Highlights
-
•
NMES might be used as adjuvant therapy to improve sitting and standing GMFM dimensions.
-
•
NMES is not better than PT alone to improve GMFM walking dimension.
-
•
Further research is still necessary to determine the precise effects of NMES on GMFM.
Keywords: Rehabilitation, Electrical stimulation, Systematic review, Child, Physical therapy modalities
Abstract
Objective
To systematically review the effectiveness of neuromuscular electrical stimulation (NMES) as an adjuvant therapy to improve gross motor function in children with spastic cerebral palsy.
Methods
MEDLINE, EMBASE, Cochrane CENTRAL, PEDro and Scopus were searched. We included randomized controlled trials examining the effects of NMES combined with other therapies on gross motor function as assessed by the Gross Motor Function Measure (GMFM) and its functional dimensions. Two reviewers independently screened, extracted data, assessed the risk of bias (PEDro) and quality of the evidence (GRADE).
Results
Six randomized controlled trials (pooled n = 174) were included in the meta-analysis. NMES combined with other therapies presented medium effect size to improve gross motor function in children with cerebral palsy in comparison with conventional physical therapy or neurodevelopmental therapy. Our sensitivity analysis showed that NMES combined with other therapies was effective to improve GMFM-sitting and standing dimensions but not GMFM-walking dimension.
Conclusion
Low-quality evidence suggests that NMES may be used as adjuvant therapy to improve sitting and standing dimensions of GMFM in children with spastic cerebral palsy.
Introduction
Cerebral palsy (CP) is a common clinical disorder affecting movements and posture.1 CP primarily reduces muscle strength,2 and impairs the selective motor control.3 This non-progressive brain injury may prevent the child from achieving gross motor functions, that are essential to perform daily activities.4, 5
Complete development of gross motor function in children is described as the acquirement of unsupported sitting, crawling, and walking.6 It is well known that a late development of gross motor function, as well as the decrease in postural control, are the most important problems observed in children with CP.6, 7 Therefore, children with spastic CP have limitations to perform several daily activities including sitting, standing and walking. Sitting is an important ability for many functional activities, mainly because it facilitates transfers and allows the independent use of upper limbs to manipulate objects.6, 7
Some scales have been developed with the purpose of evaluating gross motor function. The Gross Motor Function Measure (GMFM) is a standard tool used for clinical rehabilitation and research purposes.8 GMFM consists of 88 or 66 items grouped into five functional dimensions: GMFM-A (lying down and rolling); GMFM-B (sitting); GMFM-C (crawling and kneeling); GMFM-D (standing); GMFM-E (walking, running and jumping).9 Each GMFM subsection can be used separately in order to verify motor changes in a specific dimension of interest.9
Neuromuscular electrical stimulation (NMES) is a common type of electrical stimulation (ES) and has been used as adjuvant therapy to improve gross motor function in children with CP.10 NMES consists in the application of an electrical current with sufficient intensity to produce muscle contraction by depolarizing local motor nerves to facilitate the muscle strength and reduce spasticity.11, 12, 13
A previous systematic review and some controlled trials have shown positive effects of NMES on gross motor function of children with CP. However, the effects of NMES on functional dimensions of GMFM as sitting, standing and walking are, so far, unknown. The aim of this systematic review with meta-analysis was to verify the effectiveness of NMES combined with other therapies on gross motor function through GMFM and its different dimensions in children with spastic CP.
Methods
This systematic review was carried out in accordance with the Cochrane Collaboration15 and the Preferred Reporting Items for Systematic Review and Meta-Analyses: The PRISMA Statement.16 PICO framework: P = children with spastic cerebral palsy; I = NMES; C = conventional physical therapy or neurodevelopmental therapy; O = GMFM. The protocol was registered at the International Prospective Register of Systematic Reviews – PROSPERO and can be accessed online (CRD42018103972).
Eligibility criteria
To be included in this systematic review, studies had to be randomized controlled trials examining the effects of NMES combined with other therapies on gross motor function of children with CP. We included all trials that applied NMES on muscles of trunk or lower limbs, regardless of the ES dosage. The comparison group must not have received ES.
