Abstract
A bioartificial endocrine pancreas is proposed as a future alternative to current treatment options. Patients with insulin-secretion deficiency might benefit. This is the first systematic review that provides an overview of scaffold materials and techniques for insulin-secreting cells or cells to be differentiated into insulin-secreting cells. An electronic literature survey was conducted in PubMed/MEDLINE and Web of Science, limited to the past 10 years. A total of 197 articles investigating 60 different materials met the inclusion criteria. The extracted data on materials, cell types, study design, and transplantation sites were plotted into two evidence gap maps. Integral parts of the tissue engineering network such as fabrication technique, extracellular matrix, vascularization, immunoprotection, suitable transplantation sites, and the use of stem cells are highlighted. This systematic review provides an evidence-based structure for future studies. Accumulating evidence shows that scaffold-based tissue engineering can enhance the viability and function or differentiation of insulin-secreting cells both in vitro and in vivo.
Keywords: Tissue engineering, insulin-producing cell, artificial organ, endocrine pancreas, evidence map
Introduction
Diabetes mellitus (DM) due to loss of insulin-secreting ß-cells, because of either autoimmune processes in type I DM or surgical resection of the pancreas, represents a suitable model for cell-based therapies. Although the current gold standard for the management of DM is exogenous insulin therapy in response to elevated blood glucose levels, this treatment option is inferior to continuous endogenous insulin secretion by ß-cells.1,2 Therefore, alternative therapies are needed that restore insulin-secreting function and avoid adverse effects such as recurrent hypoglycemia and long-term complications.1,2 An alternative for patients refractory to exogenous insulin injection is islet transplantation following the Edmonton protocol.2,3 The Edmonton protocol is a state-of-the-art procedure that comprises clinical isolation of human islet cells from cadaveric donors, purification of the islets after digestion, intraportal transplantation, and a glucocorticoid-free immunosuppressive regimen for the recipient after transplantation.3,4 Despite improvements in the isolation and cell culture protocol and use of various implantation sites for the ß-cells, only 60%–85% of the patients are independent of insulin at 1 year after transplantation, and this figure decreases with the passage of time.2,4,5 Fewer than 20% of the patients remain insulin-independent for 5 years.6 The reasons for apoptosis of the transplanted allogenic islets and failure of this treatment include non-immune-related, instant blood-mediated inflammatory reactions (IBMIR), graft–host reactions, and a lack of engraftment due to insufficient oxygen supply and increased levels of toxins or pharmaceuticals at the intraportal or intrahepatic transplantation site, respectively.7–9 Another limiting factor is the global shortage of suitable donor organs. Together, these findings show the need for improvement in techniques for restoration of insulin-secreting function. The tissue engineering approaches reviewed here are intended to overcome the current limitations.
In the emerging field of tissue engineering, scaffolds replace the extracellular matrix (ECM) with the intention of mimicking native tissues to provide an optimal environment for cells. Scaffolds, cells, and growth-stimulating factors, often referred to as the tissue engineering triad,10–12 are essential to create bioartificial organs. In native tissues, the ECM contributes to viability and function of cells by (a) providing structural support, (b) ensuring mechanical stability, (c) regulating cellular activities, (d) storing and releasing growth factors, and (e) providing a degradable environment that can be remodeled on demand.13,14 To replicate each of these ECM functions, biological and synthetic materials such as porous scaffolds for cell seeding, decellularized ECM (dECM) scaffolds, scaffold cell sheets, and cell-encapsulating hydrogels are currently used to host insulin-secreting cells (ISC). The application of scaffolds ranges from the generation of ISC from stem cells and progenitor cells in scaffold-based three dimensional (3D) culture systems to the building of artificially created support systems that serve as a logistic template. These support systems are intended to prevent anoikis, protect the patient from inflammatory and immunological host reactions, and improve long-term viability. The use of scaffold-based tissue engineering in routine treatment of these patients is still hampered by (a) reduced vascularization and consequential insufficient supply and hypoxia of the containing cells, (b) immunological host–graft reactions against cells and scaffolds, and (c) a lack of scaffolding techniques that precisely mimic nature.
The aim of this publication is to provide a comprehensive systematic review of scaffolds used in combination with islet cells in the past 10 years, and so to provide impulses for further research. Although tissue engineering has been established for decades, a surge in interest, accelerated by new scaffold materials and fabrication technologies has resulted in increased numbers of publications in the last decade. This development includes pancreatic tissue engineering. The usage of a wider variety of scaffold materials, both natural and synthetic, and new techniques for scaffold fabrication are interdependent developments that have contributed to this growth in interest. In addition, a more comprehensive understanding of the embryology and biology of ISC and their interactions with the surrounding tissue has led, for example, to more efficient protocols for differentiation of stem cells into specialized ISC. We set out to address these developments and the remaining limitations in pancreatic tissue engineering. Finally, the extended research interest also led to many different approaches that may overall appear unsystematic and unclear. The creation of evidence maps yields an overview and defines areas where further research may be necessary.
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses—PRISMA Statement checklist15 was used in this systematic review.
Search strategy
A literature survey was conducted in the PubMed/MEDLINE and Web of Science databases to identify pertinent publications about scaffold materials and scaffolding techniques in combination with ISC or cells intended to be differentiated into ISC up to January 1, 2019. The search strategy on PubMed/MEDLINE was as follows (for the Web of Science search strategy, please refer to Supplemental information Appendix 2):
((islet cell(Title/Abstract)) OR (islet cell*(Title/Abstract) OR (beta cell(Title/Abstract) OR (beta cell*(Title/Abstract) OR (insulin producing cell(Title/Abstract) OR (insulin producing cell*(Title/Abstract) OR (islets of langerhans(MeSH Terms)) OR (insulin secreting cell(MeSH Terms))) AND ((tissue engineering) OR (bioprinting) OR (3D printing) OR (tissue engineering(MeSH Terms)) OR (bioprinting(MeSH Terms)) OR (printing, three dimensional(MeSH Terms)) OR (Tissue Scaffolds(MeSH Terms))) AND (“last 10 years”(PDat)) AND (English(lang)).
Study selection
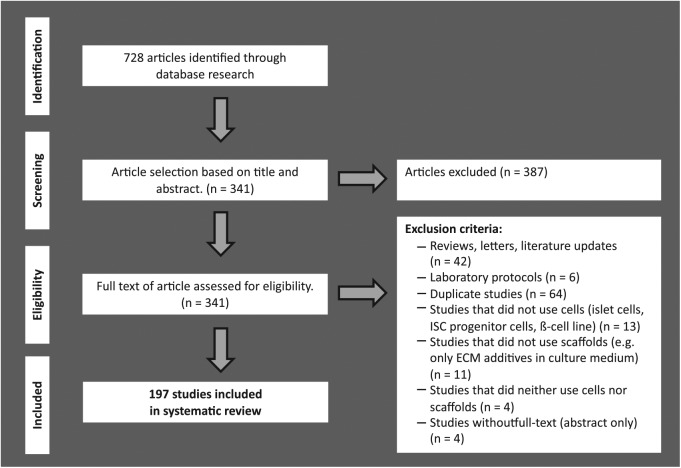
A flow chart showing the strategy used to identify and select studies to be included in this systematic review is presented in Figure 1. A total of 728 possible relevant citations were retrieved by searching the databases, and 387 of them were excluded after screening of titles and abstracts for fulfillment of the inclusion criteria: a tissue engineering approach for ISC, and combination of cells and scaffold material to achieve the intended outcome. Thus, 341 articles remained for full-text analysis. A further 144 articles were excluded after careful analysis of the complete texts under consideration of the exclusion criteria: (a) no usage of ISC or progenitor cells intended for differentiation into ISC, (b) no usage of natural, synthetic, or hybrid scaffold material, or (c) inappropriate article type (articles that studied and reviewed the literature, laboratory protocols and duplicates). The remaining 197 articles fulfilled the eligibility criteria and were included in the systematic review (see Supplemental information, Appendix 1).
Figure 1.
Flow chart of systematic literature search according to the PRISMA guidelines.
Data collection and aggregation
For each included study, the following general data were systematically recorded: publication details (authors and year); methodological details (cell type, scaffold material, study design (in vitro/in vivo), study period, scaffolding technique, supplemented signaling molecules, supplemented ECM molecules, scaffold examination methods (e.g. scanning electron microscopy, X-ray spectroscopy), cell examination methods, viability and function testing methods), and outcome assessment (for further information about the included articles, please consult Supplemental information, Appendix 1). Furthermore, for each included study that performed in vivo tests, the following methodological details were systematically recorded: animal type and transplantation site (see Supplemental information, Appendix 1).
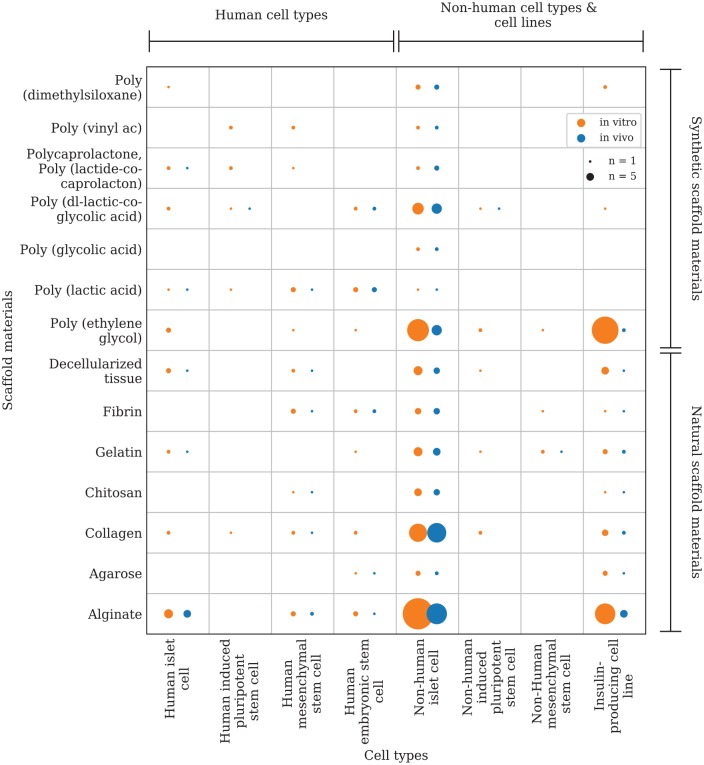
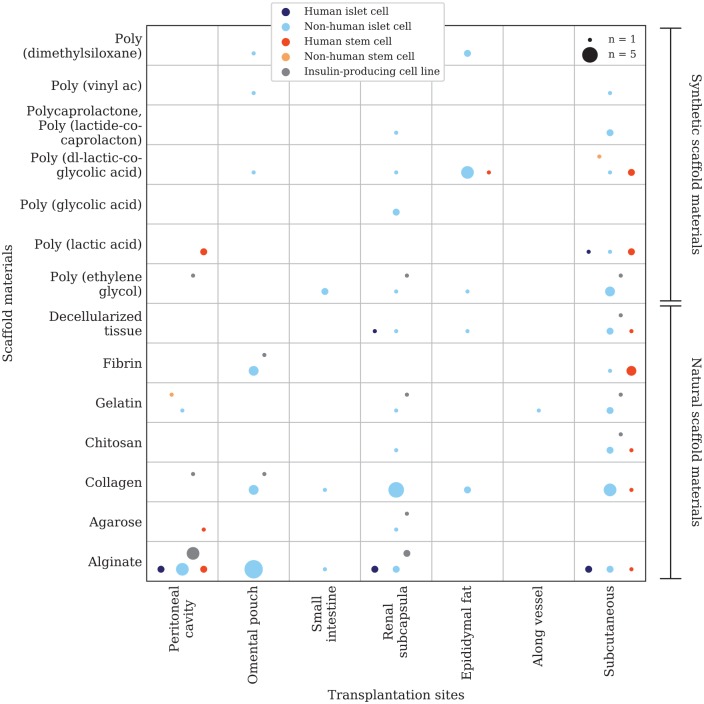
The data were analyzed with Microsoft Office Excel 2016®. Two evidence gap maps (Figure 2, Figure 7) were plotted using a python script developed for this purpose. An evidence map with a focus on the combination of scaffold materials and cell types in in vitro/in vivo trials (Figure 2) was created based on the aggregated data from 161 of the 197 articles included in this systematic review (s. Supplemental information, Appendix 1). Due to the variety of scaffold materials (n = 60), only the most frequently used materials and cell types were considered for plotting. The materials and cell types investigated in the remaining 36 articles could not be aggregated within the plotted groups and were therefore not considered in the evidence gap map. A second evidence map with a focus on scaffold materials, cell types, and their respective transplantation site in vivo (Figure 7) contains the aggregated data from 67 articles (s. Supplemental information, Appendix 1). The vertical axis represents the different scaffold materials that were investigated, and the horizontal axis represents the cell types or transplantation sites. In order to be able to plot multiple, different circles on the respective x-y intersections, separated data packages were created and tagged with a keyword (e.g. in vitro/in vivo). The tagged data packages are referenced in the script and can be distinguished by the circle color. The size of these intersection points is determined by the number of articles that investigated the setting. The first evidence gap map presents different natural and synthetic scaffold materials investigated in the included studies in comparison with the investigated cell type or species origin (human, non-human), respectively (Figure 2). The intersection points are divided into two groups depending on the study design (in vitro/in vivo) and colored differently (Figure 3). The second evidence gap map takes only in vivo studies into consideration and compares natural and synthetic scaffold materials with the transplantation site chosen in the respective study. The color of the intersection points is dependent on the cell type or species origin, respectively (Figure 7).
Figure 2.
Evidence map: Scaffold materials for tissue engineering approaches on insulin-producing cells. x-axis: cell types and species origin, y-axis: scaffold materials. The circle size is correlated to the number of studies that investigated the respective combination of scaffold material and cell type in an in vitro and/or in vivo study design (circle color). In total data from 161 included articles were mapped.
Figure 7.
Evidence gap map: transplantation sites of in vivo studies. x-axis: transplantation sites; y-axis: scaffold materials; circle color: species of cell origin and maturation status; and circle size: correlated to the number of studies that investigated the respective combination. In total data from 67 included articles were mapped.
Figure 3.
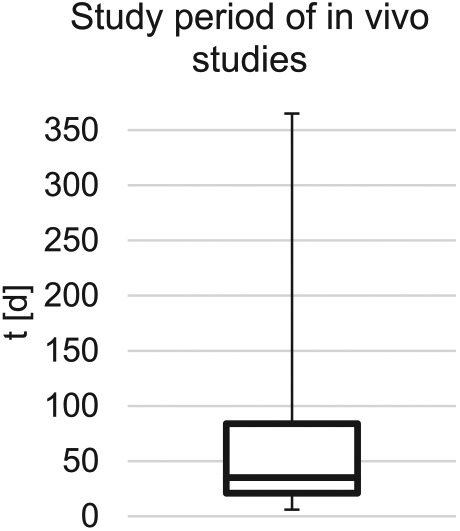
Boxplot of the aggregated study periods of included in vivo studies. Median = 35 days, minimum = 6 days, maximum = 365 days, Q1 = 6 days, and maximum Q3 = 84 days.
Results
Scaffold-based approaches in 3D cell culture systems, enhancement of cell differentiation, and clinical organ replacement therapy
The first evidence gap map shows that there is a lack of experimental evidence for several scaffold materials in combination with human islet cells (in vitro: n = 22; in vivo: n = 9) especially compared with the combination of scaffold materials with non-human islet cells (in vitro: n = 81; in vivo: n = 71) (Figure 2). This can be explained by the lower availability of human islet cells. Hence, it is important to consider the differences between human islets and non-human islets when drawing conclusions with regard to the applicability of the research objective for clinical use in humans.
