Abstract
Background
The root of Panax ginseng, a member of Araliaceae family, has been used as herbal medicine and functional food in Asia for thousands of years. According to Traditional Chinese medicine, ginseng is the most widely used “Qi-invigorating” herbs, which provides tonic and preventive effects by resisting oxidative stress, influencing energy metabolism, and improving mitochondrial function. Very few reports have systematically measured cell mitochondrial bioenergetics after ginseng treatment.
Methods
Here, H9C2 cell line, a rat cardiomyoblast, was treated with ginseng extracts having extracted using solvents of different polarity, i.e., water, 50% ethanol, and 90% ethanol, and subsequently, the oxygen consumption rate in healthy and tert-butyl hydroperoxide–treated live cultures was determined by Seahorse extracellular flux analyzer.
Results
The 90% ethanol extracts of ginseng possessed the strongest antioxidative and tonic activities to mitochondrial respiration and therefore provided the best protective effects to H9C2 cardiomyocytes. By increasing the spare respiratory capacity of stressed H9C2 cells up to three-folds of that of healthy cells, the 90% ethanol extracts of ginseng greatly improved the tolerance of myocardial cells to oxidative damage.
Conclusion
These results demonstrated that the low polarity extracts of ginseng could be the best extract, as compared with others, in regulating the oxygen consumption rate of cultured cardiomyocytes during mitochondrial respiration.
Keywords: Cell protection, Extracellular flux analyzer, Ginseng Radix, Live cell, Reactive oxygen species
1. Introduction
The root of Panax ginseng refers to Korean or Chinese ginseng, which is a highly valued herb that has been extensively used for millennia in Asian countries for diverse beneficial effects, including antiaging [1], antiinflammatory [2], antitumor, and antioxidant actions [3], cardioprotective effects [4], antihypertensive effects [5], and attenuation of peripheral vascular disease and congestive heart failure [6]. According to the theory of Traditional Chinese medicine, ginseng is one of the most widely used “Qi-invigorating” herbs [7]. Therefore, the intake of ginseng is beneficial for the patients by supplementing “Qi” to promote blood circulation and to enhance the medicinal efficacy of benefiting vital energy [8].
In clinical applications, ginseng extracts reduced oxidative stress in patients suffering from cardiovascular diseases: these beneficial effects were proposed to be mediated by antioxidant and chelating abilities of ginsenosides [9]. The activity of ginsenosides is strongly dependent on the types of aglycone [10]. For example, ginsenoside Rg2, Rg3, and Rh2 and ginsenoside aglycones were proposed as pro-oxidative chemicals, whereas ginsenoside Rb1, Rb3, Rc, Rd, Re, Rg1, Rh1, and R1 were proposed as antioxidants [11], [12]. In addition, ginsenoside Rb2 and Rc were shown to have better inhibition effects on oxidative stress than ginsenoside Rb1, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh1, and Rh2 [13]. In contrast to ginsenosides, the phenolic compounds of ginseng displayed significant antioxidant capacities by directly neutralizing free radicals or decomposing peroxides [14], [15], [16], [17].
Mitochondria are responsible to generate adenosine triphosphate (ATP), meeting the energy demands of the cell, particularly in cardiac myocytes [18]. The demand of ATP could be increased during exercise or other physical activities. The ability of mitochondria to increase ATP generation refers to spare respiratory capacity [19]: this capacity is strongly related to cell survival. The measurement of spare respiratory capacity could be determined by treating cells with carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP), an uncoupling agent that is able to disrupt proton gradient and mitochondrial membrane potential [20]. Apart from spare respiratory capacity, the maximal respiration of cells is affected by basal respiration, which consists of proton leak and conventional ATP production. Proton leak from mitochondrial inner membrane could result in uncoupling of oxidative phosphorylation, which was shown to be cytoprotective in ischemic injury models [21]. In addition, this leakage could result to downregulate reactive oxygen species (ROS) generation [22]. However, the proton leak also represents the basal respiration not coupling to ATP production, and this could be considered as a sign of mitochondrial damage [23]. Today, the aforementioned mitochondrial bioenergetics parameters could be determined in live cells by using an extracellular flux analyzer [24].
Although the capabilities of ginseng to attenuate ROS production in cardiomyocytes have been demonstrated previously [25], the effects of ginseng in mitochondrial bioenergetics of a live cell remain ambiguous. To have a comprehensive understanding of the influences of ginseng extracts on mitochondrial bioenergetics of cardiomyocytes, those indicators of mitochondrial respiration were simultaneously measured with an extracellular flux analyzer; this analyzer provided a novel high-throughput instrument that could monitor the real-time metabolism of living cells. Therefore, the tonic and preventive effects of ginseng extracts to both healthy cells and cells under oxidative stress state could be determined and compared.
2. Materials and methods
2.1. Chemicals and preparation of ginseng extracts
HPLC-grade methanol and acetonitrile were purchased from Merck (Darmstadt, Germany). Ultra-pure water was prepared from a Milli-Q purification system (Merck Millipore, Molsheim, France). Formic acid was bought from Riedel-de Haen International (Hanover, Germany). The chemical standards of ginsenoside Rb1, ginsenoside Rd, ginsenoside Re, and ginsenoside Rg1 were obtained from the Shanghai R&D Center for Standardization of Traditional Chinese medicine (Shanghai, China). All these chemical markers were more than 98% purity. Three batches of dried raw materials of Ginseng Radix et Rhizome (ginseng; root and rhizome of P. ginseng) were purchased from Jilin Province of China and then authenticated by Dr Tina Dong at The Hong Kong University of Science and Technology (HKUST), according to the morphological characteristics. The voucher specimens were deposited in the Centre for Chinese Medicine Research and Development at HKUST. The water extracts, 50% ethanol extracts, and 90% ethanol extracts of ginseng were prepared using a standardized extraction method. To be specific, four grams of the powdered sample were refluxed in 100 mL solvent two times (each time for 2 hours), and the supernatant was combined and then dried under vacuum.
2.2. Standardization of ginseng extracts
Ginseng extracts were analyzed on an Agilent HPLC 1,200 series system (Agilent Technologies, Waldbronn, Germany), which was equipped with a degasser, a binary pump, an auto sampler, a thermostated column compartment, and a diode array detector (DAD). The mixtures were filtered with a guard column before separated on an Agilent ZORBAX Eclipse XDB-C18 column (1.8 mm id, 50 mm × 4.6 mm). The mobile phase was composed of 0.1% formic acid in acetonitrile (A) and 0.1% formic acid in water (B) according to the preset gradient program: 0–14 min, linear gradient 20.0–42.0% (A); 14–17 min, linear gradient 42.0–75.0% (A); 17–18 min, isocratic gradient 75.0–75.0% (A); 18–25 min, linear gradient 75.0–85.0% (A). A prebalance period of 8 min was used between each run. The flow rate was set at 0.4 mL/min, and the injection volume was 5 μL. To get the fingerprints of the ginseng extracts, the wavelength of the UV detector was set to 330 nm with full spectral scanning from 190 nm to 400 nm. Then, the effluent was directed into the MS for further analysis. Mass spectrometry was performed on an Agilent triple quadrupole tandem mass spectrometry (Agilent QQQ-MS/MS) (6410A), which was equipped with an electrospray ionization (ESI) ion source. The drying gas was set to 10 L/min at a temperature of 325°C under negative ion mode. The capillary voltage was set to 4,000 V, and the delta electro multiplier voltage was set to 400 V. For the MS/MS analysis, two transition pairs were chosen for acquisition in multiple reaction monitoring (MRM) mode for ginsenoside Rb1, Rd, Re, and Rg1. The collision energy values and fragmentor voltage were optimized in advance to obtain the highest abundance.
2.3. Cell cultures
H9C2 cells, routinely used as a cardiomyocyte cell line (rat embryonic cardiomyoblasts), were purchased from the American Type Culture Collection (Manassas, VA). H9C2 cells were cultured in high-glucose Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, 100 units/mL penicillin/streptomycin at 37°C in a 5% CO2 humidified incubator. The culture reagents were purchased from Invitrogen Technologies (Carlsbad, CA). The medium was replaced every 2–3 days, and the cells were grown up to 80–90% confluence for experimental use.
