Abstract
PURPOSE
The primary aim of this clinical trial was to prioritize bevacizumab or temsirolimus for additional investigation in rhabdomyosarcoma (RMS) when administered in combination with cytotoxic chemotherapy to patients with RMS in first relapse with unfavorable prognosis.
PATIENTS AND METHODS
Patients were randomly assigned to receive bevacizumab on day 1 or temsirolimus on days 1, 8, and 15 of each 21-day treatment cycle, together with vinorelbine on days 1 and 8, and cyclophosphamide on day 1 for a maximum of 12 cycles. Local tumor control with surgery and/or radiation therapy was permitted after 6 weeks of treatment. The primary end point was event-free survival (EFS). Radiographic response was assessed at 6 weeks. The study had a phase II selection that was design to detect a 15% difference between the two regimens (α = .2; 1-β = 0.8; two sided test).
RESULTS
Eighty-seven of 100 planned patients were enrolled when the trial was closed after the second interim analysis after 46 events occurred in 68 patients with sufficient follow-up. The O’Brien Fleming boundary at this analysis corresponded to a two-sided P value of .058 with an observed two-sided P value of .003 favoring temsirolimus. The 6-month EFS for the bevacizumab arm was 54.6% (95% CI, 39.8% to 69.3%) and 69.1% (95% CI, 55.1% to 83%) for the temsirolimus arm. Objective response rates were 28% (95% CI, 13.7% to 41.3%) and 47% (95% CI, 31.5% to 63.2%) for the bevacizumab and temsirolimus arms, respectively (P = .12) and, 28% of patients on bevacizumab and 11% on temsirolimus had progressive disease at 6 weeks.
CONCLUSION
Patients who received temsirolimus had a superior EFS compared with bevacizumab. Temsirolimus has been selected for additional investigation in newly diagnosed patients with intermediate-risk RMS.
INTRODUCTION
Rhabdomyosarcoma (RMS) is the most common soft-tissue sarcoma observed in the first two decades of life and rarely occurs beyond the third decade.1 Standard treatment includes chemotherapy, surgery, and/or radiation therapy and results in approximately two thirds of patients being cured.2,3 Patients with distant metastases at initial presentation have a less favorable prognosis,4,5 whereas those with relapsed disease have a poor prognosis, particularly those with unfavorable features.6-8 Unfavorable prognostic features identified at the time of first relapse include patients with a history of distant metastases at initial presentation, alveolar histology, primary tumor greater than 5 cm, lymph node metastases, and those treated with radiation therapy and/or at least three chemotherapy agents, including cyclophosphamide or ifosfamide. The Children’s Oncology Group (COG) Soft Tissue Sarcoma (STS) committee conducted the only previous prospective clinical trial for first relapse of RMS, but this effort with risk-adapted multiagent therapy failed to improve survival compared with historic results in patients with a history of unfavorable prognostic features as defined.7,9 To identify agents to prioritize for future front-line phase III studies and to improve outcomes for patients with recurrent RMS, the COG STS committee adopted a strategy to test molecularly targeted agents with a preclinical rationale in combination with cytotoxic chemotherapy in this population of patients with first relapse RMS with unfavorable features.
Bevacizumab and temsirolimus were the two molecularly targeted agents that were selected for study in combination with cytotoxic chemotherapy. Bevacizumab is a humanized monoclonal neutralizing antibody that binds all five isoforms of vascular endothelial growth factor (VEGF) and has an antiangiogenic effect.10 Inhibition of angiogenesis reduces tumor growth in many ex vivo models of adult and pediatric malignancies, including RMS.11-16 Bevacizumab has been approved for use as a single agent or in combination with chemotherapy for several malignancies in adults, including colorectal cancer, nonsquamous non–small-cell lung cancer, glioblastoma, renal-cell carcinoma, cervical cancer, and epithelial ovarian cancer.17-22 Several pediatric clinical trials with single-agent bevacizumab or bevacizumab in combination with chemotherapy have also been completed.23-27 Major toxicities of bevacizumab include GI perforation and non-GI fistula formation, delayed wound healing, arterial thromboembolic events, hypertension, reversible posterior leukoencephalopathy syndrome, proteinuria, and infusion reactions.28,29 Temsirolimus is a soluble ester of rapamycin, a natural product that has antifungal, immunosuppressive, and anticancer activity.30 Temsirolimus forms a complex with FK506 binding protein and prevents the activation of mammalian target of rapamycin. Its anticancer activity has been demonstrated in a variety of preclinical models.31-33 Increased mammalian target of rapamycin pathway activation has been reported in childhood RMS and is associated with decreased survival.34,35 In addition, temsirolimus has also been shown to inhibit growth in RMS xenografts, in part, through inhibition of angiogenesis.36-38 Furthermore, the Pediatric Preclinical Testing Program (PPTP) reported activity of rapamycin, both alone and in combination with vincristine or cyclophosphamide in RMS xenografts.39,40 Temsirolimus as a single agent is approved for the treatment of advanced renal-cell carcinoma and has also been studied in combination with chemotherapy in several malignancies.41-46 The main adverse effects of temsirolimus include hypersensitivity, hyperglycemia, hyperlipidemia, mucositis, anemia, thrombocytopenia, infections, interstitial pneumonitis, abnormal wound healing, and renal failure.47 A pediatric phase I clinical trial of single-agent temsirolimus did not identify a maximum tolerated dose.48
Vinorelbine and cyclophosphamide were selected for the cytotoxic chemotherapy backbone. Vinorelbine is active in patients with relapsed RMS as a single agent.49,50 Cyclophosphamide is among the most active chemotherapy agents in RMS and has been administered intravenously at various doses in the primary treatment of patients and at the time of relapse.9,51-53 The combination of vinorelbine and oral cyclophosphamide is active in patients with relapsed RMS54 and has also been investigated in the treatment of metastatic RMS.55 Preclinical data from the PPTP demonstrated synergy of rapamycin with parenterally administered vincristine and intermittent bolus infusion of cyclophosphamide.39 Vinorelbine has not been previously combined with intravenous cyclophosphamide for the treatment of RMS; however, vinorelbine has been safely combined with ifosfamide in children.56 Intravenous vincristine and cyclophosphamide are used in the standard treatment of most patients with newly diagnosed RMS; therefore, if a targeted agent was selected for additional study in treatment-naïve patients, the combination of an intravenous vinca alkaloid and oxazophorine was desirable. These data formed the basis for the design of this clinical trial (ARST0921).
