Abstract
Background
Periconceptional folic acid prevents neural tube defects (NTDs), but it is uncertain whether there are benefits for offspring neurodevelopment arising from continued maternal folic acid supplementation beyond the first trimester. We investigated the effect of folic acid supplementation during trimesters 2 and 3 of pregnancy on cognitive performance in the child.
Methods
We followed up the children of mothers who had participated in a randomized controlled trial in 2006/2007 of Folic Acid Supplementation during the Second and Third Trimesters (FASSTT) and received 400 μg/d folic acid or placebo from the 14th gestational week until the end of pregnancy. Cognitive performance of children at 7 years was evaluated using the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III) and at 3 years using the Bayley’s Scale of Infant and Toddler Development (BSITD-III).
Results
From a total of 119 potential mother-child pairs, 70 children completed the assessment at age 7 years, and 39 at age 3 years. At 7 years, the children of folic acid treated mothers scored significantly higher than the placebo group in word reasoning: mean 13.3 (95% CI 12.4–14.2) versus 11.9 (95% CI 11.0–12.8); p = 0.027; at 3 years, they scored significantly higher in cognition: 10.3 (95% CI 9.3–11.3) versus 9.5 (95% CI 8.8–10.2); p = 0.040. At both time points, greater proportions of children from folic acid treated mothers compared with placebo had cognitive scores above the median values of 10 (girls and boys) for the BSITD-III, and 24.5 (girls) and 21.5 (boys) for the WPPSI-III tests. When compared with a nationally representative sample of British children at 7 years, WPPSI-III test scores were higher in children from folic acid treated mothers for verbal IQ (p < 0.001), performance IQ (p = 0.035), general language (p = 0.002), and full scale IQ (p = 0.001), whereas comparison of the placebo group with British children showed smaller differences in scores for verbal IQ (p = 0.034) and full scale IQ (p = 0.017) and no differences for performance IQ or general language.
Conclusions
Continued folic acid supplementation in pregnancy beyond the early period recommended to prevent NTD may have beneficial effects on child cognitive development. Further randomized trials in pregnancy with follow-up in childhood are warranted.
Trial registration
ISRCTN ISRCTN19917787. Registered 15 May 2013.
Keywords: Prenatal folic acid, Pregnancy, Cognitive performance, Child, Randomized controlled trial, Public health, Wechsler Preschool and Primary Scale of Intelligence
Background
Folate plays a crucial role in pregnancy and fetal development as it is essential for cell division and tissue growth, by acting as the key co-factor in one-carbon metabolism and therefore required for nucleotide biosynthesis, amino acid metabolism, and numerous methylation reactions [1]. Conclusive scientific evidence has existed for over 25 years that periconceptional folic acid (FA; the synthetic vitamin form) can protect against neural tube defects (NTDs) [2, 3], but apart from the early pregnancy period targeted for preventing NTD, maternal folate may have other roles in offspring health and particularly in relation to neurodevelopment in the child [4–9]. Folate is recognized among key nutrients required for the formation and development of fetal brain [10] owing to its involvement in the proliferation and growth of neuronal cells and the synthesis of neurotransmitters [11]. Experimental evidence from in vivo studies shows that there is active placental transport of folate and elevated total folate concentrations are found in the brain during early fetal development [12, 13], while prenatal folate deficiency was shown in animal models to decrease progenitor cell proliferation, increase apoptosis, and provoke structural changes in the brain [14, 15]. Observational studies in humans have linked self-reported FA supplement use in the first trimester of pregnancy with better cognitive performance [6–9] and specifically with improved vocabulary and verbal skills [8] in children. Of particular note, a large study of almost 40,000 children in the USA found a lower rate of severe language delay in children at 3 years whose mothers reported taking FA in the first trimester of pregnancy [7]. Maternal FA supplement use was also associated with lower risk of child behavioral and emotional problems [16, 17]. One study, however, found no evidence in secondary analysis that maternal FA supplementation up to the eighth gestational week was associated with long-term somatic and mental development in children [18], albeit this was designed to investigate the effect of multivitamin supplementation on NTD risk and therefore focused on very early pregnancy only, possibly explaining the lack of relationship of FA use with child cognition.
Later pregnancy (i.e., 24–42 gestational weeks) is recognized to be a crucial period for brain growth [19], but far fewer human studies have examined maternal folate status at this time in relation to subsequent cognitive performance in childhood. One study over 40 years ago however reported that children born to mothers with a diagnosis of folate-related megaloblastic anemia during the third trimester of pregnancy had abnormal neurodevelopment and lower intellectual abilities compared with infants born to mothers with optimal folate status [4]. Much more recently, a longitudinal study of 256 mother-child pairs linked maternal folate deficiency diagnosed at the start of the second trimester with reduced brain volume in the children at 6–8 years, as measured using magnetic resonance imaging (MRI) [20]. Correspondingly, higher plasma folate concentrations at the 30th gestational week were associated with better cognitive performance in over 500 children in South India sampled at age 9–10 years [5]. However, the evidence is not entirely consistent, with two longitudinal observational studies from Canada and the USA, respectively, reporting no significant associations of maternal folate biomarkers sampled at several time points between the 16th and the 37th gestational weeks with infant neurodevelopment [21] or cognitive performance of children at age 5 years [19].
Although the totality of evidence generally supports an association of maternal folate during pregnancy with neurodevelopment and cognitive performance in the first decade of life, it is not clear if this relationship is causative as the evidence is drawn from observational studies, often relying on FA supplement use in pregnancy as reported by the mother; information typically collected retrospectively (at time of assessing the child) and thus with a high risk of recall bias. Therefore, it is uncertain whether there are any benefits for the offspring brain arising from continued maternal folic supplementation beyond the first trimester. Accordingly, we conducted a follow-up study of children whose mothers had participated in a randomized controlled trial (RCT) of FA during trimesters 2 and 3 of pregnancy to investigate the effect of maternal FA supplementation on the subsequent cognitive performance in the child.
