Abstract
Background
Breast cancer is a severe disease and the second leading cause of cancer death in women worldwide. To surmount this, various diagnosis and treatment options for breast cancer have been developed. One of the most effective strategies for cancer treatment is to induce apoptosis using naturally occurring compounds. Compound K (CK) is a ginseng saponin metabolite generated by human intestinal bacteria. CK has been studied for its cardioprotective, antiinflammatory, and liver-protective effects; however, the role of CK in breast cancer is not fully understood.
Methods
To investigate the anticancer effects of CK in SKBR3 and MDA-MB-231 cells, cell viability assays and flow cytometry analysis were used. In addition, the direct targets of CK anticancer activity were identified using immunoblotting analysis and overexpression experiments. Invasion, migration, and clonogenic assays were carried out to determine the effects of CK on cancer metastasis.
Results
CK-induced cell apoptosis in SKBR3 cells as determined through 3-(4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide assays, propidium iodide (PI) and annexin V staining, and morphological changes. CK increased the cleaved forms of caspase-7, caspase-8, and caspase-9, whereas the expression of Bcl-2 was reduced by CK. In assays probing the cell survival pathway, CK activated only AKT1 and not AKT2. Moreover, CK inhibited breast cancer cell invasion, migration, and colony formation. Through regulation of AKT1 activity, CK exerts anticancer effects by inducing apoptosis.
Conclusion
Our results suggest that CK could be used as a therapeutic compound for breast cancer.
Keywords: AKT1, Apoptosis, Cancer, Compound K, Panax ginseng
1. Introduction
Breast cancer is the most common type of cancer in women. Every year, approximately 500,000 deaths occur in women worldwide because of breast cancer, which is the second leading cause of global death in women [1], [2]. Although many methods of diagnosis of and treatment for breast cancer have developed and patients are offered various treatment options such as chemotherapy, radiotherapy, and endocrine therapy, the incidence of breast cancer is still increasing rapidly [3]. Breast cancer is a heterogeneous disease represented by conditions with unique histopathological and molecular characteristics. Tumors are characterized based on the responsiveness of estrogen receptor, progesterone receptor, and human epithelial growth factor receptor-2 (HER2). Approximately 15–20% of patients with breast cancer overexpress HER2, leading to a high risk of relapse and a short survival period [4]. Although many drugs targeting HER2-positive breast cancer have been developed, conventional treatments are not very successful. Therefore, it is very important to study other chemotherapeutic drugs that can effectively treat breast cancer, especially HER2-positive breast cancer.
Tumors are caused by imbalance of cell proliferation and apoptosis. Because the apoptosis mechanism induces programmed cell death, approaches to suppress the growth of cancer cells and induce cell death by upregulating the apoptosis pathway have been widely studied for cancer treatment. Numerous studies have reported a variety of cancer treatments involving activation of the apoptosis mechanism [5]. Among them are numerous natural, plant-derived compounds that have excellent cancer-suppressing activity via the apoptosis pathway [6]. This suggests that use of herbal compounds that affect the mechanism of apoptosis can be an effective strategy for development of anticancer drugs.
The roots of Korean ginseng (Panax ginseng Meyer) have been widely used as herbal medicines in East Asia. In particular, the biological and pharmacological effects of ginsenoside, a major bioactive component of ginseng, have been actively investigated [7]. Various pharmacological activities of ginsenosides, including neuroprotective, anticancer, and antiinflammatory activities, have been reported [8], [9]. Compound K (CK), an active ingredient of ginsenosides and a novel ginseng saponin metabolite, has recently been found. This compound is formed through deglycosylation of ginsenosides Rb1, Rb2, and Rc by human intestinal bacteria. CK has cardioprotective, antiinflammatory, and liver-protective effects [10], [11], [12], [13], [14], [15]. In addition, it has recently been reported that CK has in vivo anticancer activity in triple-negative breast cancer [16]. However, the activity and biological mechanism of CK in breast cancer are not fully understood. The aims of this study were to investigate the ability of CK to regulate cell proliferation and apoptosis in SKBR3 human breast cancer cells and to elucidate the molecular mechanism of its activity.
2. Materials and methods
2.1. Materials
CK was provided by Ambo Institute (Daejeon, Korea). SKBR3 cells were purchased from ATCC (Rockville, MD, USA). Roswell Park Memorial Institute 1640 medium, fetal bovine serum (FBS), and phosphate-buffered saline (PBS) were obtained from HyClone (Grand Island, NY, USA). Dimethyl sulfoxide, 3-(4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTT), hematoxylin, eosin, and sodium dodecyl sulfate were purchased from Sigma Chemical Co. (St. Louis, MO, USA). PI and annexin V staining kits were purchased from BD Biosciences (Franklin Lakes, NJ, USA). Total or phospho-specific antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA).
2.2. Cell culture and drug treatment
SKBR3 cells (HER2-positive breast cancer cell line) and MDA-MB-231 cells (HER2-negative breast cancer cell line) were cultured in Roswell Park Memorial Institute 1640 medium with 10% heat-inactivated FBS and 1% penicillin and streptomycin at 37°C and 5% CO2. For each experiment, trypsin was used to detach the SKBR3 cells, and the stock solution of CK was diluted in dimethyl sulfoxide.