Clinical trials that did not provide information regarding the magnitude of the intervention effect, either in the experimental or in the control groups, were excluded from the meta-analysis.
Search strategy
Literature search was conducted in the following electronic databases (from the inception to December 2018): MEDLINE (accessed by PubMed), EMBASE, Cochrane Central Register of Controlled Trials (Cochrane CENTRAL), Physiotherapy Evidence Database (PEDro), and Scopus. The search terms used individually or combined included ‘cerebral palsy’, ‘electrical stimulation’ and a string of words previously proposed, which yielded a high sensibility in the search for randomized controlled trials.17 To enhance the sensitivity of the search, words related to the outcomes of interest were not included. There were no language restrictions in the strategy, but non-English studies were included only when the translation was possible. References used in the published articles were identified and used as an additional source to identify other clinical trials. The complete search strategy is shown in Appendix 1.
Study selection and data extraction
Two reviewers independently screened the titles and abstracts of the identified studies. A standard screening checklist based on the eligibility criteria was used for each study. Studies that did not meet the criteria were excluded. A third reviewer solved all disagreements related to trial eligibility and assisted in the decision process of including or excluding studies. For studies without sufficient information to evaluate the eligibility, authors were contacted via email to obtain clarifications. Studies with insufficient information, even after contact with authors, were excluded.
We extracted the following data from the studies: methodological design, number of subjects, comparison groups, intervention protocol, frequency, intensity and duration of stimulation, as well as results of the outcomes. The primary outcome extracted was the gross motor function, assessed by means and functional dimensions of GMFM-88 or -66.
Risk of bias assessment
The same two reviewers also assessed the risk of bias of studies using the PEDro scale. We collected the PEDro scores directly from PEDro database when they were available. The PEDro scale assesses the methodological quality of a study based on important criteria, such as concealed allocation, intention-to-treat analysis, and the adequacy of blinding. The scale consists of 11 items. One item of the PEDro scale (regarding eligibility criteria) is related to external validity and generally is not used to calculate the method score. Then, the score ranges from 0 to 10.18 Studies were classified as follows: scores 9 or 10, excellent; scores between 6 and 8, good; scores 4 or 5, fair; and scores <4, poor. Scores between 0 and 5 correspond to a high risk of bias; scores ranging from 6 to 10 correspond to a low risk of bias.19
The overall quality of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE). This approach comprises five items: (1) presence of within-study limitations (risk of bias); (2) inconsistency of results (unexplained heterogeneity of results); (3) indirectness of evidence (target population, intervention, and outcomes of interest); (4) imprecision of the effect estimates (regarding the confidence interval, i.e. confidence in the estimates of effect); (5) Publication bias (systematic underestimation or an over-estimation of the underlying beneficial or harmful effect due to the selective publication of studies). Each non-satisfied item downgraded the overall quality of evidence for each outcome. The quality of the evidence was classified into four categories: high, moderate, low, and very low.19 We created the Evidence Profile and Summary of Findings Table for each population using the GRADE's electronic tool GRADEpro GDT (available at www.gradepro.org).
Data analysis
For quantitative synthesis, pooled-effect estimates were obtained comparing the change between baseline and follow-ups of studies in each group. For those studies with more than one time-point, we used the time point immediately after the intervention. If the change score was not available, we calculated the delta score (change) based on data from post and baseline evaluations. Results were presented as the weighted mean difference (MD with 95% confidence intervals (95% CIs). Statistical heterogeneity was assessed using I2 statistic. I2 values below 25% were considered indicative of low heterogeneity, between 25% and 50% moderate heterogeneity and I2 values above 50% were considered as high heterogeneity. Calculations were performed using the random effects method.
