Abstract
PURPOSE
Chemotherapy-induced thrombocytopenia (CIT) leads to delay or reduction in cancer treatment. There is no approved treatment.
METHODS
We conducted a phase II randomized trial of romiplostim versus untreated observation in patients with solid tumors with CIT. Before enrollment, patients had platelets less than 100,000/μL for at least 4 weeks, despite delay or dose reduction of chemotherapy. Patients received weekly titrated romiplostim with a target platelet count of 100,000/μL or more, or were monitored with usual care. The primary end point was correction of platelet count within 3 weeks. Twenty-three patients were treated in a randomization phase, and an additional 37 patients were treated in a single-arm, romiplostim phase. Resumption of chemotherapy without recurrent CIT was a secondary end point.
RESULTS
The mean platelet count at enrollment was 62,000/μL. In the randomization phase, 14 of 15 romiplostim-treated patients (93%) experienced correction of their platelet count within 3 weeks, compared with one of eight control patients (12.5%; P < .001). Including all romiplostim-treated patients (N = 52), the mean platelet count at 2 weeks of treatment was 141,000/μL. The mean platelet count in the eight observation patients at 3 weeks was 57,000/μL. Forty-four patients who achieved platelet correction with romiplostim resumed chemotherapy with weekly romiplostim. Only three patients (6.8%) experienced recurrent reduction or delay of chemotherapy because of isolated CIT.
CONCLUSION
This prospective trial evaluated treatment of CIT with romiplostim. Romiplostim is effective in correcting CIT, and maintenance allows for resumption of chemotherapy without recurrence of CIT in most patients.
INTRODUCTION
Suppression of hematopoiesis is a common adverse effect of chemotherapy, resulting in delay or dose reduction of cancer treatment. Although there are multiple reasons for delay or dose reduction of chemotherapy, marrow suppression remains a major cause.1 In one report, 22.7% of patients experienced chemotherapy dose delay because of inadequate blood counts.2 Neutropenia can be effectively treated with filgrastim3; however, there is no available treatment of chemotherapy-induced thrombocytopenia (CIT). Retrospective cohort studies have reported treatment-related thrombocytopenia (≤ 100,000/μL) rates of 16.5% and 21.8%,2,4 and more than 30% for platinum- or gemcitabine-based regimens.2 The major consequence of CIT is reduction in relative dose intensity (RDI). Reduced RDI may result from a number of factors besides CIT, but thrombocytopenia is an important contributor.1,5,6 Reduction in RDI affects progression-free or overall survival in studies of colorectal, ovarian, and breast cancers.7-10
Platelet transfusions provide only transient and unpredictable improvement in platelet counts and are not practical to maintain platelet counts through cycles of chemotherapy nadir.11 Thrombopoietin (TPO) is the primary growth factor that stimulates megakaryocytes and platelet production.12 Recombinant TPO and a polyethylene glycol–modified variant were both found to raise platelet counts in test patients.12 However, therapeutic development was halted after the development of antidrug antibodies that cross-reacted with endogenous TPO, causing thrombocytopenia.12,13 There are no approved treatments for CIT, and this remains an important unmet need.
More recently, TPO receptor agonists have been developed. Romiplostim is a subcutaneously administered Fc-peptide fusion protein, which contains two 14 amino-acid peptides, stabilized by linkage to the immunoglobulin G Fc domain, that binds to and activates the TPO receptor.12 Romiplostim does not contain the peptide sequence of endogenous TPO, and cross-reactive antibodies have not been reported. Romiplostim is approved and widely used to treat immune thrombocytopenia.12 Several oral, small-molecule TPO receptor agonists have also been developed, but none are approved for treatment of CIT.12,14,15
Several small studies, presented only as abstracts, demonstrated that administration of romiplostim both before and after chemotherapy was effective in improving platelet counts, but did not lead to an impact on ongoing cancer therapy or ongoing RDI.16,17 We previously published a case series of 20 patients with solid tumors with CIT (< 100,000/μL) treated with weekly romiplostim, suggesting romiplostim may treat CIT and allow for resumption of chemotherapy.18 A more recent retrospective case series showed similar findings.19 We report the results of a prospective, phase II clinical trial of romiplostim for CIT in patients with solid tumors.
METHODS
Patients
Eligible patients were 18 years or older at the time of enrollment with active nonhematologic cancer and CIT (defined as a platelet count < 100,000/μL) for at least 4 weeks, despite dose reduction or delay of prior chemotherapy. Past chemotherapy included a nucleoside analog, carboplatin or cisplatin, an anthracycline, an alkylating agent, or other cytotoxic chemotherapy agent with thrombocytopenia as a known common toxicity, but no chemotherapy in the prior 14 days. Patients had an Eastern Cooperative Oncology Group performance status of 2 or below.