The primary aims of ARST0921 were to determine the feasibility of administering bevacizumab or temsirolimus in combination with intravenous vinorelbine and cyclophosphamide chemotherapy in first relapse of RMS, and to estimate the event-free survival (EFS) of patients treated with bevacizumab compared with those treated with temsirolimus.
PATIENTS AND METHODS
Patient Eligibility
Patients who were eligible for ARST0921 included those with biopsy-proven RMS—embryonal, alveolar, or not otherwise specified—at first relapse or disease progression and younger than age 30 years at the time of enrollment with an Eastern Cooperative Oncology Group performance status of 0, 1 or 2 and a life expectancy of at least 8 weeks. Patients with primary refractory disease, defined as first progression after at least one cycle of cyclophosphamide or ifosfamide-containing chemotherapy without a prior response to chemotherapy were also eligible. Other protocol-specific patient eligibility criteria are included in the Data Supplement. Written informed consent was required from all participants and/or their parents/legal guardians after all institutional, US Food and Drug Administration, and National Cancer Institute (NCI) requirements for human studies were met.
Clinical Trial Design
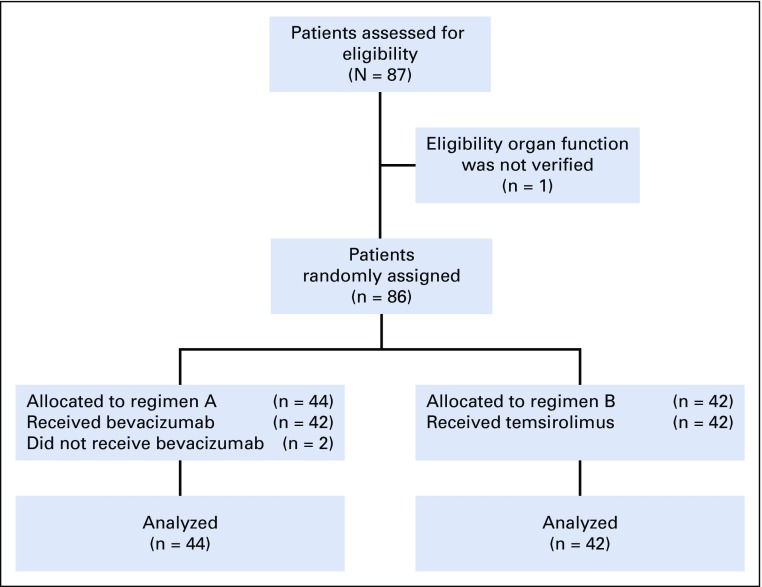
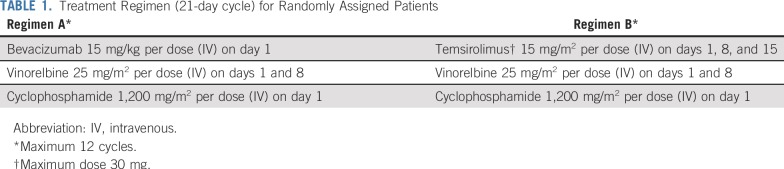
The overall experimental design for ARST0921 is shown in Figure 1. Patients were randomly assigned to either Regimen A (bevacizumab) or Regimen B (temsirolimus). The schedule and route of administration of drugs is depicted in Table 1. Treatment was administered in 21-day cycles and a maximum of 12 cycles was allowed in the absence of disease progression. Myeloid growth factor was administered starting on day 9 of each cycle. Surgery and radiation were not allowed during the first 6 weeks of treatment and patients were taken off protocol therapy if they underwent surgery or received radiation therapy in the first 6 weeks of treatment. Patients with measurable disease as defined by NCI Response Evaluation Criteria in Solid Tumors (RECIST) were evaluable for response.58 Disease response was assessed after 6 weeks of treatment at the end of cycle 2 and subsequently after cycles 4, 6, 9, and 12. Patients who achieved complete response (CR), partial response (PR), or stable disease could continue with the assigned treatment until disease progression or completion of 12 cycles of treatment. Bevacizumab was withheld 4 weeks before and 4 weeks after a surgical procedure. Temsirolimus was not withheld before surgery and was resumed at the same time as chemotherapy after surgery. Both bevacizumab and temsirolimus were withheld during radiation therapy. Patients were taken off protocol therapy if bevacizumab or temsirolimus was interrupted for more than 9 weeks.
FIG 1.
CONSORT diagram for ARST0921.
TABLE 1.
Treatment Regimen (21-day cycle) for Randomly Assigned Patients
Statistical Analysis
The current study was designed as a randomized phase II selection trial59 to determine whether bevacizumab or temsirolimus should be chosen for additional evaluation in RMS. This analysis describes the toxicities and compares EFS, overall survival (OS), and response rate of patients who were treated with bevacizumab and temsirolimus. Anticipating a median EFS of 20% at 2 years, a sample size of 100 patients with 79 expected failures was required to detect a difference in the relative risk of failure between the two treatment groups of 0.62:1.00, corresponding to EFSs of 50% and 65% at 6 months (α = .2; 1-β = 0.8; two sided log-rank test). EFS was defined as the time from study enrollment to disease progression, disease recurrence, second malignant neoplasm, or death from any cause, whichever occurred first. OS was defined as the time from study enrollment to death from any cause. EFS and OS were censored at the patient’s last contact date. EFS and OS were estimated using the Kaplan-Meier method60 and curves were compared using the log-rank test. CIs for EFS and OS were estimated using the Peto-Peto method.61 Interim monitoring of EFS was performed using an alpha-spending approach with an O’Brien-Fleming boundary with monitoring beginning after 30% of the expected events with additional analysis performed at approximately 50% and 75% of the expected events. As histology has been shown to be prognostic in first relapse RMS6 and not stratified for at the time of random assignment, a Cox proportional hazards regression model was used to calculate the hazard ratio for treatment failure after adjusting for histology. The assumption of proportional hazards was checked graphically by plotting log[-log(EFS)] by log(time).
