Abstract
Background
Ginsenoside Re (Re) is one of the major components of Panax ginseng Meyer. Ginsenoside Rk3 (Rk3) is a secondary metabolite of Re. The aim of this study was to investigate and compare the effects and underlying mechanisms of Re and Rk3 on cyclophosphamide-induced myelosuppression.
Methods
The mice myelosuppression model was established by intraperitoneal (i.p.) injection of cyclophosphamide. Peripheral blood cells, bone marrow nucleated cells, and colony yield of hematopoietic progenitor cells in vitro were counted. The levels of erythropoietin, thrombopoietin, and granulocyte macrophage colony-stimulating factor in plasma were measured by enzyme-linked immunosorbent assay. Bone marrow cell cycle was performed by flow cytometry. The expression of apoptotic protein bcl-2, bax, and caspase-3 was detected by Western blotting.
Results
Both Re and Rk3 could improve peripheral blood cells, bone marrow nucleated cell counts, thymus index, and spleen index. Furthermore, they could enhance the yield of colonies cultured in vitro and make the levels of granulocyte macrophage colony-stimulating factor, erythropoietin, and thrombopoietin normal, reduce the ratio of G0/G1 phase cells, and increase the proliferation index. Finally, Re and Rk3 could upregulate the expression of bcl-2, whereas they could downregulate the expression of bax and caspase-3.
Conclusion
Re and Rk3 could improve the hematopoietic function of myelosuppressed mice. The effect of Rk3 was superior to that of Re at any dose. Regulating the levels of cytokines, promoting cells enter the normal cell cycle, regulating the balance of bcl-2/bax, and inhibiting the expression of caspase-3 may be the effects of Re and Rk3 on myelosuppression.
Keywords: Chemotherapy, Ginsenoside Re, Ginsenoside Rk3, Myelosuppression
1. Introduction
Cancer has emerged as a serious global threat. Chemotherapy is one of the most effective treatments for cancer. It is a systemic treatment, but at the same time, it often causes myelosuppression due to its toxicity to cells. Myelosuppression often first manifests as a decline in white blood cells, followed by a series of hematopoietic impairments [1], [2]. Its essence is the decrease of precursor blood cell activity, which affects the ability of hematopoietic stem cells to proliferate and differentiate [3], [4]. Myelosuppression can limit the progress of chemotherapy; severe myelosuppression also leads to secondary infections or bleeding, which in turn threatens the lives of patients [5], [6] Therefore, improving the symptoms of myelosuppression during the treatment of cancer has become the key to ensure the efficacy of chemotherapy.
Ginseng is the root of Panax ginseng Meyer. It has been used as a traditional Chinese medicine in China for more than 4,000 years. Total ginsenosides derived from ginseng possess various biological activities [7]. Among the total ginsenosides, ginsenoside Re (Re) is regarded as the main active components. Modern medical research shows that Re plays an important role in resisting cancer [8], improving immunity [9], enhancing memory [10], and improving cardiovascular system [11]. Ginsenoside Rk3 (Rk3) is a secondary metabolite of Re. Previous experiments showed that Rk3 possesses anticancer [12], immunomodulatory [13], and anti–platelet-aggregating [14] activities. Re is a kind of polar ginsenoside, whereas Rk3 is a kind of nonpolar ginsenoside. Rk3 can be obtained from Re by hydrolysis of the sugar moieties [15]. Compared with polar ginsenosides, nonpolar ginsenosides are more easily absorbed and have better pharmacological activity due to its lower polarity [15].
Based on the traditional efficacy of ginseng and previous studies, we speculated that Re and Rk3 can improve bone marrow hematopoietic function and can be used for the treatment of chemotherapy-induced myelosuppression. To validate the speculation and compare the two, we investigated the effects of Re and Rk3 on hematopoietic function of bone marrow, including peripheral blood, bone marrow nucleated cell (BMNC) counts, and thymus/spleen index in cyclophosphamide (CTX)-induced myelosuppression mice. The mechanism of action is explored at the cellular and molecular levels by detecting the proliferation and differentiation of hematopoietic progenitor cells, bone marrow cell cycle, cell growth factor content, and bone marrow cell apoptosis protein expression.
2. Materials and methods
2.1. Materials
Re and Rk3 were purchased from College of Fundamental Medical, Jilin University (Changchun, China). All standards were at least 98% pure, as confirmed by HPLC. HPLC-grade acetonitrile and methanol were purchased from Fisher (USA). Cyclophosphamide was obtained from Jiangsu Shengdi Pharmaceutical Co., Ltd. (Lianyungang, Jiangsu, China). Mouse G-CSF, erythropoietin (EPO), thrombopoietin (TPO), and interleukin-2 (IL-2) were supplied by novoprotein Scientific Inc. (Shanghai, China). We purchased enzyme-linked immunosorbent assay kits for mouse G-CSF, EPO, and TPO from R&D systems (Minneapolis, MN, USA). Rabbit monoclonal anti-bax, anti-bcl-2, and anti-caspase-3 antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Propidium iodide used in current experiments was purchased from Tianjin Sungene Biotech Co., Ltd. (Tianjin, China). All other chemicals were of the highest grade from a commercial source.
2.2. Animals
Male Balb/c mice with body weights ranging from 18 to 22 g were obtained from the Laboratory Animal Quality Testing Center of Jilin Province (Certificate No. SCXK-2016-0003) and maintained at controlled temperature (22 ± 2°C) and humidity (50% ± 10%) with a 12 h/12 h light/dark cycle. All efforts were made to minimize the pain of animals. The animal procedures were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee of China Academy of Chinese Medical Sciences.