Exclusion criteria included a history of hematologic malignancy, confirmed by fluorescence in situ hybridization (FISH) from bone marrow aspirate and biopsy or peripheral blood test in the prior 3 months. Exclusion criteria also included a hemoglobin level of less than 8.0 gm/dL despite red cell transfusion or an absolute neutrophil count of less than 1,000/μL despite use of granulocyte colony stimulating factor. In the presence of primary or metastatic liver cancer, patients were excluded if transaminases or total bilirubin were greater than five times the upper limit of normal. Patients without liver involvement by cancer were excluded if transaminases or total bilirubin were greater than three times the upper limit of normal. A history of a prior symptomatic venous thromboembolic event (VTE) or arterial event, such as myocardial infarction, ischemic stroke, or transient ischemic attack, was acceptable as long as the patient had completed a prescribed course of anticoagulation or was tolerating ongoing anticoagulation.Other reasons for exclusion included any serious concomitant medical condition that could interfere with the conduct of the clinical trial, such as unstable angina, renal failure requiring hemodialysis, or active infection requiring intravenous antibiotics. Pregnant women, lactating mothers, and patients unwilling to use contraception were excluded. Patients with bone metastases sufficient to result in cortical bone destruction on imaging studies were excluded. In our preliminary experience, it was apparent that patients with extensive bone metastases did not respond to romiplostim, and we did not want to enroll patients in whom the drug was known to be futile.
Trial Design and Treatment
This was an open-label phase II trial of romiplostim versus observation only in patients with CIT with locally advanced or metastatic solid tumors with thrombocytopenia after known marrow-suppressive chemotherapy (ClinicalTrials.gov identifier: NCT02052882). Patients were enrolled at Memorial Sloan Kettering Cancer Center (MSK) from May 13, 2014, through February 21, 2018. Patients had thrombocytopenia (< 100,000/μL) for at least 4 weeks and no platelet count greater than 100,000/μL in the prior 6-week period, despite dose delay or reduction of chemotherapeutic regimen. Patients had not received chemotherapy for at least 14 days before enrollment. Furthermore, patients with a hemoglobin level below 8.0 gm/dL or absolute neutrophil count below 1,000/μL, despite standard supportive measures of transfusions and/or white cell growth factor support, were considered to have pancytopenia, not CIT, and therefore were ineligible.
The underlying hypothesis was that romiplostim would more effectively correct platelet counts (≥ 100,000/μL) within 3 weeks compared with observation control. The initial design was a 2:1 randomization to weekly, titrated romiplostim or untreated observation. The initial romiplostim dose was 2.0 µg/kg, increased by 1.0 µg/kg for up to 3 weeks, until a platelet count of 100,000/μL or more was achieved. The primary end point was reaching a platelet count of 100,000/μL or greater within 3 weeks of enrollment. Patients in the romiplostim arm whose platelet counts were corrected at or before 3 weeks successfully reached the primary end point. Observation patients whose platelet counts were corrected within 3 weeks achieved spontaneous correction, a primary end point, and completed study participation.
If a romiplostim-treated patient’s platelet count recovered to 100,000/μL or more at or before 3 weeks, chemotherapy could be resumed. During resumption of chemotherapy, weekly romiplostim was continued and titrated to maintain a target platelet count of 100,000 to 200,000/μL. The protocol allowed for romiplostim administration on the same day as chemotherapy. A prespecified secondary end point was toleration of resumption of chemotherapy for at least 8 weeks or two cycles without subsequent chemotherapy dose reduction or dose delay because of recurrent CIT. Patients who received romiplostim and demonstrated clinical benefit during resumed chemotherapy could continue romiplostim treatment as long as it was felt to be beneficial.
At 3 weeks after enrollment, observation patients whose platelet counts were not corrected were eligible to cross over to receive romiplostim. Correction of thrombocytopenia within 3 weeks of crossover romiplostim was a prespecified secondary end point.
A safety end point was development of a venous thromboembolism or other thrombotic event while receiving romiplostim treatment. A second safety end point was development of a secondary hematologic malignancy. At 16 weeks of receiving romiplostim, patients underwent cytogenetic testing and FISH from bone marrow biopsy or peripheral blood to evaluate for secondary hematologic malignancy. Patients also had CBC monitoring, including peripheral smear review, at least every 3 weeks as long as they continued to receive romiplostim to evaluate for evidence of secondary hematologic malignancy.
Because this was an open-label study, efficacy and safety were tracked in real time. An interim analysis, not planned in the initial study design, was conducted when eight observation and 15 romiplostim patients reached the primary end point. In consultation with the institutional review board, it was felt inappropriate to continue the observation arm because of the promising results observed in the romiplostim arm, and the study was converted to a single-arm, romiplostim treatment study for the remaining 37 patients.
End Point Assessment
The primary end point was reaching a platelet count of 100,000/μL or greater within 3 weeks of enrollment, either by romiplostim treatment or spontaneous correction. Toleration of resumption of chemotherapy while receiving romiplostim maintenance for at least 8 weeks or two cycles without subsequent chemotherapy dose reduction or dose delay because of recurrent isolated CIT was a secondary end point. Other secondary end points included requirement of platelet transfusion, development of venous or arterial thrombosis while receiving romiplostim, and crossover response in patients initially in the observation control group. Other toxicities and death from all causes were recorded.
Study Oversight
The study was approved by the MSK Institutional Review Board/Privacy Board. Written informed consent was obtained from all participants. The study was performed in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The MSK Data and Safety Monitoring Committee provided oversight of the study. This article was written by the authors with input from an editor employed by MSK, and all authors provided feedback. All authors reviewed the final article and confirmed that the data herein are accurate and complete.