2.4. Cell viability
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra- zolium bromide (MTT) assay. Cells were seeded in 96-well plates at a density of 1 × 104 cells per well. After 24 hours of drug treatment, cells in each well were incubated with 10 μL MTT (5 mg/mL; Invitrogen) at a final concentration of 0.5 mg/mL for 3 hours at 37°C. After the solution was removed, dimethyl sulfoxide (DMSO) was used to resuspend the purple precipitate inside the cells, and the absorbance was detected at 570 nm. The cell viability was calculated as percentage of the absorbance value of control (without drug treatment), while the value of control was 100%.
2.5. Folin–Ciocalteu assay
The total phenolic content of ginseng extracts was measured by Folin–Ciocalteu assay. In brief, 20 μL of each sample together with 40 μL 10% (v/v) Folin–Ciocalteu reagent (Sigma-Aldrich, St Louis, MO) was added into each well of the 96-well microplate. Then, 160 μL Na2CO3 (700 mM) was added into each well. The assay plates were incubated at room temperature in dark for 2 hours before the absorbance at 765 nm was recorded. Here, gallic acid (Sigma-Aldrich; > 98%) was used as the reference compound, and the total phenolic contents of each extract were expressed as the value compared with gallic acid.
2.6. 2, 2-diphenyl-1-picryl-hydrazyl-hydrate radical-scavenging assay
To measure the free radical–scavenging activity of the extracts, 50 μL of each extract with different concentrations (0.125–8 mg/mL) together with 150 μL 2, 2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) solution was added into each well of the 96-well microplate. After 10 min, the absorbance at 517 nm was recorded. The DPPH free radical–scavenging activity was calculated as an inhibition percentage based on the following equation: Inhibition (%) = [(A0−A1)/A0] × 100, where A0 is the absorbance of the control and A1 is the absorbance of the ginseng sample aliquot. Here, gallic acid (0–100 μM) was used as a positive control.
2.7. Oxidative stress assay
After optimization of herbal extracts with MTT assay, the dose of tert-butyl hydroperoxide (tBHP) (150 μM; Sigma-Aldrich) and positive control (vitamin C, 1 mM) was also optimized. Similar to the cell viability assay, the cells were cultured in a 96-well plate first. After drug treatments for 24 hours, tBHP (150 μM) was added into the wells for 3 hours before MTT in 1× PBS at a final concentration of 0.5 mg/mL was applied. After the solution was removed, the purple precipitate inside the cells was resuspended in DMSO and then measured at 570 nm absorbance.
2.8. ROS formation assay
The determination of ROS level in cell cultures was performed using oxidation-sensitive dye 2′, 7′-dichlorofluorescin diacetate (DCFH-DA). Cultured H9C2 cells (1 × 104 cells/well) in a 96-well plate were pretreated with tert-butyl-hydroxyquinone (tBHQ) or herbal extract for 24 hours and labeled by 100 μM DCFH-DA (Sigma-Aldrich) in Hanks' balanced salt solution (HBSS) for 1 hour at 37°C. After washing three times with HBSS, the cells were then treated with tBHP for 1 hour at 37°C. The amount of intracellular tBHP-induced ROS formation was detected by fluorometric measurement with excitation at 485 nm and emission at 530 nm.
2.9. Luciferase assay
To reveal the transcriptional activation of antioxidant response element (ARE), the pARE-Luc (Promega, Fitchburg, WI) DNA construct, containing four copies of ARE (5′-TGACnnnGCA-3′) that drives transcription of the luciferase reporter gene luc2P (Photinus pyralis), was transfected into cultured H9C2 cells by Lipofectamine 3,000 (Invitrogen) according to the manufacturer's instructions. Transfected H9C2 cells were treated with various concentrations of ginseng extracts for 1 day. Then, the medium was aspirated, and the cultures were lysed by a buffer containing 100 mM potassium phosphate buffer (pH 7.8), 0.2% Triton X-100, and 1 mM dithiothreitol at 4°C. After centrifugation at 16,100 rpm for 5 min, the supernatant was collected and used to perform luciferase assay. The activity was normalized as absorbance (up to 560 nm) per mg of protein. The transfection efficiency in H9C2 cells was 40%, as determined by another control plasmid having a β-galactosidase, under a cytomegalovirus enhancer promoter.
2.10. Mitochondrial bioenergetics analysis
To measure mitochondrial bioenergetics of H9C2 cells, a Seahorse Bioscience XFp extracellular flux analyzer (Agilent) was used. This device uses specialized microplates to create a closed chamber able to measure real-time oxygen consumption by mitochondria in live cells exposed to various stimuli through multiple designed injection ports. Optimal seeding density of H9C2 cells was established at 5,000 cells per well, and therefore, this density was used for all experiments. In addition, mitochondrial agents (Seahorse Bioscience Cell Mito Stress Test Kit #103010-100) were preoptimized at 1 μM oligomycin (complex V inhibitor), 3 μM FCCP (a respiratory uncoupler), and 1 μM rotenone/antimycin A (inhibitors of complex I and complex III) to elicit maximal effects on mitochondrial respiration. Background correction wells were used to normalize the data to background plate noise.
Cells were seeded on the XFp cell culture miniplates and treated with ginseng extracts overnight. The sensor cartridge for the XFp analyzer was hydrated in a 37°C non-CO2 incubator a day before experiment. During the sensor calibration, cells were incubated in the 37°C non-CO2 incubator in 180 μL assay medium (XF base medium, 10 mM glucose, 1 mM pyruvate, and 2 mM L-glutamine, pH 7.40) for 1 hour before the assay. The plate was placed onto calibrated XFp extracellular flux analyzer for Mito Stress Test. The oxygen consumption rate (OCR) was read over the course of three measurement cycles. Each measurement cycle consists of 2 min of mixing, 2 min of incubation, and 2 min of measurements. The OCR was normalized to cellular protein/well and corrected for extra mitochondrial O2 consumption. All experiments were conducted four times. On completion of extracellular flux (XF) assay, the cells were lysed with high salt lysis buffer (50 μL/well), and protein concentration was determined using the Bradford reagent (Bio-Rad, Hercules, CA). The protein concentration of a sample was derived by reference to a bovine serum albumin standard curve. The OCR data were expressed as pmol/min/μg protein. The resultant bioenergetics profile provides detailed information on the individual components of the respiratory chain. Briefly, six parameters of mitochondrial function were calculated from the bioenergetics profile: basal respiration, ATP production, proton leak, maximal respiration, spare respiration capacity, and nonmitochondrial respiration.
2.11. Statistical analysis
Quantitative data acquisition and processing were conducted using Agilent Mass Hunter workstation software, version B.01.00. Principal component analysis (PCA) of the peak areas of ginsenosides was performed using SIMCA-P, version 12.0 (Umetrics, Sweden). The resultant bioenergetics profile was analyzed on Wave Desktop 2.3.0. All data were expressed as the mean ± standard error of the mean (SEM) for n = 3 to 5, unless otherwise specified. Statistical tests were performed by one-way analysis of variance with multiple comparisons using Dunnett's test. Differences were considered significant at p < 0.05.