After seven days of acclimatization, the mice were divided into seven groups (n = 10 per group): (1) normal control group (Normal), (2) CTX treatment group (Model), (3) CTX + rhG-CSF (Positive), (4) CTX + low-dose Re (Re-L), (5) CTX + high-dose Re (Re-H), (6) CTX + low-dose Rk3 (Rk3-L), and (7) CTX + high-dose Rk3 (Rk3-H).
The mice in Groups 2–7 were intraperitoneally (i.p.) injected with a dose of CTX (100 mg kg−1, 0.1 mL 10 g−1) for 3 days, and the normal group received an equal volume of saline. After CTX treatment for 24 hours, all the drug treatment groups (Groups 3–7) were i.p. injected with rhG-CSF (11.25 μg kg−1), Re (5 and 10 mg kg−1), or Rk3 (5 and 10 mg kg−1) for 7 days. The normal and model groups were administered an equivalent volume of saline water, once a day for 7 consecutive days.
2.3. Detection of peripheral blood cells
On Day 11, blood samples were collected from the eye socket vein, and white blood cells (WBCs), red blood cells (RBCs), hemoglobin (HGB), and platelets (PLTs) were counted using a cell counter. After the last drug administration, body weights of mice were measured. Blood samples were extracted from eyeballs and collected in test tubes with ethylenediaminetetraacetic acid for further analysis.
2.4. Determinations of thymus/spleen index and bone marrow nucleated cells
After blood samples were collected, mice were euthanized by cervical vertebra dislocation and then placed in 75% ethanol for 5 min twice. Immediately, the thymus gland and spleen were excised and weighed, and the thymus and spleen indices were calculated as the thymus weight/body weight and spleen weight/body weight, respectively.
Double femurs were removed under aseptic conditions, and bone marrow cells were flushed using sterile phosphate-buffered saline (PBS) to form a single cell suspension. The suspension was taken in a centrifuge tube and centrifuged for 10 min at 1200r/min. And, 2 mL of RBC lysis buffer was added, and it was let to stand for 3 minutes after blending centrifuged for 10 min at 1200r/min. The bone marrow karyocytes were removed and rinsed twice with 1 mL of sterile PBS for 10 min at 1200r/min and then resuspended in sterile PBS and counted using an inverted microscope.
2.5. Hematopoietic progenitor cells culture
Bone marrow cells were plated at a concentration of 105/mL on Iscove's Modified Dulbecco's Medium (IMDM) medium supplemented with horse serum, 10−4M 2-mercaptoethanol, 2 mM 3% L-glutamine, 20 ng/mL of recombinant mouse (rm) IL-3, 20 U/mL of recombinant human (rh) EPO, 50 ng/mL of rm granulocyte macrophage colony-stimulating factor (GM-CSF), and 5 ng/mL of rm TPO.
Plates were cultured at 37°C in 5% CO2 atmosphere. Colony-forming unit erythroid (CFU-E) were counted under inverted phase-contrast microscope after 3 days of culture. Burst-forming unit erythroid (BFU-E), colony-forming unit granulocyte macrophage (CFU-GM), and colony-forming unit megakaryocte (CFU-Meg) were counted after 7 days of culture.
2.6. Determination of the levels of GM-CSF, EPO, and TPO in plasma
Plasma samples were prepared from the blood samples collected from the mice, and the levels of EPO, TPO, GM-CSF, and IL-6 were measured by enzyme-linked immunosorbent assay.
2.7. Cell cycle
A single cell suspension was collected in a centrifuge tube, rinsed twice with cold PBS, and centrifuged, and then, 2 mL of 70% cold ethanol was added to fix the cells overnight at 4°C. Cells were then incubated with in the dark for 30 min. Cells from all groups were measured by flow cytometry.
2.8. Analysis of protein expressions of bal-2, bax, and caspase-3 in bone marrow cells
Western blotting is used for the determination of protein content. Total protein in BMNC was extracted, and protein concentration was determined by Bradford method. Protein samples were denatured and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis, transferred to Poly(vinylidene fluoride) (PVDF) membrane by semi-dry electrophoresis, and blocked using 5% skimmed milk liquid for 1 h, and primary antibodies were added and left undisturbed at 4°C overnight. After adding a secondary antibody (1:3000) and incubating for 1 hour, the color was developed by chemiluminescence. β-Actin was used as an internal reference. After the film was scanned, the protein bands were quantitatively analyzed, and the gray ratios of the target protein band and β-actin band were calculated as the relative expression level of each target protein.
2.9. Statistical analysis
The data were expressed as mean ± standard deviation. Statistical analysis was performed by one-way analysis of variance. Statistical analysis was performed using SPSS version 13.0 (SPSS, Chicago, IL, USA) statistical software, and p < 0.05 was considered statistically significant.
3. Results
3.1. Effect of Re and Rk3 on peripheral blood cells in CTX-induced mice
The quantities of WBC, RBC, HGB, and PLT from peripheral blood are shown in Table 1. Compared with normal mice, the quantity of WBC increased (p < 0.01), whereas RBC, HGB, and PLT decreased significantly (p < 0.01) in the model group with CTX at a dose of 100 mg/kg. It also indicates that both Re and Rk3 can increase the quantities of HGB and PLT and decrease the quantity of WBC, whereas only Rk3-H can increase the quantity of RBC significantly (p < 0.05). Two high-dose groups are more effective than two low-dose groups, and Rk3 is more effective than Re at any dose group.