Statistical Analysis
The study was designed as a randomized phase II study of romiplostim compared with observation. The primary end point was a platelet count of 100,000/μL or greater within 3 weeks after randomization. A total of 60 patients were to be randomly assigned 2:1 (40 romiplostim to 20 observation alone). On the basis of preliminary data, the probability of a spontaneous recovery in the observation arm was estimated to be 60% or less; the romiplostim arm would be considered promising if the recovery probability was 90% or higher. Using these rates, the study had more than 80% power using a one-sided test of binomial proportions at an alpha level of .10. The analysis population was intent to treat. The two arms were compared at the interim analysis using Fisher’s exact test (R statistical package).
RESULTS
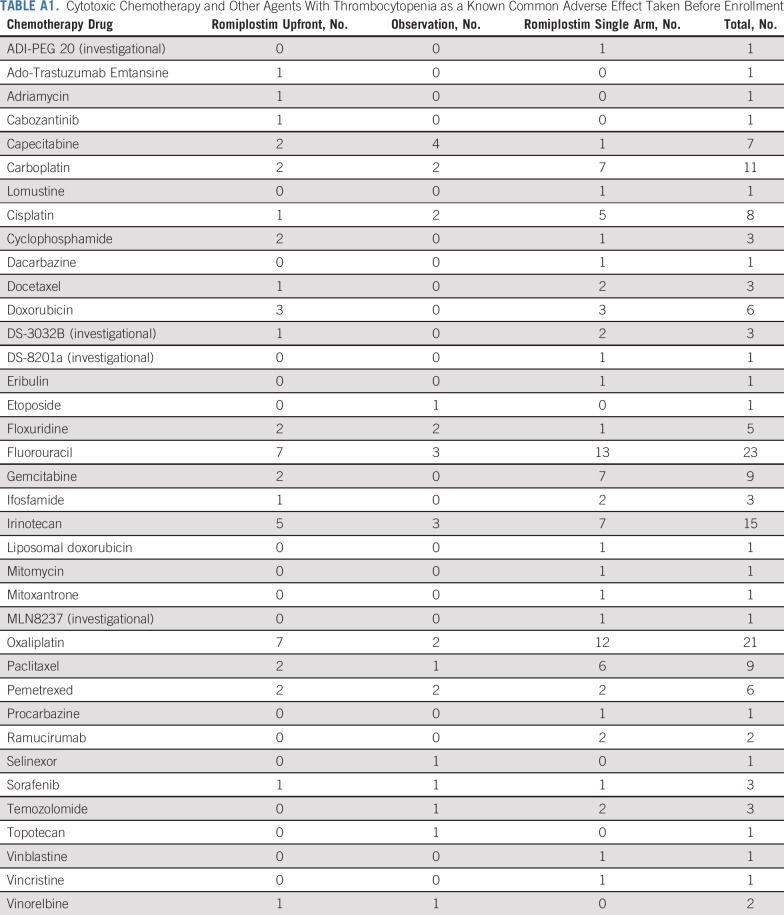
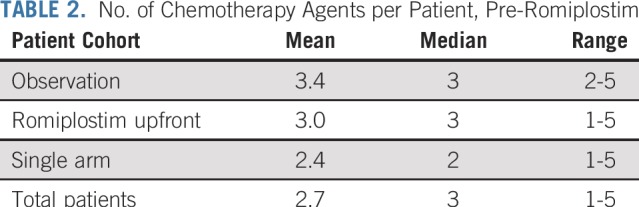
Sixty patients were enrolled. The baseline characteristics are listed in Table 1. There were equal numbers of men and women, and the median age was 58 years. More than half of the patients had a primary GI malignancy. More than three fourths had metastatic disease, and approximately half of the patients had primary or metastatic disease involving the liver. The median number of prior cytotoxic chemotherapy drugs was three and was similar between the romiplostim-treated and observation arms (Table 2). All chemotherapy drugs taken before enrollment are listed in Appendix Table A1 (online only). The spectrum of prior chemotherapy reflected the cancers being treated. No regimen seemed to be a particular risk factor for development of CIT.
TABLE 1.
Patient Characteristics
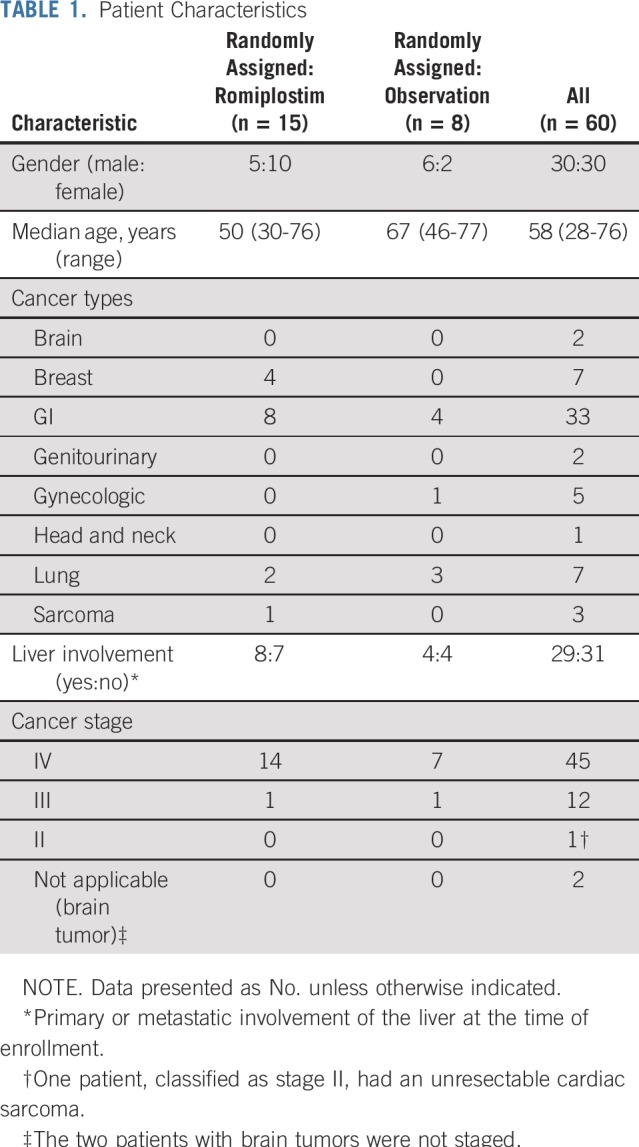
TABLE 2.
