Abstract
Aim
To histopathologically evaluate the pulpal healing after direct pulp capping with TotalFill and compared it with Neo MTA Plus in human tooth cultures.
Methods
Forty sound human premolars freshly extracted for orthodontic reasons were randomly assigned to two experimental groups; group I: TotalFill and group II: Neo MTA Plus. Standardized Class V cavities were prepared on the buccal surface of each tooth where the pulp exposure was performed with a dental explorer. The pulp-exposed teeth were immediately capped with one of the tested materials. The prepared cavities were then finally restored with glass-ionomer cement. Teeth were cultured for three weeks and three months. Then, the cultured teeth were demineralized, sectioned and stained for histopathological evaluation. Data collected and statistically analyzed by using Fisher's exact test. The significance level was set at P ≤ 0.05.
Results
Histopathological analysis showed complete dentin bridge formation and an absence of inflammatory pulp response. Statistical analysis showed no significant differences between the TotalFill and Neo MTA Plus groups during the observation periods. However, a significantly higher thickness of the dentin bridge was found in the group of teeth treated with TotalFill at three months.
Conclusions
TotalFill produced similar favorable pulp healing and repair, and were comparable to Neo MTA Plus. Furthermore, TotalFill can form a thicker dentin bridge compared with Neo MTA Plus.
Keywords: Bioceramics, Direct pulp cap, Histology, Human, Neo MTA Plus, TotalFill
1. Introduction
Direct pulp capping (DPC) has been frequently used to preserve vitality of dental pulp (Okamoto et al., 2018). DPC is defined as a procedure, in which pulpal injury is immediately sealed by placing a medicament directly on mechanical or traumatic vital pulp exposure to favor pulp healing with reparative dentin bridge formation (Katge and Patil, 2017).
Conventionally, Calcium Hydroxide (CH) has been used for direct capping technique. However, Mineral Trioxide Aggregate (MTA), a material primarily composed of calcium silicates, is considered the new ‘gold standard’ for pulp capping procedures due to its ability to deposit a thicker dentinal bridge with less pulpal inflammatory response compared to CH (Li et al., 2015, Nilsen et al., 2017). The main drawbacks of using MTA as a pulp capping material are handling difficulties and long setting time (Arias-Molis et al., 2017). Therefore, efforts have been devoted to develop bioceramic substitutes in order to overcome the shortcomings of original MTA (Tian et al., 2017).
A recent bioceramic material has been introduced to dental market, namely, Neo MTA Plus (NMP; Avalon Biomed Inc., Bradenton, Florida, USA), which is regarded as a possible substitute to MTA for pulp capping procedure (Tomás-Catalá et al., 2018). NMP consists of fine powder of calcium silicate and gel mixed to form putty-like consistency to improve the handling characteristics (Avalon Biomed, 2015). It does not cause tooth discoloration in addition to its favorable sealing ability (Tran et al., 2016) and suitable radiopacity (Camilleri, 2015).
Another recent generation of bioceramic material, calcium phosphate silicate cement has been launched where phosphate salts are added to the conventional calcium silicate cement (Shen et al., 2015). That mixture is supposed to improve the setting properties and results in a crystalline structure, which resembles tooth hydroxyapatite (Poggio et al., 2015). This cement has beneficial physical and biological properties (Aydin et al., 2018). It is available either as premixed putty or as a paste in a syringe which starts to set when in contact with moisture (Wang, 2015). Lately, this material has been marketed as TotalFill (TF; FKG Dentaire, La-Chaux-de-Fronds, Switzerland) (FKG Dentaire, 2014).
Therefore, it seems worthwhile to histopathologically evaluate the pulpal repair after DPC with TF and compare it to NMP in human tooth cultures.
2. Materials and methods
2.1. Ethical committee approval
This study was approved by the Medical Research Ethics Committee (25/08/2016) in accordance with Helsinki declaration, and written informed consent was obtained from all participants.
2.2. Tooth culture
The inclusion criteria for this research were healthy patients ranging from 15 to 25 years of age, having sound intact premolar teeth that were scheduled to be extracted for orthodontic purposes, without history of trauma, and with no periapical and periodontal pathosis. All patients were diagnosed free from systemic diseases. The exclusion criteria were clinical and radiographic signs of pulp or periodontal lesions, tooth anomaly, no response to vitality tests, and patients diagnosed with systemic diseases including diabetes and cardiac disorders.