The feasibility of administering bevacizumab or temsirolimus in combination with vinorelbine and cyclophosphamide was assessed in the first 10 randomly assigned patients in each arm during the first two cycles of therapy. Toxicities were reported using NCI Common Toxicity Criteria, version 4. Adverse events of interest that defined dose-limiting toxicities (DLTs) in both treatment arms are detailed in the Data Supplement. A DLT rate of 40% or greater during the first two cycles in the first 10 randomly assigned patients in each arm was considered unacceptable and would require a protocol amendment to reduce the dose of bevacizumab or temsirolimus. A 25% increase in the toxicity rate over baseline expected 50% grade 3 or greater nonhematologic toxicities and toxic deaths in any group would also be sufficient to suspend the study and require a protocol amendment to reduce the dose of the investigational agents.
RESULTS
Patient Population
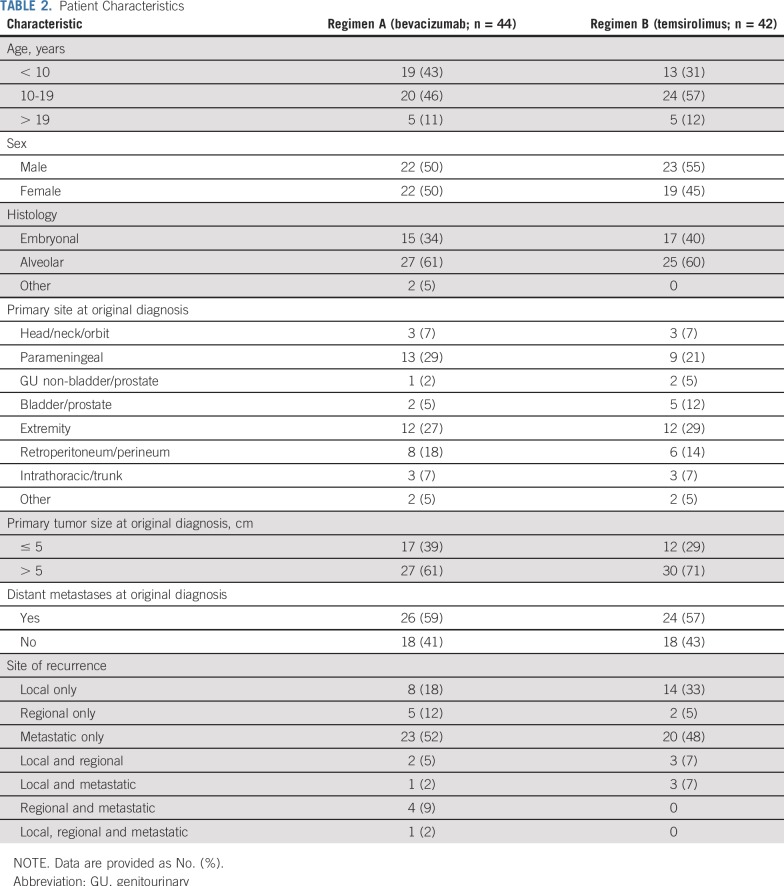
ARST0921 enrolled 87 randomly assigned patients between October 2010 and July 2013, when the trial was closed to accrual on the recommendation of the Data Safety Monitoring Committee, which was based on the second scheduled interim analysis. Patient follow-up is current through September 30, 2017, for this report. One patient who was randomly assigned to the temsirolimus arm was ineligible as a result of not meeting an inclusion criterion for organ function requirements. Two patients who were randomly assigned to the bevacizumab arm did not receive study treatment because of a rapid decline in performance status after enrollment and before initiating chemotherapy. Clinical characteristics of eligible patients are shown in Table 2. Patient populations were balanced in the two arms, including such factors as tumor histology, disease extent at original diagnosis, and pattern of recurrence.
TABLE 2.
Patient Characteristics
Survival Outcomes
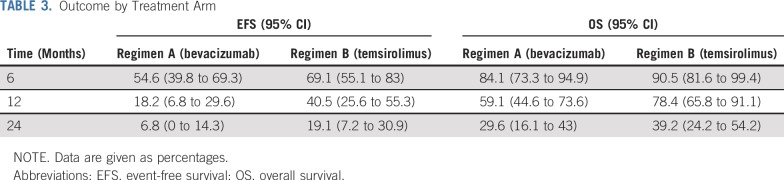
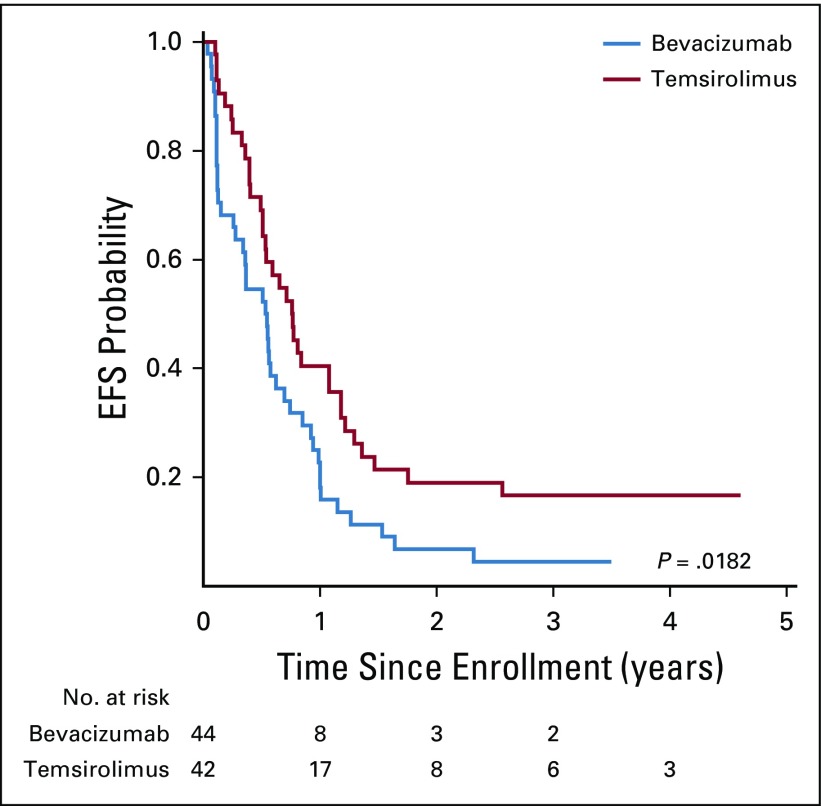
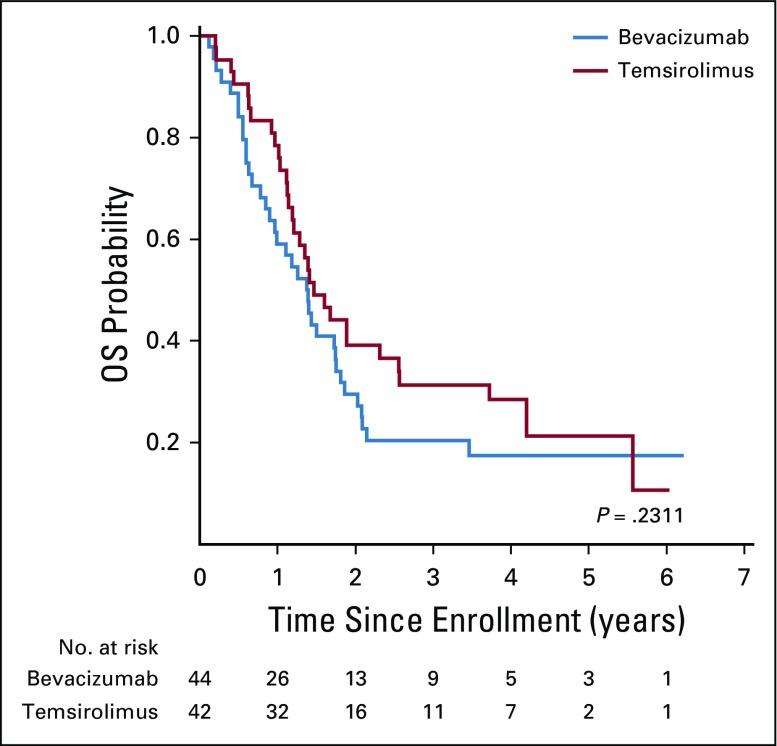
At the second interim analysis, 46 events had occurred—58% of expected—in 68 patients with sufficient follow-up. The O’Brien-Fleming boundary at this analysis corresponded to an adjusted two-sided P value of .058 with an observed two-sided P value of .003 favoring the EFS of the temsirolimus arm. The 6-month EFS in the bevacizumab arm was 50% (95% CI, 32% to 66%) and 65% (95% CI, 44% to 79%) in the temsirolimus arm. There were no deaths as a first event in either regimen. Two patients developed second malignant neoplasm—both acute myelogenous leukemia/treatment-related myelodysplastic syndrome—one each in the bevacizumab and temsirolimus arms. The 6-, 12-, and 24-month EFS and OS are shown in Table 3. After a median follow up of 4 years for surviving patients, those who were treated with vinorelbine, cyclophosphamide, and temsirolimus had significantly better EFS compared with patients who were treated in the bevacizumab arm (P = .018, two sided; Fig 2). There was no difference in OS between the two regimens (P = .23; Fig 3). The assumption of proportional hazards was upheld and the estimated hazard ratio for treatment failure (bevacizumab:temsirolimus) was 1.71 (95% CI, 1.08 to 2.69; P = .02).
