988;33(1):49–51. doi: 10.1016/0304-3959(88)90202-3. [DOI] [PubMed] [Google Scholar]
- 90.Broadbent E., Petrie K.J., Main J., Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 91.Leysen M., Nijs J., Meeus M. Clinimetric properties of illness perception questionnaire revised (IPQ-R) and brief illness perception questionnaire (brief IPQ) in patients with musculoskeletal disorders: a systematic review. Man Ther. 2015;20(1):10–17. doi: 10.1016/j.math.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 92.Giannousi Z., Manaras I., Georgoulias V., Samonis G. Illness perceptions in Greek patients with cancer: a validation of the Revised-Illness Perception Questionnaire. Psychooncology. 2010;19(1):85–92. doi: 10.1002/pon.1538. [DOI] [PubMed] [Google Scholar]
- 93.Kraaimaat F.W., Evers A.W. Pain-coping strategies in chronic pain patients: psychometric characteristics of the pain-coping inventory (PCI) Int J Behav Med. 2003;10(4):343–363. doi: 10.1207/s15327558ijbm1004_5. [DOI] [PubMed] [Google Scholar]
- 94.Brown G.K., Nicassio P.M. Development of a questionnaire for the assessment of active and passive coping strategies in chronic pain patients. Pain. 1987;31(1):53–64. doi: 10.1016/0304-3959(87)90006-6. [DOI] [PubMed] [Google Scholar]
- 95.Lundberg M., Grimby-Ekman A., Verbunt J., Simmonds M.J. Pain-related fear: a critical review of the related measures. Pain Res Treat. 2011;494196(10):15. doi: 10.1155/2011/494196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.French D.J., France C.R., Vigneau F., French J.A., Evans R.T. Fear of movement/(re)injury in chronic pain: a psychometric assessment of the original English version of the Tampa scale for kinesiophobia (TSK) Pain. 2007;127(1–2):42–51. doi: 10.1016/j.pain.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 97.Wicksell R.K., Lekander M., Sorjonen K., Olsson G.L. The Psychological Inflexibility in Pain Scale (PIPS) – statistical properties and model fit of an instrument to assess change processes in pain related disability. Eur J Pain. 2010;14(7):771. doi: 10.1016/j.ejpain.2009.11.015. e1–e14. [DOI] [PubMed] [Google Scholar]
- 98.Gros D.F., Antony M.M., Simms L.J., McCabe R.E. Psychometric properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA): comparison to the State-Trait Anxiety Inventory (STAI) Psychol Assess. 2007;19(4):369–381. doi: 10.1037/1040-3590.19.4.369. [DOI] [PubMed] [Google Scholar]
- 99.Beck A.T., Guth D., Steer R.A., Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35(8):785–791. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 100.Erol Y., Cakan A., Ergonul A.G. Psychiatric assessments in patients operated on due to lung cancer. Asian Cardiovasc Thorac Ann. 2017 doi: 10.1177/0218492317724044. 218492317724044. [DOI] [PubMed] [Google Scholar]
- 101.Wicksell R.K., Olsson G.L., Melin L. The Chronic Pain Acceptance Questionnaire (CPAQ) – further validation including a confirmatory factor analysis and a comparison with the Tampa Scale of Kinesiophobia. Eur J Pain. 2009;13(7):760–768. doi: 10.1016/j.ejpain.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 102.Roelofs J., Peters M.L., McCracken L., Vlaeyen J.W. The pain vigilance and awareness questionnaire (PVAQ): further psychometric evaluation in fibromyalgia and other chronic pain syndromes. Pain. 2003;101(3):299–306. doi: 10.1016/S0304-3959(02)00338-X. [DOI] [PubMed] [Google Scholar]