2.3. Cell viability assay
SKBR3 cells (5 × 105 cells/mL) were seeded in 96-well plates for 18 h and then incubated with varying concentrations of CK. After the indicated times, cell viability was measured using a conventional MTT assay as described previously [17].
2.4. PI and annexin V staining
SKBR3 cells were plated and incubated overnight. The cells were treated with CK for 6 h, collected, and washed with PBS. The cells were then stained with two apoptotic markers following the manufacturer's instructions.
2.5. Preparation of cell lysates and Western blot assay
The cell lysates from CK-treated SKBR3 cells were prepared using lysis buffer (20 mM Tris-HCl, pH 7.4, 2 mM EDTA, 2 mM ethylene glycol tetraacetic acid, 50 mM β-glycerol phosphate, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1% Triton X-100, 10% glycerol, 10 μg/ml aprotinin, 10 μg/ml pepstatin, 1 mM benzamide, and 2 mM phenylmethylsulfonyl fluoride (PMSF)). The protein concentrations were measured using the Bradford protein assay (Bio-Rad, USA) according to the manufacturer's instructions. Western blotting assays were conducted as previously reported [18].
2.6. Semiquantitative reverse transcriptase polymerase chain reaction
To determine the mRNA expression of Bcl2, total RNA was extracted from CK-treated SKBR3 cells using TRIzol reagent (Gibco, Gaithersburg, MD, USA) according to the manufacturer's instructions. The cDNA synthesis and quantitative reverse transcriptase polymerase chain reaction (RT-PCR) were performed as previously described [19], and the primer sequences are listed in Table 1.
Table 1.
List of primers for RT-PCR used in this study
| Gene | Sequence (5′ to 3′) |
|---|---|
| Bcl2 | |
| F | ACAACATCGCCCTGTGGATGAC |
| R | ATAGCTGATTCGACGTTTTGCC |
| GAPDH | |
| F | CACTCACGGCAAATTCAACGGCA |
| R | GACTCCACGACATACTCAGCAC |
RT-PCR, reverse transcriptase polymerase chain reaction
2.7. Generation of short hairpin RNA–expressing cell lines
The short hairpin RNA (shRNA) coding sequences against AKT1- or AKT2-containing constructs were cloned following the Addgene protocol (www.addgene.org). Nontargeting scrambled shRNA sequences and AKT1 or AKT2 shRNA sequences were cloned into the pLKO.1 vector. Lentivirus was produced using transient HEK293T cell transfection. SKBR3 cells were infected with the virus, and the infected cells were selected through puromycin treatment. The knockdown levels of AKT1 and AKT2 were confirmed by immunoblotting.
2.8. Invasion assay
To analyze the invasive ability of SKBR3 cells, invasion assays were performed as previously reported [20]. Briefly, Matrigel (BD Biosciences, San Jose, CA) was diluted with serum-free Dulbecco's Modified Eagle Medium (DMEM) (1:3) and used to coat the upper surface of the transwell chamber. The lower chamber was filled with culture medium containing 10% FBS, SKBR3 cells, and various concentration of CK and was incubated at 37°C for 48 h. The cells were fixed in 4% formaldehyde and then stained with hematoxylin and eosin. Images of the cell invasion were produced using a microscope, and the invasion area was measured using Image J software (Research Services Branch, National Institute of Mental Health, Bethesda, MD).
2.9. Clonogenic assay
SKBR3 cells (1 × 103 cells/well) were plated on 6-well plates and incubated with CK for 6 h. The CK was then removed, and the cells were incubated for at least 10 more days until colony formation. The medium was discarded, and the cells were washed once with PBS. The cells were then fixed in fixation buffer (1:7 acetic acid:methanol) for 5 min. The fixation buffer was removed via suction, and the cells were incubated with 0.5% crystal violet solution for 2 h. The stained cells were washed with tap water and dried. Images of cells were captured, and the areas of colonies were measured using Image J software.
2.10. Migration assay
SKBR3 cells (1 × 106 cells/ml) were plated. The cells were then scratched using a disposable pipette tip and carefully washed with PBS twice to remove the detached cells. The cells were treated with CK in a dose-dependent manner. At each time point (0–24 h), the wound was observed by microscopy [21].
