Abstract
Background
Korean Red Ginseng (KRG) is widely used for strengthening the immune system and fighting fatigue, especially in people with deficiency syndrome. However, there is concern that the long-term application or a high dose of KRG can cause “fireness” (上火 in Chinese) because of its “dryness” (燥性 in Chinese). The aim of this study was to assess the safety and efficacy of a 4-week treatment with KRG in participants with deficiency syndrome.
Methods
This was a 4-week, randomized, double-blind, placebo-controlled clinical trial. A total of 180 Chinese participants were randomly allocated to three groups: placebo control group, participants were given a placebo, 3.6 g/d; KRG 1.8 g and 3.6 g groups. The primary outcomes were the changes in fireness and safety evaluation (adverse events, laboratory tests, and electrocardiogram). The secondary outcomes were the efficacy of KRG on fatigue, which include the following: traditional Chinese medicine (TCM) symptom scale and fatigue self-assessment scale.
Results
Of the 180 patients, 174 completed the full study. After 4 weeks of KRG treatment, the Fire-heat symptoms score including Excess fire-heat score and Deficient fire-heat score showed no significant change as compared with placebo treatment, and no clinically significant changes in any safety parameter were observed. Based on the TCM syndrome score and fatigue self-assessment score, TCM symptoms and fatigue were greatly improved after treatment with KRG, which showed a dose- and time-dependent effect. The total effective rate was also significantly increased in the KRG groups.
Conclusion
Our study revealed that KRG has a potent antifatigue effect without significant adverse effects in people with deficiency syndrome. Although a larger sample size and longer treatment may be required for a more definite conclusion, this clinical trial is the first to disprove the common conception of “fireness” related to KRG.
Keywords: Antifatigue effect, Fireness, Korean Red Ginseng, Randomized controlled trial, Safety
1. Introduction
Ginseng (Panax ginseng Meyer) is one of the most popular herbal medicines and has been used in many Asian countries for thousands of years. The major active ingredient of ginseng is the ginsenoside, which is reported to have a wide range of pharmacological activities, including stress reduction and homeostasis maintenance, immunomodulation, antifatigue actions, platelet aggregation inhibition, and anticancer effects [1], [2], [3]. Korean Red Ginseng (KRG) is a heat-processed ginseng developed by the repeated steaming and air drying. Specific ginseng metabolites are produced by chemical transformations during the steaming process [4], [5]. Based on general knowledge, red ginseng has significantly better biological functions and fewer side effects compared with fresh and white ginseng [6]. Studies have indicated that the main biological activities of KRG include immune-enhancement effects, recovery of vital energy, alleviation of fatigue and menopausal disorder, and improvement in blood flow and memory functions [7], [8], [9], [10], [11].
Because of the diversity of its biological functions, KRG has been traditionally used in Asia for thousands of years to treat a variety of ailments. However, in the Chinese population, it is thought that the long-term application or a high dose of KRG may cause fireness [12]. The symptoms evoked by KRG are in accordance with the description of fire-heat symptoms in traditional Chinese medicine (TCM). According to its property theory, TCM shows four Qi (气 in Chinese) and five flavors. The four Qi referred to are cold, cool, warm, and hot. According to the Chinese Pharmacopoeia [13], both red ginseng and ginseng exhibit the warm property, which can replenish the body's energy. However, pathogenic fire-heat tends to consume the body fluids and leads to their deficiency, which then causes fire-heat symptoms.
Fire-heat syndrome is a traditional syndrome of Chinese medicine and refers to any feverish condition during a pathological development [14]. According to the theory of TCM, the fire-heat syndrome can be divided into excess fire-heat and deficient fire-heat. The major symptoms of excess fire-heat syndrome include scorching chest and abdomen, ulcers in the mouth and throat, painful gums, and constipation, whereas dry cough, hectic fever, night fever, and insomnia are usually considered to be characteristics of deficient fire-heat [15], [16], [17]. Although it has been used as a diagnostic marker in clinical practice for more than 1,000 years, it has not yet been clearly defined by specific physiological indicators. In Western medicine, some experts believe that fireness is usually associated with inflammation because both are characterized by “redness, swelling, fever, and pain” [18], [19], [20], [21]. Until now, the fire-heat syndrome has been diagnosed only according to the feelings of patients, without the use of modern diagnostic techniques [22].
Although it is thought that improper use of KRG can cause fireness, there has been no evidence of an association between KRG treatment and fireness, and it is still controversial whether red ginseng or ginseng will cause fireness. To date, there has been no randomized clinical trial examining the effect of KRG on patients with deficiency syndrome. The aim of the present study was to evaluate the safety, particularly in the side effect of “fireness,” and the antifatigue effect of KRG on patients with deficiency syndrome.
2. Methods
2.1. Participants
Our study was well designed according to the CONSORT 2017 statement [23]. The participants were recruited at the LongHua Hospital Shanghai University of Traditional Chinese Medicine (Shanghai, China) from March 2017 to December 2017.
Volunteers were screened according to the inclusion and exclusion criteria. The inclusion criteria were as follows: (1) syndrome differentiation of TCM is asthenia syndrome [24]; (2) men or women aged 18 to 60 years; (3) no functional foods or drugs used within 1 month; and (4) without severe disease of the heart, liver, kidney, or blood system. The exclusion criteria were as follows: (1) syndrome differentiation of TCM is sthenia syndrome [25]; (2) coughing of blood; (3) acute infection, acute stage of chronic disease; (4) not suitable for red ginseng treatment according to their physician; (5) unable to follow dietary or drug restrictions; and (6) participation in another clinical trial within the past 3 months.