Effect sizes were based on thresholds defined by Cohen, which categorize effect size as small (Standard mean difference (SMD) between 0.2 and 0.5), medium (SMD ranges from 0.5 to 0.8), and large (SMD greater than 0.8).20 A p-value ≤0.05 was considered statistically significant. All analyses were conducted using the R statistical software (version 1.0.153, package metaphor version 2.0-0).20
Results
Description of the selected studies
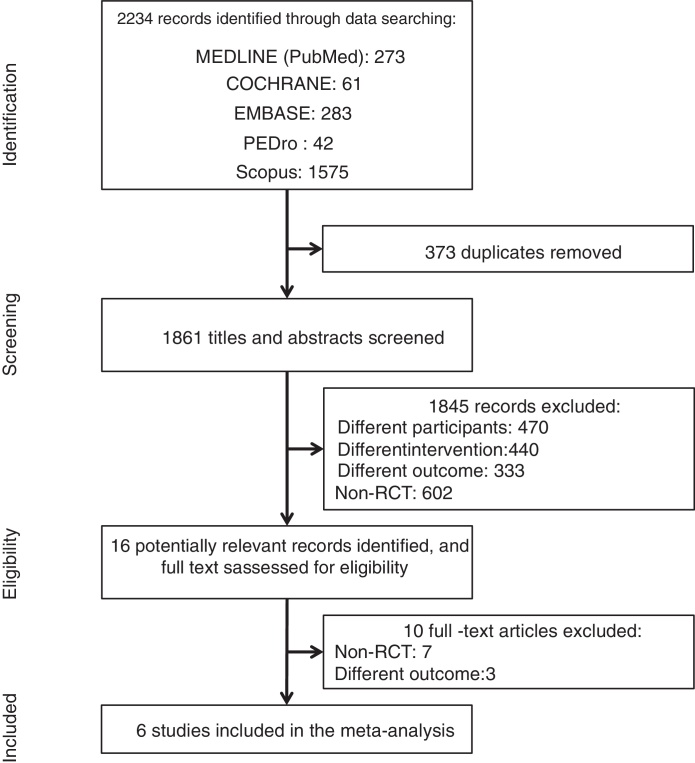
A total of 2234 articles were identified by the search strategy. After screening titles and abstracts, 16 studies were considered potentially relevant. The majority of the screened studies were not randomized controlled trials and were excluded. Six randomized trials met the eligibility criteria and were included in the meta-analysis,14, 21, 22, 23, 24, 25 providing data from 174 children with CP, of which 90 were included in the intervention group and 84 in the control group. Fig. 1 shows the flow diagram of the studies.
Figure 1.
The flow diagram of studies included in the review.
Participants
The mean age (years) of participants ranged from 1.0425 to 8.6.24 Studies included only children with spastic CP.14, 21, 22, 23, 24, 25 Regarding the topographic distribution, papers included children with unilateral asymmetric spasticity (hemiplegic), bilateral symmetric spasticity (diplegic) and bilateral symmetric spasticity (quadriplegic).26
Characteristics of the studies
Characteristics of studies and their protocols are summarized in Table 1. All studies used NMES combined with other therapies (conventional physical therapy14, 24; neurodevelopmental therapy (NDT)25; conventional physical therapy and NDT22, 23; treadmill21).
Table 1.
Summary of included studies.
| Study | Design | Participants and groups allocation | Intervention |
Outcome measures | |
|---|---|---|---|---|---|
| Frequency and duration | Parameters of stimulation | ||||
| Chan et al. (2004)21 | RCT | Participants: children with spastic diplegic or hemiplegic CP Experimental group: n: 6; age (yr): 6.3 ± 1.0; gender: 4 M, 2 F Control group: n: 6; age (yr): 6.5 ± 2.7; gender: 5 M, 1 F |
Experimental group: NMES applied on the triceps surae muscle + treadmill (0.45–0.80 m/s) 15 min, 3/wk × 4 wk Control group: Treadmill (0.45–0.80 m/s) 15 min, 3/wk × 4 wk |
Frequency: 30–35 Hz Intensity: visible muscle contraction Pulse width: not reported Time on/time off: not reported |
GMFM dimension D (standing) GMFM dimension E (walking, running and jumping) |
| Karabay et al. (2012)22 | RCT | Participants: children with spastic diplegic CP Experimental group: n: 17; age (yr): 5.4 ± 2.0; gender: not reported Control group: n: 16; age (yr): 6.5 ± 2.74; gender: not reported |
Experimental group: FES applied on lumbar and abdominal muscles + conventional physical therapy and neurodevelopmental therapy 30 min, 5/wk × 4 wk Control group: Conventional physical therapy and neurodevelopmental therapy 30 min, 5/wk × 4 wk |
Frequency: 25 Hz Intensity: 20–30 mA Pulse width: 250 μs Time on/time off: 10 s/12 s |
GMFM dimension B (sitting) (trunk control-sitting balance) |
| Karabay et al. (2016)23 | RCT | Participants: children with spastic diplegic CP Experimental group: n: 23; age (yr): 5.4 ± 2.0; gender: not reported Control group: n: 19; age (yr): 6.5 ± 2.74; gender: not reported |