2.11. Statistical analysis
All data presented in this study are expressed as mean ± standard deviation of experiments performed with three replicates. For statistical comparisons, the results were analyzed using either analysis of variance with the Scheffe post hoc test (for normally distributed data) or the Kruskal–Wallis or Mann–Whitney tests (for nonnormally distributed data). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. The induction of the cell death by CK in SKBR3 cells
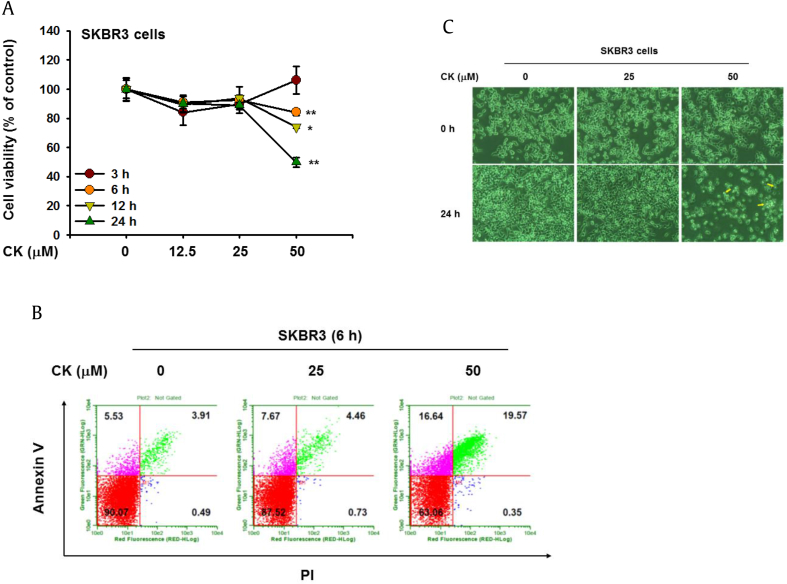
To investigate the anticancer activities of CK, we first evaluated the cell growth of SKBR3 human breast cancer cells using cell viability assay. SKBR3 cells were treated with CK (0–50 μM) for 3–24 h. The growth of SKBR3 cells was significantly inhibited by CK in a time- and dose-dependent manner (Fig. 1A). Since the treatment of CK-induced cell death, we further identified which mechanism of cell death was involved. To confirm the apoptotic effects of CK, we analyzed cell death using PI and annexin V staining (Fig. 1B). The percentage of apoptotic cells increased (from 3.91% to 19.57%) in proportion to CK concentration (0–50 μM). In contrast, the proportion of live cells decreased from 90.7% to 63.06%. Consequently, the CK-induced cell death was the result of apoptosis. To confirm the apoptotic effects of CK, we further examined the morphological changes in CK-treated SKBR3 cells. Actin cytoskeleton–mediated morphological changes, including apoptotic bodies and nuclear condensation, were seen after treatment with CK for 24 h (Fig. 1C). These results suggest that CK suppresses the proliferation of cancer cells by increasing apoptosis.
Fig. 1.
In vitro anticancer effects of CK on SKBR3 cells. (A) Viability of SKBR3 cells treated with the indicated dose of CK (0–50 μM) for 3–24 h was measured using MTT assay. (B) The antiapoptotic effects of CK were determined by measuring the numbers of apoptotic cells after staining with Annexin V and propidium iodide (PI). (C) SKBR3 cells were treated with CK (0–50 μM) for 24 h, and morphological changes were cataloged using an optical microscope. *p < 0.05, **p < 0.01 compared with control. CK, Compound K; MTT, 3-(4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide.
3.2. The apoptotic activity of CK through caspase-dependent and AKT signaling pathways in SKBR3 cells
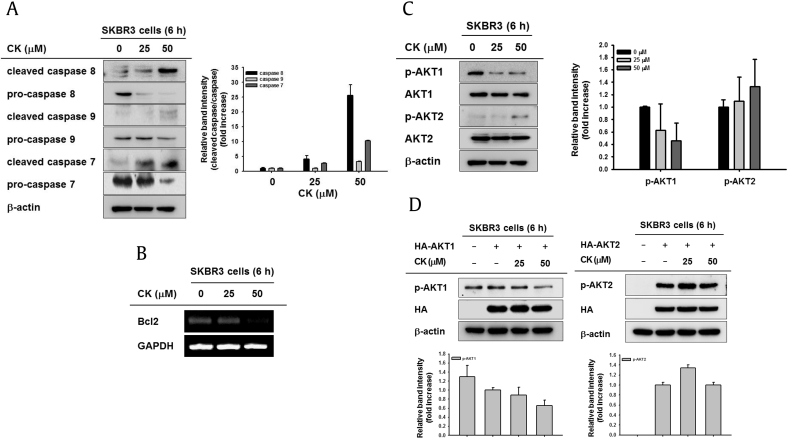
To determine the molecular mechanism underlying the proapoptotic activity of CK, we identified the active forms of caspases by Western blot analysis. The levels of cleaved caspase-7, caspase-8, and caspase-9, which are the active forms of procaspases, were increased in CK-treated SKBR3 cells, and the proforms were reduced in a dose-dependent manner (Fig. 2A). Moreover, the mRNA expression of Bcl2, an antiapoptotic protein, was suppressed by CK treatment (Fig. 2B). These results indicate that CK promotes apoptotic signaling cascades via both intrinsic and extrinsic pathways. To examine which molecules involved in activation of the apoptotic pathway are regulated by CK, we further analyzed the expression of AKT, which is a primary regulator of apoptosis. Phosphorylation of the AKT isoform AKT1 was significantly reduced in the presence of CK, but phosphorylation of AKT2 was not inhibited by CK (Fig. 2C). The Western blotting results suggest that AKT1 is a specific target of CK in human breast cancer cells. To confirm this, the AKT1 and AKT2 genes were overexpressed in SKBR3 cells treated with CK. As expected, CK clearly suppressed the phosphorylation of AKT1 in SKBR3 cells transfected with AKT1 (Fig. 2D, left panel), but phosphorylation of AKT2 was not affected by overexpression (Fig. 2D, right panel). These findings indicate that CK increases apoptosis of cancer cells via downregulation of AKT1.