Methods
Study population
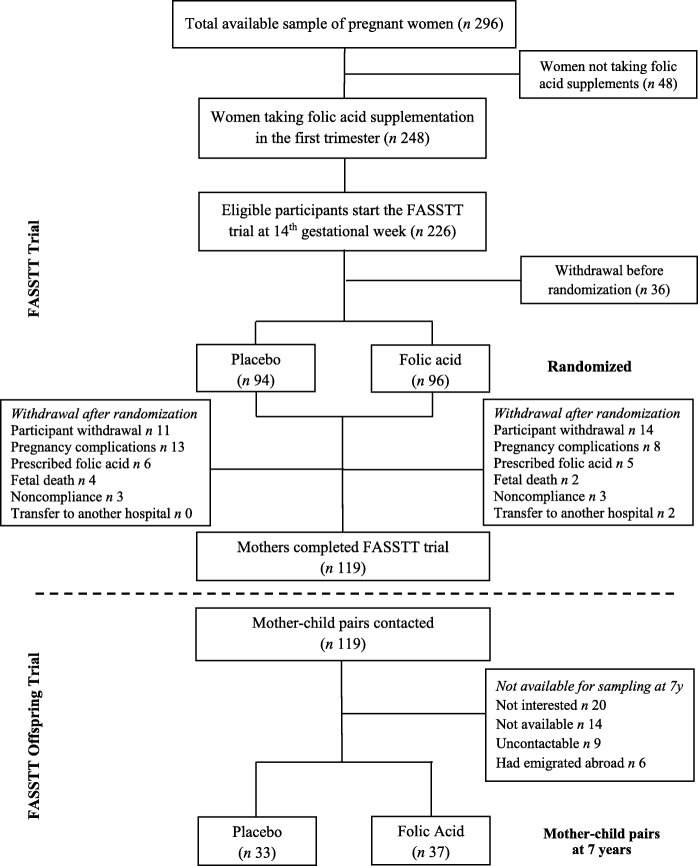
This was a follow-up investigation of children whose mothers took part in the Folic Acid Supplementation during the Second and Third Trimesters (FASSTT) trial in pregnancy. The original FASSTT trial conducted in 2006/2007 has been described in detail elsewhere [22]. Briefly for the purpose of this report, healthy pregnant women, aged 18–35 years with a singleton pregnancy and who had taken the recommended dose of 400 μg/d of FA in the first trimester, were recruited from antenatal clinics at the 14th week of gestation (Fig. 1). At the start of the second trimester, eligible participants were randomly assigned to take 400 μg/d FA or placebo for 26 weeks, of which 59 women in the treatment group and 60 in the placebo group completed the trial. FA supplements were distributed (in 7-day pillboxes) to participants every 4 weeks. Based on the recording of unused tablets, an overall participant compliance rate of 93% was estimated.
Fig. 1.
Flowchart showing the study population
The FASSTT Offspring Trial was approved by the Office for Research Ethics Committees Northern Ireland and was registered (ISRCTN19917787). Participants from the original FASSTT trial were sent an invitation letter, and in a follow-up telephone call, those who verbally consented to take part were given an appointment to attend the Nutrition Innovation Centre for Food and Health at Ulster University. In compliance with ethical requirements, signed written consent from the mother and assent from the child were obtained at the time of the appointment. All efforts were made to recruit the maximum number of participants from the original FASSTT trial. If current contact details were not readily available from our records, the new participant details were traced through health records. Those who failed to attend their appointment were offered an alternative date (up to a maximum of five appointments).
Measurements of the FASSTT Offspring Trial
Cognitive tests
Cognitive performance of the children at age 7 years was completed by a trained researcher using the Wechsler Preschool and Primary Scale of Intelligence test 3rd UK edition (WPPSI-III). This validated clinical instrument for assessing children (up to age 7 years and 3 months) provides composite scores representing intellectual functioning in specified cognitive domains (i.e., verbal IQ, performance IQ, and processing speed) and an overall score for full scale IQ. Each assessment lasted 40–50 min and was completed by the same researcher in one session. To provide an appropriate environment for assessing the child, the room was well-lit, ventilated, and free from distractions, with the researcher seated directly opposite the child and the mother outside of the child’s view. Both researcher and participants were blinded to treatment allocations in the original FASSTT trial.
Prior to conducting the main assessment of the children at 7 years, a pilot investigation of participants at 3 years was conducted using the Bayley’s Scales of Infant and Toddler Development 3rd Edition (BSITD-III), the most frequently used developmental test for infants and young children of 1–42 months, which includes an assessment of cognitive performance, along with other developmental domains such as receptive and expressive communication, and fine and gross motor skills.
Anthropometric measurements of the child
Height, weight, waist and head circumference, and body fatness were measured using standardized equipment on the day of the appointment. Height (cm) was measured using a portable, standalone stadiometer, with shoes removed. Weight (kg) was measured to the nearest 0.1 kg using electronic weighing scales, with any heavy clothing removed. Waist and head circumference (cm) were measured using a non-elastic measuring tape. Body mass index (BMI) was calculated as weight in kilograms/height in meters squared. Z-scores for BMI were calculated based on the Centre for Disease Control Growth Charts and using the LMS method, which involves the equation Z = ((BMI/M)^L − 1)/(L × S) [23]. The parameters of the formula include M which is the median BMI by age, S which corresponds to the coefficient of variation of BMI, and L which allows for age-dependent skewness in the distribution of body mass index [24]. For children aged 7 years, body fat measures were also obtained using the Tanita-305 body fat analyzer (Tanita Corp, Tokyo, Japan).
Laboratory assessment
Non-fasting blood samples, collected from the mother at 14th and 36th gestational weeks and umbilical cord blood at delivery, were analyzed for serum and red blood cell (RBC) folate by microbiological assay [25]. Methylenetetrahydrofolate reductase (MTHFR) C677T genotype was identified using polymerase chain reaction amplification, followed by HinfI digestion [26].
Statistical analyses
Estimation of the sample size for the current study was based on our pilot investigation in 39 children sampled at 3 years, where assessment scores for the cognitive domain in the BSITD-III test from children of mothers supplemented with folic acid or placebo were used. A sample size of 43 children in each group was estimated for the assessment at 7 years to detect a significant difference in cognitive performance between children of the mothers randomized to each treatment with a power of 80% at α = 0.05 [27].
Statistical analysis was performed using the Statistical Package for the Social Sciences software (version 21.0; SPSS UK Ltd. Chertsey, UK). For normalization purposes, variables were log transformed before analysis, as appropriate. Differences between placebo and treatment groups were analyzed using independent t tests for linear variables and chi-square tests for categorical variables. Raw cognitive scores were standardized for the child’s age at time of testing, and appropriate age-specific reference intervals were applied in adherence with test protocols. Analysis of covariance (ANCOVA) was used to test for differences in cognitive variables between treatment groups, with adjustment for relevant confounding factors, including maternal age and education attainment [28], child’s sex [29, 30], birth weight [31], and breastfeeding [28]. Multiple linear regression analysis was used to examine the predictors of cognitive performance in children at 7 years. Further analysis compared mean WPSSI-III composite tests scores for FASSTT Offspring Trial participants with test scores from a nationally representative sample of British children using a one-sample t test. The latter cohort, collected for the UK WPPSI-III Project, was specifically sampled to validate this test for use in the UK. During 2002–2003, children were sampled to represent the UK population according to the 2001 Census, in terms of geographic regions, sex, age, ethnicity, and parental educational level [32]. Of a total of 805 children of age 2 years 6 months to 7 years 3 months sampled for this validation project, the mean WPSSI-III scores for 41 children at age 7 years were used in the current analysis.