No. of Chemotherapy Agents per Patient, Pre-Romiplostim
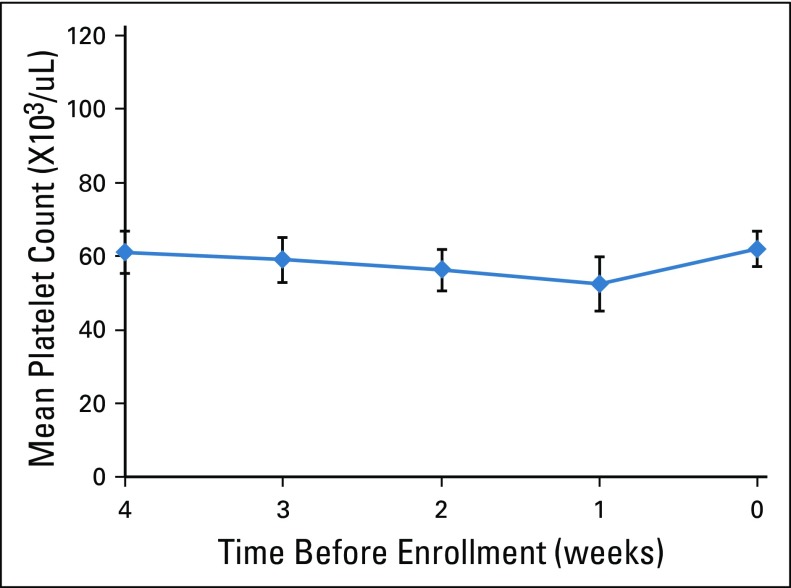
The mean platelet counts in the 4 weeks before enrollment are shown in Figure 1. Patients had been off chemotherapy for at least 14 days, with no evidence of spontaneous improvement. The eligibility criteria were designed to identify patients with thrombocytopenia leading to chemotherapy dose modification, as opposed to pancytopenia. At enrollment, the mean absolute neutrophil count was 3,200/μL (range, 1,100 to 10,400/μL), and the mean hemoglobin was 11.7 gm/dL (range, 8.2 to 15.3/μL).
FIG 1.
Platelet counts before enrollment. Counts are mean and 95% CI.
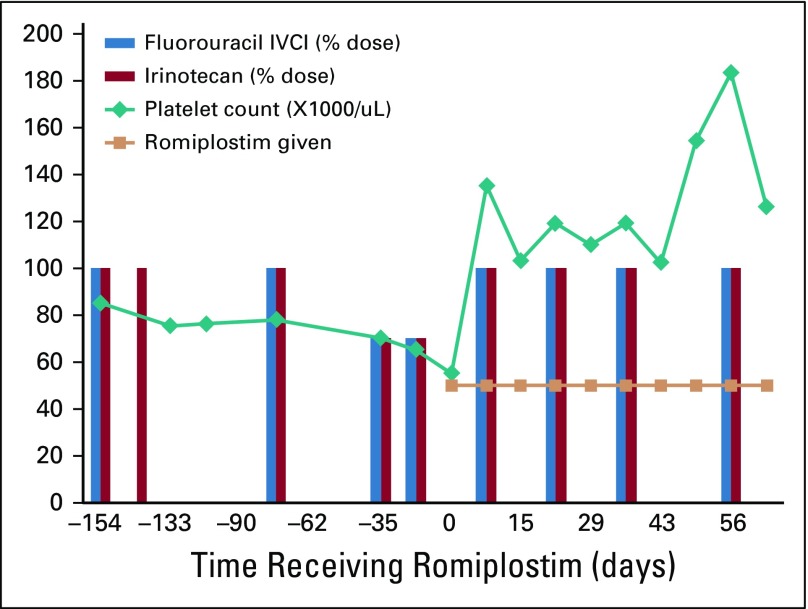
During the initial randomization phase of the trial, patients were randomly assigned 2:1 to weekly, dose-titrated romiplostim or observation control. Romiplostim was titrated with a target of 100,000 to 200,000/μL. In patients who responded to romiplostim (≥ 100,000/μL), weekly romiplostim was maintained to allow for resumption of chemotherapy as a secondary end point. The course of one representative romiplostim-treated patient is illustrated in Figure 2.