Fig. 2.
The effect of CK on activation of apoptosis-related proteins in SKBR3 cells. (A) Caspase levels of CK-treated SKBR3 cells were analyzed using Western blotting with antibodies against cleaved or full-length proteins. (B) SKBR3 cells were treated with CK (0–50 μM) for 6 h, and the mRNA level of Bcl2 was measured using semiquantitative RT-PCR. (C) The protein levels of phospho- and total AKT1 and AKT2 in whole cell lysates were determined using immunoblotting after treating SKBR3 cells with CK (0–50 μM) for 6 h. (D) SKBR3 cells overexpressing hemagglutinin (HA)-AKT1 (left panel) or HA-AKT2 (right panel) were treated with CK (0–50 μM) for 6 h. The protein levels of phospho-AKT1/2, HA, and β-actin (internal control) were identified by immunoblotting. Quantification of the band intensity was carried out using Image J. CK, Compound K; RT-PCR, reverse transcriptase polymerase chain reaction.
3.3. The effects of CK on AKT activation and metastasis in SKBR3 cells
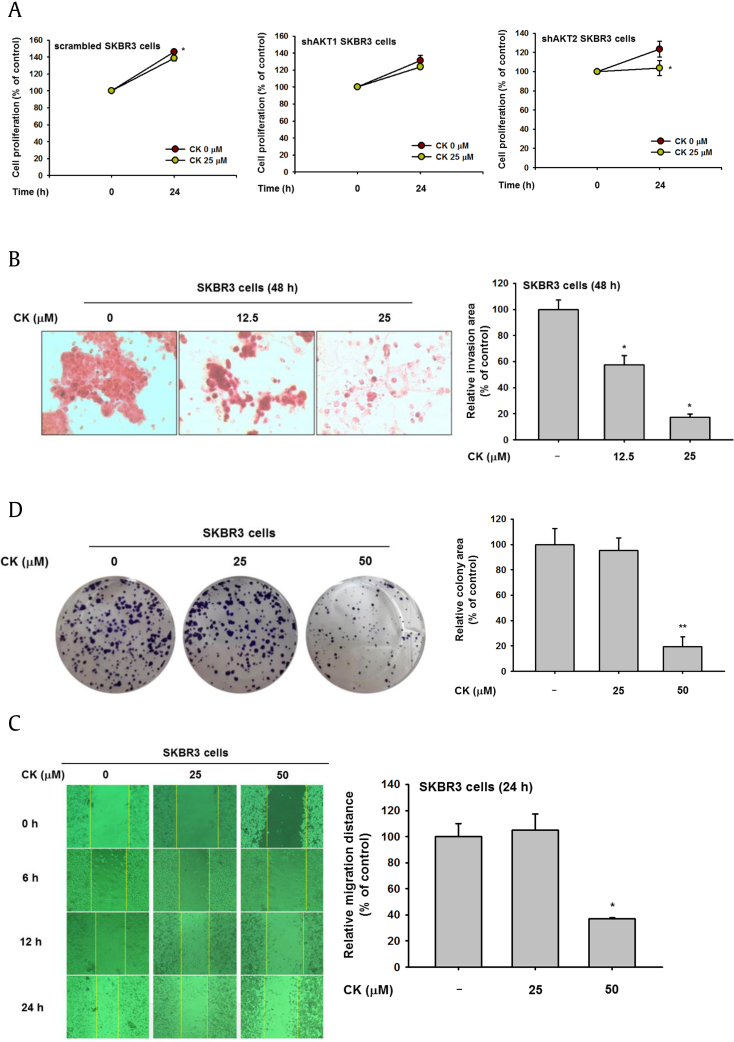
To confirm the regulation of AKT1 and AKT2 by CK at the cellular level, cellular proliferation of AKT1-knockdown and AKT2-knockdown SKBR3 cells was examined using MTT assays. Cell viability was decreased only in shAKT2 cells treated with CK (25 μM) (Fig. 3A). These results confirm that CK is involved in cancer cell death through regulation of AKT1. Because cancer metastasis is often fatal and cancer cell migration is one of the important markers of cancer cell invasion and metastasis [21], we analyzed the suppressive effects of CK on metastasis using invasion and migration assays. In the invasion assay, CK dose-dependently reduced the invasion area of SKBR3 cells after treatment for 48 h (Fig. 3B). For the cell migration assay, SKBR3 cells were scratched and then treated with varying concentrations of CK, and the cell migration ability was observed at several time points over 24 h [22]. The cell migration rate was inhibited with 50 μM CK (Fig. 3C). Taken together, the invasion and migration assays reveal that CK reduces cancer cell invasion and metastasis. Clonogenic (or colony formation) assays are typically used to validate cytotoxic agents or anticancer therapeutics [23], [24]. The number and area of SKBR3 colonies were greatly decreased by CK (50 μM) (Fig. 3D), confirming its possible use as an anticancer compound.