Experimental group: NMES applied on lumbar and abdominal muscles + conventional physical therapy and neurodevelopmental therapy 30 min, 5/wk × 4 wk Control group: Conventional physical therapy and neurodevelopmental therapy 30 min, 5/wk × 4 wk |
Frequency: 25 Hz Intensity: 20 to 30 mA Pulse width: 250 μs Time on/time off: 10 s/12 s |
GMFM dimension B (sitting) (trunk control-sitting balance) |
| Linden et al. (2003)24 | RCT | Participants: children with hemiplegic diplegic and quadriplegic CP. Experimental group: n: 11; age (yr): 8.6 ± 2.10; gender: not reported Control group: n: 11; age (yr): 8.2 ± 2.11; gender: not reported |
Experimental group: NMES applied on the gluteus maximus of the most affected leg + conventional physical therapy and home exercise therapy 60 min, 6/wk × 8 wk (adaptation for 2 wk) Control group: Conventional physical therapy and home exercise therapy |
Frequency: Week 1–10 Hz (60 min) Week 2–30 Hz (30 min); 10 Hz (30 min) Week 3–8 – 30 Hz (60 min). Intensity: not reported Pulse width: week 1 – 75 μs (60 min) Week 2 – 100 μs (30 min); 75 μs (30 min) Week 3–8 – 100 μs (60 min). Time on/time off: week 1 – 5 s/10 s (60 min) Week 2 – 5 s/15 s (30 min); 5 s/10 s (30 min) Week 3–8 – 5 s/15 s (60 min) |
GMFM dimension E (walking, running and jumping) |
| Mohanty et al. (2016)14 | RCT | Participants: children with spastic diplegic CP. Experimental group: n: 20; age (yr): not reported separately; Gender: not reported Control group: n: 20; age (yr): not reported separately; Gender: not reported |
Experimental group: NMES applied on the gluteus maximus (lying position) and quadriceps (sitting position) + conventional physical therapy (strengthening exercises) 15 min each muscle (30 min total), 5/wk × 6 wk (adaptation for 2 wk) Control group: Conventional physical therapy (strengthening exercises) 30 min, 5/wk × 6 wk |
Frequency: 50 Hz. Intensity: maximum tolerable used to produce a visible contraction but as tolerated by the child Pulse width: 300 μs Time on/time off: 5 s/15 s |
GMFM dimension D (standing) GMFM dimension E (walking, running and jumping) |
| Park et al. (2001)25 | RCT | Participants: children with spastic diplegic CP. Experimental group: n: 14; age (yr): 1.13 ± 0.36; gender: 9 M, 5 F Control group: n: 12; age (yr): 1.04 ± 0.31; gender: 8 M, 4 F |
Experimental group: FES applied on the abdomen and posterior back muscles + neurodevelopmental therapy 30 min, 6/wk × 6 wk Control group: Neurodevelopmental therapy 30 min, 5/wk × 6 wk |
Frequency: 35 Hz. Intensity: 25–30 mA Pulse width: 250 μs Time on/time off: 5 s/15 s |
GMFM dimension D (standing) |
Abbreviations: CP, cerebral palsy; FES, functional electrical stimulation; GMFM, gross motor function measure; M, male; F, female; NMES, neuromuscular electrical stimulation; RCT, randomized controlled trial; yr, year; wk, week.
Considering the stimulation parameters, the frequencies ranged from 10 Hz24 to 50 Hz14 and the pulse width from 75 μs24 to 300 μs.14 The intensity of stimulation ranged from 20 mA to 30 mA.22, 23, 25 Two studies reported that intensity was set as the highest tolerable intensity to produce muscle contraction.14, 21 One trial did not report the pulse width21 and another one did not present the intensity of stimulation.24 Three studies applied ES on trunk muscles (abdominal and paravertebral)22, 23, 25 and three on lower limbs muscles (triceps surae,21 quadriceps,14 and gluteus14, 24).