3. Results
3.1. Quantitative analysis of major ingredients
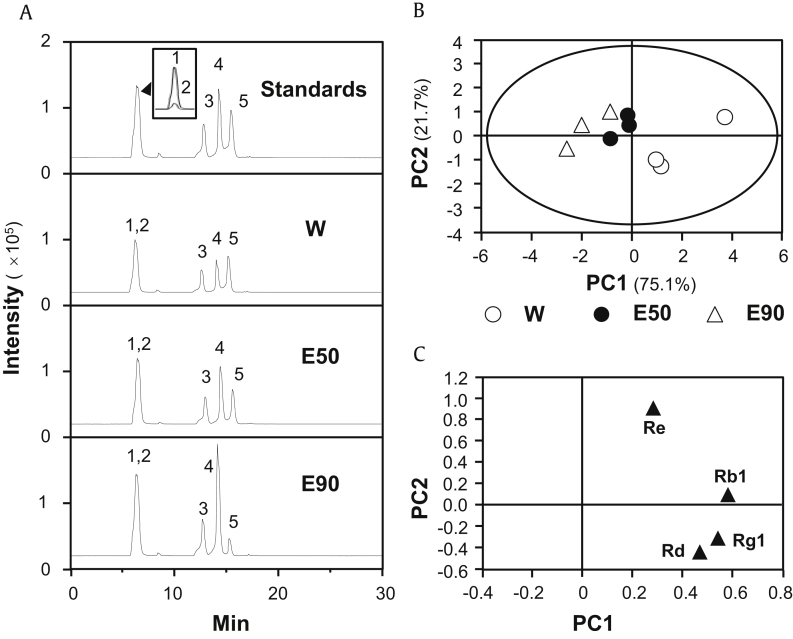
The contents of four abundant ginsenoside, Rb1, Rd, Re, and Rg1 in water extracts, 50% ethanol extracts, and 90% ethanol extracts of ginseng were determined by an optimized analytical method (Table S1) in accord with the previous report [26]. The established method provided adequate stability, sensitivity, precision, linearity, and accuracy for simultaneous determination of ginsenosides in ginseng extracts, as summarized in Table S2. The amounts of ginsenosides in different ginseng extracts from three batches of herbs were quantified by LC-MS (Fig. 1A), and the contents of ginsenosides were recorded in Table 1. The ginsenoside content served as quality control parameters. PCA of the contents of ginsenosides was conducted to differentiate ginseng samples, extracted with different solvents. The two ranking principal components (PCs), PC1 and PC2, described ∼75% and ∼22% of total variability, respectively, which accounted for 96.8% of total variance. The score plot showed that ginseng extracts could be obviously classified into three distinct groups according to their extracting solvents used (Fig. 1B). The loading plots for PC1 versus PC2 were shown in Fig. 1C, showing the role of each variable (ginsenoside Rb1, Rd, Re, and Rg1) in discriminating the extracts.
Fig. 1.
PCA of ginsenosides in different ginseng extracts. (A) Identification of ginsenoside Rg1 (1), Re (2), Rb1 (3), Rd (5), and astragaloside IV (4; internal control marker) was made by an MS detector. Representative chromatograms of standard markers (Standards), water extract (W), 50% ethanol extract (E50), and 90% ethanol extract (E90) under MRM mode were shown. (B) The scoring plot of different ginseng extracts by comparing the contents of chosen ginsenosides. PC1 and PC2 described ∼75% and ∼22% of total variability, respectively. (C) The loading plot of PC1 versus PC2 for four ginsenosides, from LC-MS profiles, of three ginseng extracts was shown. All values were from n = 3.
PCA, principal component analysis; PC1, principal component 1; PC2, principal component 2.
Table 1.
Quantitative assessment of four ginsenosides in different ginseng extracts
| Ginsenoside | RS-11) |
RS-2 |
RS-3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| W2) | E50 | E90 | W | E50 | E90 | W | E50 | E90 | |
| Rb1 | 2.347 ± 0.0163) | 2.888 ± 0.025 | 3.518 ± 0.069 | 4.048 ± 0.025 | 3.447 ± 0.027 | 2.735 ± 0.023 | 2.410 ± 0.036 | 3.283 ± 0.021 | 2.215 ± 0.021 |
| Rd | 1.335 ± 0.014 | 0.626 ± 0.011 | 0.857 ± 0.009 | 1.923 ± 0.027 | 0.726 ± 0.011 | 0.222 ± 0.019 | 1.366 ± 0.014 | 0.714 ± 0.002 | 0.513 ± 0.011 |
| Re | 0.808 ± 0.023 | 0.704 ± 0.011 | 0.703 ± 0.012 | 1.025 ± 0.016 | 0.713 ± 0.021 | 0.577 ± 0.016 | 0.767 ± 0.011 | 0.746 ± 0.012 | 0.492 ± 0.002 |
| Rg1 | 0.587 ± 0.017 | 0.330 ± 0.009 | 0.088 ± 0.002 | 0.730 ± 0.013 | 0.344 ± 0.009 | 0.084 ± 0.001 | 0.570 ± 0.019 | 0.353 ± 0.03 | 0.073 ± 0.002 |
Three batches of ginseng purchased from Jilin province of China were used in the present study
W, water extracts of ginseng; E50, 50% ethanol extracts of ginseng; E90, 90% ethanol extracts of ginseng
Values are expressed in mg/g of dried powder of ginseng, mean ± SD, n = 3
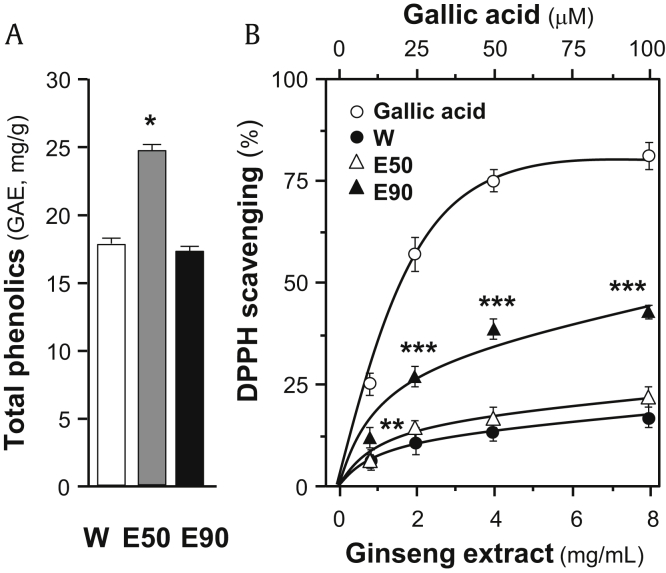
To have a comprehensive view of antioxidant effect of ginseng extracts, total phenolic compounds together with free radical–scavenging activity of the extract were determined. Gallic acid was used as a reference compound, and total phenolic compounds of each extract were expressed as the value in reference of gallic acid. As shown in Fig. 2A, the 50% ethanol extract of ginseng showed significant higher content of phenolic compounds, i.e., equivalent to ∼25 mg gallic acid/g of sample. The water extract and the 90% ethanol extract showed ∼17 mg gallic acid/g and ∼16 mg gallic acid/g, respectively. However, the 90% ethanol extract showed significantly higher activity, at least double, in the maximum free radical–scavenging activity, than other ginseng extracts (Fig. 2B). Therefore, the relationship between total phenolic compounds and free radical–scavenging activity of ginseng extracts was relatively low, indicating that the phenolic compounds are not the only antioxidant substance within the extracts.
Fig. 2.
Comparison of total phenolic contents and DPPH radical-scavenging activity of different ginseng extracts. (A) Total phenolic contents of ginseng extracts were determined using Folin–Ciocalteu assay. Gallic acid was used as a reference compound, and total phenolic content of each extract was expressed as the value of gallic acid equivalent (i.e., GAE in mg/g). (B) Antioxidant effects of samples were determined using DPPH radical-scavenging assays. Gallic acid was used as a positive control. All data are expressed as mean ± SD, n = 5. Statistical comparison was made with the sample with the lowest value of corresponding concentration, *p < 0.05, **p < 0.01, ***p < 0.001.
SD, standard deviation.