Fig. 3.
The effects of CK on AKT activation and metastasis. (A) SKBR3 cells were treated with shAKT1 or shAKT2 in the presence or absence of CK (25 μM) for 24 h, and cell proliferation was determined by MTT assay. (B) The invasion capacity of SKBR3 cells treated with various dosages of CK (0–25 μM) for 24 h was analyzed by hematoxylin and eosin staining. Quantification of invasion capacity was performed using Image J software. (C) The effects of CK on cell migration were evaluated by wound healing assay. A wound was created by scraping the cell monolayer using a pipette tip. Photographs were captured using a digital camera after 24 h of treatment with CK (0–25 μM). (D) In the clonogenic assay, SKBR3 cells were seeded on plates and incubated until the colonies formed. The colonies were stained with 0.5% crystal violet, and their areas were measured using Image J software. *p < 0.05, **p < 0.01 compared with control. CK, Compound K; MTT, 3-(4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide.
4. Discussion
Ginsenosides, the major bioactive components of ginseng, have been extensively researched, in particular for their anticancer activity in angiogenesis and metastasis [25], [26], [27]. In addition, CK, which was recently discovered as an active ingredient of ginsenosides, has been studied for its pharmacological activities such as antiproliferative and neuroprotective effects [28], [29]. Specifically, recent studies have shown that CK exerts antitumor effects through induction of apoptosis in leukemia and prostate cancer cells [30], [31], [32]. However, there are few reports on the anticancer activity of CK in breast cancer, and the molecular mechanisms of this compound have not been fully elucidated. SKBR3 is a hormone-independent human breast cancer cell line. Because SKBR3 overexpresses the HER2 gene (Neu/ErbB-2), an important regulator in the progression of certain types of breast cancer, this cell line is widely used for the development of breast cancer therapies targeting HER2 [33]. Thus, we aimed to investigate the antitumor activities of CK in human breast cancer SKBR3 cells and its molecular mechanism.
Apoptosis is an important biological response that prevents excessive cell growth, but defects in apoptosis cause various diseases including cancer [34]. For this reason, cancer therapy strategies inducing apoptosis have been widely used [35]. In the present study, we first investigated whether CK induces apoptosis in breast cancer cells. Cell viability assays confirmed that the growth of SKBR3 cells treated with CK was inhibited in a dose- and time-dependent manner (Fig. 1A). In annexin V/PI fluorescent staining, early apoptotic cells are only positive for annexin V, and cells in which apoptosis has progressed are annexin V- and PI-positive [36]. Using these characteristics, the cell death of SKBR3 cells was measured by flow cytometry analysis. Treatment with CK dose-dependently increased the number of both early apoptotic and late apoptotic SKBR3 cells (Fig. 1B). When apoptosis occurs, apoptotic bodies appear along with morphological changes such as nuclear shrinkage and chromatin condensation [37]. The number of apoptotic bodies was increased in 50 μM CK-treated SKBR3 cells (Fig. 1C). These results suggest that CK leads to cell death through apoptosis in SKBR3 cells.
We further identified the molecular mechanism of the apoptotic activity of CK. Apoptosis has two major signaling pathways, the extrinsic pathway and the intrinsic pathway [38], and the related key molecules are in the caspase family. Caspase-8 and caspase-9 are representative caspases involved in the extrinsic and intrinsic pathways, respectively [39]. Both cleaved caspase-8 and cleaved caspase-9, which are the active forms of procaspases, were increased in CK-treated SKBR3 cells. Caspase-7, a downstream executive enzyme, was also increased by CK in a dose-dependent manner (Fig. 2A). Furthermore, the mRNA expression of Bcl2, which plays a role as an apoptosis inhibitor, was suppressed by CK (Fig. 2B). Taken together, these results indicate that CK induces the caspase-dependent apoptotic pathway.
AKT is a primary kinase involved in cell survival, and it is important in cancer because it plays a role in the balance between cell proliferation and apoptosis [40], [41]. AKT is activated by phosphorylation and promotes cell growth through inhibition of apoptosis mechanisms [42]. Thus, suppression of AKT could be an effective strategy for cancer treatment. AKT isoforms, including AKT1, AKT2, and AKT3, are highly homologous, but they have different expression patterns and functions [43], [44]. The knockout of AKT1 is defective in growth, whereas the knockout of AKT2 is reported to have a problem in glucose homeostasis. In addition, it has been reported that AKT1 regulates local tumor growth and AKT2 regulates distant tumor dissemination in breast cancer progression [45]. As shown in Fig. 2C and D, CK specifically blocked the activation of AKT1, an isoform of AKT. In particular, cell growth was clearly reduced in AKT2-knockdown SKBR3 cells treated with CK, but there was no effect on AKT1-knockdown cells (Fig. 3A), implying that CK inhibits cell proliferation and induces apoptosis by targeting AKT1. Because the AKT signaling pathway is involved in cancer metastasis, we investigated the antimetastatic activity of CK using migration and invasion assays. CK significantly inhibited the invasion and migration of SKBR3 cells in a dose-dependent manner (Fig. 3B and C). Because metastasis determines the prognosis of patients with cancer, suppression of metastasis is crucial to cure cancer. In this study, we ascertained inhibitory effect of CK only in vitro not in vivo. Lee et al [16] reported that that CK has in vivo anticancer activity in triple-negative breast cancer. CK significantly reduced the tumor mass and metastasis of colon cancer cells [46]. Based on previous studies, CK has in vivo anticancer activities, so we could expect that CK inhibits the cancer growth and metastasis in HER2-positive breast cancer.