The sample size was calculated using PASS 15 (Power Analysis and Sample Size Software (2017). NCSS, LLC. Kaysville, Utah, USA, ncss. com/software/pass). A total sample size of 153 provided a test power of 0.8 to detect difference using a two-sided t test when the relative margin of equivalence was 0.15 and the significance level was 0.05. We used a parallel-controlled design with an equal number of participates in each group. However, considering a 15% withdrawal rate, 180 participants were recruited, and 174 participants completed the study [26].
All participants gave written informed consent before beginning the study. This study was approved by the Institutional Review Board of the LongHua Hospital Shanghai University of Traditional Chinese Medicine (2017LCSY007). The protocol was registered at www.chictr.org.cn (ChiCTR-IPR-17012151).
2.2. Study design
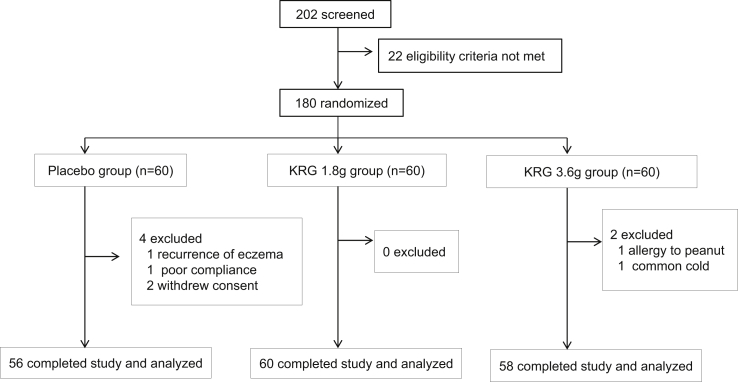
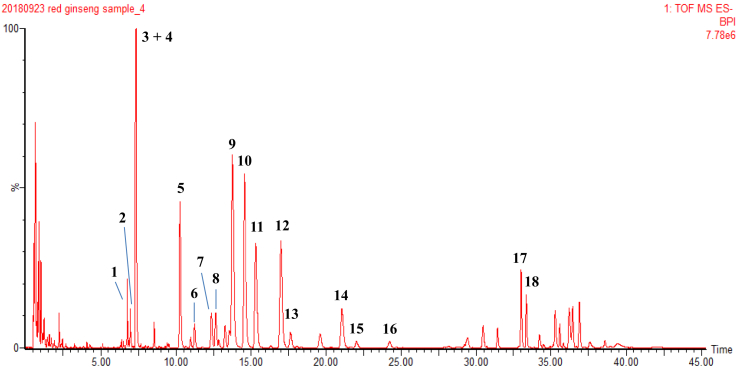
A total of 180 health volunteers with deficiency syndrome were recruited, and those with excess syndrome were excluded (Fig. 1). The participants were then randomized into 3 groups. Participants in the placebo control group received placebo capsules (lactose, microcrystalline cellulose, coloring agent, silica, magnesium stearate, and food flavors) for 4 weeks. In the KRG 1.8 g group, the participants were given 1.8 g KRG each day; whereas in the KRG 3.6 g group, the participants were given 3.6 g KRG each day. The KRG capsules were manufactured by the Korea Ginseng Corporation (Seoul, Korea), the amount of total saponins is 3.5∼4.8 g/100 g, and the major ginsenosides were identified by ultrahigh performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UHPLC-QTOF-MS) (Fig. 2 and supplementary table 1). Placebo capsules, identical in size, weight, color, and taste, were also provided by the Korea Ginseng Corporation. Randomization was performed by a third party using a computerized method of random list generation. All participants, investigators, pharmacists, and study personnel were blinded to treatment allocation by ensuring both KRG and placebo medication were delivered in identical capsules and boxes.
Fig. 1.
Flowchart of the study design. KRG, Korean Red Ginseng.
Fig. 2.
UHPLC–QTOF–MS chromatogram of KRG. KRG was extracted by ultrasonic-assisted solid/liquid extraction (100 mg in 300 mL) using 70% aqueous methanol for 60 min at room temperature. The extracted solutions were analyzed by UHPLC–QTOF–MS analysis. The compounds were identified as follows: ginsenoside Re2 or isomer (1); quinquenoside L17 or isomer (2); ginsenoside Re (3); ginsenoside Rf or isomer (4); ginsenoside Rf (5); ginsenoside F3 or isomer (6); ginsenoside Rg2 (7); 20S-ginsenoside Rh1 (8); ginsenoside Rb1 (9); ginsenoside Ro (10); ginsenoside Rc (11); ginsenoside Rb2 (12); ginsenoside Rb3 (13); ginsenoside Rd (14); ginsenoside Rs1 or isomer (15, 16) ; 20S-ginsenoside Rg3 or isomer (17); 20S-ginsenoside Rg3 (18). KRG, Korean Red Ginseng.
2.3. Outcome measures
After treatment for 4 weeks, the safety index and therapeutic index were determined. For the therapeutic index, the TCM symptom scale and fatigue self-assessment scale were used, whereas the fire-heat symptoms scale, adverse events, electrocardiogram, and other laboratory tests (white blood cells, red blood cells, hemoglobin, hematocrit, platelet count, total protein, albumin, alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, creatinine, and so on) were evaluated to determine the safety index.
2.3.1. Safety assessments
Safety assessments included the following: fire-heat symptom scale, electrocardiogram, and laboratory tests.
The diagnostic score table of heatiness, a tool for assessing fireness [21], was modified, and a fire-heat symptom scale was developed. The fire-heat symptom scale is a 43-item scale that has two subscales: excess fire-heat (29 items) and deficiency fire-heat (14 items) [27]. Each item has 4 response categories on a 0–3 scale, and each subscale is scored individually. The scores are then added to obtain an overall score, with a higher score reflecting more fireness.
