Abstract
Context
Prostate-specific antigen (PSA) changes during testosterone treatment of older hypogonadal men have not been rigorously evaluated.
Design
Double-blinded, placebo-controlled trial.
Setting
Twelve US academic medical centers.
Participants
Seven hundred ninety hypogonadal men ≥65 years of age with average testosterone levels ≤275 ng/dL. Men at high risk for prostate cancer were excluded.
Interventions
Testosterone or placebo gel for 12 months.
Main Outcomes
Percentile changes in PSA during testosterone treatment of 12 months.
Results
Testosterone treatment that increased testosterone levels from 232 ± 63 ng/dL to midnormal was associated with a small but substantially greater increase (P < 0.001) in PSA levels than placebo treatment. Serum PSA levels increased from 1.14 ± 0.86 ng/mL (mean ± SD) at baseline by 0.47 ± 1.1 ng/mL at 12 months in the testosterone group and from 1.25 ± 0.86 ng/mL by 0.06 ± 0.72 ng/mL in the placebo group. Five percent of men treated with testosterone had an increase ≥1.7 ng/mL and 2.5% of men had an increase of ≥3.4 ng/mL. A confirmed absolute PSA >4.0 ng/mL at 12 months was observed in 1.9% of men in the testosterone group and 0.3% in the placebo group. Four men were diagnosed with prostate cancer; two were Gleason 8.
Conclusions
When hypogonadal older men with normal baseline PSA are treated with testosterone, 5% had an increase in PSA ≥1.7 ng/mL, and 2.5% had an increase ≥3.4 ng/mL.
Older hypogonadal men were treated with testosterone or placebo gel for 12 months. Testosterone treatment increased PSA levels ≥1.7 ng/mL in 5% of men and ≥3.4 ng/mL in 2.5% of men.
Testosterone treatment of hypogonadal older men has beneficial effects on sexual function, body composition, bone density, and anemia (1–6). This treatment, however, requires discussion of risks as well as benefits with the patient and the patient should share in the decision to treat and in plans for monitoring treatment. Although there is no definitive evidence that testosterone treatment causes prostate cancer, it potentially increases the risk of detection of prostate cancer. Testosterone therapy often increases serum prostate-specific antigen (PSA) levels, which in turn may lead to urological referral, prostate biopsy, and detection of prostate cancer. Overtreatment of subclinical, indolent prostate cancers has been and continues to be a major concern, especially in men over 65 years of age (7).
Clinicians who prescribe testosterone must monitor PSA and consider if an increase is within the range expected or sufficiently higher to warrant urological referral. Absolute PSA values >4.0 ng/mL (8) or >3.0 ng/mL (9, 10) are widely used for urological referral of eugonadal men. The Endocrine Society Guidelines for testosterone treatment of hypogonadal men recommend a monitoring plan that aims to minimize the risk of unnecessary prostate biopsies while enabling detection of clinically important prostate cancers. However, because of limited clinical trial data about the PSA response to testosterone treatment in older men, the thresholds for urological referral suggested by the Endocrine Society Guidelines were based largely upon the distribution of PSA increases in the placebo arm of a finasteride trial in men with benign prostatic hyperplasia (11, 12) and on absolute PSA levels recommended by urologists (8–10).
In previous placebo-controlled trials, hypogonadal men treated with testosterone were more likely to have increases in PSA than men treated with placebo, but the increases in PSA were variable and of small magnitude (13–17). In addition, most prior trials have been relatively small, of short duration, did not maintain testosterone levels in the normal range, and did not use a standardized PSA monitoring plan.
To determine the magnitude of the increase in PSA that can be expected from testosterone treatment of older men with low testosterone, we evaluated the PSA responses of men in The Testosterone Trials (TTrials). The PSA and prostate outcomes were ascertained using a prespecified plan, and the criteria for urological referral for consideration of prostate biopsy were established a priori.
Methods
Study design
The TTrials are seven coordinated trials of the efficacy of testosterone in older men who have low testosterone concentrations. They were conducted at 12 clinical trial sites in the United States. Participants were allocated to receive testosterone or placebo gel for one year and serum testosterone was assessed every three months.
The protocol and consent forms were approved by the institutional review boards of the University of Pennsylvania and all trial sites. All participants provided written informed consent. A data and safety monitoring board monitored unblinded safety data every three months and reviewed overall trial progress every six months.
Participants
The study design, eligibility criteria and recruitment process for the TTrials have been published (1, 18, 19). The inclusion criteria included age ≥65 years, an initial early morning fasting testosterone <275 ng/dL, a second value <300 ng/dL, and an average <275 ng/dL, measured using liquid chromatography tandem mass spectrometry in a central laboratory (18). Men with a history of prostate cancer were excluded. We measured PSA levels and performed a digital rectal examination of the prostate at screening. We used the prostate cancer risk calculator to determine eligibility because it includes known risk factors for prostate cancer (PSA, age, race, and family history of prostate cancer), allowing exclusion of men with high risk of prostate (20). We excluded men with >35% life-time risk of any prostate cancer and >7% life-time risk of high-grade cancer (values chosen in consultation with Dr. Ian Thompson) (20). We assessed urinary symptoms with the International Prostate Symptom Score and excluded men with scores >19.
