Abstract
PURPOSE
Off-label use of vemurafenib (VMF) to treat BRAFV600E mutation–positive, refractory, childhood Langerhans cell histiocytosis (LCH) was evaluated.
PATIENTS AND METHODS
Fifty-four patients from 12 countries took VMF 20 mg/kg/d. They were classified according to risk organ involvement: liver, spleen, and/or blood cytopenia. The main evaluation criteria were adverse events (Common Terminology Criteria for Adverse Events [version 4.3]) and therapeutic responses according to Disease Activity Score.
RESULTS
LCH extent was distributed as follows: 44 with positive and 10 with negative risk organ involvement. Median age at diagnosis was 0.9 years (range, 0.1 to 6.5 years). Median age at VMF initiation was 1.8 years (range, 0.18 to 14 years), with a median follow-up of 22 months (range, 4.3 to 57 months), whereas median treatment duration was 13.9 months (for 855 patient-months). At 8 weeks, 38 complete responses and 16 partial responses had been achieved, with the median Disease Activity Score decreasing from 7 at diagnosis to 0 (P < .001). Skin rash, the most frequent adverse event, affected 74% of patients. No secondary skin cancer was observed. Therapeutic plasma VMF concentrations (range, 10 to 20 mg/L) seemed to be safe and effective. VMF discontinuation for 30 patients led to 24 LCH reactivations. The blood BRAFV600E allele load, assessed as circulating cell-free DNA, decreased after starting VMF but remained positive (median, 3.6% at diagnosis, and 1.6% during VMF treatment; P < .001) and was associated with a higher risk of reactivation at VMF discontinuation. None of the various empirical therapies (hematopoietic stem-cell transplantation, cladribine and cytarabine, anti-MEK agent, vinblastine, etc) used for maintenance could eradicate the BRAFV600E clone.
CONCLUSION
VMF seemed safe and effective in children with refractory BRAFV600E-positive LCH. Additional studies are needed to find effective maintenance therapy approaches.
INTRODUCTION
Langerhans cell histiocytosis (LCH) is a rare disease characterized by inflammatory lesions that contain abundant CD1a+CD207+ histiocytes.1,2 Its natural course is heterogeneous and ranges from self-healing lesions to multi-organ disease with life-threatening consequences.3 LCH refractory to standard chemotherapy has a very poor prognosis,4 which can be improved only by highly toxic second-line chemotherapy5 or hematopoietic stem-cell transplantation (HSCT).6 Patients with refractory LCH are usually younger than 2 years of age, have life-threatening involvement at diagnosis, and frequently harbor the BRAFV600E mutation.7 Vemurafenib (VMF), a BRAF (v-RAF murine sarcoma viral oncogene homolog B) inhibitor originally licensed for metastatic melanoma,8 was previously given to two young children with refractory LCH.9,10 Faced with critically ill children and the regulatory challenges of organizing a phase I/II study in infants in a timely fashion, individual physicians applied for permission to use VMF off label. The European Medicines Agency approved VMF as an orphan drug for this indication.11 Herein, we report the results of an observational study of European and Mediterranean VMF-treated children with LCH.
PATIENTS AND METHODS
Patients
This study included 54 children treated for biopsy-proven, BRAFV600E-mutated LCH considered refractory.12 Three patients were reported previously,9,10,13 and their follow-up was extended. Patients were classified as risk organ (RO) positive (liver, spleen, and/or blood cytopenia12) or negative according to LCH extent. Macrophage activation syndrome was defined according to the literature.14 Patients with sclerosing cholangitis (n = 4)15 or CNS neurodegeneration (n = 7)16,17 were excluded from this analysis because they usually have irreversible anatomic lesions. Patients with RO-negative LCH had to have experienced at least two treatment line failures, including at least one intensive salvage regimen, on the basis of combinations of cladribine, cytarabine, or clofarabine. Failure of first-line therapy was characterized by disease progression in one or more ROs after six or more vinblastine (VBL) doses (one per week) and 28 days of prednisolone (minimum dose, 40 mg/m2/d), with or without the adjunction of a third drug. RO-positive patients were considered to have refractory LCH when one or more ROs showed no improvement after first-line therapy. Failure could occur at LCH onset or during its evolution in a patient whose initial disease response was followed by reactivation in one or more ROs.
Patients were included in their respective national LCH registries (Commission Nationale d’Informatique et des Libertés number in France 909027 and LCH-IV [ClinicalTrials.gov identifier: NCT02205762] elsewhere),3 and parents gave informed consent for enrollment in this observational study. In addition, after explaining to the parents the use of an off-label medication in the absence of an effective therapeutic alternative, informed consent was obtained before prescribing VMF. This procedure is in agreement with the Declaration of Helsinki.
VMF Dosing
Off-label VMF monotherapy was administered orally (10 mg/kg twice a day) for at least 8 weeks.9 For children younger than 2 years of age, the tablets (240 mg each) were split, crushed, and dissolved in water or milk for oral administration.9 Dose and duration were adjusted while taking into account tolerance, pharmacokinetics (PK), and efficacy.