2.3.2. Efficacy assessments
Fatigue was assessed by the fatigue self-assessment scale and TCM syndrome questionnaire. Based on the study of fatigue assessment in both China and overseas countries, Wang and Xue [28] developed the fatigue self-assessment scale with consideration of the background of Chinese culture. The scale consists of 23 items and is used to assess the type and severity of fatigue (including three subscales of measuring physical fatigue, mental fatigue, and the consequences of fatigue). Each scale is directly transformed into a 0–4 scale based on the severity of fatigue (0 = no fatigue).
According to the theory of TCM, fatigue meets the diagnostic standards of Qi and Xue deficiency [29]. Based on the Guideline of Clinical Research of TCM New Drugs [30], the TCM syndrome questionnaire was used in our study. The questionnaire consists of 10 items categorized as major and secondary symptoms, and each symptom is directly transformed into a 0–3 scale based on the severity of disease. The instrument yields a summed total score ranging between 0 and 30 (0 = no fatigue). The curative rate was determined based on total integral symptom scores [29].
2.4. Statistical analysis
Descriptive data for means and standard deviations were based on independent measurements and used to describe the baseline and outcome characteristics of the study groups. Differences in mean change from baseline between the treatment groups were examined using a mixed-effects model. A paired t test was used to determine the significance of change from baseline within each treatment group. All p values shown are two-tailed. A p value < 0.05 was considered significant. Statistical analysis was performed using SPSS (version 21.0.0; SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Baseline characteristics
Among the 202 participants screened, 22 participants were excluded because of laboratory test results consistent with the exclusion criteria. There was no seasonal difference in the time of the patients entering the study. The remaining 180 participants were assigned to a placebo group (n = 30) or a KRG group based on each dose (1.8 or 3.6 g of KRG, n = 30 for each group) using a computer-generated randomization schedule. General characteristics of the participants are shown in Table 1. There were no significant differences among the three groups in baseline demographic characteristics including sex, age, body mass index, blood pressure, or heart rate.
Table 1.
Baseline characteristics of the study participants
| Group | Total | Placebo | KRG 1.8 g | KRG 3.6 g |
|---|---|---|---|---|
| Participant, no. (%) | 180 | 60 | 60 | 60 |
| Female sex (%) | 125 (69.4) | 39 (65) | 43 (71.7) | 43 (71.7) |
| Age (yr) | 36.13±11.35 | 37.72±10.88 | 34.88±11.07 | 35.8±0.16 |
| Height (cm) | 165.29±8.18 | 165.51±8.08 | 166.02±8.40 | 164.34±0.73 |
| Body weight (kg) | 59.9±10.03 | 60.87±11.67 | 60.38±11.18 | 58.46±0.81 |
| Body mass index (kg/m2) | 21.92±2.70 | 22.05±2.90 | 21.77±2.88 | 21.95±0.59 |
| Body temperature | 36.75±0.15 | 36.76±0.23 | 36.75±0.20 | 36.73±0.71 |
| Breath (/min) | 17.94±0.42 | 17.92±0.63 | 17.93±0.81 | 17.98±0.87 |
| Heart rate (/min) | 70.08±6.46 | 69.67±7.47 | 69.6±7.87 | 70.98±0.96 |
| Diastolic pressure | 116.98±14.79 | 118.37±8.73 | 117.27±9.54 | 115.3±0.62 |
| Systolic pressure | 72.27±5.4 | 72.2±5.55 | 72.33±6.33 | 72.27±0.89 |
All values are presented as mean ± standard deviation.
3.2. Patient withdrawal
Among the 180 participants, a total of 6 participants dropped out. Four participants dropped out of the placebo group (1 due to the recurrence of eczema, 1 due to low compliance of dietary restrictions, and 2 due to withdrawal of consent); 2 participants dropped out of the KRG 3.6 g group (1 due to allergic reaction to peanuts and 1 due to drug treatment for common cold). Thus, a total of 174 participants completed the 4-week treatment without major protocol violations and had a compliance rate of more than 90%.
3.3. Changes on fireness and safety evaluation
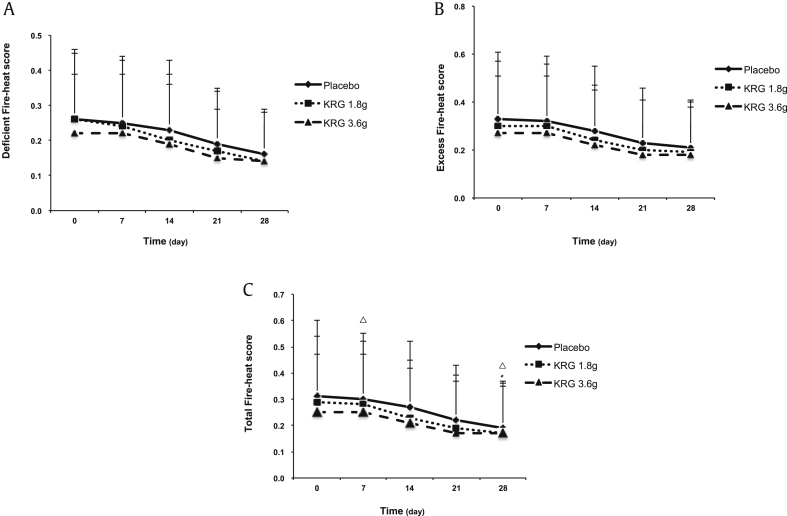
The fire-heat symptoms scale contained excess fire-heat score (EFS) and deficient fire-heat score (DFS). The initial scores in the placebo and KRG 1.8 g and 3.6 g groups were 0.31 ± 0.29, 0.29 ± 0.25, and 0.25 ± 0.22 for total fire-heat score; 0.33 ± 0.28, 0.30 ± 0.27, and 0.27 ± 0.24 for EFS; and 0.26 ± 0.19, 0.26 ± 0.20, and 0.22 ± 0.17 for DFS, respectively. For the difference in within-group treatment, the results showed that the total fire-heat score as well as EFS and DFS was gradually decreased in all three groups (Fig. 3). After treatment with both placebo and KRG for 4 weeks, the total fire-heat score showed a statistically significant difference as compared with initial scores within the same groups. As for the difference in the changes among the three groups, our results (Table 2) showed that the reduction of total fire-heat score was −0.11 ± 0.09 and −0.11 ± 0.09 after the treatment of 1.8 g and 3.6 g KRG, respectively, which showed no significant reduction as compared with the placebo group (mean of changed value −0.09 ± 0.10). Similar results were observed for the EFS and DFS, which also showed no significant reduction after 4 weeks of treatment with KRG as compared with placebo treatment.