Results
Of 119 participants in the original FASSTT trial, 70 mother-child pairs completed the FASSTT Offspring Trial at 7 years, representing a 59% response rate. Of the 70 children sampled, 50% (n = 34) was previously also sampled for the pilot study at 3 years, along with 5 other children who were not available for the 7-year follow-up (providing a total of 39 children for assessment at 3 years). A comparison of responders and non-responders to follow-up at 7 years showed no significant differences in any general characteristic, including maternal age (p = 0.207), weeks of gestation at labor (p = 0.587), parity (p = 0.198), birth weight (p = 0.642), Apgar score at 5th minute (p = 0.760), or % breast fed from birth (p = 0.415). Likewise, there were no significant differences in general characteristics between treatment groups among non-responders (Additional file 1: Table S1). No adverse events were reported at any time during the FASSTT trial or at either of the follow-up phases of the study.
Study cohort characteristics and folate biomarkers in mothers and newborns
There were no significant differences between the placebo and treatment groups in general maternal or child characteristics (Table 1 and Additional file 1: Table S2). Pre-intervention (i.e., at the 14th gestational week), neither serum nor RBC folate, showed significant differences between the treatment groups, but both biomarkers were significantly higher in the FA group compared with placebo following intervention (Table 2). Analysis of the cord blood at delivery also showed significantly higher folate concentrations in the FA treatment group. The frequency of the MTHFR 677TT genotype (variant genotype for a common folate polymorphism) was not significantly different between the treatment groups among mothers or children.
Table 1.
General characteristics of FASSTT Offspring Trial participants at age 3 and 7 years
| Participants at 3 years (n = 39) | Participants at 7 years (n = 70) | |||
|---|---|---|---|---|
| Placebo (n = 16) | Folic acid (n = 23) | Placebo (n = 33) | Folic acid (n = 37) | |
| Maternal characteristics | ||||
| Age, yearsa | 27.1 (25.1, 29.0) | 28.8 (27.2, 30.4) | 28.0 (26.4, 29.6) | 29.4 (28.2, 30.7) |
| BMI, kg/m2 | 25.0 (22.9, 27.1) | 25.7 (22.8, 28.6) | 24.3 (23.1, 25.6) | 25.3 (23.5, 27.2) |
| Smoking in pregnancy, % | 38 | 22 | 12 | 19 |
| Alcohol use, % | 6 | 0 | 3 | 3 |
| Duration of folic acid use at time of enrolment, weeks | 13.5 (8.4, 18.6) | 12.0 (9.0, 15.0) | 13.6 (10.7, 16.4) | 12.8 (10.5, 15.1) |
| Iron supplement use, % | 25 | 22 | 18 | 27 |
| Married, % | 73 | 91 | 88 | 95 |
| Education attainment, yearsb | 19.2 (17.8, 20.6) | 19.7 (18.5, 20.9) | 19.6 (18.5, 20.6) | 19.5 (18.5, 20.4) |
| Homeowner, % | 60 | 76 | 79 | 76 |
| Parity (n) | 2.9 (2.5, 3.3) | 3.2 (2.7, 3.6) | 2.6 (2.3, 2.9) | 2.8 (2.5, 3.1) |
| Week of gestation at labor | 40.0 (39.3, 40.7) | 40.0 (39.3, 40.6) | 40.2 (40.0, 40.7) | 39.9 (3.94, 40.3) |
| Child characteristics at birth | ||||
| Sex, female % | 75 | 65 | 48 | 59 |
| Birth weight, g | 3392 (3144, 3640) | 3317 (3029, 3605) | 3519 (3323, 3716) | 3374 (3188, 3559) |
| Birth length, cm | 51.1 (49.9, 52.4) | 50.6 (49.6, 51.7) | 51.4 (50.4, 52.4) | 50.8 (50.1, 51.5) |
| Head circumference, cm | 34.4 (33.6, 35.2) | 34.4 (33.6, 35.2) | 34.6 (34.1, 35.2) | 34.6 (34.1, 35.1) |
| Apgar at 1st minute | 8.3 (7.6, 8.9) | 8.5 (8.2, 8.8) | 8.5 (8.1, 8.9) | 8.7 (8.5, 8.8) |
| Apgar at 5th minute | 9.0 (9.0, 9.0) | 9.0 (8.9, 9.1) | 9.0 (8.8, 9.1) | 9.0 (8.9, 9.1) |
| Breastfed from birth, % | 56 | 44 | 39 | 46 |
| Child characteristics at follow-up | ||||
| Age at assessment, years | 2.8 (2.5, 3.0) | 2.7 (2.6, 2.9) | 6.8 (6.7, 6.9) | 6.7 (6.7, 6.8) |
| BMI Z-scorec | 0.55 (− 0.39, 1.48) | 0.19 (− 0.19, 0.57) | 0.24 (− 0.11, 0.58) | − 0.08 (− 0.40, 0.23) |
Continuous measures presented as mean (95% CI), unless otherwise indicated. Categorical measures compared using Pearson’s chi-square, as appropriate
aAge of mother at enrolment to the FASSTT trial
bAge of leaving of formal education
cBody mass index (BMI) was calculated as weight in kilograms/height in meters squared. BMI Z-score was calculated based on the Centre for Disease Control Growth Charts and using the LMS method, using the equation Z = (BMI/M)^L − 1)/(L × S) [23]. The parameters of the formula include the following: M which is the median BMI by age, S which corresponds to the coefficient of variation of BMI, and L which allows for age-dependent skewness in the distribution of body mass index [24]
Table 2.
Maternal and cord blood folate biomarkers of FASSTT trial participants
| Placebo (n = 33) | Folic acid (n = 37) | p value | |
|---|---|---|---|
| 14th GW (pre-intervention) | |||
| Serum folate, nmol/L | 48.7 (40.7, 56.7) | 45.6 (39.1, 52.1) | 0.544 |
| RBC folate, nmol/L | 1109 (846, 1371) | 1223 (1025, 1421) | 0.312 |
| 36th GW (post-intervention)a | |||
| Serum folate, nmol/L | 26.0 (18.9, 33.2) | 51.1 (43.6, 58.6) | < 0.001 |
| RBC folate, nmol/L | 978 (823, 1133) | 1834 (1609, 2060) | < 0.001 |
| MTHFR 677TT genotype, % | 9 | 16 | 0.595 |
| Cord bloodb | |||
| Serum folate, nmol/L | 71.7 (60.5, 83.0) | 99.1 (86.6, 111.6) | 0.002 |
| RBC folate, nmol/L | 1535 (1260, 1810) | 2177 (1779, 2574) | 0.009 |
| MTHFR 677TT genotype, % | 14 | 9 | 0.914 |
Continuous measures presented as mean (95% CI) unless otherwise indicated. Continuous measures compared using independent sample t test. Count measures compared using Pearson’s chi-square
aPost-intervention, following supplementation with folic acid (400 μg/d) for 22 weeks in pregnancy
bCord blood collected upon delivery
Effect of maternal folic acid during pregnancy on offspring cognition
WPPSI-III composite test scores from children at age 7 years are presented in Table 3. Following adjustment for child’s sex, birth weight, breastfeeding, maternal age, and maternal education attainment, analysis showed that children born to mothers who had received FA in pregnancy scored significantly higher in word reasoning compared to children from the placebo group (mean (95% CI) 13.3 (12.4–14.2) vs 11.9 (11.0–12.8), p = 0.027). No other statistically significant differences in WPPSI-III scores were observed between the two groups (results of all subtests are provided in Additional file 1: Table S3).