Criteria Used to Assess Outcome
Because LCH is a heterogeneous systemic disease, the quantitative Disease Activity Score (DAS), which reflects overall LCH extension, was used as an evolution criterion.18 The criteria applied in Histiocyte Society (HS) trials (nonactive disease [NAD] or active disease better [ADB], stable, or worse)19,20 also were used. Finally, when present, tumors were assessed with computed tomography scans and Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.21 For RO-positive patients, complete response (CR) was defined as a null DAS, which corresponds to NAD in HS trials; the DAS declined for a partial response (PR) but remained positive below 3, which corresponds to ADB in HS trials. For RO-negative patients, CR was the disappearance of the entire tumor burden, whereas PR corresponded to a more than 50% tumor size decrease.
LCH activity was assessed at VMF initiation, 2 weeks, 8 weeks, and 12 weeks, with reactivation defined as the reappearance of disease activity after CR. Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events (version 4.3). VMF PK parameters (area under the curve, Cmin, Cmax, Tmax) were evaluated for 22 patients. For nine additional patients, only steady-state residual concentrations were determined. The assay used a previously published liquid chromatography-tandem mass spectroscopy method.22 Blood BRAFV600E load was assessed with various methods. Circulating cell-free BRAFV600E DNA (ccf-BRAFV600E) quantification was assessed for 34 patients using droplet-based digital polymerase chain reaction according to previously described techniques10,23,24 and expressed as the percentage of mutant alleles relative to the total number of alleles. The positivity threshold for the detection of allele mutants with this technique is 0.5 × 10−3.23 For three other patients, BRAFV600E was assessed by allele-specific real-time quantitative polymerase chain reaction on mononuclear cells for two and whole-blood cells for one.25
Statistical Analyses
The first criterion evaluated was VMF safety according to Common Terminology Criteria for Adverse Events (version 4.3), and the second was VMF efficacy according to the overall response rate evaluated 8 weeks after starting the drug. According to HS criteria, the overall response rate was considered favorable when LCH activity was null (ie, NAD) or decreased by more than 50% (ie, ADB).19,26 Between-group differences were compared using the Mann-Whitney U test for quantitative variables and Fisher’s exact test for qualitative variables. End points for survival analyses were any type of reactivation and death. Survival rates were estimated with the Kaplan-Meier method, and subgroups were compared with the log-rank test. All participating patients had to have started VMF before July 31, 2018. The cutoff date for this analysis was December 31, 2018.
RESULTS
Patient Characteristics
Fifty-four patients (28 males, 26 females) with multisystem LCH from 12 countries were identified (Table 1; Data Supplement): 44 patients were RO positive, including six with macrophage activation syndrome, and 10 were RO negative. Median age at LCH diagnosis was 0.9 years. VMF was started at a median age of 1.8 years; RO-positive patients were younger (1.6 years) than RO-negative patients (3.4 years). The median initial diagnosis-to-VMF initiation time was 0.7 years. Median VMF onset-to-last examination follow-up was 22.1 months for a cumulative follow-up of 1,232 months. Thirty-one patients were VMF treated at the time of first LCH episode, and 23 patients were VMF treated during reactivation. At VMF initiation, all patients were considered active disease worse (n = 53) or active disease stable (n = 1) according the HS criteria.19,20 Median DAS for all 54 children was 7 (range, 1 to 22). Induction therapy consisted of VBL plus corticosteroids (VBL + CS) for all patients (one cycle for 29 patients, two for 18 patients, and three or more for seven patients). In addition, LCH refractoriness was distributed as follows: cladribine monotherapy (five RO-negative patients and seven RO-positive patients), cladribine and cytarabine combination (eight RO-positive patients), clofarabine (two RO-positive patients), and vincristine and cytarabine (three RO-positive patients and five RO-negative patients).
TABLE 1.
Characteristics of 54 VMF-Treated Patients With Childhood LCH in the International Series
Therapeutic Intervention
Median VMF administration duration from onset to discontinuation for the 30 assessable patients was 6.1 months (range, 2 to 14.7 months). For the 24 remaining patients, VMF cessation could not be evaluated because the patients switched immediately at discontinuation to HSCT (n = 2), cladribine and cytarabine (n = 1), or VBL + CS (n = 2) or were still taking VMF at the last visit (n = 19). With consideration of all patients, including those for whom VMF was secondarily resumed, the median total VMF administration duration was 13.9 months (range, 2 to 38 months), with cumulative duration lasting 855 patient-months (Table 1; Data Supplement). The latter can be broken down as follows: 225 months for the initial administration until first stoppage, 303 months for the 30 patients who stopped VMF at least once after resuming VMF, 83 months for patients who switched to HSCT and chemotherapy, and 244 months for those still taking VMF at the last follow-up.