3.2. Against oxidative stress in H9C2 cells
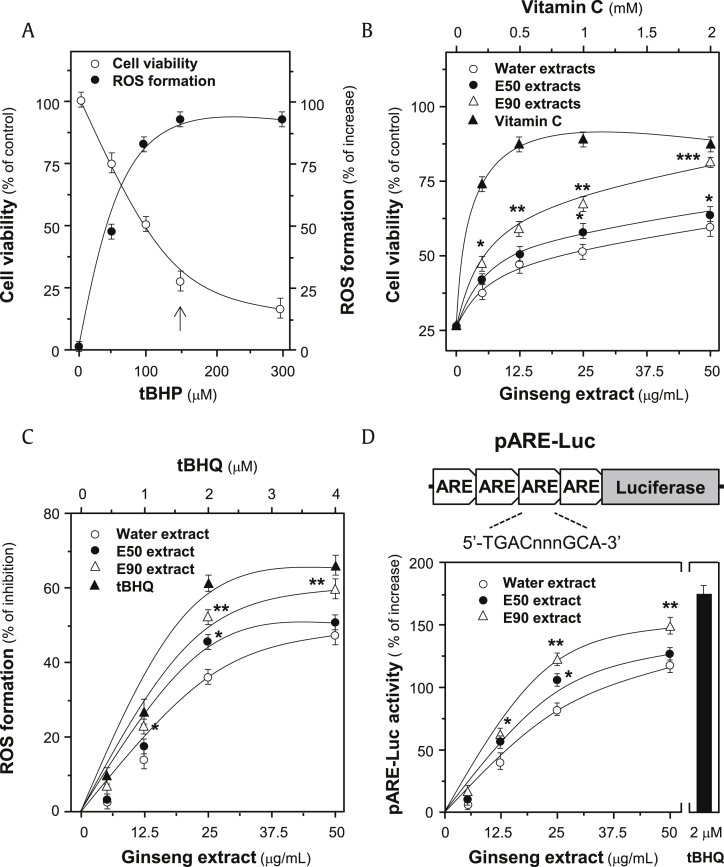
A stress inducer, tBHP, was chosen to damage cardiomyocytes, which induced cell death in a dose-dependent manner (Fig. 3A). In parallel, the applied tBHP induced ROS formation, showing a maximal induction starting at ∼100 μM (Fig. 3A). Here, 150 μM tBHP was used for subsequent tests. The cell viability, determined by MTT assay, showed an insignificant cell death up to the treatment of 50 μg/mL ginseng extracts (Fig. S1). The extracts from RS-1 batch were used for subsequent analyses. The treatment of ginseng extracts, dose dependently, protected cells against oxidative insult, and the 90% ethanol extracts showed the best protection effects to cultured H9C2 cells, with maximal protection of more than 60% as compared with control (Fig. 3B). Vitamin C served as a control showing protective effect against tBHP-induced cell death.
Fig. 3.
Protection effects of ginseng extracts to H9C2 cells against oxidative stress. (A) Cultured H9C2 cells (1 × 104 cells/well) were exposed to tBHP at various concentrations. The cell viability was determined by MTT assay after treated for 3 hours, and the level of intracellular ROS was measured by fluorescent staining after 1 hour. Cell viability was expressed as percentage of control (cells without tBHP), and ROS level was in percentage of increase (against ROS in control). tBHP at 150 μM was used for routine analysis (indicated by an arrow). (B) Dose-dependent response was performed by pretreating the cultures with ginseng extracts (batch RS-1), i.e., water extract (W), 50% ethanol extract (E50), and 90% ethanol extract (E90), for 24 hours before addition of tBHP (150 μM). Vitamin C at various concentrations served as a positive control. (C) Cultured H9C2 cells were pretreated with ginseng extracts or tBHQ for 24 hours and then exposed to tBHP (150 μM) for 1 hour. The result was in percentage of ROS formation relative to tBHP-treated control. (D) Cultured H9C2 cells, transfected with pARE-Luc, were treated with ginseng extracts or tBHQ for 24 hours. The cell lysates were then subjected to luciferase assay. Values are expressed as the percentage of increase to basal reading (untreated culture). All data are expressed as Mean ± SD, n = 5, each with triplicate samples. Statistical comparison was made with the sample with the lowest value of corresponding concentration, *p < 0.05, **p < 0.01, ***p < 0.001.
ARE, antioxidant response element; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra- zolium bromide; ROS, reactive oxygen species; SD, standard deviation; tBHP, tert-butyl hydroperoxide.
The inhibitory effect of ginseng extract to formation of ROS in tBHP-treated cultured H9C2 cells was analyzed by fluorometric measurement using DCFH-DA. By applying the ginseng extracts before tBHP application, the intracellular ROS was, dose dependently, decreased (Fig. 3C). The 90% ethanol extracts showed the greatest inhibitory effect of ∼60%, as compared with the control and other extracts, which was consistent with the enhancement effect to cell viability, as mentioned previously (Fig. 3C). Therefore, the protective effects of ginseng extracts to H9C2 cells could be mainly mediated by inhibiting the formation of ROS.
The transcriptional activity of ARE, a crucial regulator activating Nrf2 pathway to resist oxidative stress, was investigated. The luciferase reporter construct (i.e., pARE-Luc) containing four ARE DNA regulatory elements derived from the promoters of antioxidative genes, tagged upstream of a luciferase gene, was transfected into cultured H9C2 cells. The activation of pARE-Luc, triggered by ginseng extracts, was in a dose-dependent manner (Fig. 3D). The maximal induction of 90% ethanol extracts was ∼150% increase, which was relatively stronger than that of other extracts.
3.3. Mitochondrial bioenergetics analysis
A detailed profiling of mitochondrial bioenergetics is essential to have a comprehensive view of antioxidant activity and/or tonic effects of ginseng extracts in cardiomyocytes. Here, a Seahorse Bioscience XFp extracellular flux analyzer, having a real-time measurement of OCR, was used to monitor various parameters of mitochondrial bioenergetics during metabolism (Fig. S2). In cultured H9C2 cells, seeding cell density and FCCP concentration were optimized to measure the cellular metabolic functions. As shown in Fig. S3, the optimal cell density of H9C2 cells was set at 5,000 cells/well to adjust initial OCR to an appropriate range (100–160 pmol/min). Meanwhile, the concentration of FCCP was optimized to 3 μM to yield maximal OCR as shown in Fig. S3. The concentration of oligomycin (1 μM) and rotenone/antimycin A (1 μM) was optimized according to the manufacturer's guidelines and previous reports [27].