Table 3.
WPPSI-III test scores of FASSTT Offspring Trial participants at 7 years
| Composite and subtest scores | Placebo (n = 33) | Folic acid (n = 37) | Difference | p value (unadjusted)a | p value (adjusted)b |
|---|---|---|---|---|---|
| Verbal IQ | 103.4 (99.4, 107.4) | 107.7 (103.7, 111.8) | 4.3 (− 1.2, 9.9) | 0.126 | 0.120 |
| Information | 10.9 (9.9, 11.8) | 11.1 (10.3, 12.0) | 0.3 (− 1.0, 1.5) | 0.648 | 0.630 |
| Vocabulary | 9.6 (8.9, 10.4) | 10.3 (9.5, 11.1) | 0.7 (− 0.4, 1.8) | 0.221 | 0.262 |
| Word reasoning | 11.9 (11.0, 12.8) | 13.3 (12.4, 14.2) | 1.4 (0.2, 2.6) | 0.023 | 0.027 |
| Performance IQ | 100.6 (96.5, 104.6) | 104.1 (99.1, 109.1) | 3.5 (− 2.9, 9.8) | 0.274 | 0.429 |
| Processing speed | 103.9 (98.1, 109.7) | 102.5 (97.4, 107.7) | 1.4 (− 6.2, 8.9) | 0.718 | 0.712 |
| General language | 105.8 (101.1, 110.5) | 108.9 (104.5, 113.2) | 3.1 (− 3.2, 9.4) | 0.334 | 0.514 |
| Full scale IQ | 103.5 (99.3, 107.6) | 106.4 (101.7, 111.1) | 3.0 (− 3.3, 9.2) | 0.348 | 0.441 |
Data presented as mean (95% CI)
Test scores assessed by Wechsler Preschool and Primary Scale of Intelligence test, 3rd UK edition (WPPSI-III)
Data analyzed by aindependent t test and bANCOVA, with adjustment for maternal age and education attainment [28], child’s sex [29, 30], birth weight [31], and breastfeeding [28]. Results considered significant when p < 0.05
When compared with a nationally representative sample of British children at age 7 years, WPPSI-III scores were found to be higher in children from FA treated mothers for verbal IQ (107.7 vs 99.1, p < 0.001), performance IQ (104.1 vs 98.7, p = 0.035), general language (108.9 vs 101.8, p = 0.002), and full scale IQ (106.4 vs 98.3, p = 0.001) (Table 4). Comparison of the placebo group with British children however showed smaller differences in scores for verbal IQ (103.4 vs 99.1, p = 0.034) and full scale IQ (103.5 vs 98.3, p = 0.017) and no differences in performance IQ or general language scores. In neither the FA nor the placebo group were scores for processing speed found to be different from the UK mean scores for children of this age.
Table 4.
Comparison between WPPSI-III test scores of FASSTT Offspring Trial participants with a representative sample of British children
| Composite scores | UK mean (n = 41) | Placebo (n=33) | p value | Folic acid (n = 37) | p value |
|---|---|---|---|---|---|
| Verbal IQ | 99.07 | 103.4 | 0.034 | 107.7 | < 0.001 |
| Performance IQ | 98.74 | 100.6 | 0.361 | 104.1 | 0.035 |
| Processing speed | 101.32 | 103.9 | 0.373 | 102.5 | 0.636 |
| General language | 101.85 | 105.8 | 0.098 | 108.9 | 0.002 |
| Full scale IQ | 98.31 | 103.5 | 0.017 | 106.4 | 0.001 |
Data presented as mean. The p values refer to data analyzed by a one-sample t test for comparison of placebo and treatment groups with WPPSI-III test scores from a representative sample of British children [32]. Results considered significant when p < 0.05
Maternal biomarker status of folate at the 36th gestational week was found to be a significant predictor of subsequent verbal IQ (but not other WPPSI-III scores) in children at 7 years: β = 0.268 (95% CI 0.000–0.001), p = 0.027, for serum folate, after adjustment for relevant covariates, namely maternal age [28], maternal education attainment [28], child’s sex [29, 30], and breastfeeding [28] using multiple linear regression analysis (Additional file 1: Table S4). In this analysis, apart from maternal folate status at the 36th gestational week, breastfeeding was the only factor significantly related to child cognition as determined by WPPSI-III test scores for verbal IQ (β = 0.300 (95% CI 0.005–0.053), p = 0.017), general language (β = 0.369 (95% CI 3.387–16.142), p = 0.003), and full scale IQ (β = 0.314 (95% CI 1.622–14.837), p = 0.016).
In the sample of children assessed also at age 3 years, those whose mothers received FA treatment during pregnancy scored significantly higher in the cognitive domain of the BSITD-III test compared to children from placebo mothers (Table 5). No significant differences between the treatment groups in any other developmental domain of the BSITD test were observed.
Table 5.