Islet size is considerably more variable in the human species than, for example, in mice (in vitro: n = 35, in vivo: n = 31), leading to earlier central core necrosis in larger islets once the oxygen supply is insufficient.16–18 It has also been shown that murine islets contain a higher proportion of insulin-secreting beta cells than human islets. Buchwald et al. reported that insulin secretion upon glucose stimulation per islet equivalent (IEQ) was 2 to 3 times lower in human islets than in a control with murine islets.19 These differences have to be considered to avoid misaligned interpretation of studies on non-human islets, especially in terms of scaffold architecture, oxygen, and nutrient diffusion, and the potential for scaling up to reach therapeutic dosages. Even less evidence was retrieved for in vivo studies on human islet cells (n = 9). The evidence gap map further emphasizes the focus of studies on natural scaffold materials. In accordance with the idea of mimicking nature, it seems logical to use natural materials that either show similarities to the body’s ECM or are a component of the ECM itself before conducting research on synthetic materials. Poly(ethylene glycol) (PEG) and its derivatives stand out in this division (n = 53 in total). In comparison, alginate was the most often employed natural scaffold material (n = 42). PEG can be modified and functionalized with reasonable effort and is easy to fabricate into a scaffold or microcapsule.20 In particular, crosslinking by means of ultraviolet (UV) light may be useful. However, the focus of future studies may shift to the fabrication of synthetic or hybrid scaffolds, as certain techniques such as electrospinning and additive manufacturing are applicable to these materials.
Scaffold materials serve as matrices to incorporate cells for cultivation. The structure and properties of these scaffolds must be selected to ensure normal cell behavior and performance of the cultivated tissue.11,21,22 The cell-incorporating scaffolds support cellular viability and function both before implantation and during remodeling of surrounding tissue after implantation.11 First, in the reviewed articles scaffold materials are used as a cell culture template for ISC providing a three-dimensional environment, more akin to nature than two-dimensional cell culture.23,24 Second, the use of scaffold materials as cell differentiation templates to enhance maturation of stem and progenitor cells to specialized ISC is described. Eventually, scaffolds will serve as logistic templates for the clinical application of insulin replacement therapy in the future of regenerative medicine. Bioartificial insulin-secreting tissue as a treatment option for patients with a loss of endocrine pancreatic function, for example, due to type I DM or to pancreoprive diabetes after surgical resection, needs to fulfill certain requirements for in vivo usage. The reviewed in vivo studies (n = 91) had a median study period of 35 days during which the determined endpoints (e.g. metabolic control, oxygen tension, functional mass, tissue response, practical applicability, cell differentiation) were assessed. There was significant variance of the study period, with a minimum of 6 days and a maximum of 365 days. Different observation periods may be expedient, depending on the endpoints of the study. It has been shown that the graft often shows impaired function shortly after ISC transplantation but recovers after a number of days, or up to several weeks.25,26 If proof of function is one of the endpoints, it is therefore reasonable to choose a longer study period. Moreover, long-term immunological reactions and foreign body responses leading to pericapsular fibrotic overgrowth (PFO) and thus impaired graft function can only be assessed appropriately after long-term in vivo experiments. A study using a non-diabetic, non-human primate model for implantation of macroencapsulated islets proved the viability and function of the encapsulated islets over an observation period of 365 days before explantation.27
Scaffold materials
Biomaterials have been defined as follows by the American National Institute of Health:28
any substance or combination of substances, other than drugs, synthetic or natural in origin, which can be used for any period of time, which augments or replaces partially or totally any tissue, organ or function of the body, in order to maintain or improve the quality of life of the individual.
Common biomaterials for scaffold-based tissue engineering can be divided into natural, synthetic, and hybrid materials. Furthermore, the physical condition, that is, whether the biomaterial is hydrated and thus used as a hydrogel or in a material-specific solid state, must be taken into consideration. Hydrogels offer easy delivery of growth factors and encapsulate cells, thereby shielding them from the immune system of the host.29,30 The size of hydrogel capsules or the diffusion distance, the permeability of the material, and the difficult retrievability after implantation may be detrimental factors, depending on the objective. As both natural and synthetic materials have certain advantages and disadvantages, the fabrication of composite scaffolds is becoming increasingly common.10 Scaffolds provide a three-dimensional structure to facilitate the ingrowth of cells and support their proliferation and differentiation in vitro and in vivo. An ingrowth of cells can be achieved through a porous, interconnective architecture of the scaffold.10,31 The choice of the scaffold material should be made in light of the cell type to be cultured. The cell types applicable are differentiated cells derived from cultured tissue and stem cells for differentiation in a 3D culture process.11 In some studies growth factors and other stimulating substances are added to support proliferation. A variety of scaffold materials have been investigated for their eligibility for ISC and progenitor cells that are to be differentiated into ISC. Criteria such as biocompatibility, mechanical properties and stability, potential for modification and functionalization with supplementary substrates, and the fabrication of a suitable scaffold architecture within a scalable process in adherence to good manufacturing practice (GMP) have been described and should be evaluated when choosing a scaffold material.10,32 Sixty different scaffold materials were investigated in the included studies. A selection will be described briefly.
Biological scaffold materials
The idea of mimicking the structure and composition of natural tissues in order to achieve a similar functional outcome leads to the use of natural materials with structural similarities to the ECM. Biologically derived materials such as alginate (n = 42), agarose (n = 6), collagen (n = 27), fibrin (n = 11), and gelatin (n = 12) have been investigated in many tissue engineering studies and were also chosen as scaffold materials for ISC. Alginate is biologically inert with good long-term stability.33 The gelation of alginate can be achieved by addition of divalent cations (e.g. Ca2+, Ba2+).34 There are several blends available with different compositions of L-guluronate (G) and D-mannuronate (M) residues, which affect their mechanical properties.33 Alginates with high M content show higher immunogenicity and a 10-fold cytokine production compared with high G content.35 This finding has been disputed, so purification is carried out before fabrication into a hydrogel to prevent adverse immunogenicity.33 Derivatives that include peptide sequences for better cell adhesion have been synthesized using carbodiimide chemistry, and signaling molecules have been encapsulated together with cells.33 These characteristics led to the frequent usage of alginate as a 3D culture system. In comparison with Matrigel® (n = 4) (gelatinous protein mixture from mouse sarcoma), the alginate 3D culture platform can readily be translated to in vivo trials. Alginate as a 3D culture platform was, for instance, used for reaggregation of dispersed single islet cells with the intention of creating similar-sized islets, in order to overcome the problem of oxygen diffusion due to the wide size range in human islets.36
Collagen is the main structural protein for most tissues and has often been investigated due to its good mechanical properties.37 Many biological scaffold materials incorporating collagen are biologically active and show therefore good cell adhesion and proliferation properties. These materials (e.g. collagen, fibrin) mimic some features of the ECM such as the presentation of the RGD peptide (binding motif of fibronectin to molecules), which is an important sequence for cell adhesion.37–42 Gelatin is the denaturized form of collagen but differs in some important characteristics such as gelation time and the presentation of binding site sequences.43
Synthetic scaffold materials
The most frequently used synthetic material investigated in the reviewed studies was PEG and its derivatives poly(ethylene glycol) diacrylate (PEGDA) and poly(ethylene glycol) dimethacrylate (PEGDM) (n = 53 in total). The density of hydrogels fabricated from these materials is adjustable by their molecular weight, and as described above, they can be rapidly crosslinked with UV irradiation (e.g. by addition of the type I photoinitiator 2-hydroxy-4-(2-hydroxyethoxy)-2-methylpropiophenone, Irgacure 2959®, or the type II initiator eosin-Y) without the need for potentially harmful crosslinking agents, high temperatures, or unphysiologic pH.44–47 There are possibilities for functionalization of the reactive groups.29,30,47–49 Even a composite PEG/alginate hydrogel was manufactured by means of Staudinger ligation to take advantage of the positive characteristics of each material.50
Other synthetic polymers such as poly(lactic acid) (PLA) (n = 8), poly(glycolic acid) (PGA) (n = 2), their co-polymer poly(d,l-lactic-co-glycolic acid) (PLGA) (n = 13), poly(vinyl alcohol) (PVA) (n = 6), polycaprolactone (PCL) (n = 6), and others have been used as scaffold materials. They differ in elasticity and mechanical strength but are all biocompatible and already used in medical devices.51–54 Instead of hydration for the encapsulation of cells, these materials are mostly used as solid scaffolds to provide mechanical stability.52 The addition of hydrogel materials to form a hybrid scaffold can lead to easier attachment of the cells to the synthetic scaffold and can protect the hybrid construct from the immune system. The fabrication of scaffolds with a defined architecture, for example, using 3D printing or electrospinning, can be applied for many synthetic polymer materials, as the composition of the polymer itself or the composition of several polymers together is variable. Generally, due to their hydrophobic characteristics, synthetic materials require further modification prior to cell seeding to facilitate both cell adhesion and proliferation and the compatibility of the polymer with islets in general. PVA is an exception with its hydrophilic characteristics. Human islet cells are influenced in terms of function and cellular homeostasis by the choice of synthetic polymer material used for the scaffold.55 The hydrophobic properties of synthetic polymer scaffolds promote cellular outgrowth.55 Functionalization to increase the hydrophilicity can be beneficial. The surface of a PLA scaffold was thus treated with argon and oxygen plasma to increase the wettability and surface roughness and was subsequently loaded with a platelet–lysate mix.51 Heparin functionalization of a 3D-printed PCL scaffold achieved higher penetration of cells and vascular structures into the scaffold and could be additionally further modified with vascular endothelial growth factor (VEGF).52 Synthetic polymer materials are increasingly used for scaffold fabrication as they are amenable to new manufacturing techniques capable of producing fine architectures. Their eligibility for use with ISC can be further improved via various functionalization strategies to attach growth factors or ECM components.
Techniques for scaffold creation
Droplet microencapsulation
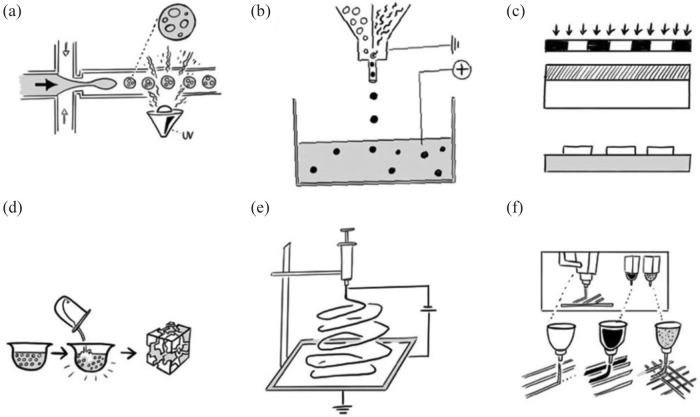
One common technique used to encapsulate ISC into a hydrogel is droplet microencapsulation. This relies on the basic concept of minimizing distances that limit oxygen and nutrient diffusion while retaining properties that prevent anoikis and immunological host-reactions and has commonly been used for in vivo studies in the reviewed literature.18,56–59 The importance of diffusion distance for the viability and function of encapsulated ISC was shown experimentally by comparison of glucose-stimulated insulin secretion by free islets, microencapsulated islets in alginate (diameter = 700 µm), and encapsulated islets in larger alginate capsules (diameter = 1800 µm). The smaller microcapsules showed only a slightly delayed and decreased insulin secretion, whereas in the larger capsules the insulin secretion was > 40% decreased and the delay time was increased due to a larger diffusion distance.19 Droplet microencapsulation is a fabrication technique used almost exclusively for hydrogels.60 The challenge is to produce inert, mechanically stable capsules that can be retrieved if necessary.61 The controllable generation of these droplets or the encapsulation of cells in general can be achieved by various techniques such as microfluidic devices (Figure 4(a)), pressure-based air jacket microencapsulation, vibrational jet flow technology, electrostatic droplet generation, or manual syringe-based extrusion into a chemical crosslinking solution (Figure 4(b)).18,19,56,57,59,62–76 Most droplet-generation methods for cell encapsulation used extrusion-based systems, where a cell-containing hydrogel solution will be extruded through a needle. Depending on the viscosity of the hydrogel, the diameter of the needle, and the flow rate or extrusion pressure, a droplet will be generated.56,62,65,77 Once the droplet reaches critical size, it falls into a cross-linking solution or hardening bath. The adjustment of the above-described parameters defines the size and shape of the cell-encapsulating hydrogel. Thus, either small-scaled hydrogel spheres or long hydrogel fibers can be produced depending on the hydrogel material and the parameters set for encapsulation.56,65 For the fabrication of very small microcapsules, further modifications of the extrusion-based systems such as the vibrational nozzle technology or the application of electrical fields with pulsing potentials are possible.18,56,62,65,73,77 The vibrating nozzle cuts the droplets into smaller pieces of uniform size.73,77
Figure 4.
Scaffold fabrication methods: (a) droplet microencapsulation using either a microfluidic device or, (b) syringe-based droplet extrusion into a chemical crosslinking solution, (c) casting of a scaffold through application of a negative scaffold and selective polymerization using photolithography, (d) particulate leaching and solvent casting using evaporating organic solutions and salts to induce a higher porosity, (e) electrospinning, and (f) 3D-printing or 3D-bioprinting, respectively, with the application of different materials (polymers, hydrogels, cells) and different extrusion systems.
Standard scaffold materials that can be applied for these manufacturing methods are alginate,18,19,56,57,59,62,63,65,66,68,69,72–75,78,79 PEG,67 or PEGDA,76 usually in combination with permselective poly-l-lysine and/or poly-l-ornithine to introduce positive charges on the hydrogel surface for potential further modifications, to influence the permeability for nutrients into the microcapsule, and to reinforce the mechanical durability of the microcapsules.62,67 ECM components and growth factors are easy to include in such a microcapsule system.80 Droplet microencapsulation was also used in an approach with the intention of shielding cells from harsh chemicals or physical stimulation (e.g. UV irradiation), that are potentially cytotoxic for crosslinking by manufacturing hydrogel microcapsules without encapsulated cells. These crosslinked microcapsules were then cocultured with ISC to replicate some 3D tissue characteristics by being the anchor for cell–cell and cell–ECM interactions.80
The different properties of these microcapsules compared with solid cell-loaded scaffolds need to be considered when choosing a transplantation site for in vivo trials. Cell-containing microcapsules are most suitable for implantation into existing anatomical cavities such as the peritoneal cavity or into an omental pouch.59 The lack of retrievability of the microcapsules is a disadvantage of this technique, so different in vivo monitoring methods have been investigated for non-invasive tracking of the microcapsules.59,69,81,82 The application of new imaging techniques can also provide information about cell morphology, spatial distribution, and oxygen supply within a scaffold or hydrogel encapsulation.83,84
Lithography and casting
Scaffold-based tissue engineering is highly dependent on the fabrication of a precise architecture. Different lithography strategies enable the fabrication of small 3D scaffold structures. For scaffold-based housing of ISC, some of the included studies used micropatterning techniques such as photolithography (Figure 4(c)),85–88 soft lithography,87 or lithographically patterned fabrication of microwell templates.89–92 There are many feasible strategies for using lithography techniques in scaffold fabrication. Buitinga et al.91 produced a poly(ethylene oxide terephtalate)/poly(butylene terephthalate) (PEOT/PBT) microwell scaffold by using a grid-patterned negative with pillared wafers. Scaffold fabrication with this lithography technique was further modified through solvent casting and particulate leaching strategies.91–93 The scaffold material is dissolved in an organic solvent, for example, chloroform.94 After addition of salts, for example, NaCl, the solvent can evaporate, creating a more porous scaffold (Figure 4(d)). Inexpensive, rapid prototyping of such microwell scaffolds was also achieved by using patterned ice templates as a negative onto which poly(dimethylsiloxane) (PDMS) was molded.95 Further modification of this method by inclusion of growth factors or ECM molecules into the aqueous droplet solution that serves as a negative for the microwell fabrication might allow easy scaffold functionalization for high-throughput screening for function-enhancing supplements.95 Conversely, a pillar-patterned PDMS template, manufactured with photolithography technique, was negative for the casting or molding of an islet-containing PEG hydrogel.85 The pillars on the PDMS template were used as a simple molding method to create microchannels. The application of lithography techniques for scaffold fabrication can be simple and inexpensive. Lithography is a printing technique and therefore designed for the manufacturing of reproducible copies in a process that can be scaled up. However, it reaches its limits when complex, delicate architectures containing potentially more than one scaffold material are needed.