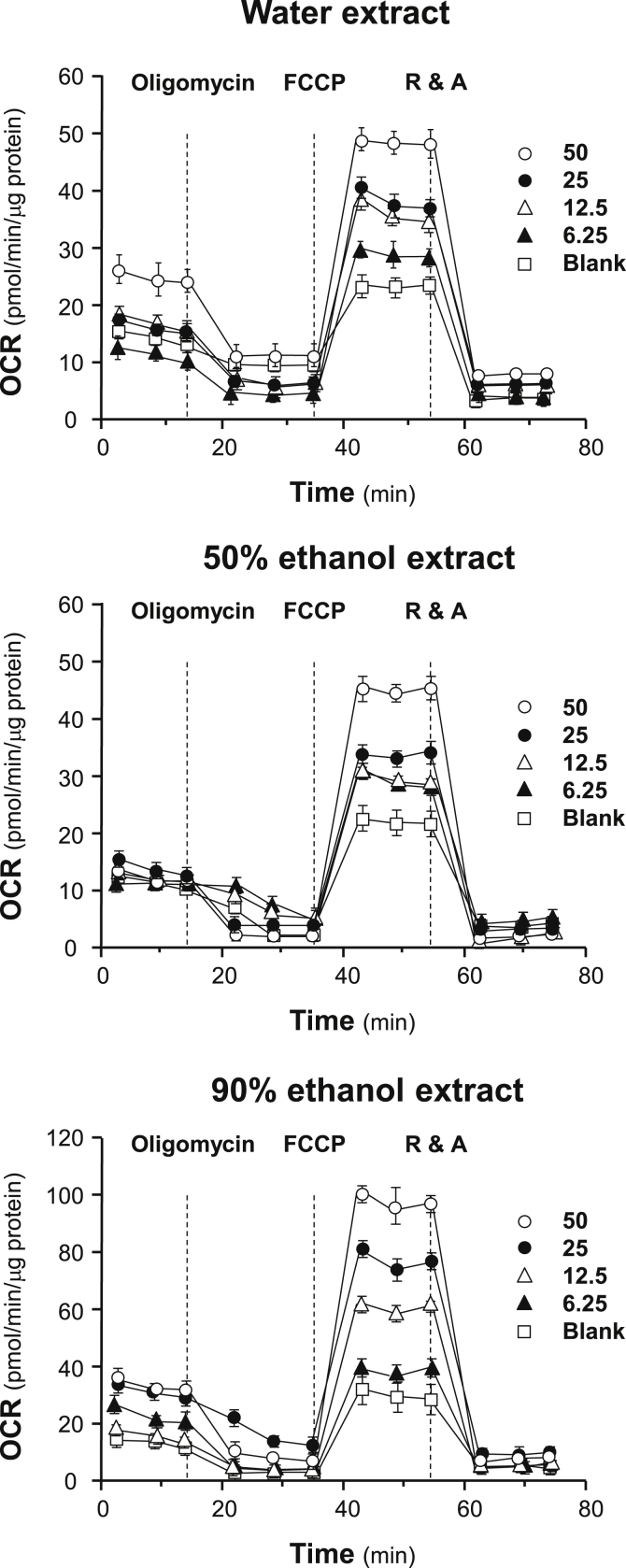
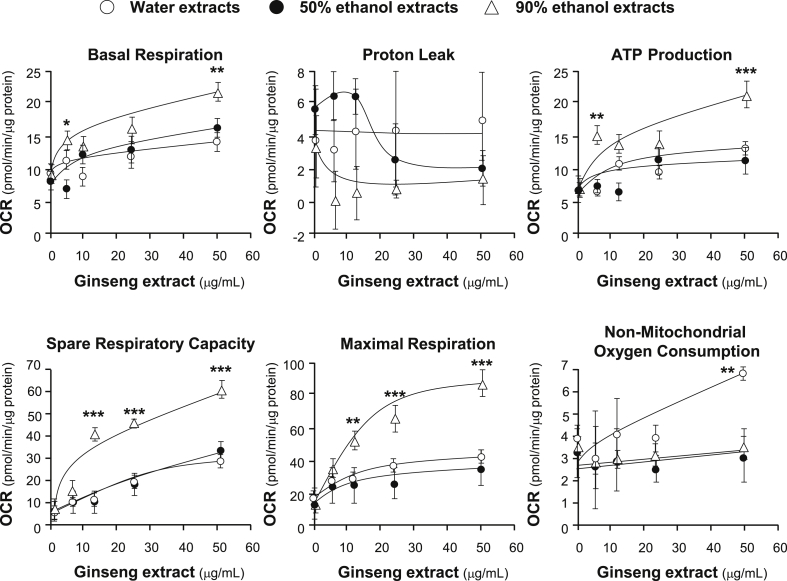
The effects of increasing concentration of ginseng extracts on the OCR of cultured H9C2 cells were plotted against time. All ginseng extracts at different solvents could dose-dependently increase basal respiration, ATP production, spare respiration capacity, and maximal respiration to different degree (Fig. 4). In contrast, the parameters of proton leak and nonmitochondrial respiration changed irregularly probably because the detection limit of the present method was not sensitive enough to measure these parameters precisely. Among other parameters, spare respiratory capacity has been considered the most important component of bioenergetics profile, which is corresponding to cell ability in supplying substrate and transport electron during an increased demand of energy consumption. Here, the 90% ethanol extracts increased spare respiratory capacity up to ∼seven-folds, as compared with background, which was significantly higher than that of the water and 50% ethanol extracts (Fig. 4).
Fig. 4.
Ginseng extracts regulate mitochondrial bioenergetics of H9C2 cells. Cultured H9C2 cells were treated with ginseng extracts (from 6.25 μg/mL to 50 μg/mL as indicated) for 24 hours before measuring the oxygen consumption rate with XFp Cell Mito Stress Test. Response of H9C2 cells after oligomycin (1 μM), FCCP (3 μM), and rotenone/antimycin A (R&A at 1 μM) applied onto the wells were recorded. Dotted lines denote times at which the three inhibitors were applied. Effects of various extracts of ginseng at various concentrations on the oxygen consumption rate (OCR) for four respiration states of H9C2 cells were shown. The OCR value was normalized with cellular protein/well by protein. Data are expressed as Mean ± SD, n = 3, each with triplicate samples. FCCP, carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone; SD, standard deviation.
By quantifying the dose responses in mitochondrial bioenergetics from the extracellular flux analyzer, different parameters could be compared among the three ginseng extracts. The ginseng extracts induced those aforementioned parameters, except proton leak, in dose-dependent manners, and maximal induction was achieved at 20 μg/mL to 40 μg/mL of the extract (Fig. 5). The 90% ethanol ginseng extracts showed the best induction in basal respiration, ATP production, spare respiration capacity, and maximal respiration, as compared with other extracts. In contrast, the water extract of ginseng showed the best induction in nonmitochondrial oxygen consumption (Fig. 5). The maximal induction, triggered by 90% ethanol ginseng extract, could increase to ∼two-folds in basal respiration, ∼three-folds in ATP production, ∼12-folds in spare respiratory capacity, and ∼four-folds in maximal respiration (Fig. 5). The 90% ethanol extracts of ginseng possessed maximum induction effects in mitochondrial bioenergetics as compared with the high polarity extracts: these results were consistent with its high content of ginsenosides. Apart from constituting maximal respiration with spare capacity respiration and proton leak, ATP production also represents a portion of ATP, produced by mitochondria, that meets the energetic needs of cells. From the dose curve shown in Fig. 5, it could be speculated that aerobic respiration was vigorously promoted by ginseng extracts. Therefore, the ginseng extracts could dose-dependently enhance both the extent and the ability of mitochondrial respiration of healthy cardiomyocytes.
Fig. 5.
Comparison of various parameters of mitochondrial respiration of ginseng-treated H9C2 cells. Cultured H9C2 cells were treated as in Fig. 4. The effects of increasing concentration of ginseng extracts to basal respiration, proton leak, ATP production, spare respiratory capacity, maximal respiration, and nonmitochondrial respiration were measured and compared among three ginseng extracts. The OCR value was normalized with cellular protein. Data are expressed as Mean ± SD, n = 3, each with triplicate samples. Statistical comparison was made with the sample with the lowest value of corresponding concentration, *p < 0.05, **p < 0.01, ***p < 0.001.
OCR, oxygen consumption rate; SD, standard deviation.