PSA adjustments
We doubled the serum PSA for any subject taking a 5-alpha reductase inhibitor to account for the known effect of this medication on PSA levels (21). Additionally, because PSA concentrations at baseline were lower than they would have been had the subjects’ testosterone concentrations been normal, we adjusted the baseline PSA concentrations upward to ensure appropriate assessment of risk of prostate cancer. We based our adjustment on data in men with serum testosterone levels <460 ng/dL in the European Male Aging Study (courtesy of Frederick Wu, MD). In this data set, the relation of testosterone to PSA was linear at values <460 ng/dL, and the slope of the regression line of association between PSA and serum testosterone was estimated as 0.00128. We used this estimate to adjust each man’s PSA to what would have been expected if his serum testosterone were 460 ng/dL. Although use of the prostate cancer risk calculator helped to reduce prostate cancer risk of participants, it reduced the number African American participants, because they are at increased risk of prostate cancer.
Participant allocation
The participants were assigned to receive testosterone or placebo gel for one year using the method of minimization (1).The balancing factors included in the minimization procedure were participation in each of the three main trials, trial site, screening testosterone ≤200 or >200 ng/dL, age ≤75 or >75 years, current antidepressant use or nonuse, and current phosphodiesterase-5 (PDE5) inhibitor use or nonuse. An automated computer algorithm assigned the treatment, providing optimal balance on the above factors with 80% probability to maintain some randomness to the assignment.
Testosterone treatment
The testosterone preparation was AndroGel 1% in a pump bottle (supplied by AbbVie, Chicago, IL, along with matching placebo). The initial dose was 5 g daily. Participants were allocated to receive AndroGel or placebo for one year. Serum testosterone concentrations were measured at baseline, one, two, three, six, and nine months in a central laboratory (Quest Clinical Trials, Valencia, CA); the dose of testosterone gel was adjusted after each measurement to attempt to keep the concentration within the normal range for young men (1).
Blinding
The participants, the study staff at the trial sites and the investigators were blinded to treatment allocation. Only the data coordinating center, the independent statistician reporting to the Data Safety and Monitoring Board (DMSB), and the central pharmacist were aware of the treatment allocation. To maintain blinding, when the dose was adjusted for a participant taking testosterone, the dose was changed simultaneously in a participant using placebo.
Assessments
At the end of the trial, the serum concentrations of total and free testosterone were measured in the Brigham Research Assay Core Laboratory, Boston, Massachusetts, on sera collected and stored at −80°C from all visits. All samples from each participant were measured in the same assay run. Total testosterone levels were measured by liquid chromatography and tandem mass spectrometry (22), and free testosterone was assayed by equilibrium dialysis (23). The lower limit for detection of testosterone was 1 ng/dL, and the interassay coefficient of variation was <7%. PSA was assayed by Quest Clinical Trials, Valencia, California, using the Access Hybritech PSA assay (Brea, CA), which is a two-site immunoenzymatic sandwich assay. The interassay coefficient of variation was 3.1% for a control with a mean value of 0.992 ng/mL
Evaluation and monitoring for prostate cancer
We evaluated PSA levels at screening, baseline, 3, 12, and 18 months and we performed a digital rectal examination of the prostate at screening, 3, and 12 months. Detection of a prostate nodule on digital rectal examination or a PSA increase of >1.0 ng/mL above the baseline PSA value (both values adjusted for low testosterone) and confirmed by a repeat determination required referral for urologic evaluation. We used a cutoff of 1.0 ng/mL rather than 1.4 ng/mL as suggested in the Endocrine Society Guidelines (12) because we adjusted the baseline PSA for low testosterone levels, and we wanted to be cautious in evaluating participants for prostate cancer. Treatment was discontinued for any participant who was diagnosed as having prostate cancer during the trial.
Statistical analyses
The sample size for the study was determined by power calculations for the primary efficacy trials comprising the TTrials (18). Monitoring for increases in PSA values >1 ng/mL at months 3 and 12 used the PSA values adjusted for use of a 5-alpha reductase inhibitor and low testosterone, as the criterion for further assessment was based on the adjusted values; all other PSA analyses used PSA values unadjusted for low testosterone levels. Calculations of means and percentile changes in PSA used unadjusted values. When a retest was done to confirm an increase in the adjusted PSA >1.0 ng/mL, analyses of observed values over time used the average of the initial value and the retest value. Denominators for proportions of men with a specified increase or observed value at a given time were the number of men with an observed value at that time. Analysis of PSA change was performed with a linear mixed effects model for longitudinal data that included age, race (black or other), baseline testosterone, baseline PSA, visit time (baseline, 3 months, or 12 months) and treatment group. Assessment of interactions between treatment and baseline covariates with respect to changes in PSA over time were done in mixed model analyses with each covariate evaluated in separate models. All statistical analyses, including calculations of means, medians, and other statistics pertinent to the distributions of the variables of interest, as well as creation of plots, were done using the statistical software SAS (version 9.4, Cary, NC).