TABLE 3.
Outcome by Treatment Arm
FIG 2.
Event-free survival (EFS) by treatment regimen.
FIG 3.
Overall survival (OS) by treatment regimen.
Tumor Response
Seventy-eight of 86 eligible patients were evaluable for response after 6 weeks of treatment in the bevacizumab arm (n = 40) and temsirolimus arm (n = 38). There were four CRs and seven PRs in the bevacizumab arm versus five CRs and 13 PRs in the temsirolimus arm. There was no significant difference between the objective response rate (CR + PR) in the bevacizumab arm (28%; 95% CI, 13.7% to 41.3%) compared with the temsirolimus arm (47%; 95% CI, 31.5% to 63.2%; P = .12). Eleven patients (28%) in the bevacizumab arm and four patients (11%) in the temsirolimus arms experienced progressive disease after 6 weeks of therapy.
Toxicity
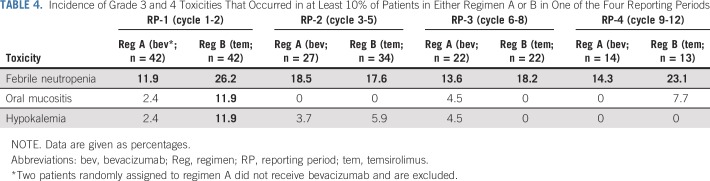
There were no toxic deaths and no unexpected toxicities. Patients who were treated with temsirolimus had a higher incidence of grade 3 and 4 toxicities compared with those who received bevacizumab; however, the difference was not statistically significant (data not shown). There were three DLTs in the bevacizumab arm, including one grade 3 hypertension, one grade 3 bleeding, and one grade 3 oral mucositis. There were eight DLTs in the temsirolimus arm, including four grade 3 oral mucositis, two grade 3 hypertriglyceridemia, one grade 3 pneumonitis, and one grade 3 elevation of ALT that did not resolve to less than grade 1 in 14 days. Table 4 displays the grade three or greater toxicities that occurred in at least 10% of patients in either the bevacizumab arm or temsirolimus arm in each of the four reporting periods, together with the corresponding incidence in both treatment arms. Febrile neutropenia was the most common toxicity reported. Oral mucositis and hypokalemia were observed almost exclusively in the temsirolimus arm in the first two cycles of treatment. In the bevacizumab arm, bleeding or thrombotic events, hypertension, cardiac toxicity, fistula or leak (GI or other organ), proteinuria, intra-abdominal infection/abscess, wound complications, reversible posterior encephalopathy, microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura were toxicities of particular interest. Of these, the highest incidence of grade 3 or greater nonhematologic toxicities noted across any reporting period were hypertension (2.4%), bleeding (4.5%), and wound infection (4.5%). Targeted toxicities in the temsirolimus arm were infusion reactions, hyperglycemia, hypertriglyceridemia, hypercholesterolemia, mucositis, cardiac toxicity, pneumonitis, liver enzyme elevation, wound complications, and GI perforation, fistula or obstruction. Of these, the highest incidence of grade 3 or greater nonhematologic toxicities noted across any reporting period were hypertriglyceridemia (9.5%), mucositis (11.9%), pneumonitis (2.4%), and liver enzyme elevation (4.8%). In addition, one patient in the temsirolimus arm suffered acute kidney injury that was attributed to temsirolimus.