The time of intervention ranged from 1521 to 60 min24 of stimulation. Weekly frequency ranged from three21 to six times per week,25 during four,21, 22, 23 six14, 25 or eight24 weeks.
Risk of bias of the studies
The median PEDro score of studies was 6 (ranged from 4 to 8) (Table 2). All studies were randomized, had similar groups at baseline, showed between-group differences, reported and pointed out estimate and variability. The majority of studies conducted adequate follow-up (83%) and assessors were blinding (67%). However, many studies did not conceal the allocation list (83%) or carry out intention-to-treat analysis (67%). None of the studies blinded neither participants nor therapists.
Table 2.
PEDro scores of included studies.
| Study | Random allocation | Concealed allocation | Groups similar at baseline | Participant blinding | Therapist blinding | Assessor blinding | Adequate follow-up | Intention-to-treat analysis | Between-group difference reported | Point estimate and variability reported | Total (0–10) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chan et al. (2004)21 | √ | √ | √ | √ | 4 | ||||||
| Karabay et al. (2012)22 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Karabay et. (2016)23 | √ | √ | √ | √ | √ | 5 | |||||
| Linden et al. (2003)24 | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Mohanty et al. (2016)14 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Park et al. (2001)25 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Median (range) | 6 (4–7) | ||||||||||
The overall quality of evidence was downgraded in two GRADE domains: (a) inconsistency of results due to high heterogeneity (87%) evidenced in the meta-analysis; (b) imprecision of the effect estimates because confidence intervals were large (ranged from 1.57 to 7.79). For these reasons, the quality of evidence was downgraded to low-quality evidence for the effectiveness of NMES on gross motor function. The evidence profile is presented in (Table 3).
Table 3.
Quality of evidence assessment (GRADE).
| Quality assessment (GRADE) |
Summary of findings |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence | Number of participants |
Effect |
||
| NMES | Control | Relative (95% CI) | Absolute (95% CI) | |||||||
| Gross motor function | ||||||||||
| 6 RCTs | Not serious | Very seriousa | Not serious | Seriousb | None | ≍≍○○ Low | 90 | 84 | – | MD 4.68 (1.57–7.79) |
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment Development and Evaluation; MD, mean difference; NMES, neuromuscular electrical stimulation; RCTs, randomized controlled trials.
I2 = 87%.
Large CI (see Fig. 2).
Effect of electrical stimulation on gross motor function
NMES combined with other therapies presented medium effect size (SMD = 0.76) to improve gross motor function in children with CP in comparison with conventional physical therapy or NDT (MD = 4.68 [95% CI, 1.57–7.79; I2 73%, p = 0.003]) (Fig. 2).
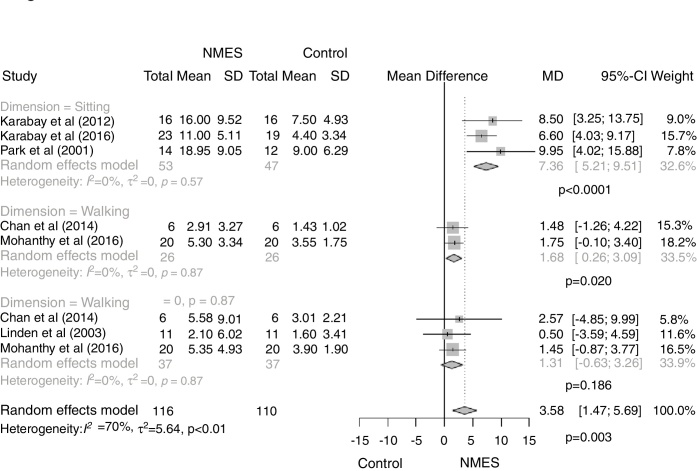
Figure 2.
Effect of NMES on gross motor function (GMFM dimensions) in children with CP compared with conventional physical therapy or neurodevelopmental therapy. NMES = neuromuscular electrical stimulation; CP = cerebral palsy.