Fig. 3.
Comparison of fire-heat symptoms score before and after KRG treatment within the group. Data are expressed as mean ± standard deviation. The within-treatment difference was analyzed by the paired t test; △P < 0.05 compared before and after placebo treatment. The within-treatment difference was analyzed by the paired t test; *P < 0.05 compared before and after treatment with 1.8 g KRG. KRG, Korean Red Ginseng.
Table 2.
Between-group comparison of fireness changes using the fire-heat symptoms scale after specified treatments
| Variable | Change during Week 1 | Change during Week 2 | Change during Week 3 | Change during Week 4 | |
|---|---|---|---|---|---|
| Deficient fire-heat score | A | −0.01±0.03 | −0.03±0.04 | −0.07±0.07 | −0.10±0.09 |
| B | −0.02±0.04 | −0.06±0.05 | −0.09±0.07 | −0.11±0.09 | |
| C | −0.01±0.03 | −0.04±0.04 | −0.07±0.06 | −0.08±0.07 | |
| Excess fire-heat score | A | −0.01±0.04 | −0.05±0.05 | −0.1±0.07 | −0.12±0.09 |
| B | 0.00±0.04 | −0.06±0.05 | −0.09±0.08 | −0.11±0.10 | |
| C | −0.01±0.04 | −0.06±0.07 | −0.09±0.10 | −0.09±0.11 | |
| Total fire-heat score | A | −0.01±0.03 | −0.05±0.05 | −0.09±0.07 | −0.11±0.09 |
| B | 0.00±0.04 | −0.06±0.05 | −0.09±0.07 | −0.11±0.09 | |
| C | −0.01±0.04 | −0.05±0.06 | −0.09±0.09 | −0.09±0.10 | |
KRG, Korean Red Ginseng.
Data are expressed as mean ± standard deviation.
A: participants were given placebo 3.6 g/d; B: participants were given KRG 1.8 g/d; C: participants were given KRG 3.6 g/d.
No moderate or serious adverse events were reported during the 4-week study period. The evaluation was also expanded to include laboratory tests and the electrocardiogram. The results of these tests were also in the normal range, so our results indicated that treatment with KRG for 4 weeks showed no significant adverse effects in people with deficiency syndrome.
3.4. Impact of KRG on fatigue
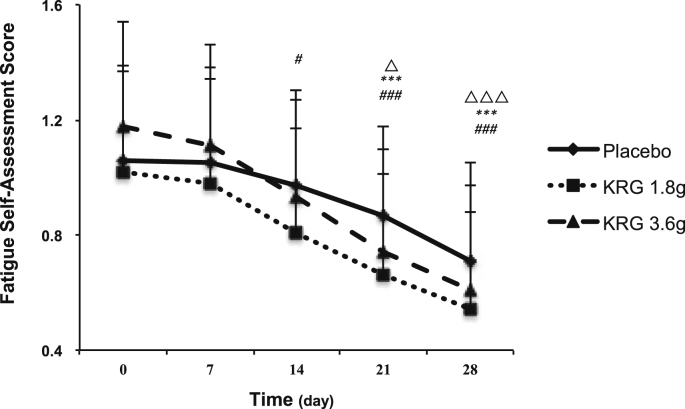
The mean baseline of the fatigue self-assessment score was 1.06 ± 0.31, 1.02 ± 0.37, and 1.18 ± 0.36 for the placebo control, KRG 1.8 g, and KRG 3.6 g groups, respectively. After treatment, although the scores decreased in all three groups, the improvement was greater after treatment with KRG (Fig. 4). The difference of the changes among the three groups was also analyzed. Our results indicated that the scores decreased significantly after the administration of 3.6 g KRG (−0.1 ± 0.07) for 1 week as compared with those of the placebo group (−0.01 ± 0.03), whereas administration of 1.8 g KRG showed a significant reduction after 2 weeks of treatment. The reduction also showed a time-dependent effect in both the 1.8 and 3.6 g KRG groups. After treatment with 1.8 g KRG, the reduction was increased from −0.06 ± 0.04 to −0.48 ± 0.19, whereas the reduction was increased from −0.1 ± 0.07 to −0.58 ± 0.20 in the KRG 3.6 g group (Table 3).
Fig. 4.
Comparison of the fatigue self-assessment score before and after KRG treatment within the group. Data are expressed as mean ± standard deviation. The within-treatment difference was analyzed by the paired t test; △P < 0.05 and △△△P < 0.001 compared before and after placebo treatment. The within-treatment difference was analyzed by the paired t test; ***P < 0.001 compared before and after treatment with 1.8 g KRG. The within-treatment difference was analyzed by the paired t test; #P <0.05 and ###P < 0.001 compared before and after treatment with 3.6 g KRG. KRG, Korean Red Ginseng; TCM, traditional Chinese medicine.