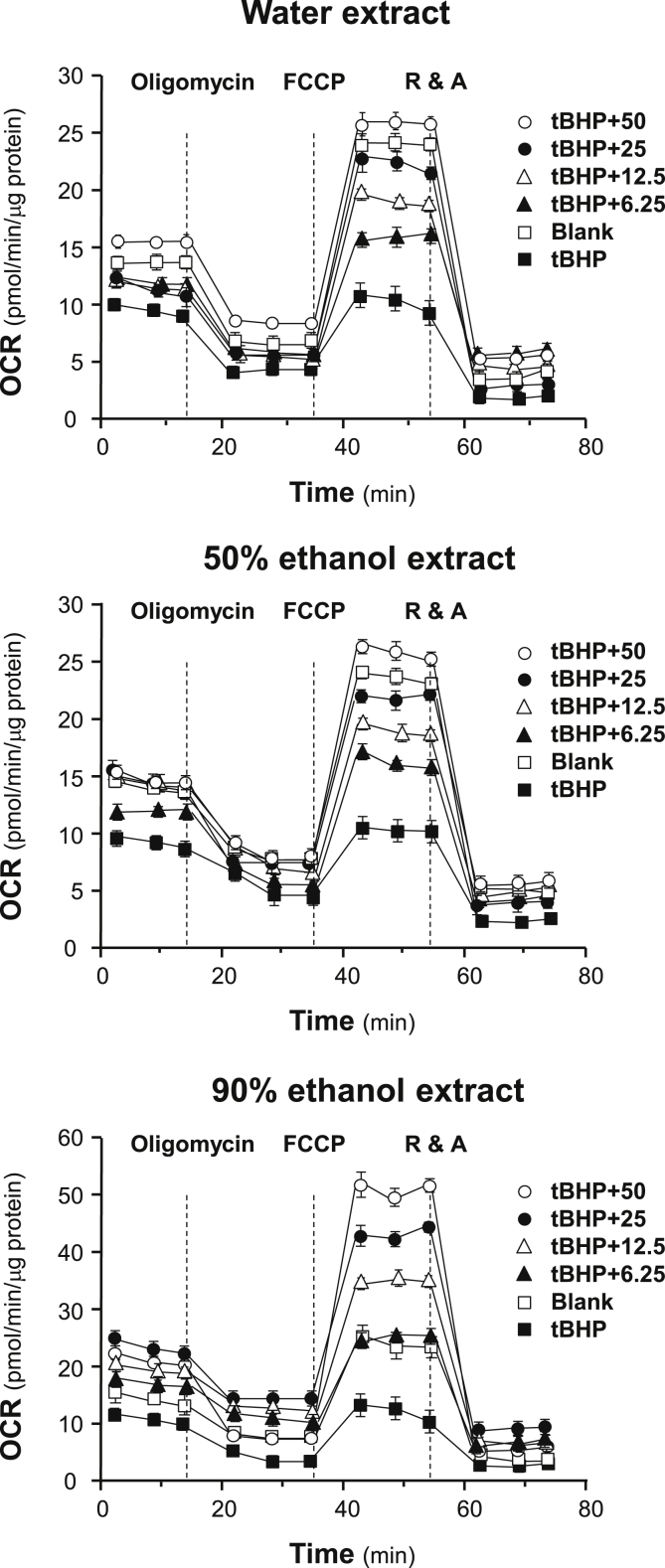
To determine the tonic effects of ginseng extracts to cells under oxidative stress, tBHP was used to simulate the damage. Application of tBHP in cultured H9C2 cells decreased the initial OCR in a dose-dependent manner, and the dosage of tBHP was optimized to 30 μM to obtain measurable OCR (Fig. 6). Compared with healthy cells, tBHP-treated H9C2 cells showed severe mitochondrial dysfunction, resulting in more than ∼10-fold reduction in spare respiratory capacity and ∼3-fold reduction in maximal respiration (Fig. 6). In parallel, the respiration of H9C2 cells significantly declined to adapt strong oxidizing environment. However, the pretreatment with ginseng extracts in tBHP-treated H9C2 cells preserved mitochondrial OCR by preventing the decline. Water extracts and 50% ethanol extracts could bring the stressed cells back to healthy status by increasing spare respiratory capacity and maximal respiration to healthy level, whereas the 90% ethanol extracts could increase the values to two to three folds of normal state (Fig. 6). Meanwhile, the cells being pretreated with ginseng extracts performed stronger production of ATP, suggesting the potential of ginseng extracts to protect mitochondria against oxidant, which could prevent the cells from oxidative damage.
Fig. 6.
Protection effects of ginseng extracts to tBHP-treated H9C2 cells against oxidative stress. Cultured H9C2 cells were pretreated with ginseng extracts (from 6.25 μg/mL to 50 μg/mL, as indicated) for 24 hours before exposed to tBHP (30 μM) for 1 hour. Effects of various extracts of ginseng at various concentrations on the oxygen consumption rate (OCR) for four respiration states of tBHP-treated H9C2 cells were determined as in Fig. 4. The OCR value was normalized with cellular protein. Data are expressed as mean ± SD, n = 3, each with triplicate samples.
FCCP, carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone; R&A, rotenone/antimycin A; SD, standard deviation; tBHP, tert-butyl hydroperoxide.
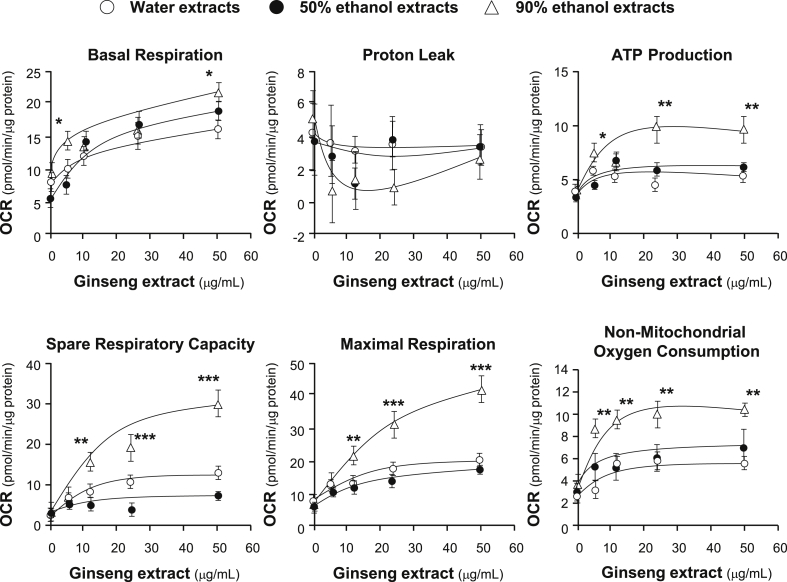
The 90% ethanol extracts obviously possessed the most significant beneficial effects to improve mitochondrial ATP level and spare respiratory capacity in oxidative stressed cells, as compared with the water extracts and the 50% ethanol extracts, similar to that in healthy cells (Fig. 7). Apart from mitochondrial ATP level, the dose-dependent trend of nonmitochondrial oxygen consumption was obvious in the stressed cell (Fig. 7), which might be due to the influence of ginseng extracts to oxidized cells that was stronger than that in healthy cells. The change in proton leak was still unintelligible, and it was not judicious to ascribe the situation to large deviation of the present method or recklessly judged as the effects of ginseng extracts. Therefore, ginseng extracts could not only provide tonic effects to healthy cells but also possess excellent defensive effects on cellular respiration and energy metabolism of cardiomyocytes on exposure to oxidative damage.
Fig. 7.
Mitochondrial respiration of tBHP-treated H9C2 cells after the pretreatment of ginseng. Cultured H9C2 cells were treated as in Fig. 6. The basal respiration, proton leak, ATP production, spare respiratory capacity, maximal respiration, and nonmitochondrial respiration were measured. The OCR value was normalized with cellular protein/well by Bradford's method. Data are expressed as Mean ± SD, n = 3, each with triplicate samples. Statistical comparison was made with the sample with the lowest value of corresponding concentration, *p < 0.05, **p < 0.01, ***p < 0.001.
OCR, oxygen consumption rate; SD, standard deviation; tBHP, tert-butyl hydroperoxide.
4. Discussion
Ginseng is among the most widely used “Qi-invigorating” herbs for its tonic and prevention effects [28]. Apart from acting like agonists of glucocorticoid and estrogen receptors [29], [30], ginsenosides showed cardio-protective effects by eliminating ROS generated from metabolic processes [31]. Another major antioxidant in ginseng is polyphenol. However, the polarities of ginsenosides and polyphenols do not allow us to obtain the herbal extracts with abundance of both compounds. To provide a comprehensive comparison of antioxidant profile of different ginseng extracts, quantitative analysis of four typical ginsenosides, Rb1, Rd, Re, and Rg1, in the three extracts, i.e., water, 50% ethanol, and 90% ethanol, of ginseng indicated that the content of ginsenosides was dependent on the extraction solvents. Moreover, the PCA scoring plot of peak areas of ginsenosides showed that ginseng extracts could be obviously classified according to their extraction solvent used; in particular, the contents of ginsenosides could be a critical parameter. Therefore, the extraction solvent could be a major factor in determining chemical composition of the ginseng extract.