Developmental scores of FASSTT Offspring Trial participants at 3 years
| Developmental domains | Placebo (n = 16) | Folic acid (n = 23) | Difference | p value (unadjusted)a | p value (adjusted)b |
|---|---|---|---|---|---|
| Cognitivea | 9.5 (8.8, 10.2) | 10.3 (9.3, 11.3) | 0.8 (− 0.5, 2.1) | 0.223 | 0.040 |
| Receptive communication | 10.3 (9.0, 11.5) | 10.5 (9.4, 11.6) | 0.2 (− 1.4, 1.8) | 0.775 | 0.395 |
| Expressive communication | 11.3 (10.0, 12.5) | 10.3 (9.1, 11.5) | 1.0 (− 0.7, 2.7) | 0.257 | 0.634 |
| Fine motor | 9.8 (8.9, 10.7) | 10.4 (9.1, 11.6) | 0.5 (− 1.1, 2.2) | 0.515 | 0.302 |
| Gross motor | 8.6 (7.5, 9.6) | 8.6 (7.7, 9.4) | 0.0 (− 1.3, 1.3) | 0.997 | 0.828 |
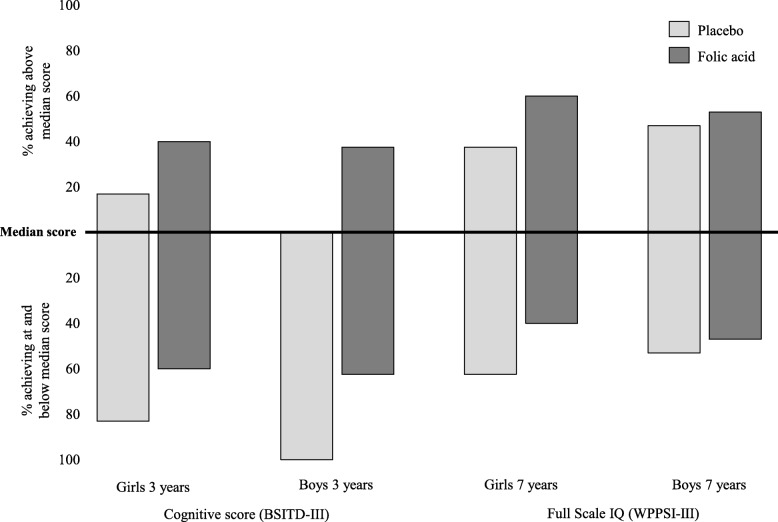
In both assessments (performed at 3 and 7 years), greater proportions of girls and boys from folic acid treated mothers compared with placebo had cognitive scores above the median value of 10 (girls and boys) for the BSITD-III, and 24.5 (girls) and 21.5 (boys) for WPPSI-III (Fig. 2).
Fig. 2.
Percentage of FASSTT Offspring participants at 3 and 7 years achieving above average cognitive performance. In children at 3 years, cognitive performance was assessed by the BSITD-III test and at 7 years by the WPPSI-III test. Values are shown as % of children at 3 or 7 years, by treatment group of the mother, who achieved above the median cognitive score for same-sex children at that age versus those who scored at and below the median scores of 10 (for girls and boys) in the BSITD-III test; 24.5 (for girls) and 21.5 (for boys) in the WPPSI-III test. Total numbers at each age and sex group are as follows: girls at 3 years, n = 27; boys at 3 years, n = 12; girls at 7 years, n = 38; and boys at 7 years, n = 32
Discussion
As a follow-up study of children whose mothers had participated in an RCT during trimesters 2 and 3 of pregnancy, the FASSTT Offspring study provides the first randomized trial evidence that continuing FA supplementation throughout pregnancy, well beyond the early period known to be protective against NTDs, can influence cognitive development of the child up to 7 years of age. Using validated and internationally recognized tools to measure cognitive performance in children, we show that the children of FA treated mothers during pregnancy had a higher score in the cognitive domain of developmental assessment at 3 years and in word reasoning testing at 7 years. When compared with a nationally representative sample of British children at age 7 years, children from FA treated mothers scored higher in verbal IQ, general language, and full scale IQ.
Apart from its well-established role in preventing NTD in very early pregnancy, folate is known to have other essential roles throughout pregnancy, with impacts in early life and beyond. Folate after the first trimester of pregnancy is likely to be essential for the developing brain as areas such as the hippocampus, striatum, and auditory and visual cortices are undergoing rapid growth to become functionally active at this time [33]. There is evidence that children of mothers diagnosed in late pregnancy with megaloblastic anemia (owing to severe folate deficiency) had abnormal neurodevelopment and lower intellectual abilities [4]. Likewise, previous studies reported reduced psychomotor and cognitive ability, or hyperactivity and peer problems, in children of mothers with low biomarker folate status at preconception or at the 14th gestational week of pregnancy [34, 35]. Other studies have linked higher maternal folate status or self-reported FA use in early pregnancy with improved cognitive performance [6–9] and behavior [16, 17] or reduced risk of severe language delay [7] in children. Despite differences in study design, timing of maternal sampling during pregnancy, and the tests used to assess cognition, the current findings are in broad agreement with the aforementioned studies. The majority of previous studies have however relied on records of pregnancy usage of FA supplements as reported by mothers, and have predominantly focused on the first trimester of pregnancy where official recommendations for FA supplementation to prevent NTD are in place worldwide. In contrast to most previous studies, our study focused on the effects of FA after the period recommended for NTD prevention (but in women who had taken FA in the first trimester, as per the original FASSTT study design), and the findings now provide convincing evidence that better folate status throughout pregnancy may lead to improved cognitive health outcomes in childhood.
The wider public health relevance of our findings is suggested by the supporting data from comparison of the cognitive performance of the FASSTT Offspring Trial participants with that of a nationally representative sample of British children of the same age [32]. When compared with British children at age 7 years, WPPSI-III scores for verbal IQ, general language, and full scale IQ were each higher in children from FA treated mothers. Furthermore, the consistency of our results in relation to cognitive function as measured at 3 years and at 7 years, despite the use of different assessment tools at these time points (and smaller sample at 3 years), strengthens our findings. At both time points, greater proportions of children from folic acid treated mothers compared with placebo achieved cognitive scores above the median value for same-sex children at that age. The cognitive domain of the BSITD-III and the word reasoning test of the WPPSI-III tool measure similar aspects of the brain including verbal comprehension, concept formation, and sensorimotor skills. Our results therefore indicate that the effect of maternal folate may be specific to the verbal domain and not across the broad range of cognition assessed at 7 years, or across developmental domains other than cognitive performance assessed at 3 years. Our findings on cognition are important, not only because achievement of full cognitive potential of every child is considered paramount for future academic attainment, but also because evidence suggests that higher intelligence in childhood is a prerequisite for better cognitive reserves in adulthood that could in turn help to delay cognitive decline in later life [36–38].
The biological mechanisms explaining our findings are not clear. They are likely however to relate to the role of folate within one-carbon metabolism and specifically folate-mediated alterations in methylation which would result in differential expression of proteins related to production of neurotransmitters, myelination, or synaptic formation in the central nervous system [39, 40]. The developing brain is particularly vulnerable to these folate-dependent reactions, and thus, low folate during pregnancy could impair optimal brain development. Furthermore, epigenetic modifications in utero can affect offspring health in later life, with emerging evidence showing that maternal folate can exert epigenetic effects in pregnancy via DNA methylation which could in turn underlie fetal programming and fetal brain development [40–42]. We previously reported folate-mediated epigenetic changes in genes related to brain development and function in the current FASSTT Offspring cohort when they were newborns [43, 44], and this potentially offers a plausible biological basis to link maternal folate during pregnancy with the cognitive effects in childhood found in this study. In this regard, however, the question of optimal FA dose for beneficial effects is somewhat unclear. The presence of plasma unmetabolized FA is reported to arise from higher dose FA supplements in pregnant and non-pregnant women [45]; however, it remains to be established whether there are any associated adverse metabolic or clinical impacts. One recent prospective cohort study (n = 2213) showed that 1-year-old children born to mothers reporting to consume FA doses of 5000 μg/d or greater had lower psychomotor development compared to those of mothers who consumed doses of 400–1000 μg/d [45], whereas another study observed beneficial effects on neurodevelopment in 18-month-old children of 5000 μg/d FA taken in early pregnancy compared with no supplementation [8]. Therefore, the effects of exposure to high-dose FA in pregnancy on outcomes in the offspring are unclear and require further investigation. In the meantime, given the uncertainty regarding long-term effects of exposure to high-dose FA, it seems prudent to recommend doses no higher than those demonstrated here in later pregnancy, and for NTD prevention in early pregnancy, as being beneficial with no known harmful effect [46, 47].