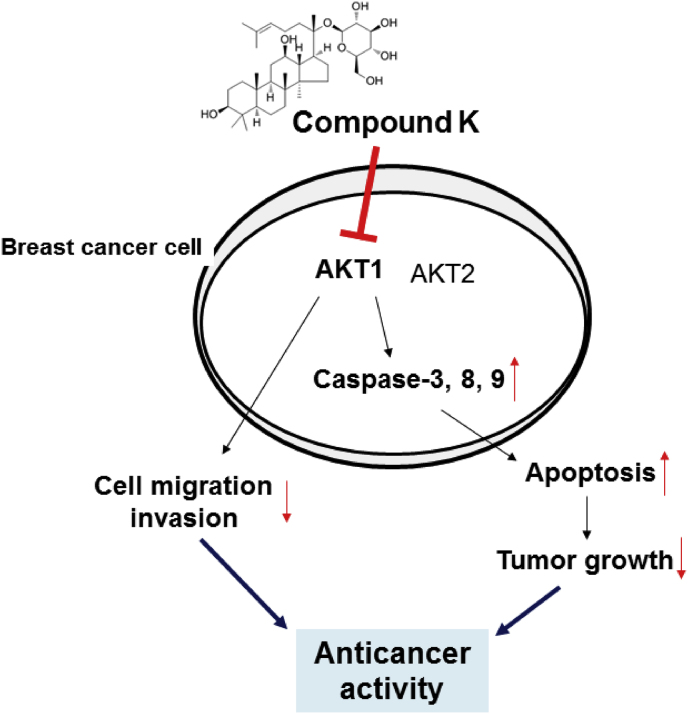
In conclusion, we demonstrated that CK strongly inhibits the proliferation and metastasis of human breast cancer SKBR3 cells by upregulating apoptosis, as summarized in Fig. 4. The proapoptotic activities of CK are related to the caspase-dependent apoptotic pathway and AKT1-mediated cell survival signaling. CK also suppresses the metastasis of SKBR3 cells through inhibition of AKT1 signaling. Thus, our findings suggest that CK has the potential to be an effective chemotherapeutic drug for breast cancer treatment.
Fig. 4.
Schematic of the anticancer mechanisms of CK in SKBR3 cells. CK, Compound K.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
This study was supported by the 2017 grant from the Korean Society of Ginseng.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.07.001.
Contributor Information
Eunju Choi, Email: cej223@naver.com.
Eunji Kim, Email: im144069@gmail.com.
Ji Hye Kim, Email: kjhmlkjhml@hanmail.net.
Keejung Yoon, Email: keejung@skku.edu.
Sunggyu Kim, Email: sukim590@skku.edu.
Jongsung Lee, Email: bioneer@skku.edu.
Jae Youl Cho, Email: jaecho@skku.edu.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. 2015. [DOI] [PubMed] [Google Scholar]
- 2.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics. CA Cancer J Clin. 2012;65:87–108. doi: 10.3322/caac.21262. 2015. [DOI] [PubMed] [Google Scholar]
- 3.Joy A., Ghosh M., Fernandes R., Clemons M. Systemic treatment approaches in her2-negative advanced breast cancer—guidance on the guidelines. Curr Oncol. 2015;22:S29. doi: 10.3747/co.22.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witton C.J., Reeves J.R., Going J.J., Cooke T.G., Bartlett J.M. Expression of the HER1–4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200:290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt C.A. Senescence, apoptosis and therapy—cutting the lifelines of cancer. Nat Rev Cancer. 2003;3:286. doi: 10.1038/nrc1044. [DOI] [PubMed] [Google Scholar]
- 6.Sreelatha S., Jeyachitra A., Padma P. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem Toxicol. 2011;49:1270–1275. doi: 10.1016/j.fct.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Z.-Z., Ming Y.-L., Chen L.-H., Zheng G.-H., Liu S.-S., Chen Q.-X. Compound K-induced apoptosis of human hepatocellular carcinoma MHCC97-H cells in vitro. Oncol Rep. 2014;32:325–331. doi: 10.3892/or.2014.3171. [DOI] [PubMed] [Google Scholar]
- 8.Shin D.-H., Leem D.-G., Shin J.-S., Kim J.-I., Kim K.-T., Choi S.Y., Lee M.-H., Choi J.-H., Lee K.-T. Compound K induced apoptosis via endoplasmic reticulum Ca2+ release through ryanodine receptor in human lung cancer cells. J Ginseng Res. 2018;42:165–174. doi: 10.1016/j.jgr.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Liu J., Zhang Z., Bi P., Qi Z., Zhang C. Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci Lett. 2011;487:70–72. doi: 10.1016/j.neulet.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 10.Zhang K., Li Y. Effects of ginsenoside compound K combined with cisplatin on the proliferation, apoptosis and epithelial mesenchymal transition in MCF-7 cells of human breast cancer. Pharm Biol. 2016;54:561–568. doi: 10.3109/13880209.2015.1101142. [DOI] [PubMed] [Google Scholar]
- 11.Lee C.S., Bae I.H., Han J., Choi Gy, Hwang K.H., Kim D.H., Yeom M.H., Park Y.H., Park M. Compound K inhibits MMP-1 expression through suppression of c-Src-dependent ERK activation in TNF-α-stimulated dermal fibroblast. Exp Dermatol. 2014;23:819–824. doi: 10.1111/exd.12536. [DOI] [PubMed] [Google Scholar]
- 12.Wu H., Chen J., Wang Q., Jia X., Song S., Yuan P., Liu K., Liu L., Zhang Y., Zhou A. Ginsenoside metabolite compound K attenuates inflammatory responses of adjuvant-induced arthritis rats. Immunopharmacol Imunotoxicol. 2014;36:124–129. doi: 10.3109/08923973.2014.880717. [DOI] [PubMed] [Google Scholar]