The measurement of total phenolic compounds and evaluation of free radical–scavenging activity are a direct and quick way to assess total antioxidant activity of unknown samples. However, the ginseng extracts containing abundant phenolic compounds did not possess better radical-scavenging activity [32], which suggested the important role of other types of compounds in resisting oxidative damage, especially ginseng saponins [33], [34]. To confirm the relationship between antioxidant activity and polarity of ginseng extracts, the protective effects of ginseng extracts to cultured H9C2 cells against tBHP-induced oxidative stress were determined. Identical with the results of DPPH radical-scavenging assay, the 90% ethanol extracts displayed the best protection effects to cardiomyocytes under oxidative stress, which were mainly realized by inhibiting the formation of ROS. By pretreating cells with ginseng extracts, especially the low polar extracts, the tBHP-induced damage could be avoided, and ginsenosides might be the reason of this prevention. This protective effect could be mediated, at least partly, by the Keap1-Nrf2-ARE signaling mechanism. This notion is supported by the current result and by the previous reports [35], [36].
Until now, there are numerous articles about the protective effects of ginsenosides to cells by inhibiting mitochondria-mediated apoptosis [37], [38]. However, most researches are focusing the effects on ATP production and intracellular ROS formation [39], [40], while the influence to other parameters of mitochondrial bioenergetics are often negated [41]. Here, all ginseng extracts showed a dose-dependent manner in enhancing spare respiratory capacity and ATP production of healthy cells, which was consistent with an increase of energy metabolism after the treatment with ginseng. In line with the healthy cells, the mitochondrial bioenergetics of tBHP-induced H9C2 cells was improved after treatment with ginseng extracts, especially the extracts with low polarity. Consistent with the present results, Wang et al (2016) showed that some ginsenosides could moderate protective effects on mitochondrial function in tBHP-treated cardiomyocytes by recovering oxygen consumption and increasing mitochondrial DNA content through activation of sirtuin 1 (SIRT1) [42]. In addition, ginseng saponin was shown to alleviate apoptosis of cardiomyocytes by reducing intracellular ROS and inhibiting mitochondria-mediated apoptosis [33]. Ginsenoside Rb1 was shown to exert a protective effect in hypoxia/ischemia-induced cell death by inhibiting GSK-3β-mediated mitochondrial permeability transition pore opening and affecting caspase-3 and caspase-9 activities [35]. Ginsenoside Rd was able to activate protein kinase B (Akt)/glycogen synthase kinase-3β (GSK-3β) signaling [36], and Rg1 was able to modulate mitofusin-2 (MFN2) and glutamate dehydrogenase (GDH) [37]. Finally, ginsenoside Re could function as an antioxidant to protect cardiomyocytes from oxidant injury [38].
Mitochondrial respiration plays critical roles in a variety of cellular processes. The experimental approach for most research in the past few years was to assess the bioenergetic parameters in detached mitochondria isolated from either heart or liver, which might result in anoikis associated with increased ROS and leading to untrustworthy results [43]. As a powerful tool to monitor real-time mitochondrial respiration in live cells, the extracellular flux analyzer has been widely used in various live cell analyses [27]. However, the application of this technology in herbal extract is still very limited [44], [45], [46]. Mu et al (2015) found that ginsenoside Rb1 could significantly improve mitochondrial respiration of mature adipocytes by increasing basal mitochondrial respiration, ATP production, and uncoupling capacity [44]. Lin et al (2015) demonstrated that Qiliqiangxin, a common prescription in Chinese herbal medicine containing ginseng, could enhance oxidative metabolism and mitochondrial uncoupling in H9C2 cells [45]. These results, together with the present research, verified the beneficial effects of ginseng, especially ginsenoside, on mitochondrial bioenergetics of different cells. In the case of unhealthy cells, Takanashi et al (2017) found that hochuekkito (Bu-Zhong-Yi-Qi-Tang in Chinese, a prescription containing ginseng) could activate both mitochondrial and glycolytic energy metabolism in influenza A/PR/8/34 (H1N1) virus (IAV)-infected cells and in noninfected cells [46], which was in line with the observed protective effect of ginseng extracts on cells under oxidative stress. In accord with previous results, our study provided a strong proof to verify the tonic and protective effects of ginseng extracts to cardiomyoblasts.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This study was supported by Hong Kong Research Grants Council Theme-based Research Scheme (T13-607/12R), ITF (UIM/254), GRF (663012, 662713, M-HKUST604/13), TUYF15SC01, The Hong Kong Jockey Club Charities Trust (HKJCCT12SC01), Foundation of The Awareness of Nature (TAON12SC01), and Shen Zhen Science and Technology Innovation (JCYJ20160229205726699, JCYJ20160229205812004 and JCYJ20160229210027564).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jgr.2018.02.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Cheng Y., Shen L.H., Zhang J.T. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 2.Park J.S., Park E.M., Kim D.H., Jung K., Jung J.S., Lee E.J., Hyun J.W., Kang J.L., Kim H.S. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J Neuroimmunol. 2009;209:40–49. doi: 10.1016/j.jneuroim.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Keum Y.S., Park K.K., Lee J.M., Chun K.S., Park J.H., Lee S.K., Kwon H., Surh Y.J. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41–48. doi: 10.1016/s0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- 4.Kim J.H. Cardiovascular diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J Ginseng Res. 2012;36:16–26. doi: 10.5142/jgr.2012.36.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek E.B., Yoo H.Y., Park S.J., Chung Y.S., Hong E.K., Kim S.J. Inhibition of arterial myogenic responses by a mixed aqueous extract of Salvia Miltiorrhiza and Panax notoginseng (PASEL) showing antihypertensive effects. Korean J Physiol Pharmacol. 2009;13:287–293. doi: 10.4196/kjpp.2009.13.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C.H., Kim J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung F.Y., Linn Y.C. Steroids in traditional Chinese medicine: what is the evidence? Singapore Med J. 2017;58(3):115–120. doi: 10.11622/smedj.2017016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J.Y., Pham D.D. Sasang constitutional medicine as a holistic tailored medicine. eCAM. 2009;6:11–19. doi: 10.1093/ecam/nep100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao Z.H., Xie J.T., Hoek T.L.V., Mehendale S., Aung H., Li C.Q., Qin Y., Schumacker P.T., Becker L.B., Yuan C.S. Antioxidant effects of American ginseng berry extract in cardiomyocytes exposed to acute oxidant stress. Biochimica et Biophysica Acta. 2004;1670:165–171. doi: 10.1016/j.bbagen.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 12.Nag S.A., Qin J.J., Wang W., Wang M.H., Wang H., Zhang R. Ginsenosides as anticancer agents: in vitro and in vivo activities, structure-activity relationships, and molecular mechanisms of action. Front Pharmacol. 2012;3:1–18. doi: 10.3389/fphar.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi L.W., Wang C.Z., Yuan C.S. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry. 2011;72:689–699. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djeridane A., Yousfi M., Nadjemi B., Boutassouna D., Stocker P., Vidal N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–660. [Google Scholar]
- 15.Kang K.S., Kim H.Y., Pyo J.S., Yokozawa T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol Pharm Bull. 2006;29:750–754. doi: 10.1248/bpb.29.750. [DOI] [PubMed] [Google Scholar]