Results
Baseline characteristics
Participants in the testosterone and placebo arms of the TTrials were similar at baseline (Table 1) in terms of age, race, body mass index (BMI), obesity, baseline testosterone, PSA, and prostate cancer risk.
Table 1.
Baseline Characteristics of Men Enrolled in the TTrials
| Characteristics | Treatment Group | |
|---|---|---|
| Placebo | Testosterone | |
| N | 395 | 395 |
| Demographics | ||
| Age, y | 72.3 ± 5.8 | 72.1 ± 5.7 |
| Race | ||
| Caucasian | 351 (88.9%) | 349 (88.4%) |
| African American | 20 (5.1%) | 21 (5.3%) |
| Other, % | 24 (6.1%) | 25 (6.3%) |
| Ethnicity | ||
| Hispanic, % | 10 (2.5%) | 18 (4.6%) |
| Non-Hispanic, % | 385 (97.5%) | 376 (95.2%) |
| College graduate, % | 198 (50.1%) | 214 (54.2%) |
| Concomitant conditions | ||
| BMI, kg/m2 | 31.0 ± 3.6 | 31.0 ± 3.5 |
| BMI > 30, % | 246 (62.3%) | 251 (63.5%) |
| Medication Use | ||
| 5-alpha reductase inhibitors, % | 18 (4.6%) | 15 (3.8%) |
| Sex Hormones | ||
| Testosterone, ng/dL | 236 ± 67 | 232 ± 63 |
| Free testosterone, pg/mL | 65.0 ± 23.4 | 62.0 ± 21.4 |
| Sex hormone binding globulin, nM | 29.5 ± 14.7 | 31.4 ± 15.2 |
| PSA | ||
| PSA, ng/dL, adjusteda,b | 1.55 ± 0.84 | 1.45 ± 0.86 |
| PSA, ng/dLa | 1.25 ± 0.86 | 1.14 ± 0.86 |
| Prostate cancer risk (12) | ||
| Risk, % of all prostate cancerc | 17.6 ± 6.0 | 17.3 ± 6.0 |
| Risk, % of high-grade prostate cancerc | 3.0 ± 1.7 | 2.9 ± 1.7 |
| Lower urinary tract symptoms | ||
| International Prostate Symptom Score | 9.6 ± 5.3 | 9.0 ± 5.2 |
PSA values were doubled for men taking a 5-alpha reductase inhibitor.
PSA values were adjusted for low testosterone levels. Adjusted PSA = PSA + (460 − testosterone level) × 0.00128.
Risk was determined by the Prostate Risk Calculator (20).
Testosterone treatment
Testosterone treatment increased serum total and free testosterone levels into the midnormal range for men 19 to 40 years of age (1).
Changes in serum PSA levels
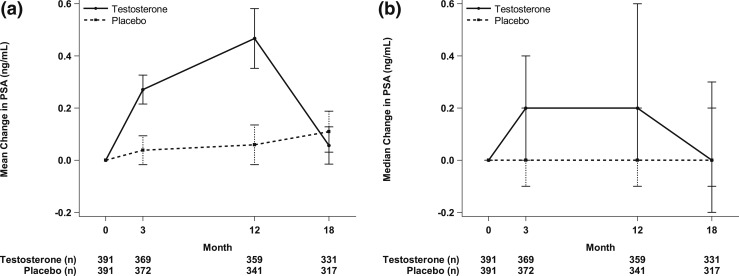
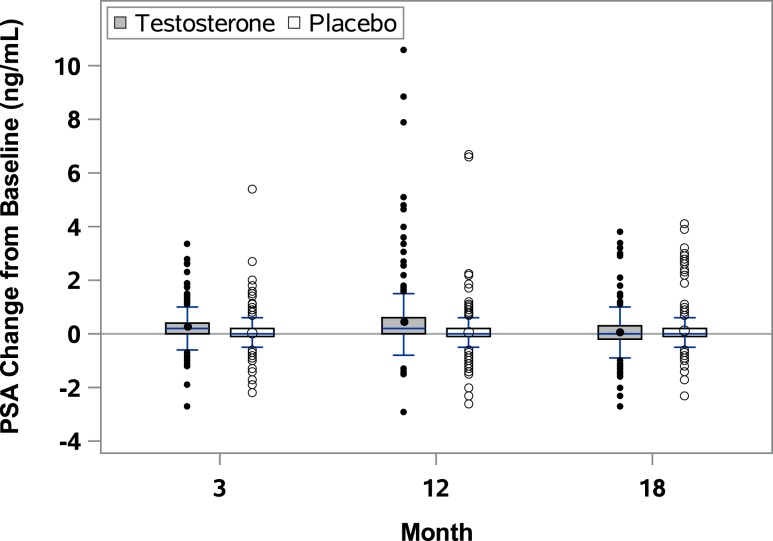
Testosterone treatment was associated with a small but substantially greater increase in serum PSA levels over one year than placebo treatment (P < 0.001). At baseline PSA values were 1.1 ± 0.9 ng/mL (mean ± SD) in the testosterone group and 1.3 ± 0.9 ng/mL in the placebo group (Table 1). PSA levels increased on average by 0.3 ± 0.5 ng/mL at 3 months and by 0.5 ± 1.1 ng/mL at 12 months in the testosterone group (Figs. 1A and 2). Five percent of men treated with testosterone had an increase ≥1.7 ng/mL and 2.5% of men had an increase of ≥3.4 ng/mL at month 12 (Table 2). The median increment in PSA values in the testosterone group at both 3 and 12 months was 0.2 ng/mL (Table 2;Figs. 1B and 2).