Electrospinning
Another technique that is being increasingly applied for tissue engineering of ISC is electrospinning (Figure 4(e)). This mode of fabrication is based on the application of high voltages (e.g. 23 kV,53 30 kV96) to a polymer solution. A high-intensity electric field between two electrodes, one in the polymer solution, the other one on the collector, forces the extruded polymer solution to overcome surface tensions and results in an ejected jet of the material. The polymer solution in the spinneret will be charged and accelerated toward a collecting plate, on which the fabricated polymer fibers will form a scaffold mesh.97 The resulting electrical forces produce polymer fibers ranging in diameter from 2 nm up to several micrometers.97 The spun fibers offer the possibility of fabricating scaffolds with a high surface to volume ratio, adaptable porosity properties, and applicability for a broad spectrum of materials.97,98 Researchers used this relatively simple technique to produce polymer fibers from PLA,99–101 PVA,53,99,100,102 PCL,53,96,103,104 polyglecaprone (PGC),96 PEOT/PBT,90 gelatin,105 polyethersulfone,106,107 silk fibroin, and collagen. One disadvantage of this fabrication method might be the difficulty of scaling up these scaffolds to allow therapeutic human dosages. For example, a scaffold made from electrospun PEOT/PBT microwells containing human islets would require a diameter of 40 cm to reach the capacity for therapeutic islet dosages.90 In addition, the irregularity of the fiber mesh leads to irregular cell distribution after seeding. Normal, passive cell seeding can thus result in a lack of cell migration and penetration into the scaffold.97 This effect was shown to be increased with decreasing diameter of the fibers, as thinner fibers displayed more fiber-to-fiber contacts and consequentially smaller pore sizes.108 Blackstone et al. actually showed a positive correlation between increased fiber diameter and inter-fiber distance with viability and function of the seeded cells.105 They produced an electrospun scaffold from gelatin as a 3D cell culture platform.105 Mostly, the electrospinning technique was applied for the fabrication of 3D cell culture templates to achieve enhanced differentiation of human adipose tissue-derived stem cells (hADSC) and human-induced pluripotent stem cells (hiPSC) into ISC53,99,101–103,106,107 but also for the in vivo transplantation of murine islets on electrospun PCL/PGC to the intrarenal transplantation site, with greater proliferation and functional output than the control without a scaffold.96
Electrospinning is a fabrication technique with good control over the porosity, pore size, and fiber diameter of the produced scaffold.98 However, for clinical application in organ replacement the cell-seeding capacity needs to be improved and penetration of the cells into the whole scaffold should be ensured, especially if whole islets of Langerhans are used. A composite of different materials including sacrificial scaffold materials that can be dissolved after fabrication to enlarge the pore sizes might be beneficial.
3D printing and 3D bioprinting
The development of scaffold materials as part of the tissue engineering triad10–12 for the creation of bioartificial organs is dependent on the characteristics of the material itself but also from the technique used to create a scaffold. The standard in the past was to seed cells onto scaffolds in order to adhere and proliferate. In this scenario, randomly distributed cells will start 3D tissue formation in a natural process with the scaffold as a basic guide. The limits of this technique are obvious if it comes to more complex multicellular structures that require sophisticated vascular trees. Biological input has stimulated engineering in the development of new technologies to leverage the scaffold materials for usage in tissue engineering and regenerative medicine. 3D Bioprinting is an example, in which additive layer-by-layer manufacturing methods are refined to create 3D tissue-like structures directly (Figure 4(f)).109 In addition to producing scaffolds alone, this manufacturing method allows the integration of precise, reproducible spatial deposition of cells or bio-inks, respectively.110 Bio-ink is the term used for any scaffold material that contains living cells in bioprinting. Deposition of different cell types, bio-inks, and other materials simultaneously by using several printer heads and extrusion techniques allows to print complex tissue structures. The fabrication of an architecture that allows islet cells to be maintained in close proximity to vascular structure with 3D printing was shown with PLA as a scaffold material.51 The scaffold was then loaded with cells and growth factor-enriched platelet hydrogel.51 3D Bioprinting, in comparison with conventional 3D printing, is able to integrate these cell-containing hydrogels in the printing process to build more complex architectures.
The development of scalable manufacturing processes to GMP standard is critically important in ensuring successful translation of tissue engineering strategies to clinical practice.31,111 A study in which cell-containing alginate hydrogels were 3D-plotted showed that they had a greater surface area than bulk hydrogels and still maintained the soft-tissue like properties of the pancreas.112 Although high viability of the cells could be achieved, they showed a low glucose diffusion coefficient and impaired functionality due to insufficient diffusion capacity of the viscous hydrogel.112 It can be concluded that the alginate/gelatin composite hydrogel that was plotted is not the ideal biomaterial for the purpose of engineering an insulin-secreting construct with this technique. A study by Daoud et al.82,113 used PLGA to 3D-print a scaffold matrix. A cell–gel composite containing human islet cells, collagen I, collagen IV, and fibronectin was then added.113 This in vitro trial showed improved viability and function of the cells when seeded onto the PLGA scaffold, implying potential usage for long-term in vitro cell culture but also for further in vivo studies.113
This fabrication method requires expensive equipment and is currently only applicable to a limited range of materials, but offers excellent control over scaffold architecture and porosity. With the use of support materials that can be dissolved in water after the printing process, there are practically no limitations on scaffold architecture.
Significance of ECM components in scaffold-based tissue engineering
The use of scaffolds to host ISC should replicate the natural cell environment. In healthy pancreatic tissue ISC are also surrounded by a basic support structure, namely the ECM, which can be seen as a natural scaffold. The significance of specific cell–ECM interactions for cell viability and function and the additional roles of the ECM network in tissue homeostasis and mechanical stability have been described previously and are commonly accepted.38,114–125 After loss of the natural ECM environment in the digestion and isolation process, islet cells show signs of apoptosis but also signs of redifferentiation to immature progenitors, thus leading to multihormonal cells.126 As a logical consequence, polymers and proteins that are found in natural ECM networks have been used for building an artificial construct to host the cells.127 Collagen is frequently used, either alone in the form of a hydrogel or in combination with other materials. A hydrogel made of rat collagen as a 3D culture medium proved to be a better platform than a conventional two dimensional (2D) suspension culture when used for the differentiation of hiPSC into ISC.128 The coculture of cells with the collagen hydrogel in the differentiation period between day 7 and day 16 showed a higher expression of mRNA for pancreatic transcription factors and hormones in the cells.128 ECM compositions differ depending on the natural target tissue. The basement membrane that surrounds human islets consists predominantly of collagen IV, laminin, and fibronectin.38,120 The restoration of the natural ECM environment using these basement membrane proteins is beneficial for cell function.117,120,129–134 Improved survival rates, better retention of morphological structure, and the development of vasculature in islet cells seeded in basement membrane extracts including collagen IV, laminin, and heparin sulfate proteoglycan verified the hypothesis.120 In another study, collagen IV from porcine tendon was processed to form a macroporous upper layer on top of decellularized porcine pericardium as a stronger bottom layer. A significantly higher proportion of mice returned to normoglycemia, compared with the controls without the scaffold, and these animals had stable blood glucose levels for up to 300 days.129 However, this study also demonstrates the difficulty that future clinical application would involve if it comes to reproducible, precise manufacturing of those bilaminar scaffolds, especially once it will be necessary to scale up for use in humans. Considering the fact that ISC show improved function in scaffolds that mimic the natural islet cell environment as closely as possible, it is not surprising that some studies focused on providing exactly this extracellular milieu as a scaffold for cell seeding. Thus, the decellularization of organs such as lung,135,136 kidney,137 spleen,138 liver,139,140 or pancreas,80,115,133,136,141–148 and their recellularization with ISC was investigated. Two in vitro studies used dECM of rat and mouse liver as a scaffold for iPSC with mouse islet cells injected through the portal vein and showed higher insulin gene expression than in 2D controls.139,140 Other studies investigated dECM pancreas as a scaffold material and confirmed its applicability. dECM pancreas scaffolds were biocompatible over a period of 120 days,142 were functional upon glucose stimulation or showed up-regulated insulin gene expression,142,144,145,148 accelerated the differentiation of ß-progenitor cells into ISC,143 and could be further improved by using alginate encapsulation strategies.115 After sufficient decellularization, the scaffold itself should not cause immune responses of the host in allo- or xenotransplantation and already includes a decellularized vascular tree.146 Among the studies using dECM scaffolds, only one tried to recellularize this vascular network with an endothelial cell (EC) line in vitro, which resulted in a primitive vascular network.146 Though pure dECM scaffolds lack reproducibility and are more difficult to scale up to the volumes necessary for clinical application in humans, the strongest argument against their use for clinical organ replacement therapy is impracticability within a streamlined process for wide application in future medical treatments if not combined with additional scaffold materials. It also remains unclear whether the complexity of the complete pancreatic ECM is necessary—at least in the period until a new ECM has been built in the weeks after implantation—for successful engraftment of the ISC-loaded scaffold in vivo. However, for research applications, dECM pancreas scaffolds might be a suitable platform for cell proliferation and differentiation studies or complex disease modeling.
A significant effect of ECM components on ISC viability and function in scaffolds was also found when synthetic scaffold materials were used. With the exception of use of a collagen hydrogel or whole dECM organ, these studies employed a combination of different materials or ECM component additives in order to amalgamate the favorable characteristics of each material. This could help to solve some difficulties experienced when using an ECM-derived scaffold alone, for example, by enabling production of scaffolds with higher mechanical stability, influencing the degradation time after implantation, and improving precision and reproducibility. A study that investigated the effects of additional peptide sequences that can be found in fibronectin, laminin-1, and/or collagen IV, namely RGD (Arg-Gly-Asp), IKLLI (Ile-Lys-Leu-Leu-IIe), IKVAV (Ile-Lys-Val-Ala-Val), and YIGSR (Tyr-Ile-Gly-Ser-Arg) functionalized onto scaffold materials showed their beneficial effect on insulin production upon glucose stimulation of a beta cell line in vitro.38 The functionalization of the RGD and the YIGSR sequence onto the scaffold resulted in the greatest enhancement of cell functionality.38 These results were supported by another study that modified microporous PLGA scaffolds with collagen IV, fibronectin, and laminin. After 72 h of in vitro culture, the functionality of murine islets seeded on the three ECM-modified scaffolds was significantly higher than in the control group with PLGA alone. The subsequent in vivo trial showed a faster return to normoglycemia especially for the collagen IV-containing PLGA scaffold.149 The question of whether addition of a combination of relevant ECM components to the basic scaffold can lead to a synergistic effect was also investigated.30,117,150 An in vitro study that added collagen IV and laminin in different compositions to a protein–polymer hydrogel showed no synergistic effect on glucose-stimulated insulin secretion compared with, for example, addition of collagen IV alone.150 This unexpected finding may be explained by the influence of the scaffold material itself on the cell–ECM interactions and the possible allocation of binding sites.150,151 This hypothesis is supported by other studies that indeed showed synergy, first with simultaneous application of RGD, IKVAV and glucagon-like peptide 1 (GLP-1) agents and second with rationally designed collagen IV and laminin (in a 25:75 ratio), compared with the effects of the ECM components alone.30,117 The addition of ECM components for improved viability and function of ISC within scaffolds is more complex and depends on factors such as the scaffold material, the investigated cell type and maturation status, the functionalization method for correct presentation of the protein or peptide sequence to the cells, and the substitution rate of ECM components on the scaffold.132,152 Different collagen IV and laminin ECM components in alginate-encapsulated human embryonic stem cells (hESC), for example, showed no significant effect compared with the control and did not positively influence the cell differentiation into ISC.153
Vascularization for oxygen and nutrient supply
Although the endocrine pancreas represents only 1%–2% of the total organ volume, pancreatic islets receive 5%–15% of the total blood flow.154,155 During isolation of islets, both in clinical islet transplantation and tissue engineering techniques, islets will be separated from their surrounding exocrine tissue and consequently lose their connection to the vasculature.113 Prolonged absence of blood supply, hypoxia, and accumulation of metabolic waste and catabolic products lead to shortened survival of the islet cell.46 In all tissue engineering approaches, the restoration of oxygen and nutrient supply through adequate blood supply is therefore essential. A sufficient supply of blood can be achieved through (a) modulation of the scaffold structure itself, for instance, in wall thickness, porosity, interconnectivity, or channel networks to allow diffusion and blood vessel ingrowth,46,51,112,156 (b) neoangiogenesis or vasculogenesis occurring naturally in the host organism or through additional stimulation by growth factors, or (c) the addition of other cell types that enhance vessel formation41,157 (Figure 5).
Figure 5.
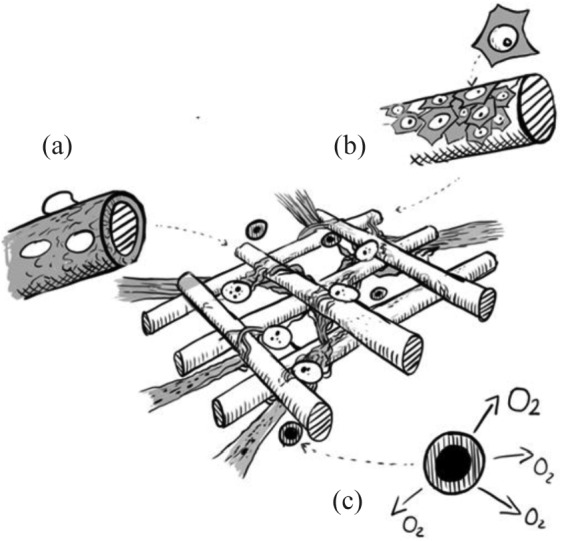
Vascularization strategies: (a) modifications of the scaffold material by, for example, coating with covalent bound heparin and association of growth factors and signaling molecules; (b) coculturing of other cell types such as endothelial cells or fibroblasts; and (c) the addition of oxygen producing or releasing particles were described to promote vascularization into the bioartificial construct.
The fabrication of the scaffold structure that incorporates ISC needs to consider the closest connection to the external environment. The most commonly accepted maximum distance from the closest pore or microchannel that will allow sufficient oxygen diffusion is 200 µm.73,89,158–160 In one study, even though the required proximity to the external environment as was achieved, sufficient diffusion could sometimes not be maintained and there was loss of functionality.112 In addition to the distance to the external environment, the size of the pore or connecting structure, and thus the diffusion capacity, also needs to meet the requirements of the encapsulated cell types. Landers et al. recommend that the pore size should be 5–10 times the diameter of the encapsulated cell.161 As human islets vary in size from 50 to 500 µm,16,19 the required pore size would not allow protection from the immune system.