VMF PK Parameters
The residual VMF level measured at least 14 days after starting the drug (steady state) seemed to coincide with toxicity and efficacy. The 95% CI for the plasma VMF concentration was 11.6 to 20.6 mg/L for a median dose of 21 mg/kg/d (range, 13 to 41 mg/kg/d; mean, 23 mg/kg/d), and seems to be VMF’s therapeutic range for pediatric patients with LCH (Simon et al, manuscript in preparation). The dose was modified for 12 patients because of mild skin AEs (three reduced doses), physician choice (three increased doses), PK analysis (three increased doses), or reactivation 3 months after VMF onset (three increased doses). Three patients’ PRs were associated with insufficient plasma VMF concentrations less than 10 mg/L; when their doses were doubled, those concentrations reached the therapeutic range, which led to clinical CR.
AEs
Fifty-four AEs occurred in 40 patients (Data Supplement). AEs were predominantly dermatologic (37 of 40; 92%), with only eight nondermatologic. Most AEs (33 of 40; 82%) were grade 1 or 2 and transient or had no permanent sequelae: The 37 dermatologic AEs were skin rashes for all 37 patients, with exacerbated skin photosensitivity in 13 or panniculitis in seven. Two had transiently abnormal nails in addition to skin rashes. The eight nondermatologic AEs were two transient grade 1 QT-interval prolongations at higher doses; grade 1 transient joint pain for two and grade 2 for a third; and one each of transient mild liver cytolysis, grade 3 transient clonus of the neck and limbs, and grade 3 tumor necrosis–related nose bleeds. Finally, VMF patient #3000068 died as a result of sepsis and pancytopenia associated with concomitant clofarabine use (Data Supplement).
AEs seemed to be more frequent when residual (after 24 hours) plasma VMF concentrations were high. For example, grade 1 QT-interval prolongations occurred with plasma VMF at 37 and 72 mg/L, and the three patients with grade 3 panniculitis had levels of 40 to 74 mg/L (#1509554, 42.9 mg/L; #1506637, 40.2 mg/L; #1509564, 74.1 mg/L).
For all patients with grade 3 toxicity, lowering of the VMF dose led to AE regression or disappearance. No secondary malignancy or other blood or immune toxicity was observed. Even though follow-up was short, no growth delay was noted, but several patients developed community infections, like chicken pox or influenza, without complications.
Therapeutic Responses
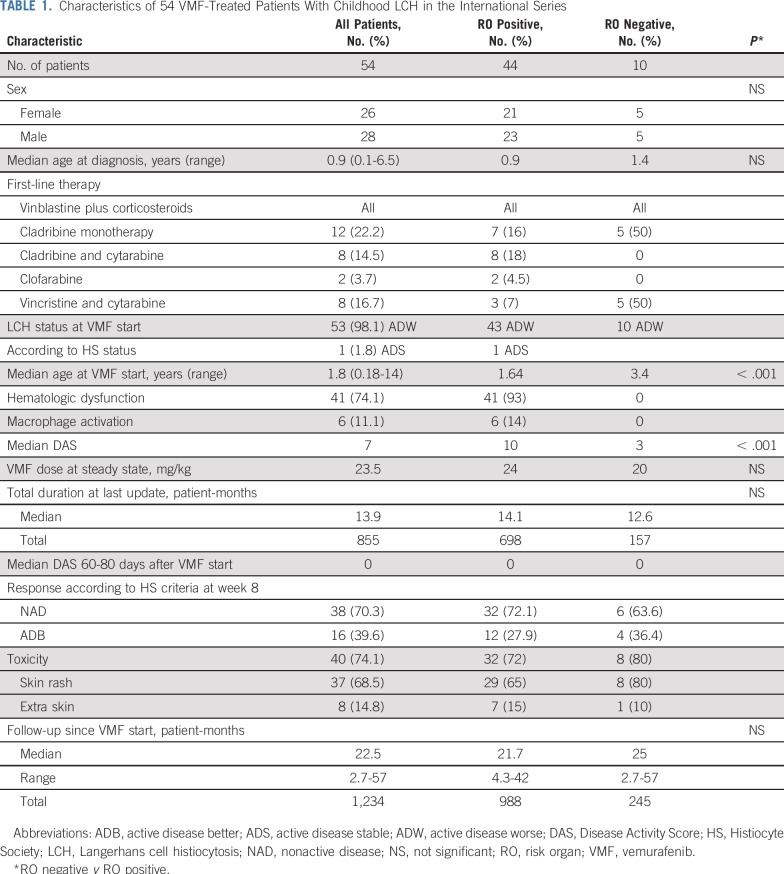
At 8 weeks, 38 patients had CRs (NAD) and 16 PRs (ADB; Fig 1A). The median DAS decreased from 7 at VMF initiation to 0 on day 60 (P < .001; Fig 1B), and that decline was more remarkable (10 to 0) for RO-positive than for RO-negative patients (3 v 0; Fig 1A). The 16 patients with persistent PR had minimal clinically active disease with mild spleen enlargement (n = 6), mild liver enlargement (n = 3), or skin lesions (n = 7). Of note, responses appeared rapidly (Fig 1C). Two patients’ soft tissue tumor masses became necrotic, and one developed severe epistaxis as a result of pharyngeal lesion necrosis on day 60. In addition, responses were assessed with computed tomography scan (RECIST version 1.1),21 with planar evaluation for 11 assessable patients with multisystem LCH. Tumors in RO-negative patients showed a more than 70% decrease compared with pre-VMF, and those patients benefited from bone remodeling (Fig 1D).