Figure 1.
Serum PSA values at baseline, months 3 and 12 of testosterone treatment, and 6 months posttreatment. (a) Values represent mean changes and SDs. (b) Values represent median changes and interquartile ranges.
Figure 2.
Changes in PSA values during and after testosterone treatment. Boxes cover the interquartile ranges, and the horizontal lines inside the box are the medians. The short vertical lines indicate the 95th percentiles, and the circles outside the boxes are the values outside the 95th percentiles.
Table 2.
Distribution of Changes From Baseline in PSA by Visit and Treatment
| Group/Visit | N | 25th Percentile (ng/mL) | Median (ng/mL) | 75th Percentile (ng/mL) | 90th Percentile (ng/mL) | 95th Percentile (ng/mL) | 97.5th Percentile (ng/mL) | Max (ng/mL) |
|---|---|---|---|---|---|---|---|---|
| Testosterone | ||||||||
| Month 3 | 369 | 0.0 | 0.2 | 0.4 | 0.8 | 1.2 | 1.5 | 3.4 |
| Month 12 | 359 | 0.0 | 0.2 | 0.6 | 1.2 | 1.7 | 3.4 | 10.6 |
| Month 18 | 331 | −0.2 | 0.0 | 0.3 | 0.7 | 1.0 | 1.4 | 3.8 |
| Placebo | ||||||||
| Month 3 | 372 | −0.1 | 0.0 | 0.2 | 0.5 | 0.6 | 1.1 | 5.4 |
| Month 12 | 341 | −0.1 | 0.0 | 0.2 | 0.6 | 0.8 | 1.1 | 6.7 |
| Month 18 | 317 | −0.1 | 0.0 | 0.2 | 0.6 | 1.1 | 2.7 | 4.1 |
In the testosterone group, six men (1.6%) had confirmed increases of adjusted PSA levels >1.0 ng/mL at 3 months and 13 (3.6%) at 12 months (Table 3). Two of the men in the testosterone group at 3 months are included in the 13 men at 12 months. Approximately 50% of the observed increases in adjusted PSA >1.0 ng/mL were confirmed by the second test; 27% of men with an observed increase of >1.0 ng/mL had no retest.
Table 3.
PSA Changes, Prostate Nodules, Prostate Biopsies, and Prostate Cancers Diagnosed During and 6 Mo After Stopping Testosterone Treatment
| Event | Month 3 | Month 12 | Month 18 | |||
|---|---|---|---|---|---|---|
| Testosterone | Placebo | Testosterone | Placebo | Testosterone | Placebo | |
| N | 371 | 373 | 362 | 343 | 335 | 318 |
| Confirmed PSA increase >1.0 (ng/mL)a | 6/371 (1.6%) | 4/372 (1.1%) | 13/361 (3.6%) | 5/343 (1.5%) | 5/335 (1.5%) | 5/318 (1.6%) |
| PSA >4.0 (ng/mL) | 8/371 (2.2%) | 6/373 (1.6%) | 16/362 (4.4%) | 6/343 (1.7%) | 8/335 (2.4%) | 13/318 (4.1%) |
| Prostate nodule but no PSA increase | 0 | 0 | 0 | 0 | 0 | 1 |
| Prostate biopsies | 1 | 0 | 1 | 1 | 2 | 1 |
| Prostate cancer | 1 | 0 | 0 | 0 | 2 | 1 |
PSA values were adjusted for low testosterone at months 3 and 12 (during testosterone treatment) but not month 18 (6 months after completion of treatment).
An absolute PSA value >4.0 ng/mL was observed in 2.2% and 4.4% of men in the testosterone group at 3 and 12 months, respectively, and in 1.6% and 1.7% men in the placebo group (Table 3).
Urological referrals for consideration of prostate biopsy and rates of prostate biopsy and diagnosed prostate cancer
Twenty-six of the 743 men who had at least one postbaseline PSA measurement (17 in testosterone group; 9 in placebo group) had a confirmed increase in adjusted serum PSA of >1.0 ng/mL, and 1 man had a prostate nodule but not an increase in PSA (Table 3), outcomes mandating referral to a site or personal urologist for evaluation. Twenty-two men were evaluated by a urologist, and 1 man died of a stroke prior to urological evaluation. Six men underwent prostate biopsies. Five of these men had increases in adjusted PSA >1.0 ng/mL and a PSA >4.0 ng/mL. The sixth man had a prostate nodule but not an increase in serum PSA. Reasons that the urologists did not perform a biopsy included adjusted PSA increase <1.0 ng/mL when repeated by the urologist (12; 3 treated with antibiotics), PSA less than age-related PSA criterion (1), normal digital rectal examination by urologist (1), and refusal of biopsy (1).