Following extraction, the teeth were cleaned with sterilized instruments after removing the periodontal ligament. The root of each tooth was cut perpendicular to the long axis, 3 mm from the apex, using a fissure bur in a high speed handpiece (W&H Impex Inc., Bürmoos, Austria) with sterile saline coolant. The teeth were sterilized with UV light for 20 min in a biosafety cabinet (SterilGARD III Advance; The Baker Co., Sanford, USA) to ensure decontamination. The teeth were then cultured in 12-well cell plates (Falcon; Becton Dickinson, Franklin Lakes, USA) and stored for 2 h at 4 °C in a Dulbecco’s Modified Eagle Medium (Lonza, Vervier, Belgium) augmented with penicillin, streptomycin and amphotericin B (300 UI/mL, 300 μg/mL and 0.75 μg/mL, respectively) to preserve pulp vitality (Camilleri et al., 2014).
2.3. Study design
A total number of 40 human premolars were equally divided into two main groups according to the type of capping material used (n = 20); group I: TF and group II: NMP. Each group was then further sub-divided into two subgroups (n = 10) according to the observation periods; three weeks and three months.
2.4. Cavity preparation and pulp exposure procedures
Aided with magnifying glasses (Univet, magnification 5×, Italy), Class V cavities were prepared on the buccal surfaces of teeth with a sterile #330 high speed bur (Meisinger, Germany) under air-water spray coolant. Pulpal floors were further deepened until the unexposed pulp was seen through dentin as a pink spot. The pulp was then exposed in the middle of the pulpal floor using a sterile dental explorer (Hu-Friedy, USA) (Njeh et al., 2016). Bleeding was controlled by placement of a cotton pellet moistened with sterile saline until physiologic hemostasis occurred.
2.5. Pulp capping materials application
The capping materials were manipulated and applied according to the manufacturers’ instructions. For TF group, putty was placed over the exposed pulp using a sterile plastic instrument. For NMP group, one scoop powder was mixed with one drop gel until putty-like consistency was obtained, and then applied on the exposed pulp. All cavities were then subsequently restored with GC Fuji™ IX GP (GC Corporation, Tokyo, Japan) to provide suitable conditions for pulpal repair.
2.6. Observation periods
The crown of each tooth was fixed to a sterile metallic wire using Embrace™ sealant (Pulpdent, Watertown, USA). The wire was then suspended on two adjacent plates. The apical portion of each root was dipped in 4 mL of culture medium without contacting the bottom of the culture wells to favor diffusion of the medium through the apical portion without any direct contact with restorative material. The culture medium was changed every day. The cultured teeth were incubated for three weeks and three months (Kamal et al., 2018).
2.7. Histological evaluation
At the end of each culture period, the teeth were immediately fixed in 4% formol solution, and then decalcified in 50% formic acid-sodium citrate for six to eight weeks (Accorinte et al., 2008). Following decalcification, the specimens were cleared in Xylene and embedded in paraffin wax. The paraffin-embedded specimens were then serially sectioned in facio-lingual plane to an average thickness of 5 µm using microtome (Leica Biosystems Inc. USA). The sections were stained with Hematoxylin and Eosin (H&E) for histopathological evaluation, examined by light microscope (Zeiss, Germany) and scored according to the criteria (Nowicka et al., 2016) presented in Table 1.
Table 1.
Criteria and scores for histopathological evaluation.
| a. Dentin bridge formation: | |
| 1 | = Complete dentin bridge formation |
| 2 | = Partial dentin bridge formation (extending to more than one-half of the exposure site but not completely closing the exposure site) |
| 3 | = Initial dentin bridge formation (extending to not more than one-half of the exposure site) |
| 4 | = No dentin bridge formation |
| b. Dentin bridge thickness: | |
| 1 | = >0.25 mm |
| 2 | = 0.1–0.25 mm |
| 3 | = <0.1 mm |
| 4 | = Absent bridge |
| c. Inflammatory response: | |
| 1 | = Absent or very few inflammatory cells |
| 2 | = Mild (An average of <10 inflammatory cells) |
| 3 | = Moderate (An average of 10–25 inflammatory cells) |
| 4 | = Severe (An average of >25 inflammatory cells) |
2.8. Statistical analysis
Qualitative data was presented as frequencies and percentages. Fisher's exact test was used for comparisons between groups, as well as to compare between follow-up times. Statistical analysis was performed with IBM SPSS (Statistical Packages for the Social Sciences, Inc., NY, USA) V20 for Windows. The significance level was set at P ≤ 0.05.