Other approaches tried to overcome this conflict by functionalization of the scaffold and addition of signaling molecules that promote vascularization (Figure 5(a)). Instead of direct injection of growth factors into the transplantation site, leading to leaking vessels and abnormal vessel function, controlled local release of signaling molecules from scaffold materials has proved successful.57,116 This strategy can be enabled through incorporation into hydrogels and cell-encapsulating systems, covalent binding to scaffold materials, or functionalization onto mediating molecules such as heparin29,52 (Figure 5(a)). Electrostatic binding of VEGF-A to heparin, which itself can be covalently linked to a 3D-printed PCL scaffold using carbodiimide chemistry, led to angiogenesis and resulted in enhanced viability and function of islets.52 Heparin as a mediation molecule between scaffold and growth factor retained a much higher amount of VEGF-A than the negative control without heparin. In addition, the functionalization prevented burst releases of the growth factor.52 A chicken chorioallantoic membrane (CAM) assay showed that 200 ng mL−1 VEGF-A loading of the PCL scaffold proved most effective for induction of de novo vessel formation. Higher concentrations resulted in aberrant vessels, reduced angiogenesis, or hemangioma formation.52 Another study using VEGF in concentrations of 0.5 µg mL−1 and 5 µg mL−1 confirmed the adverse effect of high VEGF concentrations and showed host reactions resulting in calcifications around the scaffold.51 A negative control without heparin/VEGF-A showed no effect on vessel formation.52 Rather than the total dose of VEGF, it is the microenvironmental gradient and release kinetics that define the therapeutic threshold, explaining discrepant findings.162 Another study fabricated a fibrin–heparin/VEGF scaffold and performed in vivo experiments showing penetration of cells and generation of new capillaries into the whole fibrin–heparin/VEGF scaffold after subcutaneous implantation into diabetic mice. In contrast, a fibrin/VEGF scaffold without heparin functionalization showed these effects only around the scaffold margins.163 EC migration to the scaffold, promoted by heparin alone, might be due to the different surface topography after heparin coating of the scaffold, but also the high protein-binding capacity of heparin. Increased serum protein absorption, and thus increased cell adhesion to the heparin-coated scaffolds, may be a separate factor leading to vessel ingrowth.52 In addition to scaffold functionalization with growth-signaling molecules, oxygen-producing materials can further improve islet survival as they decrease irreversible cell damage caused by hypoxia in the initial period after implantation163–165 (Figure 5(c)). Microparticles containing hydrogen peroxide with a functionalized PLGA shell to ensure retarded oxygen release were implanted together with the fibrin-heparin/VEGF scaffold. Compared with the controls, this approach proved to be the most successful on 30-day observation of blood glucose and body weight after transplantation into the omental pouch163 (Figure 5(c)). Other angiogenic factors were investigated in a human umbilical vein EC (HUVEC) tube formation assay comparing platelet derived growth factor (PDGF), VEGF, aFGF, and bFGF (fibroblast growth factor) on the angiogenic capacity of vascular EC.29,57,94,123 aFGF was found to be superior to the other substrates investigated in terms of angiogenic capacity.94
Angiogenic growth factors directly or indirectly stimulate a multi-step process that involves migration and proliferation of ECs. The inclusion of ECs and other cell types besides ISC into a scaffold was therefore investigated54,157 (Figure 5(b)). Especially in the in vitro culture period, scaffolds will allow ECs to grow adherently. This method proved to yield higher efficacy in the coating of islets, as these usually grow in suspension culture, leading to low attachment of the ECs in the absence of a scaffold microenvironment.54 Viability, function, VEGF expression, and microvessel density were all better in the PGA scaffold group.54 The beneficial effect on the insulin-secreting function of co-transplanted ECs was also shown when using other scaffold materials, such as collagen.157 The effect of cocultured mesenchymal stem cells (MSC) instead on the graft function was not consistent across studies.30,157,166,167 A doubling of insulin secretion after glucose stimulation was seen when MSC were cocultured in PEG hydrogels.30 In addition to effects on insulin secretion, MSC are known to have paracrine and immunoregulatory functions that might be beneficial in vivo.168 Further studies on other scaffold materials might even use the EC coating of inner surface areas, such as microchannels serving as artificial capillaries. This method might provide a solution to the conflict between larger pore sizes for diffusion and the need to maintain a barrier that protects from inflammatory host reactions. Fibroblasts have also been investigated in coculture with ISC on a scaffold, as they are known to produce growth factors (e.g. VEGF, FGF) and to produce and support ECM components such as collagens and fibronectin, and therefore play a role in tissue repair processes.121,169–171 Fibroblast coculture with islet cells significantly increased the insulin secretion upon glucose stimulation170 (Figure 5(b)). In an in vivo trial using a fibroblast-populated collagen scaffold, the marginal islet mass required to restore normoglycemia was lower by half compared with the control with free islets and by a third compared with the ISC/collagen scaffold alone.169 The synergistic effects of ECs and fibroblasts engineered in agarose molds to form a prevascularized sheet for islet cells were investigated and showed the ability to build a vascular network that connected to the host vessels in vivo.172
Whatever method is used for restoration of the islet vasculature, there is a certain formation period to be bypassed. Recent strategies have been either to implant the scaffold at least partially before addition of the cells for pre-vascularization,25,26,51,92,94,173 or to add oxygen-producing nanoparticles and substrates that ensure survival in the period after implantation when new vasculature has yet been formed165 (Figure 5(c)). The in vitro treatment of cultured islets prior to implantation with oxygen-generating disks (OxySite; solid encapsulation of calcium peroxide in PDMS disks) proved to have a positive impact on overall graft efficacy by enhancing intra-islet vascularization and decreasing the hypoxic expression of pro-inflammatory cytokines without diminishing the hypoxia inducible factor (HIF)-induced VEGF-A release.174 This pretreatment of the islets before transplantation may lead to higher viability, especially of bigger islets, and thus increased insulin-secreting function in vivo.
Immunoprotection through scaffold material
One reason why long-term independence from insulin in islet transplantation following the Edmonton protocol3,175 usually cannot be sustained is early graft loss due to IBMIR. It is estimated that a majority of transplanted islets are lost rapidly due to coagulation, platelet aggregation, complement activation, and invasion by neutrophilic granulocytes and monocytes.7,176,177 Treatment with chronic immunosuppressive regimens3 and the infusion of a higher number of islets, often retrieved from more than one donor,175 have been used to prevent further specific immune reactions and diminish graft loss. The side effects of immunosuppressive drugs178 and the preexisting shortage of donor organs have a negative impact on this treatment option. Tissue engineering approaches offer a variety of strategies to protect the islet cells to be implanted from the host immune system.39,179–182
Small molecules can still pass through the membrane to ensure inflow of oxygen, glucose, and nutrients and outflow of insulin and metabolic waste products12 (Figure 6). Although the severity of immediate inflammatory reactions against the grafts can be reduced by semipermeable encapsulation strategies and the selection of implantation sites other than originally proposed in the Edmonton protocol,3 long-term reactions of the immune system, which go along with cell migration toward the implant and consequent PFO, are an additional diffusion barrier that leads to hypoxia and a lack of nutrient supply. Various strategies to avoid PFO have been investigated, including local controlled release of a non-steroidal anti-inflammatory drug (NSAID) through linkage with the encapsulating hydrogel (e.g. Na-Alg).67 Although the functionalization of the drug avoided burst releases shortly after implantation, it could only be sustained for the first 14 days of an overall observation period of 30 days.67 In similar approaches, glucocorticoids183,184 and other immunosuppressive agents (e.g. tacrolimus,185 tumor necrosis factor α (TNFα)-antagonizing peptide,186 interleukin (IL)-4,184 inhibitory peptide sequence for ISC surface IL-1 receptor,187 monocyte chemoattractant protein-1 (MCP-1) binding peptides,188 and transforming growth factor (TGF-ß))189 were linked to the scaffold material (PDMS,183 PLGA-PEG-DOPA nanoparticles,185 silk,184 PLG,189 and PEG).186–188 At 30 days after implantation, only low dosages of the glucocorticoid showed improved engraftment of the islet-containing scaffold through modulation of the inflammatory response; higher dosages showed decreased engraftment and function.183 The general idea of linking immunosuppressive drugs to the scaffold material may seem contradictory, as they have been demonstrated to have cytotoxic effects on islet cells.8,189–191 The proposed future investigations for different drug linkages to the scaffold, drug dosages, or combinations of drugs might turn out to be dispensable if one follows another approach where the islets are embedded in a hydrogel scaffold (chemically triazole-thiomorpholine dioxide-modified alginate), mitigating the immune response itself.65 The bioartificial organ provided glycemic control when implanted intraperitoneally into immune-competent mice until removal at day 174.65
Figure 6.
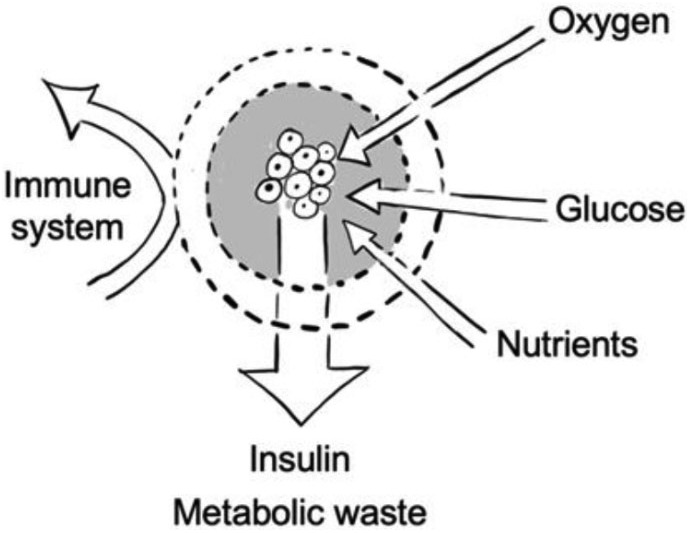
Encapsulation of insulin-secreting cells by a semi-permeable membrane.
Further strategies that proved beneficial for long-term immune protection include the coculturing of islets with other cell types, such as indoleamine 2,3-dioxygenase (IDO)-expressing (enzyme in tryptophan catabolism pathway) fibroblasts, through increasing the population of regulatory T-cells and preventing T-cell infiltration.192,193 Interestingly, the coculture of fibroblasts with ISC on a collagen scaffold did not result in significant PFO.169
In addition to the findings above, it is conceivable that physical properties of the scaffold such as size and shape may also be relevant for the dimension of the foreign body response. An in vivo study of rat islet-containing alginate capsules showed that the foreign body response is inversely correlated to the size of the scaffold (0.5 mm vs 1.5 mm, results normalized to surface area) and is further diminished when the bioartificial organ shows a spherical shape.65,194 The resulting conflict between small microcapsules for sufficient oxygen and nutrient supply, as described in the section above, and the increasing foreign body response and fibrotic overgrowth for small microcapsules is one of the challenges for current ISC tissue engineering approaches.
Cell differentiation
The approach to integration of fully differentiated, adult cells, such as pancreatic islet cells, into suitable scaffolds for clinical transplantation into patients has several disadvantages and might only be a milestone on the road to creation of bioartificial organs for regenerative medicine. A global shortage of donor organs, immunological graft–host reactions against non-autologous tissue, and the islet isolation process itself, which is stressful and potentially damaging, are not optimal preconditions for broad application. For the future of organ creation, stem cells as an unlimited source to form new tissue are favored. 3D cell culture systems are a novel technique for studying cell differentiation both in vitro and in vivo and were shown to resolve the limitations of expensive and time-consuming 2D in vitro protocols.31,53,93,102,106,195–197 Furthermore, it was shown that successful differentiation into early ISC is possible in vitro; maturation, however, requires in vivo conditions.31,101,196–200 There has been extensive research on the differentiation of insulin-secreting beta cells from human pluri- and multipotent stem cells (n = 47 studies), such as hiPSC,53,106,107,201,202 hESC,93,195,197–199,203–206 and MSC.31,100–102,207–210 Again, these approaches are based on mimicking the processes of natural pancreas embryology by stepwise reproduction with the addition of growth factors and other molecules. This process can be controlled by monitoring gene expression along the developmental stages. To verify early pancreatic progenitor stages the expression of pancreatic duodenal homeobox 1 (Pdx1) has often been detected; for later stages, NKX6.1, a transcription factor in early beta cell development; and in insulin-secreting mature states, MafA, a transcription factor in adult beta cells that is described as critical for insulin production, has been used as a marker molecule.42,93,138,195,198,199,204,207
A 10-day differentiation protocol was able to produce hESC-derived PDX1+, NKX6.1− early pancreatic progenitor cells in more than 80% of the cell population.195 Together with mesenchymal and ECs, organoids were formed in 3D cell culture with Matrigel®. After implantation into the peritoneal cavity of nude mice within a 3D-printed PLA scaffold that contained Matrigel® and collagen I, the cells further differentiated into ISC in vivo. Vascularization of the device and cell–ECM interactions supported this process, and after 90 days in vivo 38.5% of the cells were insulin-positive.195 In contrast to ESC, which can be subject to ethical issues, hADSC can be obtained repeatedly using minimally invasive techniques and are thus eligible for autologous procedures.100 The electrospun PLA/PVA 3D culture platform enhanced the differentiation of those cells into ISC. The successful differentiation of hADSC into ISC was proved by higher gene expression of PDX1, glucagon, and insulin as well as a physiological insulin response after glucose stimulation in comparison with 2D culture.100 One challenge in many differentiation protocols is the production of monohormonal ISC.204 Exenedin-4, a GLP-1 receptor agonist, was shown to not only promote the sphere formation of progenitor cells but also significantly increase the proportion of monohormonal ISC after transplantation within a PCL scaffold.100,201
Although the approach is intriguing, further research is necessary before it can be successfully implemented as a transplantation concept. In general, the reviewed literature showed a dual-track strategy. On the one hand, there are approaches that focus on the development of a suitable scaffold for mature ISC, taking the points discussed above into consideration. On the other hand, there are approaches that particularly focus on the differentiation of ISC from stem cells. Promising results from both strategies are then brought together, thus reducing the variables that could lead to failure.
Transplantation site
In contrast to the initial intraportal transplantation in the Edmonton protocol, various other implantation sites can be used for scaffold-based organ replacement treatments. The liver and the portal vein may not be ideal transplantation sites due to IBMIR, low oxygen tension, and the many toxins and drugs that are processed in the liver.8,9 Scaffold-based implants allow the choice between a wider spectrum of transplantation sites. A dense vascular network for sufficient oxygen and nutrient supply, real-time access to physiologically representative blood glucose levels, low immune response rates, but also easy surgical access and the possibility to retrieve the implant, are general criteria when choosing a suitable implantation site for the cell-loaded scaffold.211 In addition to these factors, more specific criteria may apply depending on the nature of the cell-loaded scaffold itself. Scaffold size, mechanical stability, and diffusion distances affect the choice further. Among the in vivo studies included (n = 91) the ISC/scaffold construct was transplanted into subcutaneous locations (n = 31),27,51,69,92–94,112,115,120,133,135,145,147,156,157,171,212–225 the peritoneal cavity (n = 19),18,26,56,63,65–68,101,134,135,173,195,226–232 an omental pouch (n = 14),57,59,81,118,119,163,174,225,233–238 the renal capsule (n = 18),54,67,69,75,96,120,121,131,135,169,185,192,193,228,239–242 the epididymal fat pad (n = 9),85,91,129,149,183,189,201,236,243 the small intestinal mesentery (n = 1),116 alongside a vessel (n = 3)25,26,244 and other sites (Figure 7). The evidence gap map shows that most of the studies conducting in vivo experiments focused on the subcutaneous site for implantation. Compared with the first evidence gap map (Figure 2), the evidence map on transplantation sites, which included only in vivo studies, presents a higher proportion of studies that used stem cells or cells intended for differentiation into ISC. This can be explained by the findings above showing that many differentiation protocols to produce ISC require both in vitro and in vivo settings.198–200 The tendency toward the use of natural materials for scaffold fabrication is comparable with the first evidence gap map (Figure 2). The only study that used a synthetic scaffold material (PLA) together with human islet cells, published in 2017, also used 3D printing technology to fabricate the scaffold.51 This supports the assumption that there might be a shift in future research toward the use of synthetic scaffold materials or composite scaffolds due to the access to new manufacturing techniques such as 3D (bio)printing.