Table 1.
Effect of Re and Rk3 on peripheral blood cells in CTX-induced mice
| Groups | WBC (×109/L) | RBC (×1012/L) | HGB (g/L) | PLT (×109/L) |
|---|---|---|---|---|
| Normal | 7.30 ± 0.75 | 9.76 ± 0.27 | 131.00 ± 4.59 | 917.10 ± 74.65 |
| Model | 10.34 ± 1.40## | 8.63 ± 0.68## | 113.20 ± 6.66## | 628.50 ± 66.09## |
| Positive | 8.47 ± 0.87∗∗ | 9.46 ± 0.41∗∗ | 126.70 ± 5.70∗∗ | 881.10 ± 76.87∗∗ |
| Re-L | 9.40 ± 1.28 | 8.80 ± 0.58 | 118.90 ± 8.67 | 790.00 ± 97.66∗∗ |
| Re-H | 8.81 ± 0.89∗∗ | 8.95 ± 0.75 | 121.10 ± 6.31∗∗ | 833.10 ± 68.04∗∗ |
| Rk3-L | 9.2 ± 1.29∗ | 8.85 ± 1.02 | 122.00 ± 5.29∗∗ | 799.90 ± 72.21∗∗ |
| Rk3-H | 8.51 ± 0.97∗∗ | 9.25 ± 0.59∗ | 127.40 ± 3.92∗∗a | 853.60 ± 76.65∗∗ |
Data are expressed as mean ± SD (n = 10)
##p < 0.01 as compared with the normal group. ∗∗p < 0.01 as compared with the model group. *p < 0.05 as compared with the model group. ap < 0.05 as compared with the Re-H group
CTX, cyclophosphamide; HGB, hemoglobin; PLT, platelet; RBC, red blood cell; SD, standard deviation; WBC, white blood cell
3.2. Effect of Re and Rk3 on the thymus/spleen index in CTX-induced mice
As shown in Table 2, compared with the normal group, the thymus index and spleen index of the mice in the model group changed significantly (p < 0.01). The thymus index showed a decrease, and the spleen index is opposite to thymus index. Re and Rk3 can increase the thymus index significantly (p < 0.01) while reduce the spleen index, but the effect on the spleen is not significant.
Table 2.
Effects of Re and Rk3 on the thymus/spleen index in CTX-induced mice
| Groups | Thymus index (mg/g) | Spleen index (mg/g) |
|---|---|---|
| Normal | 1.14 ± 0.14 | 4.02 ± 0.38 |
| Model | 0.28 ± 0.05## | 8.94 ± 1.10## |
| Positive | 0.60 ± 0.08∗∗ | 8.23 ± 1.36 |
| Re-L | 0.49 ± 0.06∗∗ | 8.70 ± 0.86 |
| Re-H | 0.57 ± 0.07∗∗ | 8.55 ± 0.96 |
| Rk3-L | 0.52 ± 0.07∗∗ | 8.60 ± 1.64 |
| Rk3-H | 0.65 ± 0.09∗∗a | 8.25 ± 0.67 |
Data are expressed as mean ± SD (n = 10)
##p < 0.01 as compared with the normal group. ∗∗p < 0.01 as compared with the model group. ap < 0.05 as compared with the Re-H group
CTX, cyclophosphamide; SD, standard deviation
3.3. Effect of Re and Rk3 on the BMNC counts in CTX-induced mice
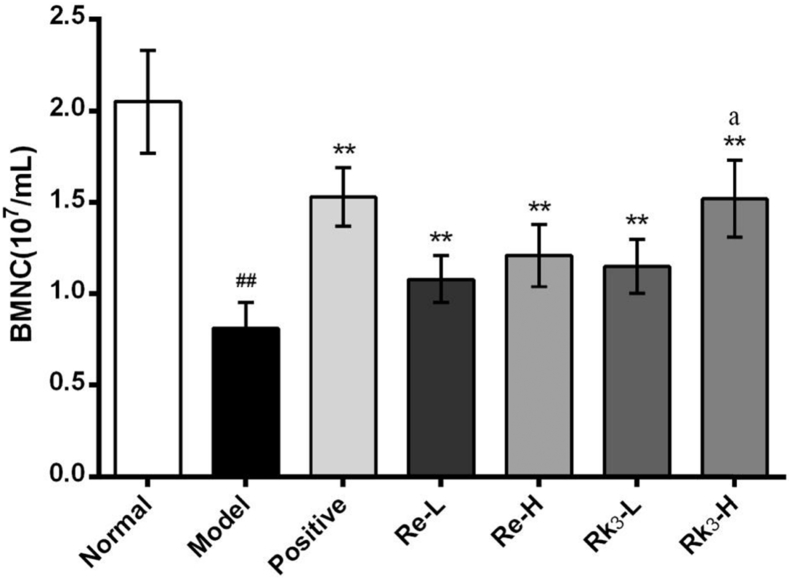
The quantity of BMNC was shown in Fig. 1. Compared with the mice in the normal group, the quantity of BMNC decreased significantly (p < 0.01) in the model group. Both Re and Rk3 can increase the quantity of BMNC significantly (p < 0.01) no matter high dose or low dose. We can also indicate that Rk3-H showed significant effects compared with the other three groups (p < 0.05).
Fig. 1.