3. Results
The summary of histopathological results presented in Table 2, Table 3, Table 4. Histopathological results showed that both capping materials were well tolerated by pulp tissue.
Table 2.
Comparison between dentin bridge formation in the two groups and the changes within each group.
| Time | Dentin bridge formation | TF |
NMP |
P-value (Between groups) |
||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| 3 weeks | Complete | 0 | 0 | 0 | 0 | 1.000 |
| Partial | 9 | 90 | 8 | 80 | ||
| Initial | 1 | 10 | 2 | 20 | ||
| No dentin bridge | 0 | 0 | 0 | 0 | ||
| 3 months | Complete | 10 | 100 | 10 | 100 | NC** |
| Partial | 0 | 0 | 0 | 0 | ||
| Initial | 0 | 0 | 0 | 0 | ||
| No dentin bridge | 0 | 0 | 0 | 0 | ||
| P-value (Within group) | <0.001* | <0.001* | ||||
Significant at P ≤ 0.05.
NC: Not Computed because the variable is constant.
Table 3.
Comparison between dentin bridge thickness in the two groups and the changes within each group.
| Time | Dentin bridge thickness | TF |
NMP |
P-value (Between groups) |
||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| 3 weeks | >0.25 mm | 0 | 0 | 0 | 0 | NC** |
| 0.1–0.25 mm | 10 | 100 | 10 | 100 | ||
| <0.1 mm | 0 | 0 | 0 | 0 | ||
| Absent bridge | 0 | 0 | 0 | 0 | ||
| 3 months | >0.25 mm | 10 | 100 | 6 | 60 | 0.042* |
| 0.1–0.25 mm | 0 | 0 | 4 | 40 | ||
| <0.1 mm | 0 | 0 | 0 | 0 | ||
| Absent bridge | 0 | 0 | 0 | 0 | ||
| P-value (Within group) | <0.001* | 0.011* | ||||
Significant at P ≤ 0.05.
NC: Not Computed because the variable is constant.
Table 4.
Comparison between pulpal inflammation in the two groups and the changes within each group.
| Time | Pulpal inflammation | TF |
NMP |
P-value (Between groups) |
||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| 3 weeks | Absent | 8 | 80 | 7 | 70 | 1.000 |
| Mild | 2 | 20 | 3 | 30 | ||
| Moderate | 0 | 0 | 0 | 0 | ||
| Severe | 0 | 0 | 0 | 0 | ||
| 3 months | Absent | 10 | 100 | 10 | 100 | NC** |
| Mild | 0 | 0 | 0 | 0 | ||
| Moderate | 0 | 0 | 0 | 0 | ||
| Severe | 0 | 0 | 0 | 0 | ||
| P-value (Within group) | 0.474 | 0.211 | ||||
*Significant at P ≤ 0.05.
NC: Not Computed because the variable is constant.