FIG 2.
Platelet counts and relative dose intensity of chemotherapy in a representative patient before and during romiplostim treatment. Patient No. 12 was a 29-year-old man with metastatic colon cancer involving the liver and lungs. Before enrollment, he experienced chemotherapy-induced thrombocytopenia despite reduction in doses and delay of fluorouracil and irinotecan. His platelet count improved from 55,000 to 135,000 per μL after 1 week of romiplostim treatment, and he resumed full-dose fluorouracil and irinotecan without recurrent chemotherapy-induced thrombocytopenia for 34 months while receiving treatment. At 3 years of romiplostim treatment with ongoing chemotherapy, he died as a result of his cancer. IVCI, continuous infusion.
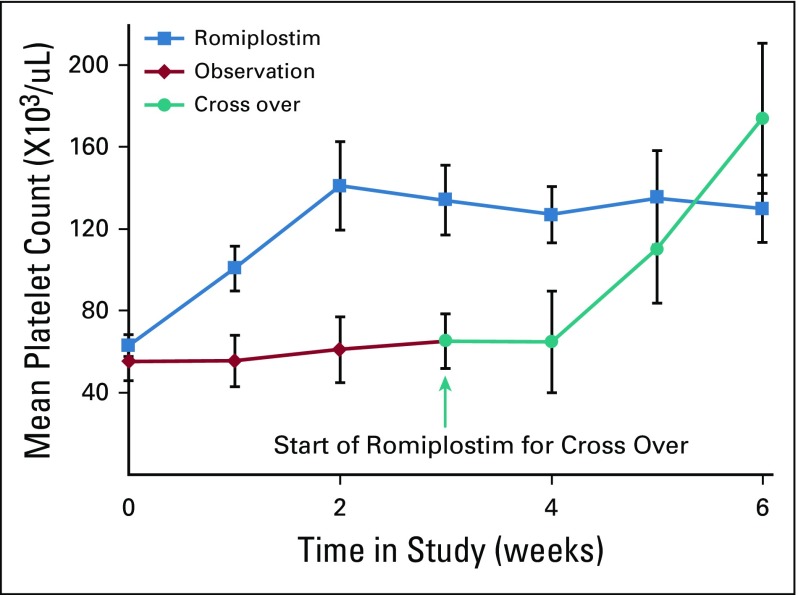
When eight observation and 15 romiplostim patients reached the primary end point, an interim analysis was performed. Fourteen of the 15 romiplostim patients (93%) achieved the primary end point of corrected platelet counts (≥ 100,000/μL) within 3 weeks. Only one of the eight observation patients (12.5%) had a spontaneously corrected platelet count. The platelet responses in the romiplostim and observation arms are listed in Table 3 and shown in Figure 3. In the romiplostim patients, the mean platelet count increased from 63 to 141,000/μL within 2 weeks, well within the range to allow for safe resumption of chemotherapy. There was no additional increase in the mean platelet count after the second week, because subsequent values reflected romiplostim dose titration as well as resumption of chemotherapy.
TABLE 3.
Primary End Point (ITT): Interim Analysis, Randomization Phase

FIG 3.
Platelet counts during the trial, 0 to 6 weeks; counts are mean and 95% CI. These results are for all romiplostim-treated patients, including those in the randomization phase as well as the single-arm phase. The platelet counts of romiplostim-treated and observation-control patients diverged by 1 week, and the differences persisted for 3 weeks, through the primary end point. Once the control patients crossed over to romiplostim, their platelet counts increased at a rate similar to romiplostim upfront.
There was no significant increase in platelet counts in the observation arm during the initial 3 weeks after enrollment. Two of the observation patients required platelet transfusions during the 3 weeks of the observation period, whereas none of the romiplostim-treated patients required platelet transfusions.
One observation patient with metastatic cervical cancer experienced spontaneous correction of her platelet count (103,000/μL) within the first 3 weeks after enrollment. Per protocol, as she reached the primary end point of spontaneous correction, she completed study participation. She later resumed chemotherapy but rapidly had recurrence of CIT. The remaining seven observation patients, who had failed to achieve the primary end point of platelet correction within 3 weeks, then received romiplostim. Six of the seven crossover patients achieved correction of their platelet counts within 3 weeks (Fig 3). One patient did not complete 3 weeks of romiplostim after crossover because of acute clinical deterioration and death.
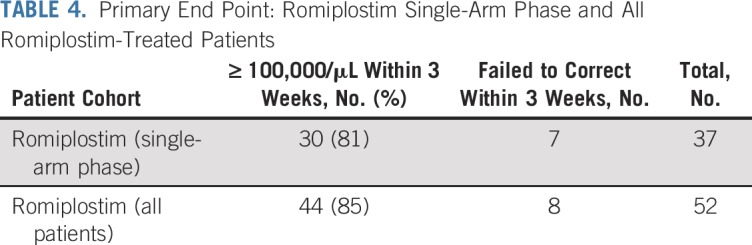
On the basis of strong significance of benefit with romiplostim (P < .001), the lack of evidence that with additional time the untreated control patients would experience corrected thrombocytopenia, and the differential requirements for platelet transfusions, in consultation with and approval by the institutional review board, the study was converted to a single-arm, romiplostim, open-label study. The final primary end point data are listed in Table 4. Eighty-five percent of all patients treated with romiplostim (44 of 52) achieved corrected platelet counts within 3 weeks. Of note, of the eight patients who were treated with romiplostim and who failed to achieve corrected platelet counts within 3 weeks, one developed sepsis before completing 3 weeks of treatment and two were in violation of protocol (chemotherapy was inappropriately resumed before achievement of the primary end point).