Of the four men treated with testosterone who underwent biopsy, three had cancer, two of which were Gleason 4+4 = 8 (International Society of Urological Pathology Grade Group 4). Of the two men treated with placebo who underwent biopsy, one had cancer.
Discussion
In older symptomatic hypogonadal men with unequivocally low serum testosterone concentrations, testosterone treatment that normalized testosterone levels for one year was associated with a small, but statistically significantly greater increase in PSA levels than placebo treatment. On average, testosterone treatment that increased serum testosterone from 232 ng/dL to ∼500 ng/dL (1) increased PSA levels from baseline levels by 0.47 ng/mL at 12 months. At 12 months, 5% had an increase in PSA ≥1.7 ng/mL, 2.5% had an increase ≥3.4 ng/mL, and 1.9% had absolute value >4.0 ng/mL.
Screening for prostate cancer is controversial and evolving. Professional societies recommend informing patients of the benefits and risks of screening and having patients share in the decision to screen (24, 25). The US Preventive Services Task Force recommended in 2012 not screening men of any age for prostate cancer (26). More recently, the US Preventive Services Task Force considered screening of men 50 to 69 years of age to have some benefit (27). The American Urological Association Guidelines recommend not screening men <40 years and those >69 years (24) [https://www.auanet.org/guidelines/prostate-cancer-early-detection-(2013-reviewed-for-currency-2018)]. The potential benefits of screening are greatest for men ages 40 to 54 at high risk for prostate cancer and men ages 50 to 69 years at average or high risk. PSA determination remains the initial screening procedure. However, PSA screening may also lead to overdiagnosis, overtreatment and biopsy- and treatment-associated complications (10, 27). It is worth noting that the majority of men enrolled in the TTrials were older than the recommended ages for routine screening.
A major goal of establishing a PSA monitoring plan during testosterone treatment of men who choose to participate in prostate cancer screening is to identify men who are at increased risk of having aggressive, potentially lethal prostate cancer, while minimizing the risk of detecting indolent prostate cancers that do not merit treatment. Absolute PSA values >4.0 ng/mL (8) or >3.0 ng/mL (9, 10) are widely used for urological referral of eugonadal men. The Endocrine Society Guidelines recommend urologic referral for men who, in the first year of testosterone treatment, have a confirmed increase of >1.4 ng/mL or a confirmed absolute value >4.0 ng/mL (or >3 ng/mL in men at high risk of prostate cancer) (12). Our analyses show that the 95th percentile values for PSA change from baseline were 1.2 and 1.7 ng/mL at 3 and 12 months, respectively. The 97.5th percentile values for PSA changes were 1.5 and 3.4 ng/mL at these intervals. Only 1.9% of the men in the TTrials, most of whom had baseline PSA levels <3.0 ng/mL, had a confirmed absolute PSA >4.0 ng/mL at 12 months.
Nearly half of the PSA elevations observed during the intervention period had resolved when the test was repeated. When the elevated PSA was confirmed, participants were to be referred to a urologist. Usually the urologist ordered another determination, and by that time an additional 50% of PSA elevations had resolved. These data suggest that PSA elevations are often the result of laboratory variability or to transient biological effects, so elevated values should be confirmed by repeating the test.
Only four prostate cancers were diagnosed during the 12 months of treatment and the 6 months of follow-up; three in the testosterone arm (two of which were high-grade prostate cancer) and one in the placebo arm. Because we used only one algorithm to screen for prostate cancer, we cannot compare the one we used to others.
Most, but not all, trials of testosterone treatment of older men have reported an increase in PSA levels (13–17, 28, 29). The heterogeneity of findings could be caused by the heterogeneity of the baseline testosterone and PSA levels, the ages of the participants, and varying testosterone delivery systems, doses, and regimens of testosterone, some resulting in supra- or subphysiological testosterone levels during treatment. Our findings are similar to those of a meta-analysis that also reported an average PSA effect size in response to testosterone of 0.3 ng/mL in younger men and 0.44 ng/mL in older men (30).