3.1. Three weeks observation period
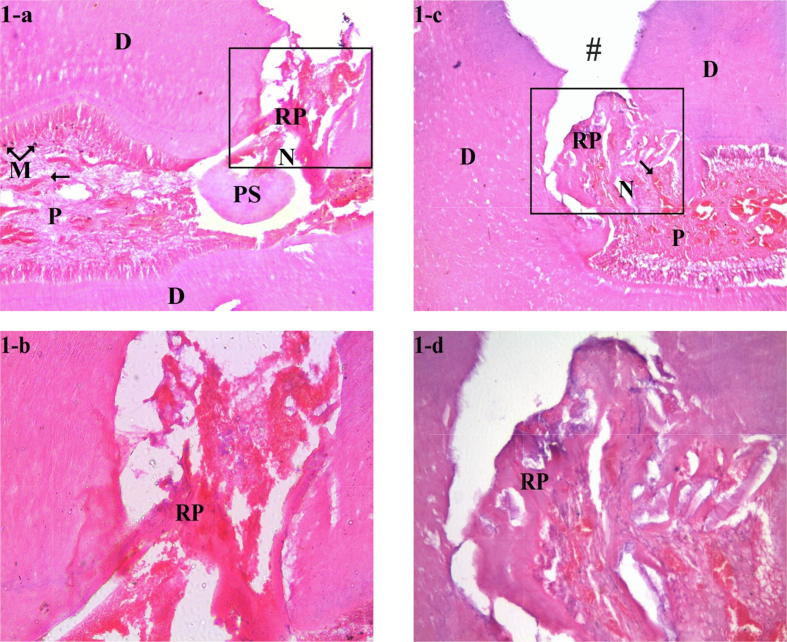
There was no statistically significant difference between TF and NMP groups in terms of dentin bridge formation, dentin bridge thickness and pulpal inflammation. The majority of specimens in both groups showed partial dentin bridge formation (score 2) and no pulpal inflammation (score 1). Only 10% of the TF group specimen and 20% of the NMP group specimens showed initial dentin bridge formation (score 3). Only 20% of the TF group specimens and 30% of the NMP group specimens showed mild pulpal inflammation (score 2). The thickness of the dentin bridges in 100% of specimens of both groups was 0.1–0.25 mm (score 2). In both groups, areas of superficial necrosis and edema could be noted. The blood vessels were dilated and congested in superficial and middle portions of the pulps. Interestingly, in TF group, V-shaped forms of reparative dentin as well as pulp stones were noticed in some specimens. Impaction of the capping material in the pulp was also observed. In NMP group, the reparative dentin had an irregular pattern Fig. 1 (1-a, 1-b, 1-c, 1-d).
Fig. 1.
Photomicrographs of pulp tissue response after three weeks, at low magnification (×40), H&E-stained sections showed superficial pulp tissue necrosis and edema, with dilated and congested blood vessels for TF and NMP groups (1-a and 1-c, respectively). At higher magnification (×100), H&E-stained sections showed V-shaped forms of reparative dentin in TF group (1-b). In contrast, the reparative dentin had a more irregular structure in NMP group (1-d).
3.2. Three months observation period
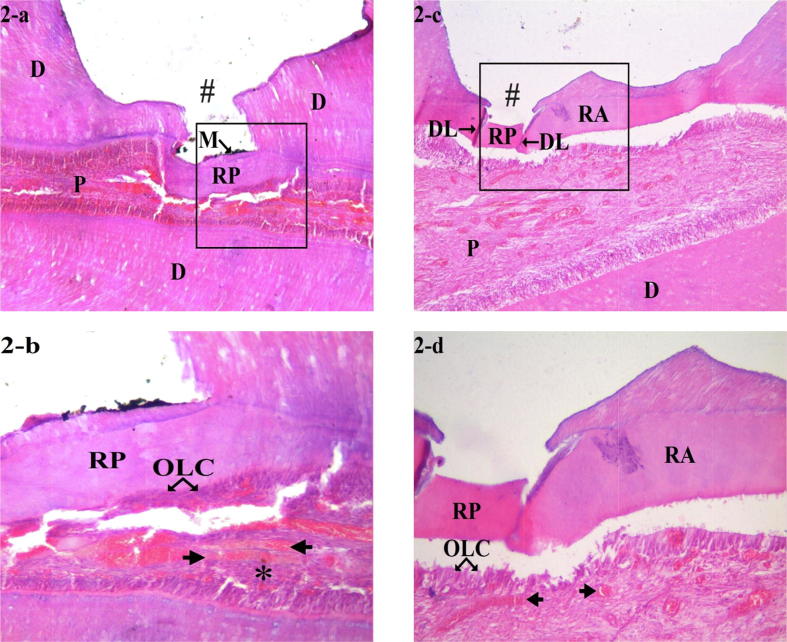
There was no statistically significant difference between TF and NMP groups in terms of dentin bridge formation and pulpal inflammation. Complete dentin bridge formation (score 1) and no pulpal inflammation (score 1) were observed in 100% of both groups specimens. As regard to dentin bridge thickness, there was statistical significant difference between both groups. It was >0.25 mm (score 1) in 100% of TF group specimens, compared to 60% of NMP group specimens. In TF group, the dentin bridges formed were very thick, of homogeneous tubular aspect in most cases. Some specimens showed a dense fibrotic pattern. In NMP group, dentin bridges were separated from surrounding dentin by demarcation line. Dilatation and congestion of blood vessels were evident in both groups Fig. 2 (2-a, 2-b, 2-c, 2-d).