Table 3.
Between-group comparison of fatigue changes using the fatigue self-assessment scale after specified treatments
| Group | Change during Week 1 | Change during Week 2 | Change during Week 3 | Change during Week 4 |
|---|---|---|---|---|
| A | −0.01±0.03 | −0.13±0.06 | −0.23±0.10 | −0.38±0.13 |
| B | −0.06±0.04 | −0.21±0.102) | −0.36±0.132) | −0.48±0.191) |
| C | −0.1±0.073) | −0.27±0.123) | −0.46±0.183) | −0.58±0.203) |
KRG, Korean Red Ginseng.
Data are expressed as mean ± standard deviation.
A: participants were given placebo 3.6 g/d; B: participants were given KRG 1.8 g/d; C: participants were given KRG 3.6 g/d.
For the difference between the changes during KRG 1.8 g versus placebo, using an effect model; P < 0.05 compared with the control.
For the difference between the changes during KRG 1.8 g versus placebo, using an effect model; P < 0.001 compared with the control.
For the difference between the changes during KRG 3.6 g versus placebo, using an effect model; P < 0.001 compared with the control.
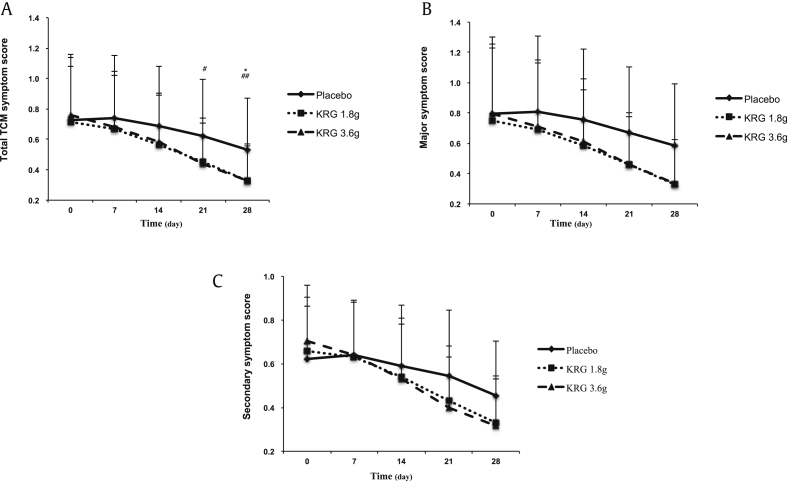
Based on per-protocol analysis, the effect of KRG on TCM symptoms is shown in Fig. 5. The TCM symptom scale contained a major symptom scale and secondary symptom scale. The total symptom scores were 0.73 ± 0.41, 0.71 ± 0.37, and 0.76 ± 0.40 in the placebo, KRG 1.8 g, and KRG 3.6 g groups, respectively, at the initial time point. After treatment, scores decreased in both the KRG and placebo groups during the trial (Fig. 5). However, the pattern of changes for the total TCM symptom score was different between the KRG treatment and placebo groups. After the 4-week treatment, the improvement was better in the KRG 1.8 g (0.33 ± 0.24) and KRG 3.6 g (0.33 ± 0.23) groups than in the placebo group (0.53 ± 0.34), and the differences were statistically significant (Fig. 5). The major symptom score and secondary symptom score also decreased in both the KRG and placebo groups; however, there was no significant within-treatment difference for all 3 groups. A multiple comparison analysis indicated that the reduction in the scores showed a dose-dependent effect (Table 4). The reduction in the total TCM symptom score was increased from −0.37 ± 0.14 with KRG 1.8 g to −0.44 ± 0.21 with KRG 3.6 g after 4 weeks of treatment. The same trend was also observed for the major symptom score and secondary symptom score. The reduction in KRG on the TCM symptom score also showed a time-dependent effect; after the treatment with 3.6 g KRG for 1 week, the reduction in the total TCM symptom scale was −0.1 ± 0.07, which was then increased to −0.44 ± 0.21 after 4 weeks of treatment (Table 4).
Fig. 5.
Comparison of the TCM symptom score before and after KRG treatment within the group. Data are expressed as mean ± standard deviation. The within-treatment difference was analyzed by the paired t test; *P < 0.05 compared before and after treatment with 1.8 g KRG. The within-treatment difference was analyzed by the paired t test; #P < 0.05 and ##P < 0.01 compared before and after treatment with 3.6 g KRG. KRG, Korean Red Ginseng; TCM, traditional Chinese medicine.
Table 4.
Between-group comparison of fatigue changes using the TCM symptom scale after specified treatments
| Variable | Change during Week 1 | Change during Week 2 | Change during Week 3 | Change during Week 4 | |
|---|---|---|---|---|---|
| Total TCM symptom score | A | −0.01±0.03 | −0.07±0.05 | −0.15±0.10 | −0.22±0.10 |
| B | −0.06±0.042) | −0.15±0.062) | −0.27±0.121) | −0.37±0.141) | |
| C | −0.1±0.074) | −0.2±0.143) | −0.33±0.174) | −0.44±0.214) | |
| Major symptoms | A | −0.02±0.03 | −0.08±0.06 | −0.17±0.11 | −0.24±0.12 |
| B | −0.06±0.031) | −0.17±0.041) | −0.29±0.11 | −0.40±0.14 | |
| C | −0.11±0.083) | −0.21±0.16 | −0.35±0.22 | −0.47±0.28 | |
| Secondary symptoms | A | 0.00±0.02 | −0.06±0.04 | −0.12±0.08 | −0.19±0.07 |
| B | −0.05±0.05 | −0.13±0.09 | −0.24±0.14 | −0.33±0.15 | |
| C | −0.08±0.06 | −0.18±0.12 | −0.31±0.09# | −0.39±0.084) | |
KRG, Korean Red Ginseng; TCM, traditional Chinese medicine.
