Abstract
Stroke is a common cause of patient morbidity and mortality, being the fifth leading cause of death in the United States. Positron emission tomography (PET) is a proven tool for oncology patients, and may have utility in patients with stroke. We demonstrate findings of stroke incidentally detected on oncologic PET/CTs using 18F-FDG, 11C-Choline, and 68Ga-DOTATATE radiotracers. Specifically, focal 11C-Choline or 68Ga-DOTATATE uptakes localized in areas of MRI confirmed ischemia, and paradoxically increased 18F-FDG activity was visualized surrounding a region of hemorrhage, in different patients. These cases demonstrate that PET may have utility in evaluating patients with stroke based on flow dynamics, metabolic activity, and receptor expression.
Keywords: Stroke, PET, 68Ga-DOTATATE, 11C-Choline, 18F-FDG
Introduction
Stroke is characterized as a neurologic deficit due to a central nervous system injury by a vascular cause. It is a very common cause of patient morbidity and mortality in the United States with approximately 795,000 people affected yearly, and it is the fifth leading cause of death [1], [2], [3]. Ischemic stroke is the most common type, accounting for approximately 87% of strokes. Hemorrhagic stroke is less common but has important distinguishing implications [3]. Because of the differing clinical management of these stroke types, imaging is crucial for diagnosis and follow-up [2]. Due to its ubiquitous nature with quick and easy access, computed tomography (CT) is the most commonly used imaging modality [4]. However, magnetic resonance imaging (MRI) has superior sensitivity compared to CT and is becoming more common as access increases and scan times become shorter [4].
Positron emission tomography (PET) is not currently used in stroke diagnosis. However, it offers unique opportunities in the evaluation of stroke pathophysiology that may not be detectable on CT and MRI [5]. 15O-labeled water PET as well as 18F-fluoromisonidazole (18F-FMISO) PET has been used to identify the hypoxic penumbra after ischemic stroke via perfusion and oxygen consumption [6], [7]. A few studies evaluated stroke detection with 18F-FDG PET have resulted in conflicting data about 18F-FDG uptake in perihematomal and peri-infarct tissues [8], [9]. 11C-Choline and 68Ga-DOTATATE PET/CT have not been specifically evaluated in the setting of stroke.
We review 3 oncological cases demonstrating strokes on restaging PET/CT and confirmed with CT and/or MRI. In each case, the patient presented for routine PET/CT follow-up and a stroke was incidentally detected on the study. Ischemic strokes were identified in 2 patients imaged with 11C-Choline or 68Ga-DOTATATE PET/CT (Case 1 and Case 2). A third patient with a hemorrhagic stroke was imaged with 18F-FDG PET/CT (Case 3).
Case 1
A 76-year-old male with prostate cancer and hypertension presented with rising prostate specific antigen (PSA). A whole body 11C-Choline PET/MRI demonstrated new abnormal focal 11C-Choline uptake within the right basal ganglia (SUVmax 2.0 vs 0.2 on the contralateral normal side). Subsequent brain MRI performed 3 days later demonstrated decreased central T1 signal intensity, increased T2 signal, and abnormal enhancement in the right globus pallidus consistent with an acute-to-subacute infarction. The patient was asymptomatic and observed. Imaging findings are presented in Fig. 1.
Fig. 1.
A 76-year-old male presented with an acute-to-subacute ischemic stroke in the right basal ganglia on 11C-Choline PET/MRI. (a) Q.Clear MIP image demonstrating faint abnormal choline activity (arrow) superior and adjacent to more intense physiologic pituitary activity. Corresponding axial (b) Q.Clear PET and (c) fused Q.Clear PET/MRI images demonstrating abnormally-increased choline activity localizing to the right basal ganglia. Subsequent diagnostic brain MR (d) FLAIR and (e) postcontrast T1-weighted images demonstrate increased signal and enhancement consistent with a subacute ischemic infarct.
Case 2
A 14-year-old female with a large right carotid sheath paraganglioma developed early postoperative left hemiparesis and dysphagia. A diagnostic head CT demonstrated thrombosis of the right middle cerebral artery with ischemic changes in the caudate, putamen, and insula. The patient was treated with intra-arterial thrombolysis resulting in partial recanalization of the middle cerebral artery. A restaging 68Ga-DOTATATE PET/CT performed 6 days later revealed abnormally-increased DOTATATE uptake (SUVmax 1.6) corresponding to the areas of subacute ischemia seen on the preintervention CT and a later MRI. Imaging findings are presented in Fig. 2.
Fig. 2.