TABLE 4.
Primary End Point: Romiplostim Single-Arm Phase and All Romiplostim-Treated Patients
The secondary efficacy end point was resumption of chemotherapy with romiplostim maintenance for at least two cycles or 8 weeks without recurrence of chemotherapy dose reduction or dose delay as a result of recurrent CIT. Forty-four patients who achieved platelet correction within 3 weeks of romiplostim treatment, either in romiplostim upfront or crossover, resumed chemotherapy with continuation of weekly romiplostim. Twenty-eight of the patients (64%) resumed the same chemotherapy regimen that had led to CIT, whereas 16 (36%) resumed a different chemotherapy regimen. Of the 19 patients who had had reduction in chemotherapy dose before enrollment, 11 (58%) resumed full-dose or increased-dose chemotherapy. Only three patients (6.8%) experienced chemotherapy dose reduction or dose delay as a result of recurrent CIT within the specified time period. Nine other patients failed to tolerate resumption of chemotherapy for at least two cycles or 8 weeks for other reasons: two had pancytopenia, one had nonhematologic dose-limiting toxicity and pancytopenia, five had nonhematologic toxicity, and one died before 8 weeks or chemotherapy. Twenty-eight of the 44 patients (64%) who resumed chemotherapy continued taking romiplostim for more than 6 months, and 12 of the 44 (27%) continued for more than 1 year. The longest exposure to romiplostim was 34 months.
The mean dose of romiplostim required to correct the platelet count was 2.6 mcg/kg (95% CI, 2.4 to 2.8 mcg/kg; range, 1.9 to 4.43 mcg/kg), and the mean dose to maintain platelet count during the resumption of chemotherapy was 3.3 mcg/kg (95% CI, 2.7 to 3.8 mcg/kg; range, 0.7 to 7.4 mcg/kg). No patient became refractory or resistant to romiplostim.
Six of the 59 patients (10.2%) developed a VTE during the first 12 months of romiplostim treatment: two had pulmonary emboli, two had proximal deep vein thrombosis, and two had calf vein deep vein thrombosis. Romiplostim was not discontinued on the development of a VTE. One patient with metastatic non–small-cell lung cancer experienced a myocardial infarction 3 months into romiplostim treatment and also developed an ischemic stroke 15 months into romiplostim treatment in the context of brain metastases. No other patient receiving romiplostim experienced a myocardial infarction or cerebral ischemic event.
Patients were monitored for development of a secondary hematologic malignancy, with cytogenetic testing and FISH from bone marrow biopsy or peripheral blood at 16 weeks in the study. None were positive. In addition, routine CBC with differential was monitored weekly while receiving romiplostim, and no patient demonstrated evidence of myelodysplasia or leukemia.
DISCUSSION
In this prospective study, we demonstrated that romiplostim is effective in correcting CIT, compared with observation. Our eligibility criteria were designed to identify patients with CIT who were most likely to benefit from romiplostim, those with adequate neutrophils and hemoglobin with standard supportive measures, yet had persistent thrombocytopenia. Patients with extensive bone metastases were also excluded. In the 4 weeks leading up to enrollment, the thrombocytopenia was stable, indicating that the patients were not simply recovering from their nadir.
In an interim analysis, the success rate of romiplostim in correcting the platelet count to 100,000/μL within 3 weeks was 93%, whereas there was little evidence of spontaneous correction with observation. On additional enrollment in the romiplostim single-arm phase, a similar response rate was observed. Romiplostim was also effective in preventing recurrence of CIT. When patients successfully achieved the primary end point of platelet count correction and then resumed chemotherapy with romiplostim support, only 6.8% of patients experienced chemotherapy dose reduction or delay as a result of recurrent CIT within the specified initial two cycles or 8 weeks of chemotherapy. The doses of romiplostim necessary to correct and maintain the platelet counts were similar to the regimens required for treatment of immune thrombocytopenic purpura.20,21 We also saw no evidence of refractoriness in the form of requirement for increasing doses of romiplostim or loss of response.
Patients with cancer, particularly those receiving active chemotherapy, are known to be at particular risk for thrombosis. Khorana et al22 reported that 12.6% of patients in a solid tumor cohort experienced a VTE within 12 months. In our study, 10.2% of patients with solid tumors experienced a VTE within 12 months. Acknowledging differences in patient characterizations between the Khorana et al22 cohort and our study patients, we believe this does not suggest a significantly increased risk of VTE with titrated romiplostim in patients with cancer.
Romiplostim may be of benefit in a wide range of solid tumor types and after a wide range of past chemotherapy. However, a key signal toward particular need is for patients with primary or metastatic involvement of the liver. Almost half of the patients enrolled in our study had liver involvement from their cancer. Because TPO is made in the liver,12 liver involvement with cancer could lead to relative TPO deficiency, thrombocytopenia, and susceptibility to CIT.