Data are expressed as mean ± standard deviation.
A: participants were given placebo 3.6 g/d;.B: participants were given KRG 1.8 g/d; C: participants were given KRG 3.6 g/d.
For the difference between the changes during KRG 1.8 g versus placebo, using an effect model; P < 0.05 compared with the control.
For the difference between the changes during KRG 1.8 g versus placebo, using an effect model; P < 0.01 compared with the control.
For the difference between the changes during KRG 3.6 g versus placebo, using an effect model; P < 0.05 compared with the control.
For the difference between the changes during KRG 3.6 g versus placebo, using an effect model; P < 0.01 compared with the control.
The curative effect of KRG was also evaluated based on total integral symptom scores. From our results, we found that the total effective rate was greatly increased after the treatment with KRG, which was 30.4%, 75%, and 84.5% in the placebo, KRG 1.8 g, and KRG 3.6 g groups, respectively (Table 5). In the placebo group, 39 participants showed no alleviation of fatigue symptoms, which was decreased to 15 and 9 for KRG 1.8 g and KRG 3.6 g, respectively.
Table 5.
Evaluation of curative effect based on total integral symptom scores
| Effectiveness evaluation1) | A |
B |
C |
|---|---|---|---|
| Case no. (%) | Case no. (%) | Case no. (%) | |
| Ineffective rate | 39 (69.6) | 15 (25.0) | 9 (15.5) |
| Effective rate | 15 (26.8) | 31 (51.7) | 29 (50.0) |
| Marked effective rate | 1 (1.8) | 7 (11.7) | 16 (27.6) |
| Curative effect rate | 1 (1.8) | 7 (11.7) | 4 (6.9) |
| Total effective rate | 17 (30.4) | 45 (75.0) | 49 (84.5) |
KRG, Korean Red Ginseng.
Marked effective: 70% ≤ symptom score decreased by <95%; effective rate: 30% ≤ symptom score decreased by <70%; ineffective: syndrome score decreased by ≤ 30%.
A: participants were given placebo 3.6 g/d; B: participants were given KRG 1.8 g/d; C: participants were given KRG 3.6 g/d.
Curative effect rate: syndrome score decreased by ≥ 95%.
4. Discussion
This study assesses the safety and efficacy of KRG treatment in participants with deficiency syndrome. First, we examined the fire-heat symptom score as our primary outcome. We found that KRG treatment was not associated with an increase in the fire-heat symptom score for people with deficiency syndrome. We then further investigated the impact of KRG treatment on fatigue alleviation. We found a statistically significant improvement in terms of fatigue-related symptoms during the KRG treatment.
Several studies have already been conducted with the aim of confirming whether red ginseng or ginseng causes fireness. Xu and Dou [31] reported that rectal temperature significantly increased, whereas saliva secretion and urine volume decreased significantly in rats treated with red ginseng and ginseng. Their study demonstrated that red ginseng and ginseng, due to their warm nature, could enhance the body's energy metabolism to produce dryness to the body. Others reported that, based on metabolomic study on mice treated with ginseng, ginseng cannot cause fire-heat syndrome in a reasonable dose, which could be used as a TCM and a nutrient [22]. It seems that the results from these two studies were controversial, and the matter of whether red ginseng causes fireness is still far from conclusive. Because the evidence from clinical trials is more direct and convincing, we conducted our study in a Chinese population. In our study, we showed that KRG treatment did not induce any increase in the fire-heat symptom score, and no abnormalities were found in laboratory tests. This finding suggests that KRG is relatively safe in people with deficiency syndrome when it is used according to the manufacturer's instructions.
The antifatigue effect of ginseng has been well demonstrated from long-term clinical experience [32]. Studies have also proved the effects of ginseng on fatigue in different in vivo studies, and the major active components are various ginsenosides, polysaccharides, and small-molecule oligopeptides [33], [34], [35]. A detailed study [36] compared the antifatigue activity between the neutral and acidic polysaccharides in an animal test and concluded that the acidic polysaccharide is more potent than the neutral polysaccharide in terms of relieving fatigue. Besides various animal studies, randomized, double-blind, placebo-controlled trials were performed to investigate antifatigue effects of the ginseng extract. The results showed that the extract treatment decreased fatigue severity in a healthy population, while adverse effects were rare, and the extract was generally well tolerated [37], [38]. However, the number of studies that have focused on exploring the antifatigue effects of ginseng or red ginseng is very limited, especially in terms of clinical trials. Our study is the first clinical study to provide systematic evidence for the antifatigue properties of red ginseng in people with deficiency syndrome. Our study indicates that symptoms related to fatigue were greatly improved after treatment with KRG. The total effective rate was also significantly increased in the KRG groups. This antifatigue effect of KRG may be due to its chemical components. Although the categories and amounts of some ginsenosides were changed during the process, the total content of ginsenosides in red ginseng is not to be changed at all, which maintains the balance of chemical materials [6]. Furthermore, the amount of acidic polysaccharides in red ginseng is three times higher than that in white ginseng because of degradation of sugar components in the processes of steaming and drying [6]. Here, we conclude that ginsenosides and a high amount of acidic polysaccharides may play a crucial pharmacological role in the antifatigue effect of KRG.