Fig. 2.
Photomicrographs of pulp tissue response after three months, at low magnification (×40), H&E-stained sections showed complete dentin bridge formation at the exposure site for TF and NMP groups (2-a and 2-c, respectively). The newly formed dentin bridges were separated from the existing dentin by demarcation line. At higher magnification (×100), H&E-stained sections showed thicker tubular reparative dentin formation in TF group (2-b) while in NMP group (2-d), the reparative dentin had thinner structure and was atubular. A new layer of odontoblast-like cells as well as dilated and congested blood vessels were also seen beneath the dentin bridge. The symbols in the histological sections indicate the following: (#) floor of the prepared cavity; (M) particles of pulp capping material; (RP) reparative dentin; (RA) reactionary dentin; (OLC) odontoblast-like cells; (N) necrosis; (arrows) dilated and congested blood vessels; (PS) pulp stone; (DL) demarcation line; (*) fibrosis; (D) dentin; (P) pulp.
4. Discussion
Histological assessment is considered the most reliable method to evaluate the condition of dental pulp, by which the degree of inflammation and the existence of necrosis are identified. Indeed, current clinical and radiographic diagnostic assessments do not present a dependable way of evaluating the pulp condition (Aaminabadi et al., 2017, Hashem et al., 2018). In the present study, two observation periods were selected, the first observation period (three weeks) to assess the initial pulpal response and second observation period (three months) for the final evaluation of the pulp capping procedure. Glass and Zander (1949) reported that, at least two weeks are required to initiate the differentiation of odontoblasts-like cells and form reparative dentin. A sharp dental explorer was used to create a mechanical pulp exposure of standardized size, which causes less pulp tissue damage than using a dental bur (Negm et al., 2017). The tooth culture model presents a predictable method for evaluating the biocompatibility of dental materials. This model permits the initial evaluation of dentin-pulp complex regeneration and pulpal tissue reactions following application of pulp capping agents. Moreover, it minimizes the number of animal experiments, which mostly require sacrificing of the animal at the end of the experiment (Téclès et al., 2008).
After three weeks, the histopathological results showed partial dentin bridge formation associated with no to mild pulpal inflammation in TF and NMP groups. These observations can be attributed to their apatite-forming capacity, which may enhances the sealing capability; prevents bacterial microleakage and pulpal inflammation, promotes the biocompatibility and the reparative ability of pulp cells by deposition of calcium phosphate minerals along the dentin-material interface (Siboni et al., 2017, Zamparini et al., 2018). The two major components of TF are hydraulic calcium silicate and calcium phosphate monobasic (Guo et al., 2016). Their setting reaction is triggered by moisture present in the dentinal tubules (Xuereb et al., 2015). When TF is hydrated, calcium silicate produces calcium silicate hydrate gel and CH; further, calcium phosphate monobasic reacts with CH to yield calcium phosphate hydroxyapatite (Han et al., 2015). The hydration reaction of NMP also produces CH in the initial phase of its setting reaction, which further reacts with phosphate ions when in contact with tissue fluids to form calcium phosphate hydroxyapatite (Camilleri, 2015). On the other hand, Gandolfi et al. (2014) observed an increase in solubility, water sorption and porosity in MTA Plus, which has a similar basic chemical composition to NMP, resulting in voids formation. This difference can be explained due to the samples of MTA Plus were immersed in distilled water; not in direct contact with tissue fluids as in the current study. The calcium ions released from NMP react with the phosphate ions of the surrounding tissue fluids, resulting in the deposition of a calcium phosphate layer able to seal the open voids.