How romiplostim treatment will fit into the wider spectrum of cancer therapy is yet to be determined, and possible options need to be considered. Our approach in this study was to identify those patients with established CIT, rather than in a prophylaxis setting. This was driven, in part, by our desire to derive a strategy in which the ratio of treatment benefit to cost was highest.
A key question for the future is whether potential improved RDI by treatment of CIT leads to improved disease control. Because there are many reasons a patient may not tolerate full-dose chemotherapy, correcting thrombocytopenia may be necessary but not sufficient to allow for improved RDI. Additional studies of romiplostim, focusing on a population with a relatively specific RDI limitation by thrombocytopenia, may lead to the most appropriate use. Reasonable target populations would be patients with primary or metastatic cancer in the liver or possibly cancers where intensive chemotherapy is associated with improved long-term survival, such as testicular cancers. Our study has provided encouraging evidence that CIT can be managed effectively with romiplostim in many patients with cancer. How to use this supportive care measure will require additional study.
APPENDIX
TABLE A1.
Cytotoxic Chemotherapy and Other Agents With Thrombocytopenia as a Known Common Adverse Effect Taken Before Enrollment
Footnotes
Supported by Amgen, Investigator Initiated Study, and National Institutes of Health/National Cancer Institute Cancer Center Support Grant No. P30 CA008748.
Clinical trial information: NCT02052882.
AUTHOR CONTRIBUTIONS
Conception and design: Gerald A. Soff, Rekha Parameswaran, Nancy E. Kemeny
Administrative support: Gemma Bendheim
Provision of study materials or patients: Gerald A. Soff, Nancy E. Kemeny
Collection and assembly of data: Gerald A. Soff, Yimei Miao, Gemma Bendheim, Jeanette Batista, Jodi V. Mones, Rekha Parameswaran, Cy R. Wilkins, Ghassan K. Abou-Alfa, Andrea Cercek, Debra M. Sarasohn
Data analysis and interpretation: Gerald A. Soff, Yimei Miao, Gemma Bendheim, Cy R. Wilkins, Sean M. Devlin, Ghassan K. Abou-Alfa, Andrea Cercek, Debra M. Sarasohn, Simon Mantha
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Romiplostim Treatment of Chemotherapy-Induced Thrombocytopenia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Gerald A. Soff
Consulting or Advisory Role: Amgen, Janssen Scientific Affairs, Dova Pharmaceuticals, Bristol-Myers Squibb, Pfizer, Bayer Pharmaceuticals
Research Funding: Amgen, Janssen Scientific Affairs
Travel, Accommodations, Expenses: Amgen, Bayer
Yimei Miao
Employment: AArete
Consulting or Advisory Role: AArete
Gemma Bendheim
Travel, Accommodations, Expenses: Amgen
Jodi V. Mones
Consulting or Advisory Role: Gerson Lehman Group, Third Bridge Consulting
Research Funding: Janssen Pharmaceuticals
Ghassan K. Abou-Alfa
Consulting or Advisory Role: Astellas Pharma, Celsion, Celgene, Sanofi, Silenseed (I), Sillajen, Boston Scientific, CASI Pharmaceuticals, Onxeo, Roche, Bristol-Myers Squibb, EMD Serono (I), Gilead Sciences (I), Vicus Therapeutics (I), Servier, Agios, Aslan Pharmaceuticals, Bayer, Delcath Systems, Eisai, Halozyme, Ipsen, Merck Serono, Sirtex Medical, AstraZeneca, Medimmune, Amgen, Antengene, Aptus Clinical, Carsgen Therapeutics, CytomX Therapeutics (I), Daiichi Sankyo, Debiopharm Group, Exelixis, Halozyme, Inovio Pharmaceuticals, PCI Biotech, Sirtex Medical, Yakult, 3DMedcare, Alignmed, BeiGene, BiolineRx (I), BridgeBio Pharma, Cipla, Genoscience Pharma, Hengrui Pharmaceutical, Jazz Pharmaceuticals, Kyowa Hakko Kirin, Janssen (I), LAM Therapeutics, Lilly, Loxo (I), Mina, Newlink Genetics (I), Novella Clinical, PCI Biotech, Pfizer (I), Pharmacyte Biotech (I), Pharmacyclics (I), Pieris Pharmaceuticals (I), QED, RedHill Biopharma, Sobi (I), Targovax (I), Tekmira, Tekmira, twoXAR, Yakult, Yiviva
Research Funding: Bayer (Inst), Exelixis (Inst), Genentech (Inst), Roche (Inst), CASI Pharmaceuticals (Inst), MedImmune (Inst), AstraZeneca (Inst), Bristol-Myers Squibb (Inst), Incyte (Inst), MabVax (Inst), Momenta Pharmaceuticals (Inst), OncoMed (Inst), Agios (Inst), Array BioPharma (Inst), Celgene (Inst), Lilly (Inst), Novartis (Inst), Acta Biologica (Inst), BeiGene (Inst), Halozyme (Inst), Polaris (Inst), OncoQuest (Inst), Puma Biotechnology (Inst), QED (Inst)
Travel, Accommodations, Expenses: Polaris
Andrea Cercek
Consulting or Advisory Role: Bayer, Proteus Digital Health
Research Funding: Bayer, AbbVie, Seattle Genetics
Nancy E. Kemeny
Research Funding: Amgen
Simon Mantha
Research Funding: Janssen
Other Relationship: Daboia Consulting
No other potential conflicts of interest were reported.