This study has several limitations. The first is that it included only individuals with deficiency syndrome. In China, many people take KRG regardless of their constitution. Therefore, because our inclusion criteria restricted patients with deficiency syndrome, it is difficult to generalize our results to all Chinese people. However, according to the theory of TCM and the Chinese Pharmacopoeia [13], red ginseng or ginseng is suitable only for people with deficiency syndrome, and it is not recommended for use in people with excess syndromes. Thus, it is reasonable for us to choose people with deficiency syndrome as our study participants. The second important limitation of our study is that we did not consider a follow-up period; all of our measurements were conducted during the 4 weeks of KRG treatment. However, our main objective is to evaluate the safety of KRG in patients with deficiency syndrome, rather than its effectiveness, and our results demonstrate a similar fire symptom score during KRG treatment and placebo treatment. Therefore, we suggest that the follow-up period will not significantly affect our results or conclusions. However, it is clear that a research design with a follow-up period will be required for further evaluation of the safety of KRG.
As mentioned earlier, fireness is a special term used in TCM that is usually based on the subjective judgment of clinicians, rather than objective diagnostic parameters. At present, research on the use of red ginseng for fireness is very limited. In summary, this study was the first randomized, double-blind, placebo-controlled clinical trial to examine the safety and efficacy of KRG in Chinese patients with deficiency syndrome. Our study showed that there was no safety concern with the consumption of KRG. In conclusion, the results of our study suggest that KRG has the potential to improve fatigue-related symptoms in people with deficiency syndrome. We believe this will be an important research study that supports the future development of clinical trials involving KRG treatment. For more comprehensive evaluation of its safety and efficacy in the Chinese population, further study of KRG on Chinese people with a larger sample size and longer treatment is required.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by the 2017 grant from the Korean Society of Ginseng, the National Natural Science Foundation of China (NSFC) grant No. 81603344, the Three-year development plan project for Traditional Chinese Medicine (Grant No. ZY2018-2020-CCCX-2001-02) and Xinglin Yong Talent Program (Dr. Li Zhang).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.05.006.
Contributor Information
Hong Fang, Email: 15000297742@163.com.
Hongxi Xu, Email: xuhongxi88@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 2.Kang S., Min H. Ginseng, the 'immunity boost': the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354–368. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofseth L.J., Wargovich M.J. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137 doi: 10.1093/jn/137.1.183S. 183s-5s. [DOI] [PubMed] [Google Scholar]
- 4.Kim D.S., Kim Y., Jeon J.Y., Kim M.G. Effect of Red Ginseng on cytochrome P450 and P-glycoprotein activities in healthy volunteers. J Ginseng Res. 2016;40:375–381. doi: 10.1016/j.jgr.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong B.M., Park M.J., Min J.W., Kim H.B., Kim S.H., Kim S.Y., Yang D.C. Physico-chemical characteristics of white, fermented and red ginseng extracts. J Ginseng Res. 2008;32:238–243. [Google Scholar]
- 6.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung C.H., Seog H.M., Choi I.W., Choi H.D., Cho H.Y. Effects of wild ginseng (Panax ginseng C.A. Meyer) leaves on lipid peroxidation levels and antioxidant enzyme activities in streptozotocin diabetic rats. J Ethnopharmacol. 2005;98:245–250. doi: 10.1016/j.jep.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Yun T.K., Choi S.Y., Yun H.Y. Epidemiological study on cancer prevention by ginseng: are all kinds of cancers preventable by ginseng? J Korean Med Sci. 2001;16:S19–S27. doi: 10.3346/jkms.2001.16.S.S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D., Yasuda T., Yu Y., Zheng P., Kawabata T., Ma Y., Okada S. Ginseng extract scavenges hydroxyl radical and protects unsaturated fatty acids from decomposition caused by iron-mediated lipid peroxidation. Free Radic Biol Med. 1996;20:145–150. doi: 10.1016/0891-5849(95)02020-9. [DOI] [PubMed] [Google Scholar]
- 10.Babiker L.B., Gadkariem E.A., Alashban R.M., Aljohar H.I. Investigation of stability of Korean ginseng in herbal drug product. American Journal of Applied Sciences. 2014;11:160–170. [Google Scholar]
- 11.Joo S.S., Won T.J., Lee D.I. Reciprocal activity of ginsenosides in the production of proinflammatory repertoire, and their potential roles in neuroprotection in vivo. Planta Med. 2005;71:476–481. doi: 10.1055/s-2005-864145. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y.F. A case report of ginseng abuse induced side effects. Chin J Clin. 1981;4:21. [Google Scholar]
- 13.Chinese Pharmacopocia Commission . vol. I. Chinese Medical Science and Technology Press; Beijing: 2015. pp. 153–154. (Chinese pharmacopocia). [Google Scholar]
- 14.Chong S.K., Oberholzer V.G. Ginseng--is there a use in clinical medicine? Postgrad Med J. 1988;64:841–846. doi: 10.1136/pgmj.64.757.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J.F., Liang R., Wang S.H., Wang Z.P., Ren J.P. A retrospective survey:" excessive internal heat. World Science and Technology / Modernization of Traditional Chinese Medicine and Materia Medica. 2007;9:39–44. [Google Scholar]