- 13.Hong Y.H., Kim D., Nam G., Yoo S., Han S.Y., Jeong S.G., Kim E., Jeong D., Yoon K., Kim S. Photoaging protective effects of BIOGF1K, a compound-K-rich fraction prepared from Panax ginseng. J Ginseng Res. 2018;42:81–89. doi: 10.1016/j.jgr.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim E., Kim D., Yoo S., Hong Y.H., Han S.Y., Jeong S., Jeong D., Kim J.H., Cho J.Y., Park J. The skin protective effects of compound K, a metabolite of ginsenoside Rb1 from Panax ginseng. J Ginseng Res. 2018;42:218–224. doi: 10.1016/j.jgr.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim E., Yi Y.S., Son Y.J., Han S.Y., Kim D.H., Nam G., Hossain M.A., Kim J.H., Park J., Cho J.Y. BIOGF1K, a compound K-rich fraction of ginseng, plays an antiinflammatory role by targeting an activator protein-1 signaling pathway in RAW264.7 macrophage-like cells. J Ginseng Res. 2018;42:233–237. doi: 10.1016/j.jgr.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S.J., Lee J.S., Lee E., Lim T.-G., Byun S. The ginsenoside metabolite compound K inhibits hormone-independent breast cancer through downregulation of cyclin D1. J Funct Foods. 2018;46:159–166. [Google Scholar]
- 17.Baek K.-S., Hong Y.D., Kim Y., Sung N.Y., Yang S., Lee K.M., Park J.Y., Park J.S., Rho H.S., Shin S.S. Anti-inflammatory activity of AP-SF, a ginsenoside-enriched fraction, from Korean ginseng. J Ginseng Res. 2015;39:155–161. doi: 10.1016/j.jgr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park J.G., Kang W.-S., Park K.T., Park D.J., Aravinthan A., Kim J.-H., Cho J.Y. Anticancer effect of joboksansam, Korean wild ginseng germinated from bird feces. J Ginseng Res. 2016;40:304–308. doi: 10.1016/j.jgr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sohn S.-H., Kim S.-K., Kim Y.-O., Kim H.-D., Shin Y.-S., Yang S.-O., Kim S.-Y., Lee S.-W. A comparison of antioxidant activity of Korean White and Red Ginsengs on H2O2-induced oxidative stress in HepG2 hepatoma cells. J Ginseng Res. 2013;37:442. doi: 10.5142/jgr.2013.37.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J.H., Lee Y.G., Yoo S., Oh J., Jeong D., Song W.K., Yoo B.C., Rhee M.H., Park J., Cha S-h. Involvement of Src and the actin cytoskeleton in the antitumorigenic action of adenosine dialdehyde. Biochem Pharmacol. 2013;85:1042–1056. doi: 10.1016/j.bcp.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi H., Wyckoff J., Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Liu J., He Y., Zhang D., Cai Y., Zhang C., Zhang P., Zhu H., Xu N., Liang S. In vitro anticancer effects of two novel phenanthroindolizidine alkaloid compounds on human colon and liver cancer cells. Mol Med Rep. 2017;16:2595–2603. doi: 10.3892/mmr.2017.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafehi H., Orlowski C., Georgiadis G.T., Ververis K., El-Osta A., Karagiannis T.C. Clonogenic assay: adherent cells. J Vis Exp. 2011;13:pii2573. doi: 10.3791/2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franken N.A., Rodermond H.M., Stap J., Haveman J., Van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 25.Yue P.Y., Wong D.Y., Wu P., Leung P., Mak N., Yeung H., Liu L., Cai Z., Jiang Z.-H., Fan T. The angiosuppressive effects of 20 (R)-ginsenoside Rg3. Biochem Pharmacol. 2006;72:437–445. doi: 10.1016/j.bcp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 26.Law C.K.-M., Kwok H.-H., Poon P.-Y., Lau C.-C., Jiang Z.-H., Tai W.C.-S., Hsiao W.W.-L., Mak N.-K., Yue P.Y.-K., Wong R.N.-S. Ginsenoside compound K induces apoptosis in nasopharyngeal carcinoma cells via activation of apoptosis-inducing factor. Chin Med. 2014;9:11. doi: 10.1186/1749-8546-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu T-m, Cui M-h, Ying X., Gu L-p, Jiang X., Su M-m, Wang D-d, Wang W-j. Inhibitory effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin Med J. 2008;121:1394–1397. [PubMed] [Google Scholar]
- 28.Park J.-S., Park E.-M., Kim D.-H., Jung K., Jung J.-S., Lee E.-J., Hyun J.-W., Kang J.L., Kim H.-S. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J Neuroimmunol. 2009;209:40–49. doi: 10.1016/j.jneuroim.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Rausch W.-D., Liu S., Gille G., Radad K. Neuroprotective effects of ginsenosides. Acta Neurobiol Exp (Wars) 2006;66:369–375. doi: 10.55782/ane-2006-1625. [DOI] [PubMed] [Google Scholar]