Strengths and limitations
The strengths of this trial include its placebo-controlled design, size, strict criteria for the diagnosis of hypogonadism, and centralized monitoring and dose adjustment during treatment to maintain normal testosterone levels. In addition, expert urological evaluation was obtained after confirmed increases in adjusted PSA of >1.0 ng/mL and detection of prostate abnormalities. This trial also has some limitations. One is that the strict inclusion and exclusion criteria prevent generalization of the results to all older men. We screened 51,085 men by telephone, of whom 3126 were excluded because of a history of prostate cancer. We screened 23,889 men at the first in-person visit, when 80.3% were excluded based on testosterone level and 7.5% by prostate cancer risk. We screened 2261 men at the second in person visit, when an additional 9.6% were excluded by testosterone level. There were many other exclusion criteria, but no others excluded as many men or likely influenced the PSA response to testosterone. Ultimately, 790 men were enrolled. Use of the prostate cancer risk calculator, which includes African American race and a first-degree relative as increasing risk, may have decreased the risk to TTrials participants but also decreased the generalizability to men overall. Testosterone treatment in the TTrials lasted for 12 months, so we do not know the effect of this treatment on PSA after that time. Most important, even though TTrials is the largest controlled trial of testosterone ever conducted, it was not nearly large enough or long enough to determine if testosterone treatment increases the risk for developing a clinically important prostate cancer.
Conclusions
One year of testosterone treatment in men ≥65 years old with unequivocally low testosterone levels and not at high risk of prostate cancer was associated with a small, but substantial increase in mean serum PSA concentration above baseline. At 12 months, 5% had an increase in PSA ≥1.7 ng/mL, 2.5% had an increase ≥3.4 ng/mL, and 1.9% had absolute values >4.0 ng/mL. Although this trial is not large enough to establish definite cutoffs, these PSA values provide information relevant to decisions for urological referral during the first year of testosterone treatment of older men.
Acknowledgments
We acknowledge with great appreciation and fondness the critical and sustained contributions of Dr. Elizabeth Barrett-Connor to the TTrials. As a member of the TTrials Steering Committee, she was instrumental in their development, conduct, and interpretation. As the principal investigator of the University of California, San Diego site, she set a high standard in the conduct of a clinical trial site.
We thank these site urologists for evaluating participants referred for elevated PSA values: Arnold Melman, Albert Einstein College of Medicine; Dov Kadmon, Baylor College of Medicine; Mark H. Katz, Brigham and Women’s Hospital; Kevin McVary, Northwestern University; Peter N. Kolettis, University of Alabama at Birmingham; Jacob Rajfer, Harbor-UCLA Medical Center; Philipp Dahm, University of Florida; Christopher Warlick, University of Minnesota; Thomas Jaffe, University of Pittsburgh Medical Center; Daniel W. Lin, VA Puget Sound Health Care System; and John Colberg, Yale University. Dr. Snyder affirms that he has listed everyone who contributed substantially to the work in this article.
Financial Support: The TTrials were supported by a grant (U01 AG030644) from the National Institute on Aging (NIA), National Institutes of Health. The trials also received funding from the National Heart, Lung, and Blood Institute, National Institute of Neurologic Disorders and Stroke, and National Institute of Child Health and Human Development. AbbVie (formerly Solvay and Abbott Laboratories) provided funding and donated Andro-Gel and placebo gel. AbbVie did not participate in the study design, the collection, analyses, and interpretation of the data, or approval of the manuscript.
The Boston site was partially supported by a grant (P30- AG013679) from the Boston Claude D. Pepper Older Americans Independence Center. The Yale Field Center was partially supported by a grant (P30-AG021342) from the Yale Claude D. Pepper Older Americans Independence Center and a grant (UL1 TR000142) from the Yale Center for Clinical Investigation. J.A.C. was supported by a grant (R01 AG37679) from the NIA. T.M.G. was the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24-AG021507) and is the recipient of an Academic Leadership Award (K07AG043587), both from the NIA. C.E.L. was supported by a grant (DK079626) from the National Institute of Diabetes and Digestive and Kidney Diseases to the University of Alabama at Birmingham Diabetes Research and Training Center.
Clinical Trial Information: ClinicalTrials.gov no. NCT00799617 (registered 1 December 2008).