The subcutaneous site offers both minimally invasive access and the space for therapeutic transplant volumes,157 and was the most frequently used transplantation site in the reviewed articles. One simple and low-risk implantation method is the subcutaneous injection of a cell-containing scaffold–hydrogel.157,213 The combination of a transient cell-containing hydrogel that can be reloaded on demand and a permanent scaffold made out of a mechanical stable synthetic polymer was described as an important step in the direction of long-term function.51 However, because the vascular density is poor, several strategies have been used to induce the vascularization that is necessary for survival of the ISC.170 A study that used an ISC/collagen scaffold compared the subcutaneous site with the renal subcapsule and showed reduced graft function for the subcutaneous site as measured by blood glucose measurement.120 The promotion of vascularization as discussed above showed positive results in several in vivo studies.51 Especially when targeting the subcutaneous transplantation site, the question needs to be addressed of whether these animal experiments are predictive for and transferable to humans. Mice made diabetic by injection of streptozotocin or alloxan shortly before the experiment do not have the secondary complications of diabetes such as angiopathy. It can be assumed that these secondary complications of diabetes will impede vascularization. A systemic vascular disease will thus affect the subcutaneous site more severely than other possible transplantation sites. In direct comparison with other transplantation sites, the subcutaneous site showed strong immune and foreign body responses against the implanted ISC/scaffold graft.69
Intraperitoneal implantation or implantation into an omental pouch of bioartificial organs in a mouse or rat model may be transferable to future human applications, although these have been reported to be pro-inflammatory environments.81,211 The peritoneum is richly vascularized, which is a basic requirement for vascular ingrowth to provide sufficient oxygen supply, and offers the space for the implantation of a therapeutic volume.233 It should be noted that revascularization after intraperitoneal implantation can only take place once the graft attaches at a defined location.57 An advantage of the peritoneal cavity, and especially the omental pouch, is the physiological venous outflow of the produced insulin directly into the liver through the portal vein. Depending on the size, architecture, and biomechanical integrity of the device, retrievability is a limiting factor for clinical applications, especially for intraperitoneal implantations.57,67 The injection or implantation of hydrogel microcapsules into the peritoneal cavity leads to difficulties if removal becomes necessary, for example, due to graft–host reactions or other medical complications. Even several peritoneal lavages may not suffice to remove all of the microcapsules. Retrievability is an important requirement for of a clinical implantation of such a medical device in humans. The high number of microcapsules necessary for the treatment of humans and the complex anatomy of the abdominal cavity make a complete retrieval of the implants practically impossible. Another issue with both intraperitoneal implantation and transplantation into an omental pouch, one that is not addressed in the in vivo studies, is the problem arising if a patient needs relaparotomy due to any other medical condition. The implanted microcapsules or other ISC/scaffold constructs would be damaged or removed in the course of surgical intervention. The reviewed articles offer different solutions to overcome this difficulty, especially when choosing the peritoneal cavity or omental pouch for implantation. The use of a 3D-printed porous PLA basket with a lockable cover would be a way of trapping ISC, hydrogel materials, and other compounds but still being able to retrieve them.195 The idea of somehow attaching the cell-containing hydrogel was also utilized in a study where a fiber made out of twisted surgical sutures was fabricated as an inner scaffold that is mechanically stable.228 This fiber was subsequently coated with a cell-containing alginate hydrogel. Complete, undamaged retrieval via laparoscopic surgery with low inflammatory tissue responses were seen in a scaled-up dog model.228 Even though the authors tested the viability and function of their device in vivo and showed safe retrievability, it remains unclear whether the geometry and size of such a structure will cause medical complications such as adhesions, obstruction, and discomfort in the long term. The qualified eligibility of the peritoneal cavity and omental pouches as transplantation sites with regard to the discussed problem of retrievability only goes to emphasize the importance of adjustment of scaffold materials, scaffold architecture, and designated transplantation site. Epididymal fat pad transplantations (n = 9) in rodent models were used to prove the concepts of the respective studies in vivo. As this anatomical location does not exist in humans, with intraperitoneal fatty tissue being the analog, the same considerations as for the intraperitoneal cavity or omental pouch sites apply.
Interestingly, there is evidence that a higher number of IEQ is necessary to restore normoglycemia, accompanied by a later onset of insulin-secreting function for the omental transplantation site than for the renal subcapsular or intrahepatic transplantation site.118,238,245 In direct comparison, the renal subcapsule showed the smallest marginal mass of IEQ to restore normoglycemia, the fastest onset of graft function, and the strictest glucose control in a mouse model.245 Furthermore, the renal subcapsule has also been described as an immunoprivileged transplantation site.69 Although this transplantation site has been chosen frequently in rodents (n = 18), clinical applicability in humans is not feasible. The human renal subcapsule is inelastic and does not offer the space necessary for the implantation of therapeutic dosage.245 In contrast to subcutaneous and intraperitoneal implantation there is not enough space to host macrocapsules and scaffold constructs larger than microcapsules (<150 µm).211
One research group investigated the transplantation of an ISC-containing scaffold directly along a vessel.25,26,244 The devices containing islet cells, scaffold material, and growth factor supplements were implanted along epigastric vessels or the femoral neurovascular pedicle.25,26,244 This transplantation site (n = 3) is expected not only to show the advantage of a sufficient vascular supply for the islets in terms of oxygen and nutrient diffusion to maintain viability, but also provide real-time access to the blood glucose concentrations and show a direct hormonal delivery pathway.26 In contrast to the conventional hepatic transplantation site or intraportal injection, the ISC-containing scaffold is not directly exposed to the bloodstream and therefore IBMIR is minimal.26 In these studies, the cells were implanted 3–4 weeks after implantation of the scaffold to avoid initial foreign body responses and bypass the period before sufficient vascularization was developed. The epigastric vessels were encased with a silicone tubing that included Matrigel® and bFGF. In a second step, a gelatin sponge with the loaded islet cells was implanted between silicone tubing and vessel.26 Although Matrigel® cannot be translated into clinical application and the choice of a peripheral site shows significant delays before graft function can be measured, this study showed promising results for the use of this transplantation site. A subsequent study of the same group without Matrigel® again used a gelatin sponge with supplemented nerve growth factor (NGF) for implantation alongside the femoral neurovascular pedicle.244 This modified protocol showed enhanced viability of islets on the gelatin scaffolds that were supplemented with NGF compared with the controls without NGF. Finally, the suitability of the transplantation site was shown by insulin secretion after intraperitoneal glucose injection, similar to the non-diabetic control.244
Conclusion
Scaffolds serve as biomimetic materials designed to resemble the models of nature and to imitate their function for a broad spectrum of applications in the medical industry. There is already evidence proving the eligibility of scaffolds as 3D culture platforms and also as platforms for enhancing differentiation of immature cells to ISC. The approach to create an insulin-secreting organ can be seen as a perfect example for other future applications in tissue engineering. The substitution of a single cell type, namely the insulin-secreting beta cells, may be an alternative future treatment for patients with a loss of endocrine pancreatic function and an advance in bioartificial organ transplantation medicine. However, much needs to be done to overcome the current limitations, specifically with regard to long-term survival and function of the cells, the use of suitable scaffold materials, and the design and fabrication of a scaffold architecture that allows sufficient oxygen and nutrient supply without compromising protection of the graft from the host immune system. These problems have to be solved before clinical application of any bioartificial organ can become reality.
Direct comparison of the tissue engineering approaches using scaffold materials on ISC is currently impossible due to the variety of factors that need to be considered. Even direct comparison of transplantation sites is difficult. Different scaffold materials, scaffold architectures, species, and maturation status of the donor cells, as well as the different parameters defined to evaluate the outcome (e.g. metabolic control, oxygen tension, functional mass, tissue response, practical applicability, cell differentiation), all preclude direct comparison. There is therefore an urgent need for structured, evidence-based research. We have shown that evidence gap maps can provide a synopsis of research data, including experimental studies, to display complex, multi-parameter objectives. The evidence gap maps yield an easily accessible quantitative and qualitative overview and allow determination of high-interest and low-interest areas in published research. This method allows us not only to identify gaps, but also to aggregate related evidence and thus come up with new findings. The reviewed literature shows promising results for of ISC/scaffold constructs but not yet a final solution for clinical application.
Both insufficient nutrient and oxygen supply due to perfusion limitations in scaled-up scaffold macrocapsules and, in contrast, the increasing host reactions against smaller-sized microcapsules will eventually lead to graft failure.194 Generally, the microencapsulation strategy might not be a feasible approach for clinical application of ISC/scaffold bioartificial organs. However, implantation of the number of hydrogel droplets required to achieve therapeutic dosages for human patients seems impractical, and the transplantation procedure and follow-up will be impeded by, for example, impaired retrievability due to the intrinsic properties of the microcapsules. This conflict may only be resolved by following a complex, multi-dimensional strategy in which scaffold materials, advanced scaffold manufacturing techniques, cells, and signaling molecules will be adapted. Both hydrogel scaffold materials and polymer-based scaffold materials can provide only some of the required scaffold characteristics. The combination of several scaffold materials to form a hybrid device seems to be a reasonable objective for future investigations. New scaffolding technologies such as 3D bioprinting may offer the possibility for combined fabrication of several materials into a designed 3D architecture. This 3D architecture can have higher complexity, for example, with microchannels that mimic natural vascular trees rather than microcapsules or bulked scaffold sheets. The inclusion of simplified vascular trees or other routes for perfusion to the scaffold core can ensure sufficient oxygen and nutrient supply for all ISC, no matter their location within the construct. The significance of ECM components for ISC viability, function, and successful differentiation of immature progenitor cells was proved in the reviewed literature and should be integrated into future investigations.
The creation of an implantation site with close proximity of the bioartificial tissue to blood vessels might improve the long-term viability and function. Only three articles that met the inclusion criteria of this systematic review focused on this transplantation site.25,26,244 As the subcutaneous site, the renal subcapsule, the epididymal fat pad, and, in the majority of cases, also the peritoneal cavity and omentum or the creation of omental pouches each have specific disadvantages we strongly recommend research into prospective new transplantation sites. Further developments in stem cell technologies and their differentiation to insulin-secreting cells, DNA editing methods, and new manufacturing techniques and materials may combine to enable the vision of a bioartificial pancreas to become reality.
Supplemental Material
Supplemental material, PRISMA_2009_checklist for The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells by Gabriel Alexander Salg, Nathalia A Giese, Miriam Schenk, Felix J Hüttner, Klaus Felix, Pascal Probst, Markus K Diener, Thilo Hackert and Hannes Götz Kenngott in Journal of Tissue Engineering
Supplemental Material
Supplemental material, SupplementaryInformation1 for The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells by Gabriel Alexander Salg, Nathalia A Giese, Miriam Schenk, Felix J Hüttner, Klaus Felix, Pascal Probst, Markus K Diener, Thilo Hackert and Hannes Götz Kenngott in Journal of Tissue Engineering
Supplemental Material
Supplemental material, SupplementaryInformation2 for The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells by Gabriel Alexander Salg, Nathalia A Giese, Miriam Schenk, Felix J Hüttner, Klaus Felix, Pascal Probst, Markus K Diener, Thilo Hackert and Hannes Götz Kenngott in Journal of Tissue Engineering
Acknowledgments
Evidence gap map plotting was performed with the kind assistance of Daniel Schmitt, New Technologies and Data Science, Heidelberg University Hospital, Germany.
Footnotes
Author contributions: G.A.S. and H.G.K. are responsible for the conception and design of this study and developed the search strategy together with P.P. and M.K.D. G.A.S. performed the literature analysis, data extraction, data interpretation, created the evidence gap maps and wrote the first draft of the report. N.A.G., F.J.H., K.F., P.P., M.K.D., T.H., and H.G.K. reviewed and edited the article. P.P. and M.K.D. initiated the usage of evidence gap maps for systematic reviews. G.A.S. and H.G.K. coordinated the editing process.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funding from EU Horizon 2020 Eurostars-2 (E!12021). Half of the article processing charge was covered by Deutsche Forschungsgemeinschaft within the funding program Open Access Publishing, by the Baden-Württemberg Ministry of Science, Research and the Arts and by Ruprecht-Karls-Universität Heidelberg.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014; 383: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fiorina P, Shapiro AM, Ricordi C, et al. The clinical impact of islet transplantation. Am J Transplant 2008; 8: 1990–1997. [DOI] [PubMed] [Google Scholar]
- 3. Shapiro AMJ, Lakey JRT, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343(4): 230–238. [DOI] [PubMed] [Google Scholar]
- 4. Hering BJ, Clarke WR, Bridges ND, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016; 39(7): 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cross SE, Vaughan RH, Willcox AJ, et al. Key matrix proteins within the pancreatic islet basement membrane are differentially digested during human islet isolation. Am J Transplant 2017; 17(2): 451–461. [DOI] [PubMed] [Google Scholar]
- 6. Desai T, Shea LD. Advances in islet encapsulation technologies. Nat Rev Drug Discov 2017; 16: 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Korsgren O, Nilsson B, Berne C, et al. Current status of clinical islet transplantation. Transplantation 2005; 79: 1289–1293. [DOI] [PubMed] [Google Scholar]
- 8. Shapiro AM, Gallant HL, Hao EG, et al. The portal immunosuppressive storm: relevance to islet transplantation. Ther Drug Monit 2005; 27(1): 35–37. [DOI] [PubMed] [Google Scholar]
- 9. Bennet W, Sundberg B, Groth CG, et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes 1999; 48(10): 1907–1914. [DOI] [PubMed] [Google Scholar]
- 10. O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today 2011; 14: 88–95. [Google Scholar]
- 11. Kolos E, Ruys AJ. Application of biomimetic scaffolds for tissue engineering. J Biomim Biomater Tissue Eng 2013; 18: e103. [Google Scholar]