- 30.Lee E.-S., Choi J.-S., Kim M.S., You H.J., Ji G.E., Kang Y.-H. Ginsenoside metabolite compound K differentially antagonizing tumor necrosis factor-α-induced monocyte–endothelial trafficking. Chem Biol Interact. 2011;194:13–22. doi: 10.1016/j.cbi.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Li W., Liu Y., Zhang J.-W., Ai C.-Z., Xiang N., Liu H.-X., Yang L. Anti-androgen-independent prostate cancer effects of ginsenoside metabolites in vitro: mechanism and possible structure-activity relationship investigation. Arch Pharm Res. 2009;32:49. doi: 10.1007/s12272-009-1117-1. [DOI] [PubMed] [Google Scholar]
- 32.Kim A., Kang K., Kim H., Kim D., Choi Y., Lee S., Hyun J. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis. 2013;4:e750. doi: 10.1038/cddis.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng P.-H., Wang Y.-C., Weng S.-C., Weng J.-R., Chen C.-S., Brueggemeier R.W., Shapiro C.L., Chen C.-Y., Dunn S.E., Pollak M. Overcoming trastuzumab resistance in HER2-overexpressing breast cancer cells by using a novel celecoxib-derived phosphoinositide-dependent kinase-1 inhibitor. Mol Pharmacol. 2006;70:1534–1541. doi: 10.1124/mol.106.023911. [DOI] [PubMed] [Google Scholar]
- 34.Fadeel B., Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med. 2005;258:479–517. doi: 10.1111/j.1365-2796.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- 35.Ghobrial I.M., Witzig T.E., Adjei A.A. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 36.Vermes I., Haanen C., Steffens-Nakken H., Reutellingsperger C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 37.Van Cruchten S., Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31:214–223. doi: 10.1046/j.1439-0264.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- 38.Tait S.W., Green D.R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 39.Budihardjo I., Oliver H., Lutter M., Luo X., Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 40.Park J.G., Son Y.-J., Lee T.H., Baek N.J., Yoon D.H., Kim T.W., Aravinthan A., Hong S., Kim J.-H., Sung G.-H. Anticancer efficacy of Cordyceps militaris ethanol extract in a xenografted leukemia model. Evid Based Complement Alternat Med. 2017;2017:8474703. doi: 10.1155/2017/8474703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Q., Dong H.-W., Sun W.-G., Liu M., Ibla J.C., Liu L.-X., Parry J.W., Han X.-H., Li M.-S., Liu J.-R. Apoptosis initiation of β-ionone in SGC-7901 gastric carcinoma cancer cells via a PI3K-AKT pathway. Arch Toxicol. 2013;87:481–490. doi: 10.1007/s00204-012-0962-8. [DOI] [PubMed] [Google Scholar]
- 42.Chia S., Gandhi S., Joy A., Edwards S., Gorr M., Hopkins S., Kondejewski J., Ayoub J., Califaretti N., Rayson D. Novel agents and associated toxicities of inhibitors of the pi3k/Akt/mtor pathway for the treatment of breast cancer. Curr Oncol. 2015;22:33. doi: 10.3747/co.22.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho H., Mu J., Kim J.K., Thorvaldsen J.L., Chu Q., Crenshaw E.B., Kaestner K.H., Bartolomei M.S., Shulman G.I., Birnbaum M.J. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 44.Song G., Ouyang G., Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riggio M., Perrone M.C., Polo M.L., Rodriguez M.J., May M., Abba M., Lanari C., Novaro V. AKT1 and AKT2 isoforms play distinct roles during breast cancer progression through the regulation of specific downstream proteins. Sci Rep. 2017;7:44244. doi: 10.1038/srep44244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X.-J., Zhang X.-J., Shui Y.-M., Wan J.-B., Gao J.-L. Anticancer activities of protopanaxadiol-and protopanaxatriol-type ginsenosides and their metabolites. Evid Based Complement Alternat Med. 2016;2016:5738694. doi: 10.1155/2016/5738694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.