Effect of Re and Rk3 on the BMNC count in CTX-induced mice. Data are expressed as mean ± SD (n = 6). ##p < 0.01 as compared with the normal group. ∗∗p < 0.01 as compared with the model group. ap < 0.05 as compared with the Re-H group.
BMNC, bone marrow nucleated cell; CTX, cyclophosphamide; SD, standard deviation.
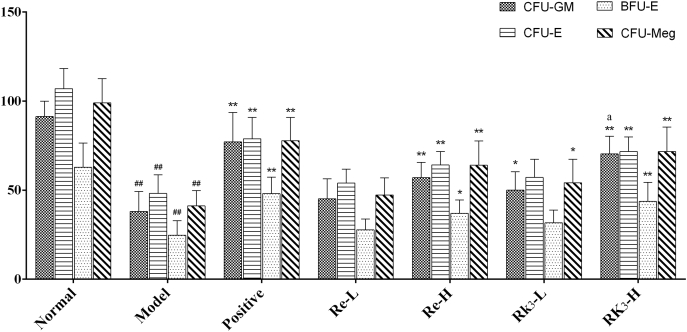
3.4. Effect of Re and Rk3 on colony yield of hematopoietic progenitor cells in vitro in CTX-induced mice
The yields of colony cultured in vitro were shown in Fig. 2. Compared with the mice in the normal group, the yields of CFU-GM, CFU-E, BFU-E, and CFU-Meg decreased significantly (p < 0.01) in the model group. Rk3-H and Re-H can increase the yields significantly (p < 0.01 or p < 0.05), whereas the effect of Rk3-L and Re-L on colony is not significant.
Fig. 2.
Effect of Re and Rk3 on colony yield of hematopoietic progenitor cells in vitro in CTX-induced mice. Data are expressed as mean ± SD (n = 6). ##p < 0.01 as compared with the normal group.∗p < 0.05 as compared with the model group. ∗∗p < 0.01 as compared with the model group. ap < 0.05 as compared with the Re-H group.
BFU-E, Burst-forming unit erythroid; CFU-E, Colony-forming unit erythroid; CFU-GM, colony-forming unit granulocyte macrophage; CFU-Meg, colony-forming unit megakaryocte; CTX, cyclophosphamide; SD, standard deviation.
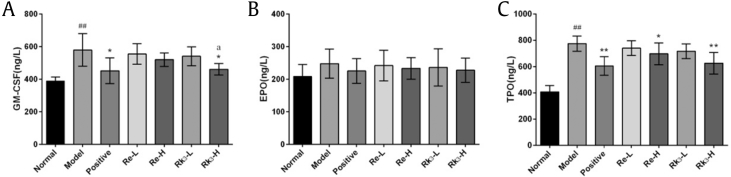
3.5. Effect of Re and Rk3 on the hematopoiesis-related cytokines in CTX-induced mice
As shown in Fig. 3, compared to the normal group, the level of GM-CSF and TPO in the model group increased significantly (p < 0.01). Both Rk3-H and Re-H can decrease the level of TPO significantly (p < 0.01 or p < 0.05). Only Rk3-H can decrease the level of GM-CSF significantly (p < 0.01).The differences between these 7 groups on the level of EPO are all not significantly.
Fig. 3.
Effect of Re and Rk3 on the hematopoiesis-related cytokines (A) GM-CSF, (B) EPO, and (C) TPO in CTX-induced mice. Data are expressed as mean ± SD (n = 10). ##p < 0.01 as compared with the normal group.∗p < 0.05 as compared with the model group. ∗∗p < 0.01 as compared with the model group. ap < 0.05 as compared with the Re-H group.
CTX, cyclophosphamide; EPO, erythropoietin; GM-CSF, granulocyte macrophage colony-stimulating factor; SD, standard deviation; TPO, thrombopoietin.
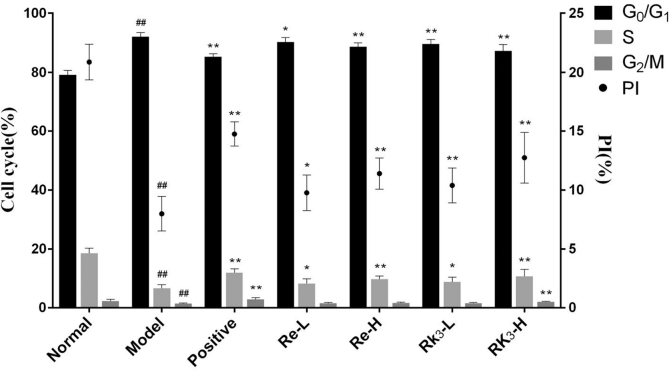
3.6. Effect of Re and Rk3 on cell cycle in CTX-induced mice
The ratios of every phase were shown in Fig. 4. The ratio of G0/G1 phase in the model group was significantly higher than that in the normal group (p < 0.01). At the same time, the ratios of S phase and G2/M phase are significantly lower (p < 0.01). Both Re and Rk3 can decrease the ratio of G0/G1 phase and increase the ratios of S phase significantly (p < 0.01 or p < 0.05). Only Rk3-H can increase the ratios of G2/M phase significantly (p < 0.05).
Fig. 4.
Effect of Re and Rk3 on cell cycle in CTX-induced mice. Data are expressed as mean ± SD (n = 6). ##p < 0.01 as compared with the normal group. ∗p < 0.05 as compared with the model group. ∗∗p < 0.01 as compared with the model group.
CTX, cyclophosphamide; SD, standard deviation.