- 16.Kang K.S., Kim H.Y., Yamabe N., Yokozawa T. Stereospecificity in hydroxyl radical scavenging activities of four ginsenosides produced by heat processing. Bioorg Med Chem Lett. 2006;16:5028–5031. doi: 10.1016/j.bmcl.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y.J., Kim H.Y., Kang K.S., Lee J.G., Yokozawa T., Park J.H. The chemical and hydroxyl radical scavenging activity changes of ginsenoside-Rb1 by heat processing. Bioorg Med Chem Lett. 2008;18:4515–4520. doi: 10.1016/j.bmcl.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 18.Cho W.C.S., Yip T.T., Chung W.S., Lee S.K.W., Leung A.W.N., Cheng C.H.K., Yue K.K.M. Altered expression of serum protein in ginsenoside Re-treated diabetic rats detected by SELDI-TOF MS. J Ethnopharmacol. 2006;108:272–279. doi: 10.1016/j.jep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Dhingra R., Kirshenbaum L.A. Succinate dehydrogenase/complex II activity obligatorily links mitochondrial reserve respiratory capacity to cell survival in cardiac myocytes. Cell Death Dis. 2015;6:1956. doi: 10.1038/cddis.2015.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholls D.G. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem Soc Trans. 2009;37:1385–1388. doi: 10.1042/BST0371385. [DOI] [PubMed] [Google Scholar]
- 21.Brookes P.S. Mitochondrial H+ leak and ROS generation: an odd couple. Free Radic Biol Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Dott W., Mistry P., Wright J., Cain K., Herbert K.E. Modulation of mitochondrial bioenergetics in a skeletal muscle cell line model of mitochondrial toxicity. Redox Biol. 2014;2:224–233. doi: 10.1016/j.redox.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu M., Neilson A., Swift A.L., Moran R., Tamagnine J., Parslow D., Armistead S., Lemire K., Orrell J., Teich J. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:125–136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 24.David A.F., Andy N., Craig B. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discovery Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Li H.X., Han S.Y., Ma X., Zhang K., Wang L., Ma Z.Z., Tu P.F. The saponin of red ginseng protects the cardiacmyocytes against ischemic injury in vitro and in vivo. Phytomedicine. 2012;19:477–483. doi: 10.1016/j.phymed.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Zhu K.Y., Fu Q., Xie H.Q., Xu S.L., Cheung A.W.H., Zheng K.Y.Z., Luk W.K.W., Choi R.C.Y., Lau D.T.W., Dong T.T.X. Quality assessment of a formulated Chinese herbal decoction, Kaixinsan, by using rapid resolution liquid chromatography coupled with mass spectrometry: a chemical evaluation of different historical formulae. J Sep Sci. 2010;33:3666–3674. doi: 10.1002/jssc.201000498. [DOI] [PubMed] [Google Scholar]
- 27.Truong J., Mailloux R.J., Chan H.M. Impact of methylmercury exposure on mitochondrial energetics in AC16 and H9C2 cardiomyocytes. Toxicol In Vitro. 2015;29:953–961. doi: 10.1016/j.tiv.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Ko K.M., Leon T.Y.Y., Mak D.H.F., Chiu P.Y., Du Y., Poon M.K.T. A characteristic pharmacological action of ‘Yang-invigorating’ Chinese tonifying herbs: enhancement of myocardial ATP-generation capacity. Phytomedicine. 2006;13:636–642. doi: 10.1016/j.phymed.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Papapetropoulos A.A. ginseng-derived oestrogen receptor β (ERβ) agonist, Rb1 ginsenoside, attenuates capillary morphogenesis. Br J Pharmacol. 2007;152:172–174. doi: 10.1038/sj.bjp.0707360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung L.W., Leung K.W., Wong C.K., Wong R.N., Wong A.S. Ginsenoside-Rg1 induces angiogenesis via non-genomic crosstalk of glucocorticoid receptor and fibroblast growth factor receptor-1. Cardiovasc Res. 2011;89:419–425. doi: 10.1093/cvr/cvq300. [DOI] [PubMed] [Google Scholar]
- 31.Zhu D., Wu L., Li C.R., Wang X.W., Ma Y.J., Zhong Z.Y., Zhao H.B., Cui J., Xun S.F., Huang X.L. Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J Cell Biochem. 2009;108:117–124. doi: 10.1002/jcb.22233. [DOI] [PubMed] [Google Scholar]
- 32.Clarke G., Ting K.N., Wiart C., Fry J. High correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all three assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants. 2013;2:1–10. doi: 10.3390/antiox2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aravinthan A., Kim J.H., Antonisamy P., Kang C.W., Choi J., Kim N.S., Kim J.H. Ginseng total saponin attenuates myocardial injury via anti-oxidative and anti-inflammatory properties. J Gins Res. 2015;39:206–212. doi: 10.1016/j.jgr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim T.H., Lee S.M. The effects of ginseng total saponin, panaxadiol and panaxatriol on ischemia/reperfusion injury in isolated rat heart. Food Chem Toxicol. 2010;48:1516–1520. doi: 10.1016/j.fct.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Kong H.L., Li Z.Q., Zhao Y.J., Zhao S.M., Zhu L., Li Tong, Fu Yao, Li H.J. Ginsenoside Rb1 protects cardiomyocytes against CoCl2 inhibiting mitochondria permeability transition pore opening. Acta Pharmacol Sinica. 2010;31:687–695. doi: 10.1038/aps.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Li X., Wang X.L., Lau W., Wang Y.J., Xing Y., Zhang X., Ma X.L., Gao F. Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury via Akt/GSK-3β signaling and inhibition of the mitochondria-dependent apoptotic pathway. Plos One. 2013;8:e70956. doi: 10.1371/journal.pone.0070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong G.T., Chen T.B., Ren X.C., Zhang Z.F., Huang W.X., Liu L., Pa Luo, Zhou H. Rg1 prevents myocardial hypoxia/reoxygenation injury by regulating mitochondrial dynamics imbalance via modulation of glutamate dehydrogenase and mitofusin 2. Mitochondrion. 2016;26:7–18. doi: 10.1016/j.mito.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Xie J.T., Shao Z.H., Vanden Hoek T.L.V., Chang W.T., Li J., Mehendale S., Wang C.Z., Hsu C.W., Becker L.B., Yin J.J. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol. 2006;532:201–207. doi: 10.1016/j.ejphar.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Luo J.Z.Q., Luo L.G. American Ginseng stimulates insulin production and prevents apoptosis through regulation of uncoupling protein-2 in cultured β cells. eCAM. 2006;3:365–372. doi: 10.1093/ecam/nel026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J.R., Zhou H., Yi X.Q., Jiang Z.H., Liu L. Total ginsenosides of Radix Ginseng modulates tricarboxylic acid cycle protein expression to enhance cardiac energy metabolism in ischemic rat heart tissues. Molecules. 2012;17:12746–12757. doi: 10.3390/molecules171112746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan W.H., Liu J.Z. Effects of Chinese herbal monomers on oxidative phosphorylation and membrane potential in cerebral mitochondria isolated from hypoxia-exposed rats in vitro. Neural Regen Res. 2012;7:2099–2106. doi: 10.3969/j.issn.1673-5374.2012.27.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y., Liang X.Y., Chen Y.Q., Zhao X.P. Screening SIRT1 activators from medicinal plants as bioactive compounds against oxidative damage in mitochondrial function. Oxid Med Cell Longev. 2016;2016:4206392. doi: 10.1155/2016/4206392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li A.E., Ito H., Rovira, Kim K.S., Takeda K., Yu Z.Y., Ferrans V.J., Finkel T. A role for reactive oxygen species in endothelial cell anoikis. Circ Res. 1999;85:304–310. doi: 10.1161/01.res.85.4.304. [DOI] [PubMed] [Google Scholar]
- 44.Mu Q.Q., Fang X., Li X.K., Zhao D.D., Mo F.F., Jiang G.J., Yu N., Zhang Y., Guo Y.B., Fu M. Ginsenoside Rb1 promotes browning through regulation of PPARγ in 3T3-L1 adipocytes. Biochem Bioph Res Co. 2015;466:530–535. doi: 10.1016/j.bbrc.2015.09.064. [DOI] [PubMed] [Google Scholar]
- 45.Lin S.H., Wu X.T., Tao L.C., Bei Y.H., Zhang H.F., Zhou Y.L., Shen S.T., Xiao J.J., Li X.L. The metabolic effects of traditional Chinese medication Qiliqiangxin on H9C2 cardiomyocytes. Cell Physiol Biochem. 2015;37:2246–2256. doi: 10.1159/000438580. [DOI] [PubMed] [Google Scholar]
- 46.Takanashi K., Dan K., Kanzaki S., Hasegawa H., Watanabe K., Ogawa K. Hochuekkito, a Japanese herbal medicine, restores metabolic homeostasis between mitochondrial and glycolytic pathways impaired by influenza A virus infection. Pharmacology. 2017;99:240–249. doi: 10.1159/000455918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.