REFERENCES
- 1.Denduluri N, Patt DA, Wang Y, et al. Dose delays, dose reductions, and relative dose intensity in patients with cancer who received adjuvant or neoadjuvant chemotherapy in community oncology practices. J Natl Compr Canc Netw. 2015;13:1383–1393. doi: 10.6004/jnccn.2015.0166. [DOI] [PubMed] [Google Scholar]
- 2.Wu Y, Aravind S, Ranganathan G, et al. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: A descriptive study of a large outpatient oncology practice database, 2000-2007. Clin Ther. 2009;31:2416–2432. doi: 10.1016/j.clinthera.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Dale DC, Crawford J, Klippel Z, et al. A systematic literature review of the efficacy, effectiveness, and safety of filgrastim. Support Care Cancer. 2018;26:7–20. doi: 10.1007/s00520-017-3854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ten Berg MJ, van den Bemt PM, Shantakumar S, et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: Results from a retrospective hospital-based cohort study. Drug Saf. 2011;34:1151–1160. doi: 10.2165/11594310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Elting LS, Rubenstein EB, Martin CG, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19:1137–1146. doi: 10.1200/JCO.2001.19.4.1137. [DOI] [PubMed] [Google Scholar]
- 6.Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: A nationwide study of community practices. J Clin Oncol. 2003;21:4524–4531. doi: 10.1200/JCO.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Aspinall SL, Good CB, Zhao X, et al. Adjuvant chemotherapy for stage III colon cancer: Relative dose intensity and survival among veterans. BMC Cancer. 2015;15:62. doi: 10.1186/s12885-015-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanna RK, Poniewierski MS, Laskey RA, et al. Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi-agent chemotherapy for epithelial ovarian cancer. Gynecol Oncol. 2013;129:74–80. doi: 10.1016/j.ygyno.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Havrilesky LJ, Reiner M, Morrow PK, et al. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol. 2015;93:203–210. doi: 10.1016/j.critrevonc.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama G, Tanaka C, Uehara K, et al. The impact of dose/time modification in irinotecan- and oxaliplatin-based chemotherapies on outcomes in metastatic colorectal cancer. Cancer Chemother Pharmacol. 2014;73:847–855. doi: 10.1007/s00280-014-2416-x. [DOI] [PubMed] [Google Scholar]
- 11. Goodnough LT, DiPersio JF: Issues in the management of cancer-related thrombocytopenia. Oncology (Williston Park) 16:1558-1567, 2002. [PubMed]
- 12.Kuter DJ. Biology and chemistry of thrombopoietic agents. Semin Hematol. 2010;47:243–248. doi: 10.1053/j.seminhematol.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Yang C, Xia Y, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98:3241–3248. doi: 10.1182/blood.v98.12.3241. [DOI] [PubMed] [Google Scholar]
- 14.Kim ES. Lusutrombopag: First global approval. Drugs. 2016;76:155–158. doi: 10.1007/s40265-015-0525-4. [DOI] [PubMed] [Google Scholar]
- 15.Shirley M. Avatrombopag: First global approval. Drugs. 2018;78:1163–1168. doi: 10.1007/s40265-018-0949-8. [DOI] [PubMed] [Google Scholar]
- 16.Vadhan-Raj S, Hagemeister F, Fayad LE, et al. Randomized, double-blind, placebo-controlled, dose and schedule-finding study of AMG 531 in chemotherapy-induced thrombocytopenia (CIT): Results of a phase I/II study. Blood. 2010;116:1544. [Google Scholar]
- 17. Vadhan-Raj S, Trent J, Araujo DM, et al: Evaluation of AMG 531 in chemotherapy-induced thrombocytopenia (CIT): Results of a phase I/II study. J Clin Oncol 27, 2009 (abstr e20616) [Google Scholar]
- 18.Parameswaran R, Lunning M, Mantha S, et al. Romiplostim for management of chemotherapy-induced thrombocytopenia. Support Care Cancer. 2014;22:1217–1222. doi: 10.1007/s00520-013-2074-2. [DOI] [PubMed] [Google Scholar]
- 19.Al-Samkari H, Marshall AL, Goodarzi K, et al. The use of romiplostim in treating chemotherapy-induced thrombocytopenia in patients with solid tumors. Haematologica. 2018;103:e169–e172. doi: 10.3324/haematol.2017.180166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bussel JB, Hsieh L, Buchanan GR, et al. Long-term use of the thrombopoietin-mimetic romiplostim in children with severe chronic immune thrombocytopenia (ITP) Pediatr Blood Cancer. 2015;62:208–213. doi: 10.1002/pbc.25136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cines DB, Gernsheimer T, Wasser J, et al. Integrated analysis of long-term safety in patients with chronic immune thrombocytopaenia (ITP) treated with the thrombopoietin (TPO) receptor agonist romiplostim. Int J Hematol. 2015;102:259–270. doi: 10.1007/s12185-015-1837-6. [DOI] [PubMed] [Google Scholar]
- 22.Khorana AA, Dalal M, Lin J, et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648–655. doi: 10.1002/cncr.27772. [DOI] [PubMed] [Google Scholar]