- 16.Xiao N., DeFranco D.B. Overexpression of unliganded steroid receptors activates endogenous heat shock factor. Mol Endocrinol. 1997;11:1365–1374. doi: 10.1210/mend.11.9.9976. [DOI] [PubMed] [Google Scholar]
- 17.Wang S., Diller K.R., Aggarwal S.J. Kinetics study of endogenous heat shock protein 70 expression. J Biomech Eng. 2003;125:794–797. doi: 10.1115/1.1632522. [DOI] [PubMed] [Google Scholar]
- 18.Sun G.R., Tong Y., Chen W. China Press of Traditional Chinese Medicine; Beijing: 2008. Basic theory of traditional Chinese medicine. [Google Scholar]
- 19.Guan S.X., Chen J.F., Ma Y.L. Clinical study on the relationship between HUO-RE (fire-heat) syndrome and plasma immune cell factor level changes at initial condition of acute cerebral infarction. Fang She Mian Yi Za Zhi. 2000;13:331–333. [Google Scholar]
- 20.Li J.W., Deng T.T. People's Medical Publishing; Beijing: 1995. Dictionary of traditional Chinese medicine; p. 942. [Google Scholar]
- 21.Liu S., Huang Z., Wu Q., Huang Z., Wu L., Yan W., Chang D.L., Yang Z., Wang Z. Quantization and diagnosis of Shanghuo (Heatiness) in Chinese medicine using a diagnostic scoring scheme and salivary biochemical parameters. Chin Med. 2014;9:2. doi: 10.1186/1749-8546-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Ma L., Sun Y., Yang L., Yue H., Liu S. Metabonomics study on the hot syndrome of traditional Chinese medicine by rapid resolution liquid chromatography combined with quadrupole time-of-flight tandem mass spectrometry. Arch Pharm Res. 2014;37:899–906. doi: 10.1007/s12272-013-0250-z. [DOI] [PubMed] [Google Scholar]
- 23.Cheng C.W., Wu T.X., Shang H.C., Li Y.P., Altman D.G., Moher D., Bian Z.X., CONSORT-CHM Formulas 2017 CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration (traditional Chinese version) Ann Intern Med. 2017;167:112–121. doi: 10.7326/IsTranslatedFrom_M17-2977_1. [DOI] [PubMed] [Google Scholar]
- 24.Sheng Z.Y., Wang W.J. Reference standard for syndrome differentiation of deficiency syndrome in TCM. Chinese Journal of Integrated Traditional and Western Medicine. 1986;10:598. [Google Scholar]
- 25.Deng T.T. Chapter third, section third. Diagnostics of Chinese medicine. ed. People's Medical Publishing House; Beijing: 2008. Deficiency and excess. [Google Scholar]
- 26.Chow S.C., Shao J., Wang H.S. 2nd ed. Chapman & Hall/CRC; Boca Raton, FL: 2008. Sample size calculations in clinical research; pp. 99–100. [Google Scholar]
- 27.Lin L., Xiong J., Li W.Y., Xing B.F. Evaluation Fire-heat Symptoms and adverse reaction of different ginseng species in oral administration for healthy subject: a pilot randomized. Placebo controlled trail. International Symposium of Food Safety and Efficacy in Ginseng. 13 September 2012 [Guanzhou, China] [Google Scholar]
- 28.Wang T.F., Xue X.L. Fatigue self-assessment scale (FSAS) China Journal of Traditional Chinese Medicine and Pharmacy. 2009;24:348–349. [Google Scholar]
- 29.Hu X.J., Liao W., Wu J.H., Huang C., Li K., Li R.C. Clinical observation on treatment of postoperative fatigue syndrome with Ci Lan anti fatigue oral liquid, acupuncture blue anti fatigue oral liquid. Science and Technology Innovation Herald. 2012;1:219–220. [Google Scholar]
- 30.Zheng X.Y. ed. China Medical Science and Technology Press; Beijing: 2002. Guidelines for clinical research of Traditional Chinese Medicin. Part one; pp. 378–383. [Google Scholar]
- 31.Xu X., Dou D. The ginseng's fireness is associated with the lowering activity of liver Na(+)-K(+)-ATPase. J Ethnopharmacol. 2016;190:241–250. doi: 10.1016/j.jep.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Bahrke M.S., Morgan W.R. Evaluation of the ergogenic properties of ginseng: an update. Sports Med. 2000;29:113–133. doi: 10.2165/00007256-200029020-00004. [DOI] [PubMed] [Google Scholar]
- 33.Tan S., Zhou F., Li N., Dong Q., Zhang X., Ye X., Guo J., Chen B., Yu Z. Anti-fatigue effect of ginsenoside Rb1 on postoperative fatigue syndrome induced by major small intestinal resection in rat. Biol Pharm Bull. 2013;36:1634–1639. doi: 10.1248/bpb.b13-00522. [DOI] [PubMed] [Google Scholar]
- 34.Oh H.A., Kim D.E., Choi H.J., Kim N.J., Kim D.H. Anti-fatigue effects of 20(S)-Protopanaxadiol and 20(S)-Protopanaxatriol in mice. Biol Pharm Bull. 2015;38:1415–1419. doi: 10.1248/bpb.b15-00230. [DOI] [PubMed] [Google Scholar]
- 35.Bao L., Cai X., Wang J., Zhang Y., Sun B., Li Y. Anti-fatigue effects of small molecule oligopeptides isolated from Panax ginseng C. A. Meyer in mice. Nutrients. 2016;8 doi: 10.3390/nu8120807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J., Li S., Fan Y., Chen Y., Liu D., Cheng H., Gao X., Zhou Y. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. J Ethnopharmacol. 2010;130:421–423. doi: 10.1016/j.jep.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 37.Lee N., Lee S.H., Yoo H.R., Yoo H.S. Anti-fatigue effects of enzyme-modified ginseng extract: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2016;22:859–864. doi: 10.1089/acm.2016.0057. [DOI] [PubMed] [Google Scholar]
- 38.Kim H.G., Cho J.H., Yoo S.R., Lee J.S., Han J.M., Lee N.H., Ahn Y.C., Son C.G. Antifatigue effects of Panax ginseng C.A. Meyer: a randomised, double-blind, placebo-controlled trial. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061271. e61271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.