A 14-year-old female with a large carotid body paraganglioma presented with an early postoperative ischemic stroke in the right middle cerebral artery territory. Subsequent restaging 68Ga-DOTATATE PET/CT demonstrates mildly-increased activity in the right caudate on the (a) Q.Clear MIP image, (b) Q.Clear PET axial image, and (c) fused Q.Clear PET/CT axial images. Follow-up brain MRI demonstrated (d) restriction diffusion in this region (orthodontic hardware susceptibility artifacts are present) as well as increased T2 signal on (e) FLAIR.
Case 3
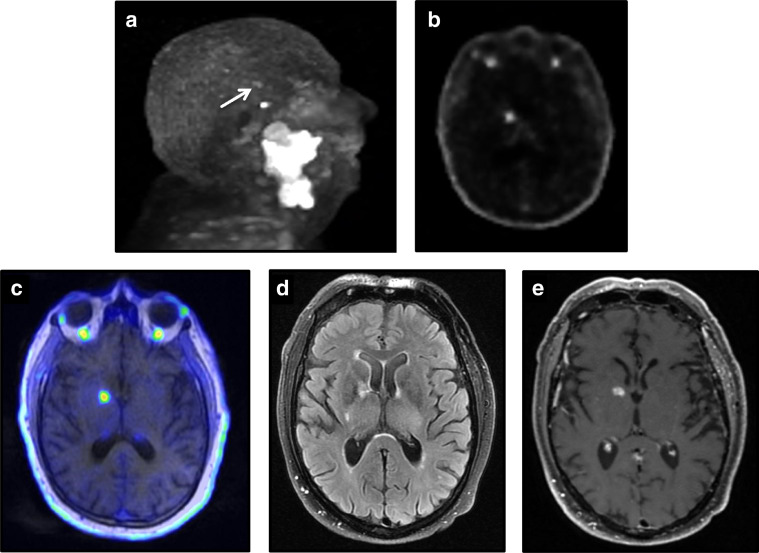
An asymptomatic 64-year-old male with lymphoma presented for a restaging 18F-FDG PET/CT after 6 cycles of hyper-CVAD chemotherapy. His past medical history included hypertension, hypercholesterolemia, diabetes mellitus type 2, and a remote hemorrhagic stroke in the right basal ganglia. On the PET/CT, a large right hemispheric intraparenchymal hemorrhage with ventricular extension was detected on the CT images. The PET images demonstrated intense 18F-FDG uptake (SUVmax 15.5) in the adjacent right hemispheric cerebral parenchyma surrounding the hemorrhage. The patient was observed and treated by medical management without surgical intervention. Follow-up anatomic imaging remained stable. Imaging findings are presented in Fig. 3.
Fig. 3.
A 64-year-old asymptomatic male presented with acute hemorrhagic stroke on a restaging oncologic 18F-FDG PET/CT. Marked increase right cerebral FDG activity is visualized on the (a) MIP image. (b) Corresponding unenhanced low-dose fusion CT axial image of the head demonstrates a right hemispheric parenchymal hemorrhage with extension into the right lateral ventricle. (c) The corresponding fused PET/CT axial image demonstrates marked FDG activity (SUVmax 15.5) in the cerebral parenchyma surrounding the hemorrhage. (d) Short-term follow-up diagnostic head CT demonstrates a stable intraparenchymal hemorrhage.
Discussion
Our cases suggest that regions of acute/subacute ischemic stroke demonstrate increased 11C-Choline and 68Ga-DOTATATE uptake, and regions adjacent to hemorrhagic stroke demonstrate increased 18F-FDG uptake.
11C-Choline PET is FDA approved for metastatic prostate cancer, but also has an emerging application for parathyroid adenoma assessment. The mechanism of choline uptake is not fully understood, however, choline kinase is upregulated in tumor cells [10]. Choline kinase activity is noted to increase under hypoxic conditions in prostate cancer cells [10], [11]. Delaunay et al observed increased 18F-Choline uptake in acute ischemic stroke in a prostate cancer patient [12]. They suggested the concept of choline precursors promoting repair in neurologic diseases, and increased choline uptake during inflammatory and repair process in acute ischemic stroke may relate to this [12]. Scremin et al demonstrated increased extracellular choline concentration in ischemia, which may support the role of choline or choline precursors in ischemia repair [13]. These mechanisms may explain the uptake we observed in the basal ganglia stroke.