FIG 1.
Langerhans cell histiocytosis (LCH) evolution on vemurafenib (VMF) according to various criteria. (A) Waterfall figure of the Disease Activity Score (DAS) change between day 1 and week 8 after starting VMF. (B) DAS evolution between VMF day 1 and week 8 according to the LCH extent of risk organ (RO) involvement (RO positive, RO negative). The differences were significant for the two groups (P < .001), but the amplitude was much more pronounced for the RO-positive group. (C) Female patient #1509231 with massive cervical lymph nodes viewed from the back on day –1 (DAS = 3; left) and after their disappearance on day 14 (right) of VMF administration. Her multisystemic RO-positive LCH was initially treated with two cycles of vinblastine plus corticosteroids (VBL + CS) that obtained good responses before reactivation, which was then retreated unsuccessfully with VBL + CS and then cladribine before further worsening. (D) Imaging of left-side temporal bone lesion in male patient #1509707. After an initial good response to standard VBL + CS induction, his initially RO-positive LCH reactivated locally on the first maintenance regimen. VBL + CS was prescribed again, but disease progression led to left-sided facial palsy. The first computed tomography scan (left) shows left-side temporal bone destruction and soft tissue involvement, whereas the computed tomography scan at week 6 on VMF (right) shows that almost all the initial lesions had disappeared and bone was partially remodeled.
VMF Discontinuation, Reactivation, Long-Term Outcomes, and Maintenance
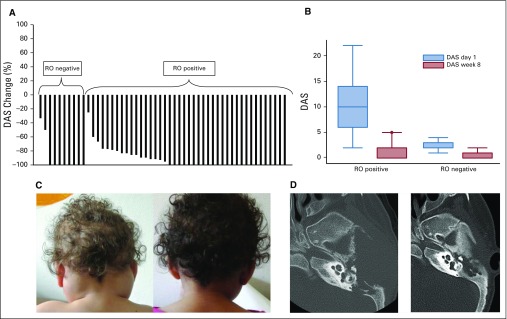
The response to VMF persisted as long as the patient remained on treatment, with three requiring dose adaptation. VMF was stopped after a median of 6.1 months (range, 2 to 14.7 months) for 30 patients without any maintenance therapy. Twenty-four of those patients rapidly experienced reactivations (median, 0.9 months; range, 0.1 to 7.3 months) after discontinuation (Data Supplement). The 6- and 12-month reactivation rates were 72% (95% CI, 56% to 88%) and 84% (95% CI, 68% to 95%), respectively (Fig 2A). RO status determined the reactivation pattern. Among the 30 patients who stopped VMF, 22 were RO-positive patients who developed 20 reactivations (RO-positive reactivations for 18 patients), whereas four reactivations among the eight RO-negative patients were RO negative (three with skin rash, one with pituitary, and/or three with bone). The reactivation rate was higher for RO-positive patients than for RO-negative patients (Fig 2B) and for patients positive, but not negative, for ccf-BRAFV600E (Fig 2C). The 12-month reactivation rate was 95% for RO-positive patients v 57% for RO-negative patients (P < .001) and 100% for patients positive for ccf-BRAFV600E v 33% for those negative for ccf-BRAFV600E on VMF (P = .006).
FIG 2.
Kaplan-Meier plots of Langerhans cell histiocytosis (LCH) reactivation and survival rates. (A) Thirty assessable patients after vemurafenib (VMF) withdrawal with 95% CIs. (B) According to initial LCH risk organ (RO) involvement (RO positive, RO negative). The probability of reactivation was significantly higher for patients with RO-positive LCH (P = .0041). (C) According to circulating cell-free BRAFV600E loads in plasma of 13 assessable patients after stopping VMF as determined by polymerase chain reaction. Despite the small number of available values, the probability of reactivation was significantly higher when the cell-free BRAFV600E load was positive (P < .001) on VMF (P = .0124). (D) Survival rate with 95% CI since VMF onset for the 54 children with LCH.
Among the 20 RO-positive reactivations, 18 were treated effectively by reintroducing VMF; the patient given VMF and clofarabine experienced major toxicity (death), and the patient with only skin-localized involvement was left untreated (Data Supplement). The four RO-negative reactivations were treated with VMF in two patients; one patient was given VBL, and the other was left untreated. Later, among the 18 VMF-treated RO-positive reactivations, two patients underwent HSCT, one after unsuccessful VBL + CS reintroduction; two were prescribed VMF and cobimetinib; and one received VBL + CS alone. Those maintenance strategies failed to control LCH because the disease always reactivated when VMF was withdrawn.