After three months, the histopathological results for the TF group were overall similar to those observed in the NMP group. Complete dentin bridge formation and absence of pulp tissue inflammation were detected in all specimens of both groups. These findings can be attributed to their excellent biocompatibility and bioactivity as well as superior sealing ability and antibacterial efficacy (Siboni et al., 2017, Poggio et al., 2015, Tanomaru-Filho et al., 2017b). NMP has been shown to increase alkaline phosphatase (ALP) activity in human osteoblast-like cells. ALP is an enzyme expressed during the initial maturation of osteoblasts, permits estimation of the bioactive properties of dental materials and their ability to enhance healing with mineralized tissue production. In an Alizarin red assay, NMP also induced more mineralized nodules than MTA and TSC/Ta2O5 experimental tricalcium silicate cement with tantalum oxide (Tanomaru-Filho et al., 2017a). Siboni et al. (2017) observed a continual and prolonged release of calcium ions over 28 days from NMP, which has been described to be a primary factor of enhancing tissue regeneration, that will promote the bioactivity and biocompatibility of the material (Prati and Gandolfi, 2015).
The only statistically significant difference between the two groups in the current study was related to the dentin bridge thickness in the TF group after three months, which was greater than the NMP group. This result may be due to the high calcium leaching amount and the potent alkalizing effect of TF (Zamparini et al., 2018), which were more pronounced than NMP, thus producing more favorable conditions for pulp repair (Gandolfi et al., 2014). The ability to release calcium and hydroxyl ions is a primary factor to determine the ability of a material to induce mineralization. Calcium ions are potent signalling regulators for mineralizing stem cells. Hydroxyl ions release during the hydration reaction gives rise to an increase in the local pH, resulting in an alkaline microenvironment, which is responsible for the antibacterial effect (Xuereb et al., 2015). Furthermore, alkaline pH induced by hydroxyl ions stimulates the apatite production (Zamparini et al., 2018). In a micro-computed tomography study, Okamoto et al. (2018) suggested that, iRoot BP Plus which has a similar composition to TF, was a more predictable pulp capping agent than ProRoot MTA, owing to its ability to induce higher quality reparative dentin. Moreover, the smaller particle size of TF permits a better hydration reaction and subsequent calcium and hydroxyl ions release, leading to increased mineralization and promotes high calcium phosphate deposition, accompanied by formation of tag-like structures that extended into dentinal tubules creating micromechanical anchorage (Kadić et al., 2018).
Although the results of the present research favor the use of TF and NMP as DPC materials, it should be considered that, the present research was conducted under optimal circumstances where all teeth used were intact as well as the capped pulps were healthy when exposed. Therefore, future investigations using larger sample size and longer observation intervals include carious teeth complicated with pulpal inflammation are needed to confirm the current results.
5. Conclusions
TF and NMP are innovative bioceramic materials with excellent bioactivities and optimal handling characteristics. The findings of the present study demonstrated that, TF and NMP used as pulp capping agents induced complete dentin bridge formation with no pulpal inflammation. Furthermore, TF has thicker dentin bridge compared with NMP.
On the basis of these results, it can be concluded that, TF produced similar favorable pulp healing and repair, and were comparable to NMP. Furthermore, TF can form a thicker dentin bridge compared with NMP.
6. Ethical Committee Approval
This study was approved by the Medical Research Ethics Committee, Al-Azhar University, Cairo, Egypt (25/08/2016) in accordance with Helsinki declaration. All procedures were conducted with the understanding and written informed consent.
Acknowledgments
Acknowledgements
None.
Declaration of Competing Interest
All authors have no competing interests to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aaminabadi N.A., Parto M., Emamverdizadeh P., Jamali Z., Shirazi S. Pulp bleeding color is an indicator of clinical and histohematologic status of primary teeth. Clin. Oral Investig. 2017;21:1831–1841. doi: 10.1007/s00784-017-2098-y. [DOI] [PubMed] [Google Scholar]
- Accorinte M.L.R., Loguercio A.D., Reis A., Carneiro E., Grande R.H.M., Murata S.S. Response of human dental pulp capped with MTA and calcium hydroxide powder. Oper. Dent. 2008;33:488–495. doi: 10.2341/07-143. [DOI] [PubMed] [Google Scholar]
- Arias-Moliz M.T., Farrugia C., Lung C.Y., Wismayer P.S., Camilleri J. Antimicrobial and biological activity of leachate from light curable pulp capping materials. J. Dent. 2017;64:45–51. doi: 10.1016/j.jdent.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Avalon Biomed., 2015. Neo MTA Plus. Available at: http://avalonbiomed.com/neomta/.