- 12. Noh I. Biomimetic medical materials: from nanotechnology to 3D bioprinting. Singapore: Springer, 2018. [Google Scholar]
- 13. Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J 2008; 17(Suppl. 4): 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang XD, Zou XH, Dai ZW, et al. Effects of oligo(3-hydroxyalkanoates) on the viability and insulin secretion of murine beta cells. J Biomater Sci Polym Ed 2009; 20(12): 1729–1746. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Komatsu H, Cook C, Wang CH, et al. Oxygen environment and islet size are the primary limiting factors of isolated pancreatic islet survival. PLoS ONE 2017; 12(8): e0183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skiles ML, Fancy R, Topiwala P, et al. Correlating hypoxia with insulin secretion using a fluorescent hypoxia detection system. J Biomed Mater Res B Appl Biomater 2011; 97(1): 148–155. [DOI] [PubMed] [Google Scholar]
- 18. O’Sullivan ES, Johnson AS, Omer A, et al. Rat islet cell aggregates are superior to islets for transplantation in microcapsules. Diabetologia 2010; 53(5): 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buchwald P, Tamayo-Garcia A, Manzoli V, et al. Glucose-stimulated insulin release: parallel perifusion studies of free and hydrogel encapsulated human pancreatic islets. Biotechnol Bioeng 2018; 115(1): 232–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ito M, Taguchi T. Enhanced insulin secretion of physically crosslinked pancreatic beta-cells by using a poly(ethylene glycol) derivative with oleyl groups. Acta Biomater 2009; 5(8): 2945–2952. [DOI] [PubMed] [Google Scholar]
- 21. Kojima N, Takeuchi S, Sakai Y. Engineering of pseudoislets: effect on insulin secretion activity by cell number, cell population, and microchannel networks. Transplant Proc 2014; 46(4): 1161–1165. [DOI] [PubMed] [Google Scholar]
- 22. Vermesh U, Vermesh O, Wang J, et al. High-density, multiplexed patterning of cells at single-cell resolution for tissue engineering and other applications. Angew Chem Int Ed Engl 2011; 50(32): 7378–7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Jalili RB, Warnock GL, et al. Three-dimensional scaffolds reduce islet amyloid formation and enhance survival and function of cultured human islets. Am J Pathol 2012; 181(4): 1296–1305. [DOI] [PubMed] [Google Scholar]
- 24. Zhao M, Song C, Zhang W, et al. The three-dimensional nanofiber scaffold culture condition improves viability and function of islets. J Biomed Mater Res A 2010; 94(3): 667–672. [DOI] [PubMed] [Google Scholar]
- 25. Hussey AJ, Winardi M, Han XL, et al. Seeding of pancreatic islets into prevascularized tissue engineering chambers. Tissue Eng Part A 2009; 15(12): 3823–3833. [DOI] [PubMed] [Google Scholar]
- 26. Forster NA, Penington AJ, Hardikar AA, et al. A prevascularized tissue engineering chamber supports growth and function of islets and progenitor cells in diabetic mice. Islets 2011; 3(5): 271–283. [DOI] [PubMed] [Google Scholar]
- 27. Sasikala M, Rao GV, Vijayalakshmi V, et al. Long-term functions of encapsulated islets grafted in nonhuman primates without immunosuppression. Transplantation 2013; 96(7): 624–632. [DOI] [PubMed] [Google Scholar]
- 28. Clinical Applications of Biomaterials. NIH Consens Statement 1982; 4: 1–19. [Google Scholar]
- 29. Marchioli G, Zellner L, Oliveira C, et al. Layered PEGDA hydrogel for islet of Langerhans encapsulation and improvement of vascularization. J Mater Sci Mater Med 2017; 28(12): 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bal T, Nazli C, Okcu A, et al. Mesenchymal stem cells and ligand incorporation in biomimetic poly(ethylene glycol) hydrogels significantly improve insulin secretion from pancreatic islets. J Tissue Eng Regen Med 2017; 11(3): 694–703. [DOI] [PubMed] [Google Scholar]
- 31. Sabek OM, Farina M, Fraga DW, et al. Three-dimensional printed polymeric system to encapsulate human mesenchymal stem cells differentiated into islet-like insulin-producing aggregates for diabetes treatment. J Tissue Eng 2016; 7: 2041731416638198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Silva AI, Mateus M. Development of a polysulfone hollow fiber vascular bio-artificial pancreas device for in vitro studies. J Biotechnol 2009; 139(3): 236–249. [DOI] [PubMed] [Google Scholar]
- 33. Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci 2012; 37: 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Espona-Noguera A, Ciriza J, Canibano-Hernandez A, et al. Tunable injectable alginate-based hydrogel for cell therapy in Type 1 Diabetes Mellitus. Int J Biol Macromol 2018; 107(Pt A): 1261–1269. [DOI] [PubMed] [Google Scholar]
- 35. Otterlei M, Ostgaard K, Skjak-Braek G, et al. Induction of cytokine production from human monocytes stimulated with alginate. J Immunother (1991) 1991; 10(4): 286–291 [DOI] [PubMed] [Google Scholar]
- 36. Li N, Sun G, Wang S, et al. Engineering islet for improved performance by optimized reaggregation in alginate gel beads. Biotechnol Appl Biochem 2017; 64(3): 400–405. [DOI] [PubMed] [Google Scholar]
- 37. Montalbano G, Toumpaniari S, Popov A, et al. Synthesis of bioinspired collagen/alginate/fibrin based hydrogels for soft tissue engineering. Mater Sci Eng C Mater Biol Appl 2018; 91: 236–246. [DOI] [PubMed] [Google Scholar]
- 38. Lim DJ, Antipenko SV, Vines JB, et al. Improved MIN6 beta-cell function on self-assembled peptide amphiphile nanomatrix inscribed with extracellular matrix-derived cell adhesive ligands. Macromol Biosci 2013; 13(10): 1404–1412. [DOI] [PubMed] [Google Scholar]
- 39. Kuehn C, Fulop T, Lakey JR, et al. Young porcine endocrine pancreatic islets cultured in fibrin and alginate gels show improved resistance towards human monocytes. Pathol Biol 2014; 62(6): 354–364. [DOI] [PubMed] [Google Scholar]
- 40. Raza A, Lin CC. The influence of matrix degradation and functionality on cell survival and morphogenesis in PEG-based hydrogels. Macromol Biosci 2013; 13(8): 1048–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mason MN, Mahoney MJ. A novel composite construct increases the vascularization potential of PEG hydrogels through the incorporation of large fibrin ribbons. J Biomed Mater Res A 2010; 95(1): 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aloy-Reverte C, Moreno-Amador JL, Nacher M, et al. Use of RGD-functionalized sandwich cultures to promote redifferentiation of human pancreatic beta cells after in vitro expansion. Tissue Eng Part A 2018; 24(5–6): 394–406. [DOI] [PubMed] [Google Scholar]
- 43. Steele JA, Barron AE, Carmona E, et al. Encapsulation of protein microfiber networks supporting pancreatic islets. J Biomed Mater Res A 2012; 100(12): 3384–3391. [DOI] [PubMed] [Google Scholar]
- 44. Skiles ML, Sahai S, Blanchette JO. Tracking hypoxic signaling within encapsulated cell aggregates. J Vis Exp 2011; 58: 3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shih H, Lin CC. Visible-light-mediated thiol-ene hydrogelation using eosin-Y as the only photoinitiator. Macromol Rapid Commun 2013; 34(3): 269–273. [DOI] [PubMed] [Google Scholar]
- 46. Blanchette JO, Langer SJ, Sahai S, et al. Use of integrin-linked kinase to extend function of encapsulated pancreatic tissue. Biomed Mater 2010; 5(6): 061001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin CC, Anseth KS. Cell-cell communication mimicry with poly(ethylene glycol) hydrogels for enhancing beta-cell function. Proc Natl Acad Sci U S A 2011; 108(16): 6380–6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shih H, Liu HY, Lin CC. Improving gelation efficiency and cytocompatibility of visible light polymerized thiol-norbornene hydrogels via addition of soluble tyrosine. Biomater Sci 2017; 5(3): 589–599. [DOI] [PubMed] [Google Scholar]
- 49. Muthyala S, Bhonde RR, Nair PD. Cytocompatibility studies of mouse pancreatic islets on gelatin—PVP semi IPN scaffolds in vitro: potential implication towards pancreatic tissue engineering. Islets 2010; 2(6): 357–366. [DOI] [PubMed] [Google Scholar]
- 50. Hall KK, Gattas-Asfura KM, Stabler CL. Microencapsulation of islets within alginate/poly(ethylene glycol) gels cross-linked via Staudinger ligation. Acta Biomater 2011; 7(2): 614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Farina M, Ballerini A, Fraga DW, et al. 3D printed vascularized device for subcutaneous transplantation of human islets. Biotechnol J 2017; 12(9): 1–5. [DOI] [PubMed] [Google Scholar]
- 52. Marchioli G, Luca AD, de Koning E, et al. Hybrid polycaprolactone/alginate scaffolds functionalized with VEGF to promote de novo vessel formation for the transplantation of islets of Langerhans. Adv Healthc Mater 2016; 5(13): 1606–1616. [DOI] [PubMed] [Google Scholar]
- 53. Abazari MF, Soleimanifar F, NouriAleagha M, et al. PCL/PVA nanofibrous scaffold improve insulin-producing cells generation from human induced pluripotent stem cells. Gene 2018; 671: 50–57. [DOI] [PubMed] [Google Scholar]
- 54. Li Y, Fan P, Ding XM, et al. Polyglycolic acid fibrous scaffold improving endothelial cell coating and vascularization of islet. Chin Med J 2017; 130(7): 832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smink AM, de Haan BJ, Paredes-Juarez GA, et al. Selection of polymers for application in scaffolds applicable for human pancreatic islet transplantation. Biomed Mater 2016; 11(3): 035006. [DOI] [PubMed] [Google Scholar]
- 56. Tiernan AR, Thule PM, Sambanis A. Therapeutic effects of a non-beta cell bioartificial pancreas in diabetic mice. Transplantation 2014; 98(5): 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Opara EC, Mirmalek-Sani SH, Khanna O, et al. Design of a bioartificial pancreas(+). J Investig Med 2010; 58: 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weidling J, Sameni S, Lakey JR, et al. Method measuring oxygen tension and transport within subcutaneous devices. J Biomed Opt 2014; 19(8): 087006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Appel AA, Ibarra V, Somo SI, et al. Imaging of hydrogel microsphere structure and foreign body response based on endogenous X-ray phase contrast. Tissue Eng Part C Methods 2016; 22(11): 1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. de Vos P, Hamel AF, Tatarkiewicz K. Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia 2002; 45(2): 159–173. [DOI] [PubMed] [Google Scholar]
- 61. Lin CC, Raza A, Shih H. PEG hydrogels formed by thiol-ene photo-click chemistry and their effect on the formation and recovery of insulin-secreting cell spheroids. Biomaterials 2011; 32(36): 9685–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khanna O, Moya ML, Opara EC, et al. Synthesis of multilayered alginate microcapsules for the sustained release of fibroblast growth factor-1. J Biomed Mater Res A 2010; 95(2): 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tiernan AR, Sambanis A. Bioluminescence tracking of alginate micro-encapsulated cell transplants. J Tissue Eng Regen Med 2017; 11(2): 501–508. [DOI] [PubMed] [Google Scholar]
- 64. Li Z, Sun H, Zhang J, et al. Development of in vitro 3D TissueFlex® islet model for diabetic drug efficacy testing. PLoS ONE 2013; 8(8): e72612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vegas AJ, Veiseh O, Gurtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016; 22: 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Johnson AS, O’Sullivan E, D’Aoust LN, et al. Quantitative assessment of islets of Langerhans encapsulated in alginate. Tissue Eng Part C Methods 2011; 17(4): 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Noverraz F, Montanari E, Pimenta J, et al. Antifibrotic effect of ketoprofen-grafted alginate microcapsules in the transplantation of insulin producing cells. Bioconjug Chem 2018; 29(6): 1932–1941. [DOI] [PubMed] [Google Scholar]
- 68. Bloch K, Bloch O, Tarasenko I, et al. A strategy for the engineering of insulin producing cells with a broad spectrum of defense properties. Biomaterials 2011; 32(7): 1816–1825. [DOI] [PubMed] [Google Scholar]
- 69. Arifin DR, Valdeig S, Anders RA, et al. Magnetoencapsulated human islets xenotransplanted into swine: a comparison of different transplantation sites. Xenotransplantation 2016; 23(3): 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mooranian A, Negrulj R, Takechi R, et al. Electrokinetic potential-stabilization by bile acid-microencapsulating formulation of pancreatic beta-cells cultured in high ratio poly-L-ornithine-gel hydrogel colloidal dispersion: applications in cell-biomaterials, tissue engineering and biotechnological applications. Artif Cells Nanomed Biotechnol 2018; 46(6): 1156–1162. [DOI] [PubMed] [Google Scholar]
- 71. Constantinidis I, Grant SC, Simpson NE, et al. Use of magnetic nanoparticles to monitor alginate-encapsulated betaTC-tet cells. Magn Reson Med 2009; 61(2): 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ahmad HF, Sambanis A. Cryopreservation effects on recombinant myoblasts encapsulated in adhesive alginate hydrogels. Acta Biomater 2013; 9(6): 6814–6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nikravesh N, Cox SC, Ellis MJ, et al. Encapsulation and fluidization maintains the viability and glucose sensitivity of beta-cells. ACS Biomater Sci Eng 2017; 3: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 74. Liu HT, Wang H, Wei WB, et al. A microfluidic strategy for controllable generation of water-in-water droplets as biocompatible microcarriers. Small 2018; 14(36): e1801095. [DOI] [PubMed] [Google Scholar]
- 75. Onoe H, Okitsu T, Itou A, et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat Mater 2013; 12(6): 584–590. [DOI] [PubMed] [Google Scholar]
- 76. Aijaz A, Faulknor R, Berthiaume F, et al. Hydrogel microencapsulated insulin-secreting cells increase keratinocyte migration, epidermal thickness, collagen fiber density, and wound closure in a diabetic mouse model of wound healing. Tissue Eng Part A 2015; 21(21–22): 2723–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang JZ, Ding ZQ, Zhang F, et al. Recent development in cell encapsulations and their therapeutic applications. Mater Sci Eng C Mater Biol Appl 2017; 77: 1247–1260. [DOI] [PubMed] [Google Scholar]
- 78. Richardson T, Kumta PN, Banerjee I. Alginate encapsulation of human embryonic stem cells to enhance directed differentiation to pancreatic islet-like cells. Tissue Eng Part A 2014; 20(23–24): 3198–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hoesli CA, Luu M, Piret JM. A novel alginate hollow fiber bioreactor process for cellular therapy applications. Biotechnol Prog 2009; 25(6): 1740–1751. [DOI] [PubMed] [Google Scholar]
- 80. Li W, Lee S, Ma M, et al. Microbead-based biomimetic synthetic neighbors enhance survival and function of rat pancreatic beta-cells. Sci Rep 2013; 3: 2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ibarra V, Appel AA, Anastasio MA, et al. Evaluation of the tissue response to alginate encapsulated islets in an omentum pouch model. J Biomed Mater Res A 2016; 104: 1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Daoud J, Heileman K, Shapka S, et al. Dielectric spectroscopy for monitoring human pancreatic islet differentiation within cell-seeded scaffolds in a perfusion bioreactor system. Analyst 2015; 140(18): 6295–6305. [DOI] [PubMed] [Google Scholar]
- 83. GrootNibbelink M, Daoudi K, Slegers S, et al. Opening the “white box” in tissue engineering: visualization of cell aggregates in optically scattering scaffolds. Tissue Eng Part C Methods 2016; 22(6): 534–542. [DOI] [PubMed] [Google Scholar]
- 84. Suszynski TM, Avgoustiniatos ES, Stein SA, et al. Assessment of tissue-engineered islet graft viability by fluorine magnetic resonance spectroscopy. Transplant Proc 2011; 43(9): 3221–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rios PD, Zhang X, Luo X, et al. Mold-casted non-degradable, islet macro-encapsulating hydrogel devices for restoration of normoglycemia in diabetic mice. Biotechnol Bioeng 2016; 113(11): 2485–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jamal M, Kadam SS, Xiao R, et al. Bio-origami hydrogel scaffolds composed of photocrosslinked PEG bilayers. Adv Healthc Mater 2013; 2(8): 1142–1150. [DOI] [PubMed] [Google Scholar]
- 87. Mendelsohn AD, Bernards DA, Lowe RD, et al. Patterning of mono- and multilayered pancreatic beta-cell clusters. Langmuir 2010; 26(12): 9943–9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bernard AB, Lin CC, Anseth KS. A microwell cell culture platform for the aggregation of pancreatic beta-cells. Tissue Eng Part C Methods 2012; 18(8): 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Park J, Kalinin YV, Kadam S, et al. Design for a lithographically patterned bioartificial endocrine pancreas. Artif Organs 2013; 37(12): 1059–1067. [DOI] [PubMed] [Google Scholar]
- 90. Buitinga M, Truckenmuller R, Engelse MA, et al. Microwell scaffolds for the extrahepatic transplantation of islets of Langerhans. PLoS ONE 2013; 8(5): e64772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Buitinga M, Assen F, Hanegraaf M, et al. Micro-fabricated scaffolds lead to efficient remission of diabetes in mice. Biomaterials 2017; 135: 10–22. [DOI] [PubMed] [Google Scholar]