If we add the five patients who had received chemotherapy before any reactivation after VMF stoppage to the six given chemotherapy after post-VMF reactivation, four maintenance regimens could be evaluated. The findings were disappointing because two of the three patients who underwent HSCT, two of three who received cladribine and cytarabine, and four of the five treated with VBL + CS had reactivations, and clofarabine adjunction was complicated by lethal sepsis. In addition, two patients treated with VMF and cobimetinib experienced reactivations when that combination was stopped. The 2-year overall survival rate was 98% (95% CI, 88% to 100%; Fig 2D).
ccf-BRAFV600E Load as a Surrogate End Point
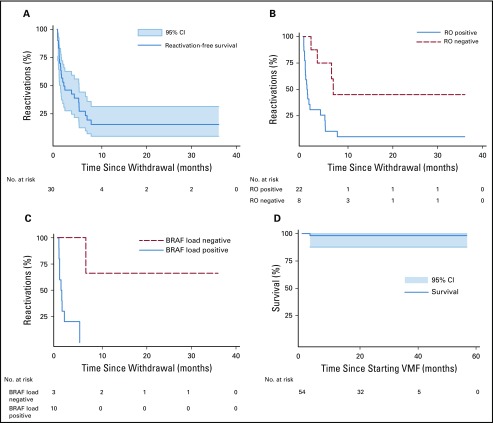
Thirty-seven patients’ plasma ccf-BRAFV600E loads were evaluated at various times. It was assessed in two patients’ whole-blood mononuclear cells, in one patient’s whole-blood cells, and in DNA extracted from plasma for the others. Although the results of the three methods seem to be comparable, only the ccf-BRAFV600E results of 34 patients were analyzed but not all at the same time. At diagnosis, a ccf-BRAFV600E load, expressed as the percentage of mutant alleles relative to the total number of alleles, was evaluated for 27 patients and detectable above the 0.5 × 10−3 threshold for 18 of the 21 RO-positive patients and one of the six RO-negative patients. Twenty-two patients (four RO negative, 18 RO positive) had plasma samples available for longitudinal follow-up; their ccf-BRAFV600E loads were tested in the same laboratory (Fig 3), as reported previously.23 ccf-BRAFV600E was detected in the 22 patients at VMF initiation, with a median load of 3.1% (range, 0.31% to 22%). At 8 weeks, the ccf-BRAFV600E load (median, 0.7%; range, 0.001% to 18%) remained within the same range as on day 15 (when tested) with no further decrease, despite good clinical responses. When the ccf-BRAFV600E load was evaluated more than 6 months after starting VMF, it remained positive for nine of 12 patients (median, 1.6%; range, 0.001% to 26%). Moreover, all patients positive for ccf-BRAFV600E 2 or more months post-VMF initiation experienced relapse soon after that agent’s discontinuation (Fig 2C). Even HSCT failed to clear the BRAFV600E clones, whose loads were still high 6 months after HSCT for two assessable patients.
FIG 3.
Circulating cell-free BRAFV600E kinetics according to time on vemurafenib and Langerhans cell histiocytosis risk organ extent (18 positive for risk organ involvement [black lines, blue diamonds]; four negative for risk organ involvement [red lines and squares]). The BRAFV600E allele load (expressed as the percentage of mutant alleles relative to the total number of alleles) is shown for each patient before vemurafenib onset (day 0), at weeks 6 to 8, and during months 3 to 6 and 9 to 12.
DISCUSSION
We report the first, to our knowledge, international series of children with refractory LCH treated with the BRAF inhibitor VMF. According to literature criteria,4,12,27 these patients had severe, life-threatening LCH manifestations because they all failed to respond to conventional therapies. Validated therapeutic options are currently cladribine and cytarabine chemotherapy5 or HSCT,6 which may reverse such situations, with 15% to 25% mortality and long hospitalization. No therapeutic trial was available for this group of infants with severely compromised organ function and at high risk of death. In the face of this difficult situation, our results show that VMF induced rapid and dramatic clinical improvement with only mild or transient toxicity. Indeed, VMF seemed to be far less toxic than cladribine and cytarabine or HSCT, which thereby allowed treatment of patients in low-income areas without access to specialized hematologic intensive care or HSCT facilities (Gaza Strip, Algeria, and Tunisia). This outcome is similar to that obtained for a US series of 21 patients, eight of whom with LCH that could be classified as refractory according the literature, even though highly heterogeneous treatments had been used (various BRAF or mitogen-activated extracellular signal–regulated kinase [MEK] inhibitors). Moreover, two of the eight patients had LCH associated with juvenile xanthogranuloma.28
VMF toxicity in children also contrasts favorably with adults treated for melanoma. Despite the small size of our series and in light of the very high risk of secondary skin tumors observed in adults with melanoma (approximately 30%),29,30 the absence of any secondary tumors in 54 patients after 855 patient-months on VMF can be considered reassuring. The frequent mild skin AEs (eg, photosensitivity, panniculitis) cannot be underestimated, but patients remain susceptible to these with dose adjustment.