Effect of electrical stimulation on functional dimensions of GMFM
We also performed a sensitivity analysis grouping studies according to the GMFM dimensions: sitting, standing or walking. Other dimensions were not studied because they were not included in the articles. NMES showed large effect size (SMD = 1.26) for improving GMFM-sitting dimension (MD = .36 [95% CI, 5.21–9.51; I2 0%, p < 0.0001]) and medium effect size for GMFM-standing dimension (standing: MD = 1.68 [95% CI, 0.26–3.09; I2 0%, p = 0.02]) when compared to conventional physical therapy or NDT (Fig. 2). On the other hand, NMES did not differ from conventional physical therapy or NDT to improve GMFM-walking dimension (walking: MD = 1.31 [95% CI, −0.63 to 3.26; I2 0%, p = 0.19]) (effect size SMD = 0.29) (Fig. 2).
Discussion
This systematic review with meta-analysis aimed to verify the effectiveness of NMES on the gross motor function in children with spastic CP. Although our meta-analysis presented high heterogeneity, we found a medium effect size for the positive effects of NMES on GMFM in comparison to conventional physical therapy or NDT in children with spastic CP. It was evidenced an enhancement of 4.68 points in the overall GMFM scale after using NMES as adjuvant therapy. This value corresponds to a clinically meaningful improvement27 and reinforces the use of NMES for CP rehabilitation.
Our manly finding agrees with a previous systematic review without meta-analysis that compared the effects of functional electrical stimulation (FES) with no-FES therapy and activity training alone. In this previous review, the authors used GMFM and walking speed as outcome measures to assess functional activity. They included five studies and concluded that FES was more effective than no-FES therapy. However, FES treatment and activity training alone produced similar effects on the selected outcomes.10 Instead, we included six randomized controlled trials in our meta-analysis and we identified improvements in specific gross motor dimensions, as assessed by a standard tool.
Our sensitivity analysis found a large effect size for the effectiveness of NMES when the GMFM-sitting dimension was analyzed. An important strength of this analysis is that all those studies included in the sensitivity analysis for sitting applied ES on abdominal and/or paravertebral muscles.22, 23, 25
Similarly, our results showed a medium effect size for the positive effects of NMES in improving GMFM-standing dimension. Possibly, as evident by previous studies, NMES does promote neural and muscular adaptations and improvement in functional outcomes. However, evidence for standing is limited, considering that only two included studies have stimulated as varied muscles as triceps surae, gluteus maximus and quadriceps.14, 21 On the other hand, we did not find positive effects (small effect size) of NMES on GMFM-walking. In our meta-analysis, the results achieved by using NMES for walking dimension were similar to those reached with conventional physical therapy or NDT. Cauraugh et al.28 conducted a meta-analysis focusing on gait outcomes categorized according to the World Health Organization International Classification of Functioning, Disability, and Health. These authors analyzed gait in terms of impairment (e.g. range of motion) and activity limitations (e.g. gross motor function), and they found positive effects of ES for improving walking problems in children with CP.28
All trials included in this review used NMES combined with conventional physical therapy and/or NDT. Previous studies have also assessed the adjuvant effect of NMES. Kang et al. verified the effects of ES combined with botulinum toxin in children with CP and equinus foot. These authors observed that ES combined with botulinum toxin induced a significant increase in ankle range of motion after two weeks of intervention when compared to botulinum toxin alone.29
Results are favorable to NMES as adjuvant therapy to improve gross motor function in children with spastic CP. However, according to PEDro scale scores, the studies included in this meta-analysis are suboptimal in terms of risk of bias. The majority of studies did not has clearly described allocation concealment or reported the patient or therapist blinding. Furthermore, two studies21, 23 did not describe the blinding of the assessors. Four studies14, 21, 22, 23 did not report if they used or not the intention-to-treat analysis.