TABLE 4.
Incidence of Grade 3 and 4 Toxicities That Occurred in at Least 10% of Patients in Either Regimen A or B in One of the Four Reporting Periods
DISCUSSION
This randomized clinical trial is only the second reported in patients with first relapse RMS. The first trial (ARST0121), also conducted by COG, demonstrated a median EFS of 50% at 6 months in first relapsed patients with unfavorable prognostic features as defined who were treated with multiagent cytotoxic chemotherapy.7,9 ARST0921 is also the first randomized clinical trial in patients with RMS to incorporate molecularly targeted therapy, together with cytotoxic chemotherapy, with the goal to prioritize a molecularly targeted agent to test in newly diagnosed patients with RMS. To that end, ARST0921 succeeded in selecting temsirolimus as a biologically active agent in RMS worthy of additional investigation.
Combination bevacizumab and chemotherapy has shown activity in some pediatric tumors, including low-grade gliomas and Wilms tumor.26,62 However, addition of bevacizumab to cytotoxic chemotherapy resulted in a 6-month EFS of 54.6% in patients with first relapse RMS with unfavorable features and was similar to the median EFS in ARST0121, which used only multiagent chemotherapy.9 Furthermore, bevacizumab has also been tested in children and adolescents with newly diagnosed metastatic soft-tissue sarcoma, where it was administered together with cytotoxic chemotherapy and did not affect survival.55 These data suggest that bevacizumab did not add to or detract from the efficacy of the vinorelbine–cyclophosphamide chemotherapy back bone. Similarly, bevacizumab did not improve outcomes when combined with chemotherapy in patients with high-grade gliomas, osteosarcoma, and neuroblastoma.63-65 These data led to questions about the role of VEGF-targeted agents in contributing to a therapeutic pathway to cure childhood cancer66; however, more recently, oral small-molecule tyrosine kinase inhibitors that target VEGF have demonstrated activity in soft-tissue sarcomas other than RMS and in osteosarcoma.67-70 Of interest, temsirolimus as a single agent had limited clinical activity in phase I and II single-agent trials, including only a 6% objective response rate in patients with recurrent RMS.48,71 Strong preclinical data generated by PPTP in RMS xenografts that supported the combination of rapamycin with chemotherapy39,40 was the main reason that led us to investigate temsirolimus in combination with vinorelbine and cyclophosphamide. This highlights the importance of considering combination trials that include molecularly targeted agents when there is a relevant biologic rationale and strong preclinical data, despite a low level of single-agent activity with the agent in early-phase clinical trials.
Therapy in ARST0921 was tolerated reasonably well. None of the toxicities noted secondary to bevacizumab or temsirolimus was unexpected. The most common serious adverse event was febrile neutropenia, observed in approximately 20% of patients. This compares favorably with the 50% nonhematologic serious adverse event rate noted in ARST0121.7 Furthermore, all therapy in ARST0921 could be administered in an ambulatory setting. Considering the similar median EFS and more favorable toxicity profile compared with ARST0121, which included therapy with vincristine/irinotecan (weeks 1 to 6, 20 to 25, and 47 to 52), interval compression with vincristine/doxorubicin/cyclophosphamide alternating with etoposide/ifosfamide (weeks 7 to 19 and 26 to 34), and vincristine/dactinomycin/cyclophosphamide (weeks 38 to 46), vinorelbine, cyclophosphamide, and temsirolimus is a reasonable alternative treatment for patients with unfavorable features at the time of first relapse RMS. In addition, this well-tolerated chemotherapy backbone of vinorelbine and cyclophosphamide can be used to investigate other promising new agents in combination; however, as vinorelbine and intravenous cyclophosphamide were not studied independently of bevacizumab or temsirolimus in this trial, these data cannot necessarily be extrapolated to the combination of vinorelbine and oral cyclophosphamide that has been more extensively studied by the European Pediatric Soft tissue sarcoma Study Group.
Local control of disease sites at the time of relapse and time to relapse after initial diagnosis of nonmetastatic RMS are prognostic factors for relapsed RMS.8,72 ARST0921 enrolled patients at first relapse with a history of both nonmetastatic disease or metastatic disease. As ARST0921 was designed solely to select a molecularly targeted agent for additional investigation, local treatment and time to relapse data were not collected in ARST0921 and their impact on outcome could not be analyzed.
The only other published positive randomized clinical trial in RMS dates backs to 197473 and confirms the benefit of adjuvant vincristine and dactinomycin chemotherapy in children with completely resected RMS. Subsequently, multiple randomized clinical trials conducted in RMS have failed to improve outcomes compared with the control arm.51-53,55,74-77 On the basis of the results of ARST0921, the COG STS committee is now conducting a randomized phase III clinical trial (ARST1431; ClinicalTrials.gov identifier: NCT02567435) investigating the addition of temsirolimus to vincristine, dactinomycin, and cyclophosphamide, alternating with the vincristine and irinotecan chemotherapy backbone in newly diagnosed patients with intermediate-risk RMS.
Footnotes
Presented in part at the 2014 American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 30-June 3, 2014.
Supported by National Cancer Institute, Children’s Oncology Group Grants No. U10-CA180886, U10-CA180899, U10-CA098543, and U10-CA098413 and St Baldrick’s Foundation.
Clinical trial information: NCT01222715.