68Ga-DOTATATE PET is primarily used for neuroendocrine tumor assessment and works by binding to somatostatin receptors (SSTRs), with specifically high affinity for SSTR type 2 (SSTR2). The mechanism for 68Ga-DOTATATE uptake in ischemia is not known; however, Vallee et al suggested that uptake may be within receptors on macrophages from the inflammatory response in addition to possible neuronal receptor activation under ischemic conditions [14]. Moreover, several studies detected SSTR2 expression in human macrophages [15], [16]; however, Tarkin et al demonstrated high levels of SSTR2 expression specifically in activated proinflammatory M1 macrophages [15], [16], [17]. They demonstrated marked 68Ga-DOTATATE activity in the coronary arteries of the patients with atherosclerotic plaques, correlating with macrophage activity [17], [18]. Strong correlation of carotid SSTR2 mRNA level with in vivo 68Ga-DOTATATE PET imaging findings has also been demonstrated [17]. Specifically, 68Ga-DOTATATE correctly differentiated culprit carotid arteries from nonculprit carotid arteries in patients with transient ischemic attack or stroke [17].
18F-FDG uptake has more commonly been evaluated in the setting of ischemic stroke than hemorrhagic stroke [19]. However, a few studies have looked at 18F-FDG uptake in hemorrhagic stroke. Zazulia et al found increased 18F-FDG uptake in perihematomal regions in the acute period (2-4 days) of intracerebral hemorrhage in 6 out of 13 patients [9]. However, they did not find any difference in the uptake during the subacute period (5-8 days) compared to baseline. Lin et al demonstrated that 18F-FDG uptake decreases in perihematomal tissues within 5 days after intracerebral hemorrhage in cat models [9]. Neither of these studies evaluated the chronic phase of stroke. The markedly increased 18F-FDG-uptake in the perihematomal regions of our patient suggests a more acute process, however, an MRI was not available for confirmation.
Although not illustrated in our cases, some investigators have proposed the use of 18F-FDG in evaluation of viable brain tissue in the setting of ischemic stroke [19], [20], [21]. Several studies on animals demonstrated a reduced 18F-FDG uptake in the presumed ischemic core regions and, while results are less consistent, transient increase has also been observed in the peri-ischemic “penumbra” region [19]. Characterization of the penumbra size helps guide clinical management in the decision to attempt revascularization. Proposed explanations of the underlying pathophysiology include activation of GLUTs, activation of hexokinase, and neuroinflammation [19].
Dedicated use of PET in the setting of stroke has several limitations that impede dedicated use, including access to radiopharmaceuticals, long uptake times, and lack of standardized protocols. However, this does not diminish the importance of identifying and understanding key characteristics that may be visualized in incidentally observed strokes. As new tracers are approved and become more commonly used in clinical practice, we must continue to identify key characteristics of ubiquitous pathology, including stroke.
Conclusion
Despite the primary role of PET in oncology, incidental strokes are not infrequently detected. Our cases demonstrate abnormally-increased uptake in both ischemic and hemorrhagic strokes using 3 different radiotracers. More work is required to understand the mechanism behind these phenomena; however, we have presented prominent theories for underlying mechanisms, and familiarity with the spectrum of stroke appearances on PET is vital for optimal patient care.
Footnotes
Declaration of Competing Interest: The authors declare that they have no conflict of interest.
References
- 1.Kochanek K.D., Murphy S.L., Xu J., Arias E. Mortality in the United States, 2013. NCHS Data Brief 178. 2014:1–8. https://www.cdc.gov/nchs/products/citations.htm. [PubMed] [Google Scholar]
- 2.Sacco R.L., Kasner S.E., Broderick J.P., Caplan L.R., Connors J.J., Culebras A. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 4.Chalela J.A., Kidwell C.S., Nentwich L.M., Luby M., Butman J.A., Demchuk A.M. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293–298. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Read S.J., Hirano T., Abbott D.F., Sachinidis J.I., Tochon-Danguy H.J., Chan J.G. Identifying hypoxic tissue after acute ischemic stroke using PET and 18F-fluoromisonidazole. Neurology. 1998;51(6):1617–1621. doi: 10.1212/wnl.51.6.1617. [DOI] [PubMed] [Google Scholar]