Limitations
When analyzing our results, it is important to consider some limitations. For instance, stimulus parameters applied by the included studies were very heterogeneous (i.e. intensity, duration, frequency and number of sessions). The frequency of stimulation ranged between low- and medium-frequencies; time of intervention varied from 15 min21 to 60 min24; and the duration of treatment ranged between 421, 22, 23 and 16 weeks. Chiu et al., also reported an important heterogeneity related to the time of intervention which ranged from 180 to 2880 min across the studies included in their research.10
Our review was restricted to the highest standard of evidence, once only randomized controlled trials were included. However, evidence is limited considering the small number of studies included, the wide variety of protocols applied and the lack of precise information about the scales used for gross motor evaluation (if GMFM-88 or GMFM-66). Further controlled studies are still needed to help clinicians make decisions about the effectiveness of ES on GMFM improvement in children with spastic CP.
Conclusion
Low-quality of evidence suggests that NMES might be used as adjuvant therapy to improve gross motor function in children with spastic CP, particularly the sitting and standing dimensions of GMFM scale. Our results need to be carefully interpreted due to the small number of studies included and the reduced sample size in each study. Further research with adequate methodological quality, ample sample size, and long-term follow-up are still necessary.
Funding
This research did not receive any specific funding.
Acknowledgements
TThis study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) Finance Code 001
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bjpt.2019.01.006.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Aisen M.L., Kerkovich D., Mast J. Cerebral palsy: clinical care and neurological rehabilitation. Lancet Neurol. 2011;10(9):844–852. doi: 10.1016/S1474-4422(11)70176-4. [DOI] [PubMed] [Google Scholar]
- 2.Nooijen C., Slaman J., van der Slot W. Health-related physical fitness of ambulatory adolescents and young adults with spastic cerebral palsy. J Rehabil Med. 2014;46(7):642–647. doi: 10.2340/16501977-1821. [DOI] [PubMed] [Google Scholar]
- 3.Ostensjo S., Carlberg E.B., Vollestad N.K. Motor impairments in young children with cerebral palsy: relationship to gross motor function and everyday activities. Dev Med Child Neurol. 2004;46(9):580–589. doi: 10.1017/s0012162204000994. [DOI] [PubMed] [Google Scholar]
- 4.Keles M.N., Elbasan B., Apaydin U., Aribas Z., Bakirtas A., Kokturk N. Effects of inspiratory muscle training in children with cerebral palsy: a randomized controlled trial. Braz J Phys Ther. 2018;22(6):493–501. doi: 10.1016/j.bjpt.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross S.A., Engsberg J.R. Relationships between spasticity, strength gait, and the GMFM-66 in persons with spastic diplegia cerebral palsy. Arch Phys Med Rehabil. 2007;88(9):1114–1120. doi: 10.1016/j.apmr.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Beckung E., Carlsson G., Carlsdotter S., Uvebrant P. The natural history of gross motor development in children with cerebral palsy aged 1 to 15 years. Dev Med Child Neurol. 2007;49(10):751–756. doi: 10.1111/j.1469-8749.2007.00751.x. [DOI] [PubMed] [Google Scholar]
- 7.Elbasan B., Akaya K.U., Akyuz M., Oskay D. Effects of neuromuscular electrical stimulation and Kinesio Taping applications in children with cerebral palsy on postural control and sitting balance. J Back Musculoskelet Rehabil. 2018;31(1):49–55. doi: 10.3233/BMR-169656. [DOI] [PubMed] [Google Scholar]
- 8.Avery L.M., Russell D.J., Raina P.S., Walter S.D., Rosenbaum P.L. Rasch analysis of the Gross Motor Function Measure: validating the assumptions of the Rasch model to create an interval-level measure. Arch Phys Med Rehabil. 2003;84(5):697–705. doi: 10.1016/s0003-9993(02)04896-7. [DOI] [PubMed] [Google Scholar]
- 9.Alotaibi M., Long T., Kennedy E., Bavishi S. The efficacy of GMFM-88 and GMFM-66 to detect changes in gross motor function in children with cerebral palsy (CP): a literature review. Disabil Rehabil. 2014;36(8):617–627. doi: 10.3109/09638288.2013.805820. [DOI] [PubMed] [Google Scholar]