Disclosure Summary: G.R.C. reports personal fees from AbbVie, Apricus, Besins, Clarus Therapeutics, Endo Pharma, Ferring, Lilly, Pfizer, Repros Therapeutics outside the submitted work; S.S.E. reports grants from National Institutes of Health and AbbVie Inc during the conduct of the study; grants from AbbVie Inc. outside the submitted work; S.B. reports grants from NIA and Transition Therapeutics during the conduct of the study; grants and personal fees from Abbvie, Lilly, and Regeneron outside the submitted work; in addition, S.B. has a patent free testosterone calculator pending and has equity interest in FPT, LLC.; K.E.E. reports grants from National Institute on Aging, during the conduct of the study; C.E.L. reports grants from the NIH and AbbVie during the conduct of the study; A.M.M. reports personal fees from AbbVie, Endo, Lilly, Lipocine and Clarus, outside the submitted work; M.E.M. reports grants from the NIH and Abbott Laboratories during the conduct of the study; M.E.M. reports personal fees from Abbvie (Abbott Laboratories), Eli Lilly & Co., and Pfizer outside the submitted work; M.P. reports grants from the NIH, during the conduct of the study; R.S.S. reports grants from The Bone Trial of the Testosterone Trial during the conduct of the study; grants Clarus, Liposine, grants and Antares outside the submitted work; S.B. reports grants from Eli Lilly and Takeda Pharmaceuticals outside the submitted work; S.J.D. reports grants from National Institute on Aging during the conduct of the study; C.W. reports grants from Besins Health International and Abbvie during the conduct of the study; grants from Clarus Therapeutics, outside the submitted work; A.J.S.-S. reports grants from National Institute on Aging, NIH, and from AbbVie (formerly Solvay & Abbott Laboratory) during the conduct of the study; P.J.S. reports grants from National Institute on Aging, NIH, grants and from AbbVie (formerly Solvay and Abbott Laboratories), during the conduct of the study; personal fees from Watson Laboratories, outside the submitted work. The remaining authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Glossary
Abbreviations:
- BMI
body mass index
- PSA
prostate-specific antigen
- TTrials
The Testosterone Trials
References and Notes
- 1. Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Cifelli D, Dougar D, Fluharty L, Resnick SM, Storer TW, Anton S, Basaria S, Diem SJ, Hou X, Mohler ER III, Parsons JK, Wenger NK, Zeldow B, Landis JR, Ellenberg SS; Testosterone Trials Investigators. Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brock G, Heiselman D, Maggi M, Kim SW, Rodríguez Vallejo JM, Behre HM, McGettigan J, Dowsett SA, Hayes RP, Knorr J, Ni X, Kinchen K. Effect of testosterone solution 2% on testosterone concentration, sex drive and energy in hypogonadal men: results of a placebo controlled study. J Urol. 2016;195(3):699–705. [DOI] [PubMed] [Google Scholar]
- 3. Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, Saad F, Mannucci E, Maggi M. Therapy of endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016;174(3):R99–R116. [DOI] [PubMed] [Google Scholar]
- 4. Roy CN, Snyder PJ, Stephens-Shields AJ, Artz AS, Bhasin S, Cohen HJ, Farrar JT, Gill TM, Zeldow B, Cella D, Barrett-Connor E, Cauley JA, Crandall JP, Cunningham GR, Ensrud KE, Lewis CE, Matsumoto AM, Molitch ME, Pahor M, Swerdloff RS, Cifelli D, Hou X, Resnick SM, Walston JD, Anton S, Basaria S, Diem SJ, Wang C, Schrier SL, Ellenberg SS. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med. 2017;177(4):480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tracz MJ, Sideras K, Boloña ER, Haddad RM, Kennedy CC, Uraga MV, Caples SM, Erwin PJ, Montori VM. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91(6):2011–2016. [DOI] [PubMed] [Google Scholar]
- 6. Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, Ellenberg SS, Cauley JA, Ensrud KE, Lewis CE, Barrett-Connor E, Schwartz AV, Lee DC, Bhasin S, Cunningham GR, Gill TM, Matsumoto AM, Swerdloff RS, Basaria S, Diem SJ, Wang C, Hou X, Cifelli D, Dougar D, Zeldow B, Bauer DC, Keaveny TM. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med. 2017;177(4):471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jahn JL, Giovannucci EL, Stampfer MJ. The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the prostate-specific antigen-era. Int J Cancer. 2015;137(12):2795–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catalona WJ, Hudson MA, Scardino PT, Richie JP, Ahmann FR, Flanigan RC, DeKernion JB, Ratliff TL, Kavoussi LR, Dalkin BL, Waters WB, MacFarlane MT, Southwick PC. Selection of optimal prostate specific antigen cutoffs for early detection of prostate cancer: receiver operating characteristic curves. J Urol. 1994;152(6 Part 1):2037–2042. [DOI] [PubMed] [Google Scholar]
- 9. Carroll PR, Parsons JK, Andriole G, Bahnson RR, Castle EP, Catalona WJ, Dahl DM, Davis JW, Epstein JI, Etzioni RB, Farrington T, Hemstreet GP III, Kawachi MH, Kim S, Lange PH, Loughlin KR, Lowrance W, Maroni P, Mohler J, Morgan TM, Moses KA, Nadler RB, Poch M, Scales C, Shaneyfelt TM, Smaldone MC, Sonn G, Sprenkle P, Vickers AJ, Wake R, Shead DA, Freedman-Cass DA. NCCN Guidelines Insights: Prostate Cancer Early Detection, Version 2.2016. J Natl Compr Canc Netw. 2016;14(5):509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen-based screening for prostate cancer: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(18):1914–1931. [DOI] [PubMed] [Google Scholar]