A number of factors contribute to the strength of this study. The study design involving the follow-up of children from participants in an RCT in pregnancy [22] enabled us to demonstrate a causative link between maternal FA supplementation and subsequent cognition in the child. Maternal and newborn responses to FA intervention were measured by RBC folate, which is unaffected by recent intake and widely considered to be the best biomarker of long-term folate status [48]. The use of internationally recognized tools to measure cognitive performance in children is also a strength and enables the results from this maternal intervention with folic acid to be placed in a wider public health context for consideration along with findings from other antenatal or child interventions in relation to cognition in children [49, 50]. The main study outcomes in children sampled at 7 years are supported by the pilot data from the same children sampled at 3 years, also showing a beneficial effect of maternal FA on child cognition. Furthermore, the two sampling time points provided the opportunity to track cognitive development into childhood and the broad agreement in results at 3 and 7 years contributes some degree of internal validation to our findings. In addition, in our analysis, we controlled for common confounders including maternal age and education attainment, child’s sex, birth weight, and breastfeeding, previously reported to be strongly associated with child neurodevelopment [28–31]. This study was however not without limitations, the most significant of which was the relatively small sample size. Of 119 participants in the original FASSTT trial, 70 (59%) of the mother-child pairs completed the FASSTT Offspring Trial at 7 years; of these, 34 children were also sampled at 3 years and provided pilot data in relation to child cognition. Although we made every effort to maximize the participation rate from the original FASSTT trial, our final sample may have lacked sufficient statistical power to detect small effects in some test components of the WPPSI-III test. In addition, the sample may not be representative of children generally, in terms of ethnicity and socioeconomic status, and therefore, the results require confirmation in other populations. Future work in this area would be much enhanced by combining cognitive tests as used in the current study with non-invasive brain imaging or novel brain mapping techniques, as previously applied to study the effects of nutritional interventions in pregnancy on brain health outcomes in the child [51, 52].
Conclusions
In summary, the current findings provide the first randomized trial evidence that continued FA supplementation of mothers through the second and third trimesters of pregnancy can influence the cognitive performance of their children up to 7 years of age. The results show that there are benefits for the child of continuing maternal use of FA throughout pregnancy, whereas current recommendations in most countries worldwide advise mothers to take FA supplements from before conceiving until the end of the 12th gestational week only. If confirmed by further randomized trials in pregnancy with follow-up in childhood, these findings could have important impacts in informing future policy and practice in relation to FA recommendations in pregnancy.
Supplementary information
Additional file 1: Table S1a. Characteristics of responders and non-responders to participation in the FASSTT Offspring trial at 7 years. Table S1b. Characteristics of non-responders to participation in the FASSTT Offspring trial at 7 years by treatment group. Table S2. Anthropometric measurements of FASSTT Offspring trial participants at age 3 and 7 years. Table S3. WPPSI -III test scores of FASSTT Offspring trial participants at 7 years. Table S4a. Maternal serum folate status at 36th GW and WPPSI-III test scores of FASSTT Offspring trial participants at 7 years. Table S4b. Maternal RBC folate status at 36th GW and WPPSI-III test scores of FASSTT Offspring trial participants at 7 years.
Acknowledgements
We wish to thank the children and their mothers/guardians who volunteered to participate in the FASSTT Offspring Trial.
Abbreviations
- ANCOVA
Analysis of covariance
- BMI
Body mass index
- BSITD-III
Bayley’s Scales of Infant and Toddler Development 3rd Edition
- FA
Folic acid
- FASSTT
Folic Acid Supplementation during the Second and Third Trimesters
- GW
Gestational weeks
- IQ
Intelligence quotient
- MTHFR
Methylenetetrahydrofolate reductase
- NTDs
Neural tube defects
- RBC
Red blood cell
- RCT
Randomized controlled trial
- SPSS
Statistical Package for the Social Sciences software
- WPPSI-III
Wechsler Preschool and Primary Scale of Intelligence test 3rd UK edition
Authors’ contributions
The authors’ contributions were as follows: HM, KP, and JD conceptualized and designed the study. All authors completed the acquisition, analysis, and interpretation of the data. KP, HM, CPW, DJL-M, TC, and MM obtained the study funding. HM, KP, TC, MM, BAM, MW, JJS, and AMM were responsible for the methodology. KP, HM, JJS, MR, and TC provided study supervision. HM and KP drafted the original version of the manuscript. All authors critically revised drafts of the manuscript and approved the final version.
Funding
This study was supported by the HSC Research and Development Division of the Public Health Agency, Northern Ireland (Enabling Research Awards Scheme: STL/5043/14), and by the Economic and Social Research Council (ESRC) and Biological Sciences Research Council (BBSRC) at the UK (ES/N000323/1). The funders had no role in the study design, the data collection and analysis, the decision to publish, or the manuscript preparation.
Availability of data and materials
Data from this study are held in full compliance with Ulster University’s Research Governance and Ethics Policy for Human Research (2018) (https://internal.ulster.ac.uk/research/office/rofficeeg.php), which in turn is fully aligned with the UK’s Data Protection Act 2018. The participants of FASSTT and FASSTT Offspring Trials did not provide consent for sharing their data publicly. Data are available from the Research Governance of Ulster University (UK) for researchers who meet the criteria for access to confidential data. Please address requests to Mr. Nick Curry, Head of Research Governance at Ulster University at n.curry@ulster.ac.uk
Ethics approval and consent to participate
The Office for Research Ethics Committee for Northern Ireland (ORECNI) has granted ethical approval for the original randomized controlled FASSTT trial (ref:05/Q2008/21) and for the follow-up FASSTT Offspring Trial (12/NI/0077). Ulster University Research Ethics Committee also approved the FASSTT Offspring Trial (UUREC: 12/0121). Written informed consent from the mother and assent from the child were obtained. The trials were registered at ISRCTN.com (ISRCTN19917787).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12916-019-1432-4.