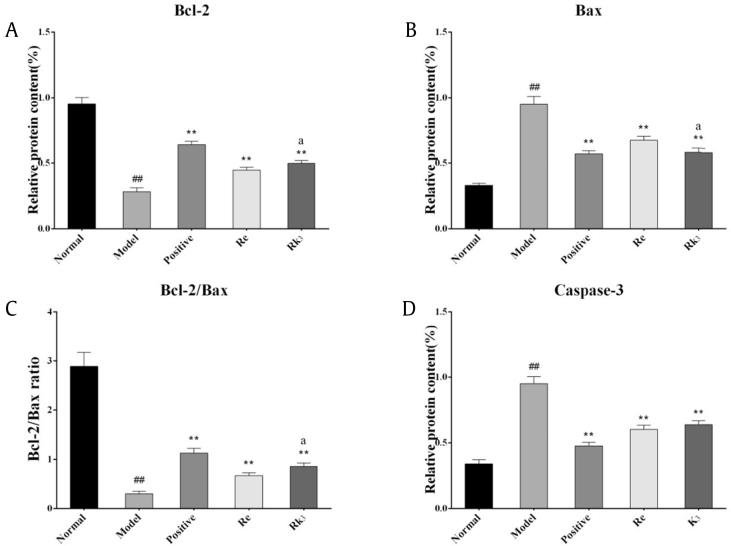
3.7. Effect of Re and Rk3 on protein expressions of bcl-2, bax, and caspase-3 in bone marrow cell in CTX-induced mice
The expressions of bcl-2, bax, and caspase-3 were shown in Fig. 5. Compared with the normal group, the expression of bcl-2 decreased significantly (p < 0.05), whereas the expression of bax and caspase-3 increased significantly (p < 0.05) in the bone marrow cells of the mice from the model group. Both Re and Rk3 can increase the expression of bcl-2 and decrease the expression of bax and caspase-3 significantly (p < 0.01 or p < 0.05). The effect of Rk3 is better than Re significantly (p < 0.05) on bcl-2 and bax while not significantly on caspase-3.
Fig. 5.
Effect of Re and Rk3 on protein expressions of (A) bcl-2, (B) bax, (C) bcl-2/bax, and (D) caspase-3 in bone marrow cell in CTX-induced mice. Data are expressed as mean ± SD (n = 3). ##p < 0.01 as compared with the normal group. ∗∗p < 0.01 as compared with the model group. ap < 0.05 as compared with the Re-H group.
CTX, cyclophosphamide; SD, standard deviation.
4. Discussion
CTX is one of the most commonly used alkylating cytotoxic chemotherapeutic agents [16]. It can directly damage intracellular DNA molecules [17], affect cell division, and can also induce senescence and apoptosis of hematopoietic stem cells [18], [19]. Owing to the lack of specificity, while killing and damaging tumor cells, CTX also affects the hematopoietic precursor stem cells [20], [21]. In this study, the model of mice with myelosuppression was established by i.p. injection of CTX. After injection, as same as the clinical symptoms of myelosuppression, the mice showed signs of reduced diet, weight loss, diarrhea, mass hair loss, and fatigue.
Myelosuppression appears as a decrease in the number of various types of blood cells in the peripheral blood [22]. Therefore, the number of WBC, RBC, HGB, and PLT from peripheral blood can directly reflect the degree of myelosuppression. The number of WBC changes most obviously because of its short life cycle [23]. The number of WBC in the mice from the model group was significantly higher than that of mice from the normal group. Some cell stores in bone marrow may be mobilized into the peripheral blood quickly after the WBC death caused by CTX. This should be the rebound of WBC [24] because these cells do not have a sound function just like WBC, but only the body's stress response to the loss of WBC. So they have no immune function. WBC will decrease rapidly after rebound which was proved in our preliminary experiments. The increase of WBC in the mice from the administration group was smaller than that from the model group. We speculated that the Re and Rk3 can improve myelosuppression to a certain extent, which reduces the degree of stress response in WBC. Thrombocytopenia can cause bleeding, while severe reduction can even lead to death. The number of PLT in the mice from the model group was significantly lower than that of mice from the normal group. PLT life cycle may become shorter because of the damage of megakaryocyte. The number of PLT in mice from the administration group increased. It shows that Re and Rk3 can promote recovery the bone marrow hematopoietic function.
The number of BMNCs is a direct indicator of bone marrow hematopoietic function [24]. The decrease in the number of BMNC in the model group indicates that CTX may cause acute damage of bone marrow and induce senescence and apoptosis of BMNC. The damage is somewhat alleviated after administration. The thymus index and spleen index were also investigated in the present study because of the close relationship between immunity and hematopoiesis [24]. The thymus is an important immune organ [25]. The maturation of lymphocytes also depends on the thymus [26]. Thymus and thymocytes not only provide a specific microenvironment but also secrete important cytokines [27]. The thymus index of mice from the model group decreased significantly. The damage of immune organs may be caused by apoptosis of thymocytes due to CTX. The increase of thymus index indicates that the Re and Rk3 can promote the recovery of this damage after administration. Spleen is not only a hematopoietic organ but also an immune organ [28]. The spleen can restore hematopoiesis and perform compensatory extramedullary hematopoiesis when the function of bone marrow is damaged [29]. The increase of spleen index in mice from the model group indicates that while CTX causes bone marrow hematopoietic damage in mice, spleen compensatory hematopoiesis leads to spleen connective tissue hyperplasia. The spleen index of the mice from the administration group is smaller than that of the model group. We speculate that Re and Rk3 may reduce the burden of the spleen while restoring bone marrow hematopoietic function [30].