- Aydın Z.U., Özyürek T., Keskin B., Baran T. Effect of chitosan nanoparticle, QMix, and EDTA on TotalFill BC sealers’ dentinal tubule penetration: a confocal laser scanning microscopy study. Odontology. 2018:1–8. doi: 10.1007/s10266-018-0359-0. [DOI] [PubMed] [Google Scholar]
- Camilleri J., Laurent P., About I. Hydration of biodentine, Theracal LC, and a prototype tricalcium silicate–based dentin replacement material after pulp capping in entire tooth cultures. J. Endod. 2014;40:1846–1854. doi: 10.1016/j.joen.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Camilleri J. Staining potential of Neo MTA Plus, MTA Plus, and Biodentine used for pulpotomy procedures. J. Endod. 2015;41:1139–1145. doi: 10.1016/j.joen.2015.02.032. [DOI] [PubMed] [Google Scholar]
- FKG Dentaire., 2014. TotalFill. Available at: https://www.fkg.ch/products/endodontics/obturation/totalfill.
- Gandolfi M.G., Siboni F., Primus C.M., Prati C. Ion release, porosity, solubility, and bioactivity of MTA Plus tricalcium silicate. J. Endod. 2014;40:1632–1637. doi: 10.1016/j.joen.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Glass R.L., Zander H.A. Pulp healing. J. Dent. Res. 1949;28:97–107. doi: 10.1177/00220345490280021101. [DOI] [PubMed] [Google Scholar]
- Guo Y.J., Du T.F., Li H.B., Shen Y., Mobuchon C., Hieawy A. Physical properties and hydration behavior of a fast setting bioceramic endodontic material. BMC Oral Health. 2016;16:23. doi: 10.1186/s12903-016-0184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Kodama S., Okiji T. Evaluation of calcium-releasing and apatite-forming abilities of fast-setting calcium silicate-based endodontic materials. Int. Endod. J. 2015;48:124–130. doi: 10.1111/iej.12290. [DOI] [PubMed] [Google Scholar]
- Hashem D., Mannocci F., Patel S., Manoharan A., Watson T.F., Banerjee A. Evaluation of the efficacy of calcium silicate vs. glass ionomer cement indirect pulp capping and restoration assessment criteria: a randomised controlled clinical trial—2-year results. Clin. Oral Investig. 2018:1–9. doi: 10.1007/s00784-018-2638-0. [DOI] [PubMed] [Google Scholar]
- Kadić S., Baraba A., Miletić I., Ionescu A., Brambilla E., Ivanišević Malčić A. Push-out bond strength of three different calcium silicate-based root-end filling materials after ultrasonic retrograde cavity preparation. Clin. Oral Investig. 2018;22:1559–1565. doi: 10.1007/s00784-017-2244-6. [DOI] [PubMed] [Google Scholar]
- Kamal E.M., Nabih S.M., Obeid R.F., Abdelhameed M.A. The reparative capacity of different bioactive dental materials for direct pulp capping. Dent. Med. Probl. 2018;55:147–152. doi: 10.17219/dmp/90257. [DOI] [PubMed] [Google Scholar]
- Katge F.A., Patil D.P. Comparative analysis of 2 calcium silicate–based cements (Biodentine and Mineral Trioxide Aggregate) as direct pulp-capping agent in young permanent molars: a split mouth study. J. Endod. 2017;43:507–513. doi: 10.1016/j.joen.2016.11.026. [DOI] [PubMed] [Google Scholar]
- Li Z., Cao L., Fan M., Xu Q. Direct pulp capping with calcium hydroxide or mineral trioxide aggregate: a meta-analysis. J. Endod. 2015;41:1412–1417. doi: 10.1016/j.joen.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Negm A.M., Hassanien E.E., Abu-Seida A.M., Nagy M.M. Biological evaluation of a new pulp capping material developed from Portland cement. Exp. Toxicol. Pathol. 2017;69:115–122. doi: 10.1016/j.etp.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Nilsen B.W., Jensen E., Örtengren U., Michelsen V.B. Analysis of organic components in resin-modified pulp capping materials: critical considerations. Eur. J. Oral Sci. 2017;125:183–194. doi: 10.1111/eos.12347. [DOI] [PubMed] [Google Scholar]
- Njeh A., Uzunoğlu E., Ardila-Osorio H., Simon S., Berdal A., Kellermann O. Reactionary and reparative dentin formation after pulp capping: Hydrogel vs. Dycal. Evid. Based Endod. 2016;1:3. [Google Scholar]