References
- 1.Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF, Mills JL, et al. Biomarkers of nutrition for development—folate review. J Nutr. 2015;147:1636S–1680S. doi: 10.3945/jn.114.206599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 3.MRC Vitamin Study Research Group Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. doi: 10.1016/0140-6736(91)90133-A. [DOI] [PubMed] [Google Scholar]
- 4.Gross R, Newberne P, Reid J. Adverse effects on infant development associated with maternal folic acid deficiency. Nutr Rep Int. 1974;10:241–248. [Google Scholar]
- 5.Veena SR, Krishnaveni GV, Srinivasan K, Wills AK, Muthayya S, Kurpad AV, et al. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10- year-old children in South India. J Nutr. 2010;140:1014–1022. doi: 10.3945/jn.109.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Julvez J, Fortuny J, Mendez M, Torrent M, Ribas-Fitó N, Sunyer J. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr Perinat Epidemiol. 2009;23:199–206. doi: 10.1111/j.1365-3016.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 7.Roth C, Magnus P, Schjølberg S, Stoltenberg C, Surén P, McKeague IW, et al. Folic acid supplements in pregnancy and severe language delay in children. JAMA. 2011;306:1566–1573. doi: 10.1001/jama.2011.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatzi L, Papadopoulou E, Koutra K, Roumeliotaki T, Georgiou V, Stratakis N, et al. Effect of high doses of folic acid supplementation in early pregnancy on child neurodevelopment at 18 months of age: the mother-child cohort “Rhea” study in Crete, Greece. Public Health Nutr. 2012;15:1728–1736. doi: 10.1017/S1368980012000067. [DOI] [PubMed] [Google Scholar]
- 9.Villamor E, Rifas-Shiman SL, Gillman MW, Oken E. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr Perinat Epidemiol. 2012;26:328–335. doi: 10.1111/j.1365-3016.2012.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyaradi A, Li J, Hickling S, Foster J, Oddy WH. The role of nutrition in children’s neurocognitive development, from pregnancy through childhood. Front Hum Neurosci. 2013;7:97. doi: 10.3389/fnhum.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85:S614–S620. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 12.Greenblatt JM, Huffman LC, Reiss AL. Folic acid in neurodevelopment and child psychiatry. Prog Neuro-Psychopharmacol Biol Psychiatry. 1994;18:647–660. doi: 10.1016/0278-5846(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 13.McClain LD, Carl GF, Bridgers WF. Distribution of folic acid coenzymes and folate dependent enzymes in mouse brain. J Neurochem. 1975;24:719–722. doi: 10.1111/j.1471-4159.1975.tb11669.x. [DOI] [PubMed] [Google Scholar]
- 14.Whitley JR, O’Dell BL, Hogan AG. Effect of diet on maze learning in second-generation rats; folic acid deficiency. J Nutr. 2018;45:153–160. doi: 10.1093/jn/45.1.153. [DOI] [PubMed] [Google Scholar]
- 15.Craciunescu CN, Brown EC, Mar M-H, Albright CD, Nadeau MR, Zeisel SH. Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. J Nutr. 2004;134:162–166. doi: 10.1093/jn/134.1.162. [DOI] [PubMed] [Google Scholar]
- 16.Roza SJ, Van Batenburg-Eddes T, Steegers EAP, Jaddoe VWV, MacKenbach JP, Hofman A, et al. Maternal folic acid supplement use in early pregnancy and child behavioural problems: the generation R study. Br J Nutr. 2010;103:445–452. doi: 10.1017/S0007114509991954. [DOI] [PubMed] [Google Scholar]
- 17.Steenweg-de Graaff J, Roza SJ, Steegers EAP, Hofman A, Verhulst FC, Jaddoe VWV, et al. Maternal folate status in early pregnancy and child emotional and behavioral problems: the generation R study. Am J Clin Nutr. 2012;95:1413–1421. doi: 10.3945/ajcn.111.030791. [DOI] [PubMed] [Google Scholar]
- 18.Dobó M, Czeizel AE. Long-term somatic and mental development of children after periconceptional multivitamin supplementation. Eur J Pediatr. 1998;157:719–723. doi: 10.1007/s004310050922. [DOI] [PubMed] [Google Scholar]
- 19.Tamura T, Goldenberg RL, Chapman VR, Johnston KE, Ramey SL, Nelson KG. Folate status of mothers during pregnancy and mental and psychomotor development of their children at five years of age. Pediatrics. 2005;116:703–708. doi: 10.1542/peds.2004-2189. [DOI] [PubMed] [Google Scholar]
- 20.Ars Charlotte L., Nijs Ilse M., Marroun Hanan E., Muetzel Ryan, Schmidt Marcus, Steenweg-de Graaff Jolien, van der Lugt Aad, Jaddoe Vincent W., Hofman Albert, Steegers Eric A., Verhulst Frank C., Tiemeier Henning, White Tonya. Prenatal folate, homocysteine and vitamin B12 levels and child brain volumes, cognitive development and psychological functioning: the Generation R Study. British Journal of Nutrition. 2016;122(s1):S1–S9. doi: 10.1017/S0007114515002081. [DOI] [PubMed] [Google Scholar]
- 21.Wu BTF, Dyer RA, King DJ, Richardson KJ, Innis SM. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS One. 2012;7:e43448. doi: 10.1371/journal.pone.0043448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNulty B, McNulty H, Marshall B, Ward M, Molloy AM, Scott JM, et al. Impact of continuing folic acid after the first trimester of pregnancy: findings of a randomized trial of folic acid supplementation in the second and third trimesters. Am J Clin Nutr. 2013;98:92–98. doi: 10.3945/ajcn.112.057489. [DOI] [PubMed] [Google Scholar]
- 23.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. National Center for Health Statistics. Vital Health Stat 11. 2002;246:1–190. [PubMed]
- 24.Cole TJ. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53. doi: 10.1016/S0076-6879(97)81007-5. [DOI] [PubMed] [Google Scholar]
- 26.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 27.DSS Research. Sample size calculator. http://www.dssresearch.com/resources/calculators/sample-size-calculator-average/. Accessed 12 Apr 2012.