To further explore the mechanism of Re and Rk3 in improving hematopoietic function, we analyzed the effect on colony yield of hematopoietic progenitor cells in vitro; the effect on TPO, EPO, and GM-CSF of plasma; and the effect on cell cycle and the expression of bcl-2, bax, and caspase-3 proteins.
The essence of myelosuppression is that the function of hematopoietic stem cell/hematopoietic progenitor cell (HSC/HPC) in the bone marrow is impaired after chemotherapy [31]. The yield of colony cultured in vitro can be used to evaluate the function of HSC/HPC. The yield of BFU-E, CFU-E, CFU-Meg, and CFU-GM is lower in the mice from the model group. It showed that CTX can cause serious damage to the proliferation and differentiation of HPC. The yield of colony in the mice from the administration group was more than that in the model group significantly. We speculate that Re and Rk3 may increase the sensitivity of the receptor on the cell surface that binds to the cytokines and enhance the proliferation and differentiation of HPC. The quantity of bone marrow cells that have been reduced by the damage of CTX is recovered so that the peripheral blood can restore.
Cytokines are important factors to stimulate the proliferation and differentiation of HSC/HPC [32]. GM-CSF has stimulative effect of the proliferation and differentiation on the process from myeloid stem cells to mature granulocytes [33]. The main physiological function of EPO is promoting the binding of erythroid progenitor cells' surface receptor, acceleration erythroid stem cells differentiate into erythrocytes and synthesis of HGB [34], [35]. TPO can boost HSC differentiation to megakaryocytes, stimulate the development and maturity of megakaryocytes, and gear up the production and release of PLT [36]. When HSC activity weakens, the number of these cytokines will be forced to rise. This is a reflection of the body's compensation mechanism [37]. Our results show that both Re and Rk3 make the number of GM-CSF, EPO, and TPO normal, indirectly demonstrating the recovery of hematopoietic stem cells' proliferative and differentiative capacity.
The cell cycle of bone marrow is one of the important indicators reflecting the hematopoietic function of the hematopoietic system, and the distribution of the various phases of the cell cycle can reflect the state of bone marrow cell proliferation [38]. The ratio of G0/G1 phase in the model group was significantly higher than that in the normal group, indicating the CTX-induced DNA damage in hematopoietic cells and that the damaged DNA stayed in G1 phase to repair. And, for DNA that cannot be repaired, an apoptotic program will be initiated. This process leads to a reduction in the ratio of cells in the S and G2/M phase and the proliferation index. The ratio of G0/G1 phase in the mice in the administration group was significantly lower than that in the model group. We speculate that Re and Rk3 may facilitate the repair of damaged DNA and may also arouse part of cells in G0 phase. Therefore, more cells can pass the G1 phase checkpoint to enter the S phase for DNA synthesis and then enter the G2/M phase to complete mitosis. Re and Rk3 can antagonize the toxicity and side effects of CTX on bone marrow cells effectively.
Bcl-2 protein (bcl-2) can inhibit many types of apoptosis. It protects cells by prolonging the survival time of cells so that it can provide time for the repair of DNA that was damaged before cell division [39]. Bax protein (bax) has the opposite biological function as bcl-2. Bax can promote apoptosis by increasing the sensitivity of apoptosis [40]. Caspase-3 protein (caspase-3) is a common downstream effector of the various apoptosis pathway [41]. It is an apoptotic executant that occupies the core position in the whole process of apoptosis [42]. It is considered a very specific and sensitive apoptotic marker [43]. The results show that Re and Rk3 can stimulate the expression of bcl-2 and inhibit the expression of bax and caspase-3. Regulating the balance of bcl-2/bax and inhibiting the expression of caspase-3 may be one of the mechanisms of Re and Rk3 in promoting myeloid bone marrow cell proliferation and recovering bone marrow hematopoietic function in mice.
5. Conclusion
In conclusion, the present study demonstrates that Re and Rk3 could relieve the symptoms of myelosuppression and improve the bone marrow hematopoietic function. It is probably attributed to three different mechanisms. First, Re and Rk3 could regulate the level of cytokines to normal. Second, Re and Rk3 could promote more cells to pass the G1 phase checkpoint to enter the normal cell cycle. Third, Re and Rk3 could regulate the balance of bcl-2/bax and inhibit the expression of caspase-3 to prevent BMNC from apoptosis. Our results showed that Rk3 in both low and high concentrations was significantly effective compared with Re. It may be due to its lower polarity, greater lipid solubility, and ease to pass through biofilms. The present study provided an experimental basis for the treatment of myelosuppression and the development of Re and Rk3. Furthermore, more precise mechanisms will be investigated in the subsequent research.
Conflicts of interest
All the authors declare that there are no conflicts of interest.
Acknowledgments
This work was financially supported by National key R&D program, Key Technology Research of Ginseng Industry and Development of Great Health Products (grant No. 2017YFC1702101).