- 10.Chiu H.C., Ada L. Effect of functional electrical stimulation on activity in children with cerebral palsy: a systematic review. Pediatr Phys Ther. 2014;26(3):283–288. doi: 10.1097/PEP.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 11.Merrill D.R. Review of electrical stimulation in cerebral palsy and recommendations for future directions. Dev Med Child Neurol. 2009;51(suppl 4):154–165. doi: 10.1111/j.1469-8749.2009.03420.x. [DOI] [PubMed] [Google Scholar]
- 12.Pool D., Elliott C., Bear N. Neuromuscular electrical stimulation-assisted gait increases muscle strength and volume in children with unilateral spastic cerebral palsy. Dev Med Child Neurol. 2016;58(5):492–501. doi: 10.1111/dmcn.12955. [DOI] [PubMed] [Google Scholar]
- 13.Nussbaum E.L., Houghton P., Anthony J., Rennie S., Shay B.L., Hoens A.M. Neuromuscular electrical stimulation for treatment of muscle impairment: critical review and recommendations for clinical practice. Physiother Can. 2017;69(5):1–76. doi: 10.3138/ptc.2015-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohanty P., Pattnaik M., Sarkar A. Effect of neuromuscular electrical stimulation on gluteus maximus and quadriceps in cerebral palsy children with crouch gait. J Neurol Disord. 2016;4(3):1–4. [Google Scholar]
- 15.Higgins J., Green S. 5th ed. John Wiley & Sons; Chichester: 2011. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Robinson K.A., Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int J Epidemiol. 2002;31(1):150–153. doi: 10.1093/ije/31.1.150. [DOI] [PubMed] [Google Scholar]
- 18.Maher C.G., Sherrington C., Herbert R.D., Moseley A.M., Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 19.Olivo S.A., Macedo L.G., Gadotti I.C., Fuentes J., Stanton T., Magee D.J. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88(2):156–175. doi: 10.2522/ptj.20070147. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. 2nd ed. L. Erlbaum Associates; Hillsdale: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 21.Chan N.N.C., Smith A.W., Lo S.K. Efficacy of neuromuscular electrical stimulation in improving ankle kinetics during walking in children with cerebral palsy. Hong Kong Physiother J. 2004;22:50–56. [Google Scholar]
- 22.Karabay I., Dogan A., Arslan M.D., Dost G., Ozgirgin N. Effects of functional electrical stimulation on trunk control in children with diplegic cerebral palsy. Disabil Rehabil. 2012;34(11):965–970. doi: 10.3109/09638288.2011.628741. [DOI] [PubMed] [Google Scholar]
- 23.Karabay I., Dogan A., Ekiz T., Koseoglu B.F., Ersoz M. Training postural control and sitting in children with cerebral palsy: Kinesio taping vs. neuromuscular electrical stimulation. Complement Therap Clin Pract. 2016;24:67–72. doi: 10.1016/j.ctcp.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Linden M., Hazlewood M., Aitchison A., Hillman S., Robb J. Electrical stimulation of gluteus maximus in children with cerebral palsy: effects on gait characteristics and muscle strength. Dev Med Child Neurol. 2003;45(6):385–390. doi: 10.1017/s0012162203000732. [DOI] [PubMed] [Google Scholar]
- 25.Park E.S., Park C.I., Lee H.J., Cho Y.S. The effect of electrical stimulation on the trunk control in young children with spastic diplegic cerebral palsy. J Korean Med Sci. 2001;16(3):347–350. doi: 10.3346/jkms.2001.16.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surveillance of Cerebral Palsy in E. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE) Dev Med Child Neurol. 2000;42(12):816–824. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- 27.Oeffinger D., Bagley A., Rogers S. Outcome tools used for ambulatory children with cerebral palsy: responsiveness and minimum clinically important differences. Dev Med Child Neurol. 2008;50(12):918–925. doi: 10.1111/j.1469-8749.2008.03150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cauraugh J.H., Naik S.K., Hsu W.H., Coombes S.A., Holt K.G. Children with cerebral palsy: a systematic review and meta-analysis on gait and electrical stimulation. Clin Rehabil. 2010;24(11):963–978. doi: 10.1177/0269215510371431. [DOI] [PubMed] [Google Scholar]
- 29.Kang B.S., Bang M.S., Jung S.H. Effects of botulinum toxin A therapy with electrical stimulation on spastic calf muscles in children with cerebral palsy. Am J Phys Med Rehabil. 2007;86(11):901–906. doi: 10.1097/PHM.0b013e3181520449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.