- 28.Victora CG, Horta BL, de Mola CL, Quevedo L, Pinheiro RT, Gigante DP, et al. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Heal. 2015;3:199–205. doi: 10.1016/S2214-109X(15)70002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- 30.Lauer JE, Yhang E, Lourenco SF. The development of gender differences in spatial reasoning: a meta-analytic review. Psychol Bull. 2019;145:537–565. doi: 10.1037/bul0000191. [DOI] [PubMed] [Google Scholar]
- 31.Matte TD, Bresnahan M, Begg MD, Susser E. Influence of variation in birth weight within normal range and within sibships on IQ at age 7 years: cohort study. BMJ. 2001;323:310–314. doi: 10.1136/bmj.323.7308.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rust J, Golombok S. In: Description of the UK study. Wechsler D, WPPSI-III UK, editor. London: Harcourt Assessment; 2003. [Google Scholar]
- 33.Thompson RA, Nelson CA. Developmental science and the media: early brain development. Am Psychol. 2001;56:5–15. doi: 10.1037/0003-066X.56.1.5. [DOI] [PubMed] [Google Scholar]
- 34.Murphy MM, Fernandez-Ballart JD, Molloy AM, Canals J. Moderately elevated maternal homocysteine at preconception is inversely associated with cognitive performance in children 4 months and 6 years after birth. Matern Child Nutr. 2016;13:e12289. doi: 10.1111/mcn.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlotz W, Jones A, Phillips DIW, Gale CR, Robinson SM, Godfrey KM. Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. J Child Psychol Psychiatry Allied Discip. 2010;51:594–602. doi: 10.1111/j.1469-7610.2009.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards M, Shipley B, Fuhrer R, Wadsworth MEJ. Cognitive ability in childhood and cognitive decline in mid-life: longitudinal birth cohort study. BMJ. 2004;328:552. doi: 10.1136/bmj.37972.513819.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun study. JAMA. 1996;275:528–532. doi: 10.1001/jama.1996.03530310034029. [DOI] [PubMed] [Google Scholar]
- 38.Murray AD, Staff RT, McNeil CJ, Salarirad S, Ahearn TS, Mustafa N, et al. The balance between cognitive reserve and brain imaging biomarkers of cerebrovascular and Alzheimer’s diseases. Brain. 2011;134:3687–3696. doi: 10.1093/brain/awr259. [DOI] [PubMed] [Google Scholar]
- 39.Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem. 2012;23:853–859. doi: 10.1016/j.jnutbio.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caffrey A, McNulty H, Irwin RE, Walsh CP, Pentieva K. Maternal folate nutrition and offspring health: evidence and current controversies. Proc Nutr Soc. 2019;78:208–220. doi: 10.1017/S0029665118002689. [DOI] [PubMed] [Google Scholar]
- 41.McGarel C, Pentieva K, Strain JJ, McNulty H. Emerging roles for folate and related B-vitamins in brain health across the lifecycle. Proc Nutr Soc. 2015;74:46–55. doi: 10.1017/S0029665114001554. [DOI] [PubMed] [Google Scholar]
- 42.Guéant JL, Namour F, Guéant-Rodriguez RM, Daval JL. Folate and fetal programming: a play in epigenomics? Trends Endocrinol Metab. 2013;24:279–289. doi: 10.1016/j.tem.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Caffrey A, Irwin RE, McNulty H, Strain JJ, Lees-Murdock DJ, McNulty BA, et al. Gene-specific DNA methylation in newborns in response to folic acid supplementation during the second and third trimesters of pregnancy: epigenetic analysis from a randomized controlled trial. Am J Clin Nutr. 2018;107:566–575. doi: 10.1093/ajcn/nqx069. [DOI] [PubMed] [Google Scholar]
- 44.Irwin RE, Thursby S-J, Ondičová M, Pentieva K, McNulty H, Richmond RC, et al. A randomized controlled trial of folic acid intervention in pregnancy highlights a putative methylation-regulated control element at ZFP57. Clin Epigenetics. 2019;11:31. doi: 10.1186/s13148-019-0618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valera-Gran D, García De La Hera M, Navarrete-Muñoz EM, Fernandez-Somoano A, Tardón A, Julvez J, et al. Folic acid supplements during pregnancy and child psychomotor development after the first year of life. JAMA Pediatr. 2014;168:e142611. doi: 10.1001/jamapediatrics.2014.2611. [DOI] [PubMed] [Google Scholar]
- 46.CDC Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep. 1992;41:1–7. [PubMed] [Google Scholar]
- 47.Pentieva K, Selhub J, Paul L, Molloy AM, McNulty B, Ward M, et al. Evidence from a randomized trial that exposure to supplemental folic acid at recommended levels during pregnancy does not lead to increased unmetabolized folic acid concentrations in maternal or cord blood. J Nutr. 2016;146:494–500. doi: 10.3945/jn.115.223644. [DOI] [PubMed] [Google Scholar]
- 48.Duffy ME, Hoey L, Hughes CF, Strain JJ, Rankin A, Souverein OW, et al. Biomarker responses to folic acid intervention in healthy adults: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:96–106. doi: 10.3945/ajcn.113.062752. [DOI] [PubMed] [Google Scholar]
- 49.Devakumar D, Fall CHD, Sachdev HS, Margetts BM, Osmond C, Wells JCK, et al. Maternal antenatal multiple micronutrient supplementation for long-term health benefits in children: a systematic review and meta-analysis. BMC Med. 2016;14:90. doi: 10.1186/s12916-016-0633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Øyen J, Kvestad I, Midtbø LK, Graff IE, Hysing M, Stormark KM, et al. Fatty fish intake and cognitive function: FINS-KIDS, a randomized controlled trial in preschool children. BMC Med. 2018;16:41. doi: 10.1186/s12916-018-1020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parra-Cabrera S, Moreno-Macias H, Mendez-Ramirez I, Schnaas L, Romieu I. Maternal dietary omega fatty acid intake and auditory brainstem-evoked potentials in Mexican infants born at term: cluster analysis. Early Hum Dev. 2008;84:51–57. doi: 10.1016/j.earlhumdev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Sizonenko SV, Babiloni C, Sijben JW, Walhovd KB. Brain imaging and human nutrition: which measures to use in intervention studies? Adv Nutr. 2013;4:554–556. doi: 10.3945/an.113.004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1a. Characteristics of responders and non-responders to participation in the FASSTT Offspring trial at 7 years. Table S1b. Characteristics of non-responders to participation in the FASSTT Offspring trial at 7 years by treatment group. Table S2. Anthropometric measurements of FASSTT Offspring trial participants at age 3 and 7 years. Table S3. WPPSI -III test scores of FASSTT Offspring trial participants at 7 years. Table S4a. Maternal serum folate status at 36th GW and WPPSI-III test scores of FASSTT Offspring trial participants at 7 years. Table S4b. Maternal RBC folate status at 36th GW and WPPSI-III test scores of FASSTT Offspring trial participants at 7 years.
Data Availability Statement
Data from this study are held in full compliance with Ulster University’s Research Governance and Ethics Policy for Human Research (2018) (https://internal.ulster.ac.uk/research/office/rofficeeg.php), which in turn is fully aligned with the UK’s Data Protection Act 2018. The participants of FASSTT and FASSTT Offspring Trials did not provide consent for sharing their data publicly. Data are available from the Research Governance of Ulster University (UK) for researchers who meet the criteria for access to confidential data. Please address requests to Mr. Nick Curry, Head of Research Governance at Ulster University at n.curry@ulster.ac.uk