References
- 1.Hoekman K., Wagstaff J., van Groeningen C.J., Vermorken J.B., Boven E., Pinedo H.M. Effects of recombinant human granulocyte-macrophage colony-stimulating factor on myelosuppression induced by multiple cycles of high-dose chemotherapy in patients with advanced breast cancer. J Natl Cancer Inst. 1991;83(21):1546–1553. doi: 10.1093/jnci/83.21.1546. [DOI] [PubMed] [Google Scholar]
- 2.Molyneux G., Andrews M., Sones W., York M., Barnett A., Quirk E., Yeung W., Turton J. Haemotoxicity of busulphan, doxorubicin, cisplatin and cyclophosphamide in the female BALB/c mouse using a brief regimen of drug administration. Cell Biol Toxicol. 2011;27(1):13–40. doi: 10.1007/s10565-010-9167-1. [DOI] [PubMed] [Google Scholar]
- 3.Toussaint O., Medrano E.E., Von Z.T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35(8):927–945. doi: 10.1016/s0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 4.Wickremasinghe R.G., Hoffbrand A.V. Biochemical and genetic control of apoptosis: relevance to normal hematopoiesis and hematological malignancies. Blood. 1999;93(11):3587–3600. [PubMed] [Google Scholar]
- 5.Friberg L.E., Karlsson M.O. Mechanistic models for myelosuppression. Invest New Drugs. 2003;21(2):183. doi: 10.1023/a:1023573429626. [DOI] [PubMed] [Google Scholar]
- 6.Rivera E., Hu S., Concha C. Ginseng and aluminium hydroxide act synergistically as vaccine adjuvants. Vaccine. 2003;21(11):1149–1157. doi: 10.1016/s0264-410x(02)00518-2. [DOI] [PubMed] [Google Scholar]
- 7.Christensen L.P. Ginsenosides chemistry, biosynthesis, analysis, and potentialhealth effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 8.Yamabe N., Kim Y.J., Lee S., Cho E.J., Park S.H., Ham J., Kim H.Y., Kang K.S. Increase in antioxidant and anticancer effects of ginsenoside Re-lysine mixture by Maillard reaction. Food Chem. 2013;138(2–3):876–883. doi: 10.1016/j.foodchem.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Su F., Yuan L., Zhang L., Hu S. Ginsenosides Rg1 and Re act as adjuvant via TLR4 signaling pathway. Vaccine. 2012;30(27):4106–4112. doi: 10.1016/j.vaccine.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 10.Liu L., Huang J., Hu X., Li K., Sun C. Simultaneous determination of ginsenoside (G-Re, G-Rg1, G-Rg2, G-F1, G-Rh1) and protopanaxatriol in human plasma and urine by LC-MS/MS and its application in a pharmacokinetics study of G-Re in volunteers. J Chromatogr B. 2011;879(22):2011–2017. doi: 10.1016/j.jchromb.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Peng L., Sun S., Xie L.H., Wicks S.M., Xie J.T. Ginsenoside Re: pharmacological effects on cardiovascular system. Cardiovasc Therapeut. 2012;30(4):e183–e188. doi: 10.1111/j.1755-5922.2011.00271.x. [DOI] [PubMed] [Google Scholar]
- 12.Duan Z., Deng J., Dong Y., Zhu C., Li W., Fan D. Anticancer effects of ginsenoside Rk3 on non-small cell lung cancer cells: in vitro and in vivo. Food Funct. 2017;8(10) doi: 10.1039/c7fo00385d. [DOI] [PubMed] [Google Scholar]
- 13.Lee J.G., Baek S.H., Lee Y.Y., Park S.Y., Park J.H. Anticomplementaryginsenosides isolated from processed ginseng. Biol Pharmaceut Bulletin. 2011;34(6):898–900. doi: 10.1248/bpb.34.898. [DOI] [PubMed] [Google Scholar]
- 14.Lee J.G., Lee Y.Y., Wu B., Kim S.Y., Lee Y.J., Yun-Choi H.S., Park J.H. Inhibitory activity of ginsenosides isolated from processed ginseng on platelet aggregation. Pharmazie. 2010;65(7):520–522. [PubMed] [Google Scholar]
- 15.Xia J., Zhang J.Q., Ruan C.C., Sun G.Z., Liu Z. Preparation of rare ginsenoside by transformation of ginsenoside Re catalyzed with aspartic acid. Chin Tradit Herb Drugs. 2016;47(19):3389–3394. [in Chinese] [Google Scholar]
- 16.Salem M.L., Al-Khami A.A., El-Nagaar S.A., Zidan A.A., Al-Sharkawi I.M., Diaz-Montero C.M., Cole D.J. Kinetics of rebounding of lymphoid and myeloid cells in mouse peripheral blood, spleen and bone marrow after treatment with cyclophosphamide. Cell Immunol. 2012;276:67–74. doi: 10.1016/j.cellimm.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan J., Qing B. Henan Medical University Press; 2000. Oncology and chemotherapy; pp. 25–50. [in Chinese] [Google Scholar]
- 18.Campisi J., Kim S.H., Lim C.S., Rubio M. Cellular senescence, cancer and aging: the telomere connection. Exp Gerontol. 2001;36:1619–1637. doi: 10.1016/s0531-5565(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 19.Marcotte R., Wang E. Replicative senescence revisited. J Gerontol A Biol Sci Med Sci. 2002;57:257–269. doi: 10.1093/gerona/57.7.b257. [DOI] [PubMed] [Google Scholar]
- 20.Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30(6):513–528. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- 21.Domen J. The role of apoptosis in regulating hematopoiesis and hematopoietic stem cells. Immunol Res. 2000;22(2–3):83–94. doi: 10.1385/IR:22:2-3:83. [DOI] [PubMed] [Google Scholar]