- 92. Smink AM, Li S, Hertsig DT, et al. The efficacy of a prevascularized, retrievable poly(D,L,-lactide-co-epsilon-caprolactone) subcutaneous scaffold as transplantation site for pancreatic islets. Transplantation 2017; 101(4): e112–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weizman A, Michael I, Wiesel-Motiuk N, et al. The effect of endothelial cells on hESC-derived pancreatic progenitors in a 3D environment. Biomater Sci 2014; 2: 1706–1714. [DOI] [PubMed] [Google Scholar]
- 94. Smink AM, Li S, Swart DH, et al. Stimulation of vascularization of a subcutaneous scaffold applicable for pancreatic islet-transplantation enhances immediate post-transplant islet graft function but not long-term normoglycemia. J Biomed Mater Res A 2017; 105: 2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Forget A, Burzava ALS, Delalat B, et al. Rapid fabrication of functionalised poly(dimethylsiloxane) microwells for cell aggregate formation. Biomater Sci 2017; 5: 828–836. [DOI] [PubMed] [Google Scholar]
- 96. Yin H, Gao M, Leoni L, et al. The therapeutic role of monocyte chemoattractant protein-1 in a renal tissue engineering strategy for diabetic patients. PLoS ONE 2013; 8(2): e57635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv 2010; 28(3): 325–347. [DOI] [PubMed] [Google Scholar]
- 98. Ding J, Zhang J, Li J, et al. Electrospun polymer biomaterials. Prog Polym Sci 2019; 90: 1–34. [Google Scholar]
- 99. Enderami SE, Kehtari M, Abazari MF, et al. Generation of insulin-producing cells from human induced pluripotent stem cells on PLLA/PVA nanofiber scaffold. Artif Cells Nanomed Biotechnol 2018; 46: 1062–1069. [DOI] [PubMed] [Google Scholar]
- 100. Ojaghi M, Soleimanifar F, Kazemi A, et al. Electrospun poly-l-lactic acid/polyvinyl alcohol nanofibers improved insulin-producing cell differentiation potential of human adipose-derived mesenchymal stem cells. J Cell Biochem 2019; 120: 9917–9926. [DOI] [PubMed] [Google Scholar]
- 101. Fazili A, Gholami S, MinaieZangi B, et al. In vivo differentiation of mesenchymal stem cells into insulin producing cells on electrospun poly-L-lactide acid scaffolds coated with matricaria chamomilla L. Cell J 2016; 18(3): 310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Enderami SE, Soleimani M, Mortazavi Y, et al. Generation of insulin-producing cells from human adipose-derived mesenchymal stem cells on PVA scaffold by optimized differentiation protocol. J Cell Physiol 2018; 233(5): 4327–4337. [DOI] [PubMed] [Google Scholar]
- 103. Hoveizi E, Khodadadi S, Tavakol S, et al. Small molecules differentiate definitive endoderm from human induced pluripotent stem cells on PCL scaffold. Appl Biochem Biotechnol 2014; 173(7): 1727–1736. [DOI] [PubMed] [Google Scholar]
- 104. Nadri S, Barati G, Mostafavi H, et al. Differentiation of conjunctiva mesenchymal stem cells into secreting islet beta cells on plasma treated electrospun nanofibrous scaffold. Artif Cells Nanomed Biotechnol 2018; 46: 178–187. [DOI] [PubMed] [Google Scholar]
- 105. Blackstone BN, Palmer AF, Rilo HR, et al. Scaffold architecture controls insulinoma clustering, viability, and insulin production. Tissue Eng Part A 2014; 20(13–14): 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mansour RN, Soleimanifar F, Abazari MF, et al. Collagen coated electrospun polyethersulfon nanofibers improved insulin producing cells differentiation potential of human induced pluripotent stem cells. Artif Cells Nanomed Biotechnol 2018: S734–S739. [DOI] [PubMed] [Google Scholar]
- 107. Mansour RN, Barati G, Soleimani M, et al. Generation of high-yield insulin producing cells from human-induced pluripotent stem cells on polyethersulfone nanofibrous scaffold. Artif Cells Nanomed Biotechnol 2018; 46(sup1): 733–739. [DOI] [PubMed] [Google Scholar]
- 108. Eichhorn SJ, Sampson WW. Statistical geometry of pores and statistics of porous nanofibrous assemblies. J R Soc Interface 2005; 2(4): 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mironov AV, Grigoryev AM, Krotova LI, et al. 3D printing of PLGA scaffolds for tissue engineering. J Biomed Mater Res A 2017; 105(1): 104–109. [DOI] [PubMed] [Google Scholar]
- 110. Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng 2013; 60(3): 691–699. [DOI] [PubMed] [Google Scholar]
- 111. Hollister SJ. Scaffold engineering: a bridge to where. Biofabrication 2009; 1(1): 012001. [DOI] [PubMed] [Google Scholar]
- 112. Marchioli G, van Gurp L, van Krieken PP, et al. Fabrication of three-dimensional bioplotted hydrogel scaffolds for islets of Langerhans transplantation. Biofabrication 2015; 7(2): 025009. [DOI] [PubMed] [Google Scholar]
- 113. Daoud JT, Petropavlovskaia MS, Patapas JM, et al. Long-term in vitro human pancreatic islet culture using three-dimensional microfabricated scaffolds. Biomaterials 2011; 32(6): 1536–1542. [DOI] [PubMed] [Google Scholar]
- 114. Daoud J, Rosenberg L, Tabrizian M. Pancreatic islet culture and preservation strategies: advances, challenges, and future outlook. Cell Transplant 2010; 19(12): 1523–1535. [DOI] [PubMed] [Google Scholar]
- 115. Chaimov D, Baruch L, Krishtul S, et al. Innovative encapsulation platform based on pancreatic extracellular matrix achieve substantial insulin delivery. J Control Release 2017; 257: 91–101. [DOI] [PubMed] [Google Scholar]
- 116. Phelps EA, Templeman KL, Thule PM, et al. Engineered VEGF-releasing PEG-MAL hydrogel for pancreatic islet vascularization. Drug Deliv Transl Res 2015; 5(2): 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Weber LM, Hayda Kirsten N, Anseth Kristi S. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A 2008; 14: 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Vernon RB, Preisinger A, Gooden MD, et al. Reversal of diabetes in mice with a bioengineered islet implant incorporating a type I collagen hydrogel and sustained release of vascular endothelial growth factor. Cell Transplant 2012; 21(10): 2099–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kin T, O’Neil JJ, Pawlick R, et al. The use of an approved biodegradable polymer scaffold as a solid support system for improvement of islet engraftment. Artif Organs 2008; 32(12): 990–993. [DOI] [PubMed] [Google Scholar]
- 120. Xu J, Miao G, Zhao Y, et al. Subcutaneous transplantation may not be an appropriate approach for the islets embedded in the collagen gel scaffolds. Transplant Proc 2011; 43: 3205–3208. [DOI] [PubMed] [Google Scholar]
- 121. Hosseini-Tabatabaei A, Jalili RB, Hartwell R, et al. Embedding islet in a liquid scaffold increases islet viability and function. Can J Diabetes 2013; 37(1): 27–35. [DOI] [PubMed] [Google Scholar]
- 122. Nyitray CE, Chavez MG, Desai TA. Compliant 3D microenvironment improves beta-cell cluster insulin expression through mechanosensing and beta-catenin signaling. Tissue Eng Part A 2014; 20(13–14): 1888–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Phelps EA, Headen DM, Taylor WR, et al. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials 2013; 34(19): 4602–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mei Y, Hollister-Lock J, Bogatyrev SR, et al. A high throughput micro-array system of polymer surfaces for the manipulation of primary pancreatic islet cells. Biomaterials 2010; 31(34): 8989–8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yuan Y, Cong C, Zhang J, et al. Self-assembling peptide nanofiber as potential substrates in islet transplantation. Transplant Proc 2008; 40(8): 2571–2574. [DOI] [PubMed] [Google Scholar]
- 126. Galli A, Maffioli E, Sogne E, et al. Cluster-assembled zirconia substrates promote long-term differentiation and functioning of human islets of Langerhans. Sci Rep 2018; 8: 9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kuehn C, Lakey JR, Lamb MW, et al. Young porcine endocrine pancreatic islets cultured in fibrin show improved resistance toward hydrogen peroxide. Islets 2013; 5(5): 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ribeiro D, Andersson EM, Heath N, et al. Human pancreatic islet-derived extracellular vesicles modulate insulin expression in 3D-differentiating iPSC clusters. PLoS ONE 2017; 12(11): e0187665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wang X, Wang K, Zhang W, et al. A bilaminated decellularized scaffold for islet transplantation: structure, properties and functions in diabetic mice. Biomaterials 2017; 138: 80–90. [DOI] [PubMed] [Google Scholar]
- 130. McEwan K, Padavan DT, Ellis C, et al. Collagen-chitosan-laminin hydrogels for the delivery of insulin-producing tissue. J Tissue Eng Regen Med 2016; 10(10): E397–E408. [DOI] [PubMed] [Google Scholar]
- 131. Fukuda Y, Akagi T, Asaoka T, et al. Layer-by-layer cell coating technique using extracellular matrix facilitates rapid fabrication and function of pancreatic beta-cell spheroids. Biomaterials 2018; 160: 82–91. [DOI] [PubMed] [Google Scholar]
- 132. Kawazoe N, Lin XT, Tateishi T, et al. Three-dimensional cultures of rat pancreatic RIN-5F cells in porous PLGA-collagen hybrid scaffolds. J Bioact Compat Pol 2009; 24: 25–42. [Google Scholar]
- 133. Sackett SD, Tremmel DM, Ma FF, et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci Rep 2018; 8(1): 10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yuan XQ, Huang Y, Guo YB, et al. Controlling the blood glucose of type 1 diabetes mice by co-culturing MIN-6 beta cells on 3D scaffold. Pediatr Transplant 2015; 19(4): 371–379. [DOI] [PubMed] [Google Scholar]
- 135. Abualhassan N, Sapozhnikov L, Pawlick RL, et al. Lung-derived microscaffolds facilitate diabetes reversal after mouse and human intraperitoneal islet transplantation. PLoS ONE 2016; 11(5): e0156053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sionov RV, Finesilver G, Sapozhnikov L, et al. Beta cells secrete significant and regulated levels of insulin for long periods when seeded onto acellular micro-scaffolds. Tissue Eng Part A 2015; 21(21–22): 2691–2702. [DOI] [PubMed] [Google Scholar]
- 137. Willenberg BJ, Oca-Cossio J, Cai Y, et al. Repurposed biological scaffolds: kidney to pancreas. Organogenesis 2015; 11(2): 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Vishwakarma SK, Lakkireddy C, Bardia A, et al. Molecular dynamics of pancreatic transcription factors in bioengineered humanized insulin producing neoorgan. Gene 2018; 675: 165–175. [DOI] [PubMed] [Google Scholar]
- 139. Zhou P, Guo Y, Huang Y, et al. The dynamic three-dimensional culture of islet-like clusters in decellularized liver scaffolds. Cell Tissue Res 2016; 365(1): 157–171. [DOI] [PubMed] [Google Scholar]
- 140. Xu T, Zhu M, Guo Y, et al. Three-dimensional culture of mouse pancreatic islet on a liver-derived perfusion-decellularized bioscaffold for potential clinical application. J Biomater Appl 2015; 30(4): 379–387. [DOI] [PubMed] [Google Scholar]
- 141. Huang YB, Mei J, Yu Y, et al. Comparative decellularization and recellularization of normal versus streptozotocin-induced diabetes mellitus rat pancreas. Artif Organs 2019; 43: 399–412. [DOI] [PubMed] [Google Scholar]
- 142. Guruswamy Damodaran R, Vermette P. Decellularized pancreas as a native extracellular matrix scaffold for pancreatic islet seeding and culture. J Tissue Eng Regen Med 2018; 12(5): 1230–1237. [DOI] [PubMed] [Google Scholar]
- 143. Wan J, Huang Y, Zhou P, et al. Culture of iPSCs derived pancreatic beta-like cells in vitro using decellularized pancreatic scaffolds: a preliminary trial. Biomed Res Int 2017; 2017: 4276928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Katsuki Y, Yagi H, Okitsu T, et al. Endocrine pancreas engineered using porcine islets and partial pancreatic scaffolds. Pancreatology 2016; 16(5): 922–930. [DOI] [PubMed] [Google Scholar]
- 145. Wu D, Wan J, Huang Y, et al. 3D culture of MIN-6 cells on decellularized pancreatic scaffold: in vitro and in vivo study. Biomed Res Int 2015; 2015: 432645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Peloso A, Urbani L, Cravedi P, et al. The human pancreas as a source of protolerogenic extracellular matrix scaffold for a new-generation bioartificial endocrine pancreas. Ann Surg 2016; 264(1): 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. DeCarlo E, Baiguera S, Conconi MT, et al. Pancreatic acellular matrix supports islet survival and function in a synthetic tubular device: in vitro and in vivo studies. Int J Mol Med 2010; 25(2): 195–202. [PubMed] [Google Scholar]
- 148. Goh SK, Bertera S, Olsen P, et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 2013; 34(28): 6760–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yap WT, Salvay DM, Silliman MA, et al. Collagen IV-modified scaffolds improve islet survival and function and reduce time to euglycemia. Tissue Eng Part A 2013; 19(21–22): 2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Beenken-Rothkopf LN, Karfeld-Sulzer LS, Davis NE, et al. The incorporation of extracellular matrix proteins in protein polymer hydrogels to improve encapsulated beta-cell function. Ann Clin Lab Sci 2013; 43(2): 111–121. [PubMed] [Google Scholar]
- 151. Davis NE, Beenken-Rothkopf LN, Mirsoian A, et al. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials 2012; 33(28): 6691–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Shalaly ND, Ria M, Johansson U, et al. Silk matrices promote formation of insulin-secreting islet-like clusters. Biomaterials 2016; 90: 50–61. [DOI] [PubMed] [Google Scholar]
- 153. Formo K, Cho CH, Vallier L, et al. Culture of hESC-derived pancreatic progenitors in alginate-based scaffolds. J Biomed Mater Res A 2015; 103(12): 3717–3726. [DOI] [PubMed] [Google Scholar]
- 154. Williams JA, Goldfine ID. The insulin-pancreatic acinar axis. Diabetes 1985; 34(10): 980–986. [DOI] [PubMed] [Google Scholar]
- 155. Jansson L, Barbu A, Bodin B, et al. Pancreatic islet blood flow and its measurement. Ups J Med Sci 2016; 121(2): 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Mao GH, Chen GA, Bai HY, et al. The reversal of hyperglycaemia in diabetic mice using PLGA scaffolds seeded with islet-like cells derived from human embryonic stem cells. Biomaterials 2009; 30(9): 1706–1714. [DOI] [PubMed] [Google Scholar]
- 157. Vlahos AE, Cober N, Sefton MV. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proc Natl Acad Sci U S A 2017; 114(35): 9337–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol 2008; 26: 434–441. [DOI] [PubMed] [Google Scholar]
- 159. Mueller-Klieser WF, Sutherland RM. Influence of convection in the growth medium on oxygen tensions in multicellular tumor spheroids. Cancer Res 1982; 42(1): 237–242. [PubMed] [Google Scholar]
- 160. Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 1993; 42(1): 12–21. [DOI] [PubMed] [Google Scholar]
- 161. Landers R, Hubner U, Schmelzeisen R, et al. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials 2002; 23(23): 4437–4447. [DOI] [PubMed] [Google Scholar]
- 162. Ozawa CR, Banfi A, Glazer NL, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest 2004; 113: 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Montazeri L, Hojjati-Emami S, Bonakdar S, et al. Improvement of islet engrafts by enhanced angiogenesis and microparticle-mediated oxygenation. Biomaterials 2016; 89: 157–165. [DOI] [PubMed] [Google Scholar]
- 164. Pedraza E, Coronel MM, Fraker CA, et al. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci U S A 2012; 109(11): 4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. McReynolds J, Wen Y, Li XF, et al. Modeling spatial distribution of oxygen in 3D culture of islet beta-cells. Biotechnol Prog 2017; 33(1): 221–228. [DOI] [PubMed] [Google Scholar]
- 166. Long R, Liu Y, Wang S, et al. Co-microencapsulation of BMSCs and mouse pancreatic beta cells for improving the efficacy of type I diabetes therapy. Int J Artif Organs 2017; 40(4): 169–175. [DOI] [PubMed] [Google Scholar]
- 167. Bhaiji T, Zhi ZL, Pickup JC. Improving cellular function and immune protection via layer-by-layer nanocoating of pancreatic islet beta-cell spheroids cocultured with mesenchymal stem cells. J Biomed Mater Res A 2012; 100(6): 1628–1636. [DOI] [PubMed] [Google Scholar]
- 168. Han Y, Li X, Zhang Y, et al. Mesenchymal stem cells for regenerative medicine. Cells 2019; 8: E886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Jalili RB, Moeen Rezakhanlou A, Hosseini-Tabatabaei A, et al. Fibroblast populated collagen matrix promotes islet survival and reduces the number of islets required for diabetes reversal. J Cell Physiol 2011; 226(7): 1813–1819. [DOI] [PubMed] [Google Scholar]
- 170. Matsushima H, Kuroki T, Adachi T, et al. Human fibroblast sheet promotes human pancreatic islet survival and function in vitro. Cell Transplant 2016; 25(8): 1525–1537. [DOI] [PubMed] [Google Scholar]