- Nowicka A., Łagocka R., Lipski M., Parafiniuk M., Grocholewicz K., Sobolewska E. Clinical and histological evaluation of direct pulp capping on human pulp tissue using a dentin adhesive system. BioMed Res. Int. 2016;ID:2591273. doi: 10.1155/2016/2591273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M., Takahashi Y., Komichi S., Ali M., Yoneda N., Ishimoto T. Novel evaluation method of dentin repair by direct pulp capping using high-resolution micro-computed tomography. Clin. Oral Invest. 2018;22:2879–2887. doi: 10.1007/s00784-018-2374-5. [DOI] [PubMed] [Google Scholar]
- Poggio C., Beltrami R., Colombo M., Ceci M., Dagna A., Chiesa M. In vitro antibacterial activity of different pulp capping materials. J. Clin. Exp. Dent. 2015;7:e584–e588. doi: 10.4317/jced.52401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prati C., Gandolfi M.G. Calcium silicate bioactive cements: biological perspectives and clinical applications. Dent. Mater. 2015;31:351–370. doi: 10.1016/j.dental.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Shen Y., Peng B., Yang Y., Ma J., Haapasalo M. What do different tests tell about the mechanical and biological properties of bioceramic materials? Endod. Topics. 2015;32:47–85. [Google Scholar]
- Siboni F., Taddei P., Prati C., Gandolfi M.G. Properties of NeoMTA plus and MTA plus cements for endodontics. Int. Endod. J. 2017;50:e83–e94. doi: 10.1111/iej.12787. [DOI] [PubMed] [Google Scholar]
- Tanomaru-Filho M., Andrade A.S., Rodrigues E.M., Viola K.S., Faria G., Camilleri J. Biocompatibility and mineralized nodule formation of Neo MTA Plus and an experimental tricalcium silicate cement containing tantalum oxide. Int. Endod. J. 2017;50:e31–e39. doi: 10.1111/iej.12780. [DOI] [PubMed] [Google Scholar]
- Tanomaru-Filho M., Torres F.F.E., Chávez-Andrade G.M., de Almeida M., Navarro L.G., Steier L. Physicochemical properties and volumetric change of silicone/bioactive glass and calcium silicate–based endodontic sealers. J. Endod. 2017;43:2097–2101. doi: 10.1016/j.joen.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Téclès O., Laurent P., Aubut V., About I. Human tooth culture: a study model for reparative dentinogenesis and direct pulp capping materials biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2008;85:180–187. doi: 10.1002/jbm.b.30933. [DOI] [PubMed] [Google Scholar]
- Tian J., Zhang Y., Lai Z., Li M., Huang Y., Jiang H. Ion release, microstructural, and biological properties of iRoot BP Plus and ProRoot MTA exposed to an acidic environment. J. Endod. 2017;1:163–168. doi: 10.1016/j.joen.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Tomás-Catalá C.J., Collado-González M., García-Bernal D., Oñate-Sánchez R.E., Forner L., Llena C. Biocompatibility of new pulp-capping materials NeoMTA plus, MTA repair HP, and biodentine on human dental pulp stem cells. J. Endod. 2018;44:126–132. doi: 10.1016/j.joen.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Tran D., He J., Glickman G.N., Woodmansey K.F. Comparative analysis of calcium silicate–based root filling materials using an open apex model. J. Endod. 2016;42:654–658. doi: 10.1016/j.joen.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Wang Z. Bioceramic materials in endodontics. Endod. Topics. 2015;32:3–30. [Google Scholar]
- Xuereb M., Vella P., Damidot D., Sammut C.V., Camilleri J. In situ assessment of the setting of tricalcium silicate-based sealers using a dentin pressure model. J. Endod. 2015;41:111–124. doi: 10.1016/j.joen.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Zamparini F., Siboni F., Prati C., Taddei P., Gandolfi M.G. Properties of calcium silicate-monobasic calcium phosphate materials for endodontics containing tantalum pentoxide and zirconium oxide. Clin. Oral Investig. 2018:1–13. doi: 10.1007/s00784-018-2453-7. [DOI] [PubMed] [Google Scholar]