- 22.Song M., Baik H.W., Hong S.G., Sung M.K. vol. 21. 2016. pp. 312–320. (Wheat bran arabinoxylan supplementationalleviates 5-fluorouracil induced mucositis andmyelosuppression in BALB/c Mice). [Google Scholar]
- 23.Li W., Zhao Y., Li X. Effect of Zishenshengxue capsule on myelosuppression in mice induced by. Cyclophosphamide. 2013;33(2):233–237. doi: 10.1016/s0254-6272(13)60131-4. [DOI] [PubMed] [Google Scholar]
- 24.Carey P.J. Drug-induced myelosuppression: diagnosis and management. Drug Safety. 2003;26(10):691–706. doi: 10.2165/00002018-200326100-00003. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi T. Effects of X-radiation and thymectomy on the immune response in the marine teleost Sebasticusmarmoratus. Dev Comp Immunol. 1986;10(4):519–528. doi: 10.1016/0145-305x(86)90173-4. [DOI] [PubMed] [Google Scholar]
- 26.Tatner M.F. Natural changes in the immune system of fish. Fish Immune Syst Org Pathog Environ. 1996:255–287. [Google Scholar]
- 27.Trede N.S., Zon L.I. Development of T-cells during fish embryogenesis. Dev Compar Immunol. 1998;22(3):253–263. doi: 10.1016/s0145-305x(98)00009-3. [DOI] [PubMed] [Google Scholar]
- 28.Lei M., Wang J.F., Wang Y.M., Pang L., Wang Y., Xu W., Xue C.H. Study of the radio-protective effect of cuttlefish ink on hemopoietic injury. Asia Pac J Clin Nutr. 2007;16:239–243. [PubMed] [Google Scholar]
- 29.Queiroz M.L., Valadares M.C., Torello C.O., Ramos A.L., Oliveira A.B., Rocha F.D., Arruda V.A., Accorci W.R. Comparative studies of the effects of Tabebuiaavellanedae bark extract and beta-lapachone on the hematopoietic response of tumour-bearing mice. J Ethnopharmacol. 2008;117(2):228–235. doi: 10.1016/j.jep.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 30.Bao H., Geng X., Jia X., Wang H., Zhi Y., Hu J., Chen H., Yu Z., Yang H. Studies on immune suppression on spleen cells of rats caused by cyclophosphamide. J Toxicol. 2014;28(3):189–194. [in Chinese] [Google Scholar]
- 31.Wang Y., Probin V., Zhou D. Cancer therapy-induced residual bone marrow injury-mechanisms of induction and implication for therapy. Curr Cancer Ther Rev. 2006;2:271–279. doi: 10.2174/157339406777934717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Y.L., Wang L.Y., Wang J.X., Wang C., Wang C.L., Zhao D.P., Wang Z.C., Zhang J.J. Protective effects of paeoniflorin and albiflorin on chemotherapy-induced myelosuppression in mice. Chin J Nat Med. 2016;14(8):599–606. doi: 10.1016/S1875-5364(16)30070-X. [DOI] [PubMed] [Google Scholar]
- 33.Kimura T., Sakabe H., Tanimukai S.H., Abe T., Urata Y., Yasukawa K., Okano A., Taga T., Sugiyama H., Kishimoto T. Simultaneous activation of signals through gp130, c-kit, andinterleukin-3 receptor promotes a trilineage blood cell production in the absence of terminally actinglineage-specific factors. Blood. 1997;90(12):4767–4778. [PubMed] [Google Scholar]
- 34.Wu H., Liu X., Jaenisch R., Lodish H.F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietinreceptor. Cell. 1995;83(1):59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 35.Lin C.S., Lim S.K., DAgati V., Costantini F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996;10(2):154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 36.Drachman J.G. Role of thrombopoietin in hematopoietic stem cell and progenitor regulation. Curr Opin Hematol. 2000;7(3):183. doi: 10.1097/00062752-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Fujiwara T., Harigae H., Kameoka J., Yokoyama H., Takahashi S., Tomiya Y., Yamada M., Ishizawa K., Imaizumi M., Sasaki T. A case of familial thrombocytosis: possible role of altered thrombopoietin production. Hematol. 2004;76(4):395. doi: 10.1002/ajh.20105. [DOI] [PubMed] [Google Scholar]
- 38.Ju G.Z., Ma S.M., Fu S.B., Liu S.Z. The influence of radiation on mouse bone marrow cell cycle and its molecular mechanism. Chin J Radiol Med Protect. 2002;22:342–343. [Google Scholar]
- 39.Kasid U., Suy S., Dent P., Whiteside T.L. Activation of Raf by ionizing radiation. Nature. 1996;382(6594):813–816. doi: 10.1038/382813a0. [DOI] [PubMed] [Google Scholar]
- 40.Delia D., Aiello A., Soligo D. Bcl-2 proto-oncogene expression in normal and neoplastic human myeloid cells. Blood. 1992;79(5):1291–1298. [PubMed] [Google Scholar]
- 41.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Cell Res. 2002;12(3):459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 42.Lu M., Cao D.M., Zhao X.X. Study on dynamic effect of acupuncture on marrow cell cycle regulatory protein cyclin D1 expression and cell cycle in mice with cyclophosphamide induced myelosuppression. Chin J Integrated Tradit Western Med. 2011;31(2):238–243. [PubMed] [Google Scholar]
- 43.Florentin A., Arama E. Caspase levels and execution efficiencies determine the apoptotic potential of the cell. J Cell Biol. 2012;196(4):513–527. doi: 10.1083/jcb.201107133. [DOI] [PMC free article] [PubMed] [Google Scholar]