AUTHOR CONTRIBUTIONS
Conception and design: Leo Mascarenhas, James R. Anderson, David A. Rodeberg, Daniel J. Indelicato, Simon C. Kao, Sheri L. Spunt, William H. Meyer, Douglas S. Hawkins
Collection and assembly of data: Leo Mascarenhas. James R. Anderson, David A. Rodeberg, Simon C. Kao, Roshni Dasgupta
Data analysis and interpretation: Leo Mascarenhas, Yueh-Yun Chi, Pooja Hingorani, James R. Anderson, Elizabeth R. Lyden, David A. Rodeberg, Daniel J. Indelicato, Roshni Dasgupta, William H. Meyer, Douglas S. Hawkins
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase II Trial of Bevacizumab or Temsirolimus in Combination With Chemotherapy for First Relapse Rhabdomyosarcoma: A Report From the Children’s Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Leo Mascarenhas
Honoraria: Bayer
Consulting or Advisory Role: Bayer, Eli Lilly (Inst)
Speakers' Bureau: Bayer
Research Funding: AstraZeneca (Inst), MedImmune (Inst)
Travel, Accommodations, Expenses: Bayer, AstraZeneca, MedImmune, Eli Lilly
James R. Anderson
Employment: Merck
Daniel J. Indelicato
Travel, Accommodations, Expenses: IBA
Sheri L. Spunt
Research Funding: Loxo (Inst)
William H. Meyer
Consulting or Advisory Role: Foundation Medicine
Travel, Accommodations, Expenses: Foundation Medicine
Douglas S. Hawkins
Research Funding: Loxo (Inst), Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Bayer (Inst), Eli Lilly (Inst), Eisai (Inst)
Travel, Accommodations, Expenses: Loxo, Bayer, Bristol-Myers Squibb, Celgene
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sultan I, Qaddoumi I, Yaser S, et al. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: An analysis of 2,600 patients. J Clin Oncol. 2009;27:3391–3397. doi: 10.1200/JCO.2008.19.7483. [DOI] [PubMed] [Google Scholar]
- 2.Malempati S, Hawkins DS. Rhabdomyosarcoma: Review of the Children’s Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer. 2012;59:5–10. doi: 10.1002/pbc.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawkins DS, Spunt SL, Skapek SX. Children’s Oncology Group’s 2013 blueprint for research: Soft tissue sarcomas. Pediatr Blood Cancer. 2013;60:1001–1008. doi: 10.1002/pbc.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carli M, Colombatti R, Oberlin O, et al. European intergroup studies (MMT4-89 and MMT4-91) on childhood metastatic rhabdomyosarcoma: Final results and analysis of prognostic factors. J Clin Oncol. 2004;22:4787–4794. doi: 10.1200/JCO.2004.04.083. [DOI] [PubMed] [Google Scholar]
- 5.Weigel BJ, Lyden E, Anderson JR, et al. Intensive multiagent therapy, including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation, in patients with high-risk rhabdomyosarcoma: A report from the Children’s Oncology Group. J Clin Oncol. 2016;34:117–122. doi: 10.1200/JCO.2015.63.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pappo AS, Anderson JR, Crist WM, et al. Survival after relapse in children and adolescents with rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. J Clin Oncol. 1999;17:3487–3493. doi: 10.1200/JCO.1999.17.11.3487. [DOI] [PubMed] [Google Scholar]
- 7.Mascarenhas L, Lyden ER, Breitfeld PP, et al. Randomized phase II window trial of two schedules of irinotecan with vincristine in patients with first relapse or progression of rhabdomyosarcoma: A report from the Children’s Oncology Group. J Clin Oncol. 2010;28:4658–4663. doi: 10.1200/JCO.2010.29.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chisholm JC, Marandet J, Rey A, et al. Prognostic factors after relapse in nonmetastatic rhabdomyosarcoma: A nomogram to better define patients who can be salvaged with further therapy. J Clin Oncol. 2011;29:1319–1325. doi: 10.1200/JCO.2010.32.1984. [DOI] [PubMed] [Google Scholar]
- 9.Mascarenhas L, Lyden ER, Breitfeld PP, et al. Risk-based treatment for patients with first relapse or progression of rhabdomyosarcoma: A report from the Children’s Oncology Group. Cancer. 2019;125:2602–2609. doi: 10.1002/cncr.32122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 11.Gerber HP, Kowalski J, Sherman D, et al. Complete inhibition of rhabdomyosarcoma xenograft growth and neovascularization requires blockade of both tumor and host vascular endothelial growth factor. Cancer Res. 2000;60:6253–6258. [PubMed] [Google Scholar]
- 12.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 13.McCrudden KW, Hopkins B, Frischer J, et al. Anti-VEGF antibody in experimental hepatoblastoma: Suppression of tumor growth and altered angiogenesis. J Pediatr Surg. 2003;38:308–314, discussion 308-314. doi: 10.1053/jpsu.2003.50099. [DOI] [PubMed] [Google Scholar]
- 14.Rowe DH, Huang J, Kayton ML, et al. Anti-VEGF antibody suppresses primary tumor growth and metastasis in an experimental model of Wilms’ tumor. J Pediatr Surg. 2000;35:30–32, discussion 32-33. doi: 10.1016/s0022-3468(00)80008-1. [DOI] [PubMed] [Google Scholar]
- 15.Rowe DH, Huang J, Li J, et al. Suppression of primary tumor growth in a mouse model of human neuroblastoma. J Pediatr Surg. 2000;35:977–981. doi: 10.1053/jpsu.2000.6946. [DOI] [PubMed] [Google Scholar]