- 171. Perez-Basterrechea M, Esteban MM, Alvarez-Viejo M, et al. Fibroblasts accelerate islet revascularization and improve long-term graft survival in a mouse model of subcutaneous islet transplantation. PLoS ONE 2017; 12(7): e0180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Rhett JM, Wang H, Bainbridge H, et al. Connexin-based therapeutics and tissue engineering approaches to the amelioration of chronic pancreatitis and type I diabetes: construction and characterization of a novel prevascularized bioartificial pancreas. J Diabetes Res 2016; 2016: 7262680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Pereira LX, Viana CTR, Orellano LAA, et al. Synthetic matrix of polyether-polyurethane as a biological platform for pancreatic regeneration. Life Sci 2017; 176: 67–74. [DOI] [PubMed] [Google Scholar]
- 174. Coronel MM, Geusz R, Stabler CL. Mitigating hypoxic stress on pancreatic islets via in situ oxygen generating biomaterial. Biomaterials 2017; 129: 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355: 1318–1330. [DOI] [PubMed] [Google Scholar]
- 176. Eich TM, Eriksson O, Lundgren T. Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N Engl J Med 2007; 356(26): 2754–2755. [DOI] [PubMed] [Google Scholar]
- 177. Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant 2011; 16(6): 620–626. [DOI] [PubMed] [Google Scholar]
- 178. Hirshberg B, Rother KI, Digon BJ, 3rd, et al. Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression: the National Institutes of Health experience. Diabetes Care 2003; 26(12): 3288–3295. [DOI] [PubMed] [Google Scholar]
- 179. Hume PS, Anseth KS. Polymerizable superoxide dismutase mimetic protects cells encapsulated in poly(ethylene glycol) hydrogels from reactive oxygen species-mediated damage. J Biomed Mater Res A 2011; 99(1): 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hume PS, Bowman CN, Anseth KS. Functionalized PEG hydrogels through reactive dip-coating for the formation of immunoactive barriers. Biomaterials 2011; 32(26): 6204–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kizilel S, Scavone A, Liu X, et al. Encapsulation of pancreatic islets within nano-thin functional polyethylene glycol coatings for enhanced insulin secretion. Tissue Eng Part A 2010; 16(7): 2217–2228. [DOI] [PubMed] [Google Scholar]
- 182. Yang KC, Wu CC, Sumi S, et al. Intramedullary cavity as an implant site for bioartificial pancreas: an in vivo study on diabetic canine. Transplantation 2010; 90(6): 604–611. [DOI] [PubMed] [Google Scholar]
- 183. Jiang K, Weaver JD, Li Y, et al. Local release of dexamethasone from macroporous scaffolds accelerates islet transplant engraftment by promotion of anti-inflammatory M2 macrophages. Biomaterials 2017; 114: 71–81. [DOI] [PubMed] [Google Scholar]
- 184. Kumar M, Nandi SK, Kaplan DL, et al. Localized immunomodulatory silk macrocapsules for islet-like spheroid formation and sustained insulin production. ACS Biomater Sci Eng 2017; 3: 2443–2456. [DOI] [PubMed] [Google Scholar]
- 185. Pham TT, Nguyen TT, Pathak S, et al. Tissue adhesive FK506-loaded polymeric nanoparticles for multi-layered nano-shielding of pancreatic islets to enhance xenograft survival in a diabetic mouse model. Biomaterials 2018; 154: 182–196. [DOI] [PubMed] [Google Scholar]
- 186. Lin CC, Metters AT, Anseth KS. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials 2009; 30(28): 4907–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Su J, Hu BH, Lowe WL, Jr, et al. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials 2010; 31(2): 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lin CC, Boyer PD, Aimetti AA, et al. Regulating MCP-1 diffusion in affinity hydrogels for enhancing immuno-isolation. J Control Release 2010; 142(3): 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Liu JMH, Zhang J, Zhang XM, et al. Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets. Biomaterials 2016; 80: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Rostambeigi N, Lanza IR, Dzeja PP, et al. Unique cellular and mitochondrial defects mediate FK506-induced islet beta-cell dysfunction. Transplantation 2011; 91(6): 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Zhang N, Su D, Qu S, et al. Sirolimus is associated with reduced islet engraftment and impaired beta-cell function. Diabetes 2006; 55(9): 2429–2436. [DOI] [PubMed] [Google Scholar]
- 192. Hosseini-Tabatabaei A, Jalili RB, Khosravi-Maharlooei M, et al. Immunoprotection and functional improvement of allogeneic islets in diabetic mice, using a stable indoleamine 2,3-dioxygenase producing scaffold. Transplantation 2015; 99(7): 1341–1348. [DOI] [PubMed] [Google Scholar]
- 193. Jalili RB, Forouzandeh F, Moeenrezakhanlou A, et al. Mouse pancreatic islets are resistant to indoleamine 2,3 dioxygenase-induced general control nonderepressible-2 kinase stress pathway and maintain normal viability and function. Am J Pathol 2009; 174(1): 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Veiseh O, Doloff JC, Ma M, et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater 2015; 14(6): 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Soltanian A, Ghezelayagh Z, Mazidi Z, et al. Generation of functional human pancreatic organoids by transplants of embryonic stem cell derivatives in a 3D-printed tissue trapper. J Cell Physiol 2019; 234: 9564–9576. [DOI] [PubMed] [Google Scholar]
- 196. Mason MN, Mahoney MJ. Selective beta-cell differentiation of dissociated embryonic pancreatic precursor cells cultured in synthetic polyethylene glycol hydrogels. Tissue Eng Part A 2009; 15(6): 1343–1352. [DOI] [PubMed] [Google Scholar]
- 197. Wang XL, Ye K. Three-dimensional differentiation of embryonic stem cells into islet-like insulin-producing clusters. Tissue Eng Part A 2009; 15(8): 1941–1952. [DOI] [PubMed] [Google Scholar]
- 198. Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012; 61(8): 2016–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32(11): 1121–1133. [DOI] [PubMed] [Google Scholar]
- 200. Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008; 26(4): 443–452. [DOI] [PubMed] [Google Scholar]
- 201. Kasputis T, Clough D, Noto F, et al. Microporous polymer scaffolds for the transplantation of embryonic stem cell derived pancreatic progenitors to a clinically translatable site for the treatment of type I diabetes. ACS Biomater Sci Eng 2018; 4(5): 1770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kuo YC, Liu YC, Rajesh R. Pancreatic differentiation of induced pluripotent stem cells in activin A-grafted gelatin-poly(lactide-co-glycolide) nanoparticle scaffolds with induction of LY294002 and retinoic acid. Mater Sci Eng C Mater Biol Appl 2017; 77: 384–393. [DOI] [PubMed] [Google Scholar]
- 203. Candiello J, Grandhi TSP, Goh SK, et al. 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials 2018; 177: 27–39. [DOI] [PubMed] [Google Scholar]
- 204. Wang W, Jin S, Ye K. Development of islet organoids from H9 human embryonic stem cells in biomimetic 3D scaffolds. Stem Cells Dev 2017; 26(6): 394–404. [DOI] [PubMed] [Google Scholar]
- 205. Mason MN, Arnold CA, Mahoney MJ. Entrapped collagen type 1 promotes differentiation of embryonic pancreatic precursor cells into glucose-responsive beta-cells when cultured in three-dimensional PEG hydrogels. Tissue Eng Part A 2009; 15: 3799–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Mason MN, Mahoney MJ. Inhibition of gamma-secretase activity promotes differentiation of embryonic pancreatic precursor cells into functional islet-like clusters in poly(ethylene glycol) hydrogel culture. Tissue Eng Part A 2010; 16(8): 2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Khorsandi L, Nejad-Dehbashi F, Ahangarpour A, et al. Three-dimensional differentiation of bone marrow-derived mesenchymal stem cells into insulin-producing cells. Tissue Cell 2015; 47(1): 66–72. [DOI] [PubMed] [Google Scholar]
- 208. Seyedi F, Farsinejad A, Nematollahi-Mahani SN. Fibrin scaffold enhances function of insulin producing cells differentiated from human umbilical cord matrix-derived stem cells. Tissue Cell 2017; 49(2 Pt B): 227–232. [DOI] [PubMed] [Google Scholar]
- 209. Niknamasl A, Ostad SN, Soleimani M, et al. A new approach for pancreatic tissue engineering: human endometrial stem cells encapsulated in fibrin gel can differentiate to pancreatic islet beta-cell. Cell Biol Int 2014; 38(10): 1174–1182. [DOI] [PubMed] [Google Scholar]
- 210. Aloysious N, Nair PD. Enhanced survival and function of islet-like clusters differentiated from adipose stem cells on a three-dimensional natural polymeric scaffold: an in vitro study. Tissue Eng Part A 2014; 20(9–10): 1508–1522. [DOI] [PubMed] [Google Scholar]
- 211. Veriter S, Gianello P, Dufrane D. Bioengineered sites for islet cell transplantation. Curr Diab Rep 2013; 13(5): 745–755. [DOI] [PubMed] [Google Scholar]
- 212. Liu L, Tan J, Li B, et al. Construction of functional pancreatic artificial islet tissue composed of fibroblast-modified polylactic- co-glycolic acid membrane and pancreatic stem cells. J Biomater Appl 2017; 32(3): 362–372. [DOI] [PubMed] [Google Scholar]
- 213. Mahou R, Zhang DKY, Vlahos AE, et al. Injectable and inherently vascularizing semi-interpenetrating polymer network for delivering cells to the subcutaneous space. Biomaterials 2017; 131: 27–35. [DOI] [PubMed] [Google Scholar]
- 214. Wang D, Ding X, Xue W, et al. A new scaffold containing small intestinal submucosa and mesenchymal stem cells improves pancreatic islet function and survival in vitro and in vivo. Int J Mol Med 2017; 39(1): 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Song J, Millman JR. Economic 3D-printing approach for transplantation of human stem cell-derived beta-like cells. Biofabrication 2016; 9(1): 015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Borg DJ, Welzel PB, Grimmer M, et al. Macroporous biohybrid cryogels for co-housing pancreatic islets with mesenchymal stromal cells. Acta Biomater 2016; 44: 178–187. [DOI] [PubMed] [Google Scholar]
- 217. Hirabaru M, Kuroki T, Adachi T, et al. A method for performing islet transplantation using tissue-engineered sheets of islets and mesenchymal stem cells. Tissue Eng Part C Methods 2015; 21(12): 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ellis CE, Vulesevic B, Suuronen E, et al. Bioengineering a highly vascularized matrix for the ectopic transplantation of islets. Islets 2013; 5(5): 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Bhang SH, Jung MJ, Shin JY, et al. Mutual effect of subcutaneously transplanted human adipose-derived stem cells and pancreatic islets within fibrin gel. Biomaterials 2013; 34(30): 7247–7256. [DOI] [PubMed] [Google Scholar]
- 220. Kaufman-Francis K, Koffler J, Weinberg N, et al. Engineered vascular beds provide key signals to pancreatic hormone-producing cells. PLoS ONE 2012; 7(7): e40741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Lee JI, Kim JY, Kim HW, et al. Long-term viability of transplanted hybrid cellular spheroids within chondrocyte sheets. Transplant Proc 2012; 44(4): 1162–1165. [DOI] [PubMed] [Google Scholar]
- 222. Deng C, Vulesevic B, Ellis C, et al. Vascularization of collagen-chitosan scaffolds with circulating progenitor cells as potential site for islet transplantation. J Control Release 2011; 152(Suppl. 1): e196–e198. [DOI] [PubMed] [Google Scholar]
- 223. Yang KC, Wu CC, Lin FH, et al. Chitosan/gelatin hydrogel as immunoisolative matrix for injectable bioartificial pancreas. Xenotransplantation 2008; 15(6): 407–416. [DOI] [PubMed] [Google Scholar]
- 224. Perez-Basterrechea M, Briones RM, Alvarez-Viejo M, et al. Plasma-fibroblast gel as scaffold for islet transplantation. Tissue Eng Part A 2009; 15(3): 569–577. [DOI] [PubMed] [Google Scholar]
- 225. Grundfest-Broniatowski SF, Tellioglu G, Rosenthal KS, et al. A new bioartificial pancreas utilizing amphiphilic membranes for the immunoisolation of porcine islets a pilot study in the canine. ASAIO J 2009; 55(4): 400–405. [DOI] [PubMed] [Google Scholar]
- 226. Kokorev OV, Khodorenko VN, Anikeev SG, et al. Dynamics of changes in metabolic and hematological parameters after transplantation of pancreatic islet cells on TiNi scaffold in experimental diabetes mellitus. Bull Exp Biol Med 2018; 165(3): 394–398. [DOI] [PubMed] [Google Scholar]
- 227. Pereira LX, Viana CTR, Orellano LAA, et al. Kinetics of pancreatic tissue proliferation in a polymeric platform in mice. Pancreatology 2018; 18(2): 221–229. [DOI] [PubMed] [Google Scholar]
- 228. An D, Chiu A, Flanders JA, et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc Natl Acad Sci U S A 2018; 115(2): E263–E272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Kokorev OV, Hodorenko VN, Chekalkin TL, et al. In vitro and in vivo evaluation of porous TiNi-based alloy as a scaffold for cell tissue engineering. Artif Cells Nanomed Biotechnol 2016; 44(2): 704–709. [DOI] [PubMed] [Google Scholar]
- 230. Chandra V, Swetha G, Muthyala S, et al. Islet-like cell aggregates generated from human adipose tissue derived stem cells ameliorate experimental diabetes in mice. PLoS ONE 2011; 6(6): e20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Muthyala S, Raj VR, Mohanty M, et al. The reversal of diabetes in rat model using mouse insulin producing cells—a combination approach of tissue engineering and macroencapsulation. Acta Biomater 2011; 7(5): 2153–2162. [DOI] [PubMed] [Google Scholar]
- 232. Kadam SS, Sudhakar M, Nair PD, et al. Reversal of experimental diabetes in mice by transplantation of neo-islets generated from human amnion-derived mesenchymal stromal cells using immuno-isolatory macrocapsules. Cytotherapy 2010; 12(8): 982–991. [DOI] [PubMed] [Google Scholar]
- 233. Berman DM, Molano D, Fotino C, et al. Bioengineering the endocrine pancreas: intraomental islet transplantation within a biologic resorbable scaffold. Diabetes 2016; 65(5): 1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Kollmer M, Appel AA, Somo SI, et al. Long-term function of alginate-encapsulated islets. Tissue Eng Part B Rev 2015; 22: 34–46. [DOI] [PubMed] [Google Scholar]
- 235. Pareta R, McQuilling JP, Sittadjody S, et al. Long-term function of islets encapsulated in a redesigned alginate microcapsule construct in omentum pouches of immune-competent diabetic rats. Pancreas 2014; 43(4): 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Gibly RF, Zhang X, Graham ML, et al. Extrahepatic islet transplantation with microporous polymer scaffolds in syngeneic mouse and allogeneic porcine models. Biomaterials 2011; 32(36): 9677–9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Gupta R, Sefton MV. Application of an endothelialized modular construct for islet transplantation in syngeneic and allogeneic immunosuppressed rat models. Tissue Eng Part A 2011; 17(15–16): 2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Berman DM, O’Neil JJ, Coffey LCK, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009; 9(1): 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Jin L, Gao D, Feng T, et al. Cells with surface expression of CD133highCD71low are enriched for tripotent colony-forming progenitor cells in the adult murine pancreas. Stem Cell Res 2016; 16(1): 40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Jaroch DB, Lu J, Madangopal R, et al. Mouse and human islets survive and function after coating by biosilicification. Am J Physiol Endocrinol Metab 2013; 305(10): E1230–E1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Kodama S, Kojima K, Furuta S, et al. Engineering functional islets from cultured cells. Tissue Eng Part A 2009; 15(11): 3321–3329. [DOI] [PubMed] [Google Scholar]
- 242. Yang KC, Qi Z, Wu CC, et al. The cytoprotection of chitosan based hydrogels in xenogeneic islet transplantation: an in vivo study in streptozotocin-induced diabetic mouse. Biochem Biophys Res Commun 2010; 393(4): 818–823. [DOI] [PubMed] [Google Scholar]
- 243. Kheradmand T, Wang S, Gibly RF, et al. Permanent protection of PLG scaffold transplanted allogeneic islet grafts in diabetic mice treated with ECDI-fixed donor splenocyte infusions. Biomaterials 2011; 32(20): 4517–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Hussey AJ, Winardi M, Wilson J, et al. Pancreatic islet transplantation using vascularised chambers containing nerve growth factor ameliorates hyperglycaemia in diabetic mice. Cells Tissues Organs 2010; 191(5): 382–393. [DOI] [PubMed] [Google Scholar]
- 245. Kim HI, Yu JE, Park CG, et al. Comparison of four pancreatic islet implantation sites. J Korean Med Sci 2010; 25(2): 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PRISMA_2009_checklist for The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells by Gabriel Alexander Salg, Nathalia A Giese, Miriam Schenk, Felix J Hüttner, Klaus Felix, Pascal Probst, Markus K Diener, Thilo Hackert and Hannes Götz Kenngott in Journal of Tissue Engineering
Supplemental material, SupplementaryInformation1 for The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells by Gabriel Alexander Salg, Nathalia A Giese, Miriam Schenk, Felix J Hüttner, Klaus Felix, Pascal Probst, Markus K Diener, Thilo Hackert and Hannes Götz Kenngott in Journal of Tissue Engineering
Supplemental material, SupplementaryInformation2 for The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells by Gabriel Alexander Salg, Nathalia A Giese, Miriam Schenk, Felix J Hüttner, Klaus Felix, Pascal Probst, Markus K Diener, Thilo Hackert and Hannes Götz Kenngott in Journal of Tissue Engineering