The absence of a pediatric VMF formulation was also a concern; it was necessary to crush tablets and use a weight-based dose adaptation. However, our PK data demonstrate the effectiveness of that approach. In addition to the good safety profile and good bioavailability of VMF, our results show that active, refractory LCH responds rapidly to the drug. The clinical response rate was higher and faster than the best observed with any previous therapies, including cladribine and cytarabine.5 Moreover, the few PRs seemed to reflect a lack of drug bioavailability and could be corrected by dose adjustment with therapeutic drug monitoring.
Our findings show that despite its good clinical efficacy, VMF was unable to eradicate the neoplastic clone. The majority of patients who discontinued VMF experienced LCH reactivations. Various strategies have been proposed to eradicate the underlying LCH clone, but none have been satisfactory. Among the three patients who underwent HSCT to eradicate LCH, two rapidly experienced reactivation, and follow-up was too short for the third. Above all, the blood BRAFV600E load was never negative after HSCT.31 As maintenance therapy, two patients received a BRAF inhibitor and MEK inhibitor combination; their blood BRAFV600E loads never changed durably. How to eradicate the BRAFV600E clone remains elusive.
The last important study-derived information was the usefulness of evaluation as a surrogate marker of LCH activity. ccf-BRAFV600E was shown previously to correlate with disease activity.23 Indeed, we observed that almost all VMF-treated patients, despite being considered clinical responders, maintained detectable BRAFV600E levels and that circulating alleles were associated with a higher reactivation risk at VMF discontinuation. Despite the seeming global efficacy of VMF, the most important limitation of this study is its observational design. The design respected some key criteria with regard to patient selection and the choice of pertinent end points for these patients with life-threatening LCH. Indeed, we are at the dawn of the development of anti-BRAF therapy for LCH.
In conclusion, VMF is at least a bridging option for patients with life-threatening, multisystem LCH. Prospective clinical trials are needed urgently to determine the appropriate treatment duration and add-on treatment options for clone eradication and LCH cure.
ACKNOWLEDGMENT
We thank the patients and their families for participation in this study and the treating physicians and nurses who contributed to it. We also thank Janet Jacobson for editorial assistance. This study was based on research carried out at the Centre de Reference des Histiocytoses (www.histiocytose.org).
Footnotes
Presented at the 34th Annual Meeting of the Histiocyte Society, Lisbon, Portugal, October 22-23, 2018.
Supported by the Association Histiocytose France and grants from Recherche Maladies Hématologiques de l’Enfant and the Conquer Cancer Foundation. Roche awarded a grant to the French Histiocytosis Study Group.
AUTHOR CONTRIBUTIONS
Conception and design: Jean Donadieu, Valerie Taly, Jean-François Emile, Sébastien Héritier
Financial support: Jean Donadieu, Valerie Taly, Jean-François Emile
Administrative support: Jean Donadieu, Sébastien Héritier
Provision of study material or patients: Jean Donadieu, Fleur Cohen, Mathilde Jehanne, Alexandra Kolenova, Pascale Schneider, Geneviève Plat, Anne Lutun, Thomas Lehrnbecher, Viktoria Efremova, Martina Ahlmann, James Nicholson, Andrej Lissat, Sarah Elitzur, Olga Slater, Milen Minkov, Jean-François Emile, Sébastien Héritier
Collection and assembly of data: Jean Donadieu, Islam Amine Larabi, Mathilde Tardieu, Johannes Visser, Caroline Hutter, Elena Sieni, Nabil Kabbara, Mohamed Barkaoui, Jean Miron, François Chalard, Paul Milne, Fleur Cohen, Zofia Hélias-Rodzewicz, Mathilde Jehanne, Alexandra Kolenova, Anne Pagnier, Nathalie Aladjidi, Pascale Schneider, Geneviève Plat, Anne Lutun, Anne Sonntagbauer, Thomas Lehrnbecher, Alina Ferster, Viktoria Efremova, Martina Ahlmann, Laurence Blanc, James Nicholson, Anne Lambilliote, Andrej Lissat, Fanette Bernard, Sarah Elitzur, Michal Golan, Dmitriy Evseev, Michael Maschan, Olga Slater, Milen Minkov, Jean-Claude Alvarez, Jean-François Emile, Sébastien Héritier
Data analysis and interpretation: Jean Donadieu, Islam Amine Larabi, Mathilde Tardieu, Johannes Visser, Paul Milne, Julien Haroche, Fleur Cohen, Zofia Hélias-Rodzewicz, Nicolas Simon, Anne Sonntagbauer, Thomas Lehrnbecher, Viktoria Efremova, Martina Ahlmann, Houda Boudiaf, Karel Svojgr, Sarah Elitzur, Michal Golan, Dmitriy Evseev, Michael Maschan, Ahmed Idbaih, Milen Minkov, Valerie Taly, Matthew Collin, Jean-Claude Alvarez, Jean-François Emile, Sébastien Héritier