- 6.Baron J.C. Mapping the ischaemic penumbra with PET: implications for acute stroke treatment. Cerebrovasc Dis. 1999;9(4):193–201. doi: 10.1159/000015955. [DOI] [PubMed] [Google Scholar]
- 7.Baron J.C. Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc Dis. 2001;11 Suppl 1:2–8. doi: 10.1159/000049119. [DOI] [PubMed] [Google Scholar]
- 8.Lin X., Tang Y., Sun B., Hou Z., Meng H., Li Z. Cerebral glucose metabolism: influence on perihematomal edema formation after intracerebral hemorrhage in cat models. Acta Radiol. 2010;51(5):549–554. doi: 10.3109/02841851003660065. [DOI] [PubMed] [Google Scholar]
- 9.Zazulia A.R., Videen T.O., Powers W.J. Transient focal increase in perihematomal glucose metabolism after acute human intracerebral hemorrhage. Stroke. 2009;40(5):1638–1643. doi: 10.1161/STROKEAHA.108.536037. [DOI] [PubMed] [Google Scholar]
- 10.Castellucci P., Fuccio C., Rubello D., Schiavina R., Santi I., Nanni C. Is there a role for 11 C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5 ng/ml? Eur J Nucl Med Mol Imaging. 2011;38(1):55–63. doi: 10.1007/s00259-010-1604-0. [DOI] [PubMed] [Google Scholar]
- 11.Glunde K., Shah T., Winnard P.T., Jr., Raman V., Takagi T., Vesuna F. Hypoxia regulates choline kinase expression through hypoxia-inducible factor-1 alpha signaling in a human prostate cancer model. Cancer Res. 2008;68(1):172–180. doi: 10.1158/0008-5472.CAN-07-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaunay K., Le Jeune F., Garin E., Devillers A., Palard-Novello X. 18F-choline uptake in acute ischemic stroke. Clin Nucl Med. 2017;42(2):e121–e122. doi: 10.1097/RLU.0000000000001439. [DOI] [PubMed] [Google Scholar]
- 13.Scremin O.U., Jenden D.J. Focal ischemia enhances choline output and decreases acetylcholine output from rat cerebral cortex. Stroke. 1989;20(1):92–95. doi: 10.1161/01.str.20.1.92. [DOI] [PubMed] [Google Scholar]
- 14.Vallée É., Paquet N., Buteau J.P., Turcotte É. 68Ga-DOTATATE uptake in ischemic stroke. Clin Nucl Med. 2018;43(1):46–47. doi: 10.1097/RLU.0000000000001894. [DOI] [PubMed] [Google Scholar]
- 15.Armani C., Catalani E., Balbarini A., Bagnoli P., Cervia D. Expression, pharmacology, and functional role of somatostatin receptor subtypes 1 and 2 in human macrophages. J Leukoc Biol. 2007;81(3):845–855. doi: 10.1189/jlb.0606417. [DOI] [PubMed] [Google Scholar]
- 16.Dalm V.A., van Hagen P.M., van Koetsveld P.M., Achilefu S., Houtsmuller A.B., Pols D.H. Expression of somatostatin, cortistatin, and somatostatin receptors in human monocytes, macrophages, and dendritic cells. Am J Physiol Endocrinol Metab. 2003;285(2):E344–E353. doi: 10.1152/ajpendo.00048.2003. [DOI] [PubMed] [Google Scholar]
- 17.Tarkin J.M., Joshi F.R., Evans N.R., Chowdhury M.M., Figg N.L., Shah A.V. Detection of atherosclerotic inflammation by 68Ga-DOTATATE PET compared to [18F] FDG PET imaging. J Am Coll Cardiol. 2017;69(14):1774–1791. doi: 10.1016/j.jacc.2017.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rominger A., Saam T., Vogl E., Übleis C., la Fougère C., Förster S. In vivo imaging of macrophage activity in the coronary arteries using 68Ga-DOTATATE PET/CT: correlation with coronary calcium burden and risk factors. J Nucl Med. 2010;51(2):193–197. doi: 10.2967/jnumed.109.070672. [DOI] [PubMed] [Google Scholar]
- 19.Bunevicius A., Yuan H., Lin W. The potential roles of 18F-FDG-PET in management of acute stroke patients. Biomed Res Int. 2013;2013 doi: 10.1155/2013/634598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobrado M., Delgado M., Fernández-Valle E., García-García L., Torres M., Sánchez-Prieto J. Longitudinal studies of ischemic penumbra by using 18F-FDG PET and MRI techniques in permanent and transient focal cerebral ischemia in rats. Neuroimage. 2011;57(1):45–54. doi: 10.1016/j.neuroimage.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Walberer M., Backes H., Rueger M.A., Neumaier B., Endepols H., Hoehn M. Potential of early [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography for identifying hypoperfusion and predicting fate of tissue in a rat embolic stroke model. Stroke. 2012;43(1):193–198. doi: 10.1161/STROKEAHA.111.624551. [DOI] [PubMed] [Google Scholar]