- 16.Myers AL, Williams RF, Ng CY, et al. Bevacizumab-induced tumor vessel remodeling in rhabdomyosarcoma xenografts increases the effectiveness of adjuvant ionizing radiation. J Pediatr Surg. 2010;45:1080–1085. doi: 10.1016/j.jpedsurg.2010.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 18.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 19.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 20.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 21.Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) Lancet. 2017;390:1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 23.Glade Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: A Children’s Oncology Group Study. J Clin Oncol. 2008;26:399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 24.Venkatramani R, Malogolowkin M, Davidson TB, et al. A phase I study of vincristine, irinotecan, temozolomide and bevacizumab (vitb) in pediatric patients with relapsed solid tumors. PLoS One. 2013;8:e68416. doi: 10.1371/journal.pone.0068416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navid F, Baker SD, McCarville MB, et al. Phase I and clinical pharmacology study of bevacizumab, sorafenib, and low-dose cyclophosphamide in children and young adults with refractory/recurrent solid tumors. Clin Cancer Res. 2013;19:236–246. doi: 10.1158/1078-0432.CCR-12-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gururangan S, Fangusaro J, Poussaint TY, et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas: A Pediatric Brain Tumor Consortium study. Neuro-oncol. 2014;16:310–317. doi: 10.1093/neuonc/not154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner L, Turpin B, Nagarajan R, et al. Pilot study of vincristine, oral irinotecan, and temozolomide (VOIT regimen) combined with bevacizumab in pediatric patients with recurrent solid tumors or brain tumors. Pediatr Blood Cancer. 2013;60:1447–1451. doi: 10.1002/pbc.24547. [DOI] [PubMed] [Google Scholar]
- 28.Sanborn RE, Sandler AB. The safety of bevacizumab. Expert Opin Drug Saf. 2006;5:289–301. doi: 10.1517/14740338.5.2.289. [DOI] [PubMed] [Google Scholar]
- 29.Fangusaro J, Gururangan S, Poussaint TY, et al. Bevacizumab (BVZ)-associated toxicities in children with recurrent central nervous system tumors treated with BVZ and irinotecan (CPT-11): A Pediatric Brain Tumor Consortium Study (PBTC-022) Cancer. 2013;119:4180–4187. doi: 10.1002/cncr.28343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rini BI. Temsirolimus, an inhibitor of mammalian target of rapamycin. Clin Cancer Res. 2008;14:1286–1290. doi: 10.1158/1078-0432.CCR-07-4719. [DOI] [PubMed] [Google Scholar]
- 31.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Podsypanina K, Lee RT, Politis C, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/- mice. Proc Natl Acad Sci USA. 2001;98:10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu K, Toral-Barza L, Discafani C, et al. mTOR, a novel target in breast cancer: The effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer. 2001;8:249–258. doi: 10.1677/erc.0.0080249. [DOI] [PubMed] [Google Scholar]
- 34.Cen L, Arnoczky KJ, Hsieh FC, et al. Phosphorylation profiles of protein kinases in alveolar and embryonal rhabdomyosarcoma. Mod Pathol. 2007;20:936–946. doi: 10.1038/modpathol.3800834. [DOI] [PubMed] [Google Scholar]
- 35.Petricoin EF, III, Espina V, Araujo RP, et al. Phosphoprotein pathway mapping: Akt/mammalian target of rapamycin activation is negatively associated with childhood rhabdomyosarcoma survival. Cancer Res. 2007;67:3431–3440. doi: 10.1158/0008-5472.CAN-06-1344. [DOI] [PubMed] [Google Scholar]
- 36.Dilling MB, Dias P, Shapiro DN, et al. Rapamycin selectively inhibits the growth of childhood rhabdomyosarcoma cells through inhibition of signaling via the type I insulin-like growth factor receptor. Cancer Res. 1994;54:903–907. [PubMed] [Google Scholar]
- 37.Wan X, Shen N, Mendoza A, et al. CCI-779 inhibits rhabdomyosarcoma xenograft growth by an antiangiogenic mechanism linked to the targeting of mTOR/Hif-1alpha/VEGF signaling. Neoplasia. 2006;8:394–401. doi: 10.1593/neo.05820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallicchio MA, van Sinderen M, Bach LA. Insulin-like growth factor binding protein-6 and CCI-779, an ester analogue of rapamycin, additively inhibit rhabdomyosarcoma growth. Horm Metab Res. 2003;35:822–827. doi: 10.1055/s-2004-814153. [DOI] [PubMed] [Google Scholar]
- 39.Houghton PJ, Morton CL, Gorlick R, et al. Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program. Mol Cancer Ther. 2010;9:101–112. doi: 10.1158/1535-7163.MCT-09-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houghton PJ, Morton CL, Kolb EA, et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:799–805. doi: 10.1002/pbc.21296. [DOI] [PubMed] [Google Scholar]
- 41.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 42.Sarkaria JN, Galanis E, Wu W, et al. Combination of temsirolimus (CCI-779) with chemoradiation in newly diagnosed glioblastoma multiforme (GBM) (NCCTG trial N027D) is associated with increased infectious risks. Clin Cancer Res. 2010;16:5573–5580. doi: 10.1158/1078-0432.CCR-10-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang-Gillam A, Thakkar N, Lockhart AC, et al. A phase I study of pegylated liposomal doxorubicin and temsirolimus in patients with refractory solid malignancies. Cancer Chemother Pharmacol. 2014;74:419–426. doi: 10.1007/s00280-014-2493-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boers-Sonderen MJ, de Geus-Oei LF, Desar IM, et al. Temsirolimus and pegylated liposomal doxorubicin (PLD) combination therapy in breast, endometrial, and ovarian cancer: Phase Ib results and prediction of clinical outcome with FDG-PET/CT. Target Oncol. 2014;9:339–347. doi: 10.1007/s11523-014-0309-x. [DOI] [PubMed] [Google Scholar]
- 45.Fury MG, Sherman E, Ho A, et al. A phase I study of temsirolimus plus carboplatin plus paclitaxel for patients with recurrent or metastatic (R/M) head and neck squamous cell cancer (HNSCC) Cancer Chemother Pharmacol. 2012;70:121–128. doi: 10.1007/s00280-012-1894-y. [DOI] [PubMed] [Google Scholar]
- 46.Kollmannsberger C, Hirte H, Siu LL, et al. Temsirolimus in combination with carboplatin and paclitaxel in patients with advanced solid tumors: A NCIC-CTG, phase I, open-label dose-escalation study (IND 179) Ann Oncol. 2012;23:238–244. doi: 10.1093/annonc/mdr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bukowski RM. Temsirolimus: A safety and efficacy review. Expert Opin Drug Saf. 2012;11:861–879. doi: 10.1517/14740338.2012.713344. [DOI] [PubMed] [Google Scholar]