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Vemurafenib for Refractory Multisystem Langerhans Cell Histiocytosis in Children: An International Observational Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Jean Donadieu
Honoraria: X4 Pharma
Consulting or Advisory Role: X4 Pharma
Research Funding: Roche (Inst), Prolong Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: X4 Pharma
Johannes Visser
Consulting or Advisory Role: Nova Laboratories
Elena Sieni
Employment: Baxter (I), Urgo Medical (I)
Honoraria: SOBI
Consulting or Advisory Role: SOBI
Speakers’ Bureau: SOBI
Travel, Accommodations, Expenses: SOBI, Urgo Medical (I)
Nicolas Simon
Consulting or Advisory Role: Indivior
Travel, Accommodations, Expenses: Lundbeck
Alexandra Kolenova
Honoraria: Novartis Slovakia, Servier Slovensko
Consulting or Advisory Role: Inova
Travel, Accommodations, Expenses: Baxalta Slovakia
Anne Pagnier
Research Funding: Novartis (Inst), Jazz Pharmaceuticals (Inst), Takeda Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Takeda Pharmaceuticals
Thomas Lehrnbecher
Honoraria: Gilead Sciences, Astellas Pharma, Merck Sharp & Dohme
Consulting or Advisory Role: Gilead Sciences, Astellas Pharma, Merck Sharp & Dohme
Speakers’ Bureau: Gilead Sciences, Astellas Pharma, Merck Sharp & Dohme
Research Funding: Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences, Astellas Pharma, Merck Sharp & Dohme
Michael Maschan
Speakers’ Bureau: Miltenyi Biotec
Ahmed Idbaih
Consulting or Advisory Role: Health Advances, Simon Partners
Research Funding: CARTHERA (Inst), Transgene (Inst), Sanofi (Inst), Air Liquide (Inst)
Other Relationship: TBWA
Olga Slater
Consulting or Advisory Role: Bayer Schering Pharma
Milen Minkov
Honoraria: Novartis
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: Novartis
Valerie Taly
Stock and Other Ownership Interests: Emulseo
Research Funding: Merieux Institute (Inst), Servier Institute (Inst)
Matthew Collin
Honoraria: Mallinckrodt
Jean-François Emile
Honoraria: Bristol-Myers Squibb, MSD Oncology, HalioDx, Pierre Fabre, Amgen
Sébastien Héritier
Honoraria: Sandoz
Research Funding: Roche (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Haroche J, Cohen-Aubart F, Rollins BJ, et al. Histiocytoses: Emerging neoplasia behind inflammation. Lancet Oncol. 2017;18:e113–e125. doi: 10.1016/S1470-2045(17)30031-1. [DOI] [PubMed] [Google Scholar]
- 2.Collin M, Bigley V, McClain KL, et al. Cell(s) of origin of Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 2015;29:825–838. doi: 10.1016/j.hoc.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rigaud C, Barkaoui MA, Thomas C, et al. Langerhans cell histiocytosis: Therapeutic strategy and outcome in a 30-year nationwide cohort of 1478 patients under 18 years of age. Br J Haematol. 2016;174:887–898. doi: 10.1111/bjh.14140. [DOI] [PubMed] [Google Scholar]
- 4.Minkov M, Grois N, Heitger A, et al. Response to initial treatment of multisystem Langerhans cell histiocytosis: An important prognostic indicator. Med Pediatr Oncol. 2002;39:581–585. doi: 10.1002/mpo.10166. [DOI] [PubMed] [Google Scholar]
- 5.Donadieu J, Bernard F, van Noesel M, et al. Cladribine and cytarabine in refractory multisystem Langerhans cell histiocytosis: Results of an international phase 2 study. Blood. 2015;126:1415–1423. doi: 10.1182/blood-2015-03-635151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veys PA, Nanduri V, Baker KS, et al. Haematopoietic stem cell transplantation for refractory Langerhans cell histiocytosis: Outcome by intensity of conditioning. Br J Haematol. 2015;169:711–718. doi: 10.1111/bjh.13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Héritier S, Emile JF, Barkaoui MA, et al. BRAF mutation correlates with high-risk Langerhans cell histiocytosis and increased resistance to first-line therapy. J Clin Oncol. 2016;34:3023–3030. doi: 10.1200/JCO.2015.65.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang S, Atkins MB. Treatment of BRAF-mutant melanoma: The role of vemurafenib and other therapies. Clin Pharmacol Ther. 2014;95:24–31. doi: 10.1038/clpt.2013.197. [DOI] [PubMed] [Google Scholar]
- 9.Héritier S, Jehanne M, Leverger G, et al. Vemurafenib use in an infant for high-risk Langerhans cell histiocytosis. JAMA Oncol. 2015;1:836–838. doi: 10.1001/jamaoncol.2015.0736. [DOI] [PubMed] [Google Scholar]
- 10.Kolenová A, Schwentner R, Jug G, et al. Targeted inhibition of the MAPK pathway: Emerging salvage option for progressive life-threatening multisystem LCH. Blood Adv. 2017;1:352–356. doi: 10.1182/bloodadvances.2016003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. European Medicines Agency: Orphan drug designation. Committee for Orphan Medicinal Products 313647. Amsterdam, the Netherlands, European Medicines Agency, 2016.