- 11. Gormley GJ, Stoner E, Bruskewitz RC, Imperato-McGinley J, Walsh PC, McConnell JD, Andriole GL, Geller J, Bracken BR, Tenover JS, Vaughan ED, Pappas F, Taylor A, Binkowitz B, Ng J; The Finasteride Study Group. The effect of finasteride in men with benign prostatic hyperplasia. N Engl J Med. 1992;327(17):1185–1191. [DOI] [PubMed] [Google Scholar]
- 12. Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone therapy in men with hypogonadism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(5):1715–1744. [DOI] [PubMed] [Google Scholar]
- 13. Kang DY, Li HJ. The effect of testosterone replacement therapy on prostate-specific antigen (PSA) levels in men being treated for hypogonadism: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94(3):e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cui Y, Zong H, Yan H, Zhang Y. The effect of testosterone replacement therapy on prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2014;17(2):132–143. [DOI] [PubMed] [Google Scholar]
- 15. Boyle P, Koechlin A, Bota M, d’Onofrio A, Zaridze DG, Perrin P, Fitzpatrick J, Burnett AL, Boniol M. Endogenous and exogenous testosterone and the risk of prostate cancer and increased prostate-specific antigen (PSA) level: a meta-analysis. BJU Int. 2016;118(5):731–741. [DOI] [PubMed] [Google Scholar]
- 16. Morgentaler A, Benesh JA, Denes BS, Kan-Dobrosky N, Harb D, Miller MG. Factors influencing prostate-specific antigen response among men treated with testosterone therapy for 6 months. J Sex Med. 2014;11(11):2818–2825. [DOI] [PubMed] [Google Scholar]
- 17. Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, Bhasin S. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60(11):1451–1457. [DOI] [PubMed] [Google Scholar]
- 18. Snyder PJ, Ellenberg SS, Cunningham GR, Matsumoto AM, Bhasin S, Barrett-Connor E, Gill TM, Farrar JT, Cella D, Rosen RC, Resnick SM, Swerdloff RS, Cauley JA, Cifelli D, Fluharty L, Pahor M, Ensrud KE, Lewis CE, Molitch ME, Crandall JP, Wang C, Budoff MJ, Wenger NK, Mohler ER, Bild DE, Cook NL, Keaveny TM, Kopperdahl DL, Lee D, Schwartz AV, Storer TW, Ershler WB, Roy CN, Raffel LJ, Romashkan S, Hadley E. The Testosterone Trials: seven coordinated trials of testosterone treatment in elderly men. Clin Trials. 2014;11(3):362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cauley JA, Fluharty L, Ellenberg SS, Gill TM, Ensrud KE, Barrett-Connor E, Cifelli D, Cunningham GR, Matsumoto AM, Bhasin S, Pahor M, Farrar JT, Cella D, Rosen RC, Resnick SM, Swerdloff RS, Lewis CE, Molitch ME, Crandall JP, Stephens-Shields AJ, Strorer TW, Wang C, Anton S, Basaria S, Diem S, Tabatabaie V, Dougar D, Hou X, Snyder PJ. Recruitment and screening for the Testosterone Trials. J Gerontol A Biol Sci Med Sci. 2015;70(9):1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, Feng Z, Parnes HL, Coltman CA Jr. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98(8):529–534. [DOI] [PubMed] [Google Scholar]
- 21. Guess HA, Gormley GJ, Stoner E, Oesterling JE. The effect of finasteride on prostate specific antigen: review of available data. J Urol. 1996;155(1):3–9. [PubMed] [Google Scholar]
- 22. Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D’Agostino R, Vasan RS. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96(8):2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sinha-Hikim I, Arver S, Beall G, Shen R, Guerrero M, Sattler F, Shikuma C, Nelson JC, Landgren BM, Mazer NA, Bhasin S. The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 1998;83(4):1312–1318. [DOI] [PubMed] [Google Scholar]
- 24. Carter HB. Prostate-specific antigen (PSA) screening for prostate cancer: revisiting the evidence. JAMA. 2018;319(18):1866–1868. 2018;319(18):1901 [DOI] [PubMed] [Google Scholar]
- 25. Tsodikov A, Gulati R, Heijnsdijk EAM, Pinsky PF, Moss SM, Qiu S, de Carvalho TM, Hugosson J, Berg CD, Auvinen A, Andriole GL, Roobol MJ, Crawford ED, Nelen V, Kwiatkowski M, Zappa M, Luján M, Villers A, Feuer EJ, de Koning HJ, Mariotto AB, Etzioni R. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med. 2017;167(7):449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134. [DOI] [PubMed] [Google Scholar]
- 27. Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, Davidson KW, Doubeni CA, Ebell M, Epling JW Jr, Kemper AR, Krist AH, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Siu AL, Tseng CW; US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901–1913. 2018;319(18):1901 [DOI] [PubMed] [Google Scholar]
- 28. Guay AT, Perez JB, Fitaihi WA, Vereb M. Testosterone treatment in hypogonadal men: prostate-specific antigen level and risk of prostate cancer. Endocr Pract. 2000;6(2):132–138. [DOI] [PubMed] [Google Scholar]
- 29. Miner MM, Bhattacharya RK, Blick G, Kushner H, Khera M. 12-month observation of testosterone replacement effectiveness in a general population of men. Postgrad Med. 2013;125(2):8–18. [DOI] [PubMed] [Google Scholar]
- 30. Bhasin S, Singh AB, Mac RP, Carter B, Lee MI, Cunningham GR. Managing the risks of prostate disease during testosterone replacement therapy in older men: recommendations for a standardized monitoring plan. J Androl. 2003;24(3):299–311. [DOI] [PubMed] [Google Scholar]