- 48.Spunt SL, Grupp SA, Vik TA, et al. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J Clin Oncol. 2011;29:2933–2940. doi: 10.1200/JCO.2010.33.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuttesch JF, Jr, Krailo MD, Madden T, et al. Phase II evaluation of intravenous vinorelbine (Navelbine) in recurrent or refractory pediatric malignancies: A Children’s Oncology Group study. Pediatr Blood Cancer. 2009;53:590–593. doi: 10.1002/pbc.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casanova M, Ferrari A, Spreafico F, et al. Vinorelbine in previously treated advanced childhood sarcomas: Evidence of activity in rhabdomyosarcoma. Cancer. 2002;94:3263–3268. doi: 10.1002/cncr.10600. [DOI] [PubMed] [Google Scholar]
- 51.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children’s Oncology Group study D9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crist W, Gehan EA, Ragab AH, et al. The third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 53.Hawkins DS, Chi YY, Anderson JR, et al. Addition of vincristine and irinotecan to vincristine, dactinomycin, and cyclophosphamide does not improve outcome for intermediate-risk rhabdomyosarcoma: A report from the Children’s Oncology Group. J Clin Oncol. 2018;36:2770–2777. doi: 10.1200/JCO.2018.77.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casanova M, Ferrari A, Bisogno G, et al. Vinorelbine and low-dose cyclophosphamide in the treatment of pediatric sarcomas: Pilot study for the upcoming European Rhabdomyosarcoma Protocol. Cancer. 2004;101:1664–1671. doi: 10.1002/cncr.20544. [DOI] [PubMed] [Google Scholar]
- 55.Chisholm JC, Merks JHM, Casanova M, et al. Open-label, multicentre, randomised, phase II study of the EpSSG and the ITCC evaluating the addition of bevacizumab to chemotherapy in childhood and adolescent patients with metastatic soft tissue sarcoma (the BERNIE study) Eur J Cancer. 2017;83:177–184. doi: 10.1016/j.ejca.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 56.Trippett TM, Schwartz CL, Guillerman RP, et al. Ifosfamide and vinorelbine is an effective reinduction regimen in children with refractory/relapsed Hodgkin lymphoma, AHOD00P1: A Children’s Oncology Group report. Pediatr Blood Cancer. 2015;62:60–64. doi: 10.1002/pbc.25205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. [Google Scholar]
- 58.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 59.Rubinstein LV, Korn EL, Freidlin B, et al. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23:7199–7206. doi: 10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
- 60.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 61.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc [Ser A] 1972;135:185–198. [Google Scholar]
- 62.Venkatramani R, Malogolowkin MH, Mascarenhas L. Treatment of multiply relapsed wilms tumor with vincristine, irinotecan, temozolomide and bevacizumab. Pediatr Blood Cancer. 2014;61:756–759. doi: 10.1002/pbc.24785. [DOI] [PubMed] [Google Scholar]
- 63.Grill J, Massimino M, Bouffet E, et al. Phase II, open-label, randomized, multicenter trial (HERBY) of bevacizumab in pediatric patients with newly diagnosed high-grade glioma. J Clin Oncol. 2018;36:951–958. doi: 10.1200/JCO.2017.76.0611. [DOI] [PubMed] [Google Scholar]
- 64.Navid F, Santana VM, Neel M, et al. A phase II trial evaluating the feasibility of adding bevacizumab to standard osteosarcoma therapy. Int J Cancer. 2017;141:1469–1477. doi: 10.1002/ijc.30841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Modak S, Kushner BH, Basu E, et al. Combination of bevacizumab, irinotecan, and temozolomide for refractory or relapsed neuroblastoma: Results of a phase II study. Pediatr Blood Cancer. 2017;64:e26448. doi: 10.1002/pbc.26448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith MA. Lessons learned from adult clinical experience to inform evaluations of VEGF pathway inhibitors in children with cancer. Pediatr Blood Cancer. 2014;61:1497–1505. doi: 10.1002/pbc.25036. [DOI] [PubMed] [Google Scholar]
- 67.Grignani G, Palmerini E, Dileo P, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: An Italian Sarcoma Group study. Ann Oncol. 2012;23:508–516. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]
- 68.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 69.Duffaud F, Mir O, Boudou-Rouquette P, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: A non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019;20:120–133. doi: 10.1016/S1470-2045(18)30742-3. [DOI] [PubMed] [Google Scholar]
- 70.Davis LE, Bolejack V, Ryan CW, et al. Randomized double-blind phase II study of regorafenib in patients with metastatic osteosarcoma. J Clin Oncol. 2019;37:1424–1431. doi: 10.1200/JCO.18.02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geoerger B, Kieran MW, Grupp S, et al. Phase II trial of temsirolimus in children with high-grade glioma, neuroblastoma and rhabdomyosarcoma. Eur J Cancer. 2012;48:253–262. doi: 10.1016/j.ejca.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dantonello TM, Int-Veen C, Schuck A, et al. Survival following disease recurrence of primary localized alveolar rhabdomyosarcoma. Pediatr Blood Cancer. 2013;60:1267–1273. doi: 10.1002/pbc.24488. [DOI] [PubMed] [Google Scholar]
- 73.Heyn RM, Holland R, Newton WA, Jr, et al. The role of combined chemotherapy in the treatment of rhabdomyosarcoma in children. Cancer. 1974;34:2128–2142. doi: 10.1002/1097-0142(197412)34:6<2128::aid-cncr2820340638>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 74.Maurer HM, Beltangady M, Gehan EA, et al. The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer. 1988;61:209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 75.Maurer HM, Gehan EA, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 76.Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 77.Bisogno G, Jenney M, Bergeron C, et al. Addition of dose-intensified doxorubicin to standard chemotherapy for rhabdomyosarcoma (EpSSG RMS 2005): A multicentre, open-label, randomised controlled, phase 3 trial. Lancet Oncol. 2018;19:1061–1071. doi: 10.1016/S1470-2045(18)30337-1. [DOI] [PubMed] [Google Scholar]