- 12.Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): Guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175–184. doi: 10.1002/pbc.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heisig A, Sörensen J, Zimmermann SY, et al. Vemurafenib in Langerhans cell histiocytosis: Report of a pediatric patient and review of the literature. Oncotarget. 2018;9:22236–22240. doi: 10.18632/oncotarget.25277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672–2681. doi: 10.1182/blood-2016-01-690636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braier J, Ciocca M, Latella A, et al. Cholestasis, sclerosing cholangitis, and liver transplantation in Langerhans cell histiocytosis. Med Pediatr Oncol. 2002;38:178–182. doi: 10.1002/mpo.1306. [DOI] [PubMed] [Google Scholar]
- 16. Grois N, Fahrner B, Arceci RJ, et al: Central nervous system disease in Langerhans cell histiocytosis. J Pediatr 156:873-881, 2010. [DOI] [PubMed]
- 17.Nanduri VR, Pritchard J, Levitt G, et al. Long term morbidity and health related quality of life after multi-system Langerhans cell histiocytosis. Eur J Cancer. 2006;42:2563–2569. doi: 10.1016/j.ejca.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 18.Donadieu J, Piguet C, Bernard F, et al. A new clinical score for disease activity in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2004;43:770–776. doi: 10.1002/pbc.20160. [DOI] [PubMed] [Google Scholar]
- 19.Gadner H, Grois N, Pötschger U, et al. Improved outcome in multisystem Langerhans cell histiocytosis is associated with therapy intensification. Blood. 2008;111:2556–2562. doi: 10.1182/blood-2007-08-106211. [DOI] [PubMed] [Google Scholar]
- 20.Gadner H, Minkov M, Grois N, et al. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood. 2013;121:5006–5014. doi: 10.1182/blood-2012-09-455774. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez JC, Funck-Brentano E, Abe E, et al. A LC/MS/MS micro-method for human plasma quantification of vemurafenib. Application to treated melanoma patients. J Pharm Biomed Anal. 2014;97:29–32. doi: 10.1016/j.jpba.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Héritier S, Hélias-Rodzewicz Z, Lapillonne H, et al. Circulating cell-free BRAFV600E as a biomarker in children with Langerhans cell histiocytosis. Br J Haematol. 2017;178:457–467. doi: 10.1111/bjh.14695. [DOI] [PubMed] [Google Scholar]
- 24.Taly V, Pekin D, Benhaim L, et al. Multiplex picodroplet digital PCR to detect KRAS mutations in circulating DNA from the plasma of colorectal cancer patients. Clin Chem. 2013;59:1722–1731. doi: 10.1373/clinchem.2013.206359. [DOI] [PubMed] [Google Scholar]
- 25.Milne P, Bigley V, Bacon CM, et al. Hematopoietic origin of Langerhans cell histiocytosis and Erdheim-Chester disease in adults. Blood. 2017;130:167–175. doi: 10.1182/blood-2016-12-757823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadner H, Grois N, Arico M, et al. A randomized trial of treatment for multisystem Langerhans’ cell histiocytosis. J Pediatr. 2001;138:728–734. doi: 10.1067/mpd.2001.111331. [DOI] [PubMed] [Google Scholar]
- 27.Weitzman S, Braier J, Donadieu J, et al. 2′-Chlorodeoxyadenosine (2-CdA) as salvage therapy for Langerhans cell histiocytosis (LCH). Results of the LCH-S-98 protocol of the Histiocyte Society. Pediatr Blood Cancer. 2009;53:1271–1276. doi: 10.1002/pbc.22229. [DOI] [PubMed] [Google Scholar]
- 28.Eckstein OS, Visser J, Rodriguez-Galindo C, et al. Clinical responses and persistent BRAF V600E+ blood cells in children with LCH treated with MAPK pathway inhibition. Blood. 2019;133:1691–1694. doi: 10.1182/blood-2018-10-878363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: A study of 42 patients. Ann Oncol. 2013;24:1691–1697. doi: 10.1093/annonc/mdt015. [DOI] [PubMed] [Google Scholar]
- 30.Ma C, Armstrong AW. Severe adverse events from the treatment of advanced melanoma: A systematic review of severe side effects associated with ipilimumab, vemurafenib, interferon alfa-2b, dacarbazine and interleukin-2. J Dermatolog Treat. 2014;25:401–408. doi: 10.3109/09546634.2013.813897. [DOI] [PubMed] [Google Scholar]
- 31. doi: 10.1038/s41390-018-0238-y. Schwentner R, Kolenova A, Jug G, et al: Longitudinal assessment of peripheral blood BRAFV600E levels in patients with Langerhans cell histiocytosis. Pediatr Res 85:856-894, 2019. [DOI] [PubMed] [Google Scholar]