Abstract
There is a growing recognition that myogenic stem cells are influenced by their microenvironment during regeneration. Several interstitial cell types have been described as supportive for myoblasts. In this role, both the pericyte as a possible progenitor for mesenchymal stem cells, and interstitial cells in the endomysium have been discussed. We have applied immunohistochemistry on normal and pathological human skeletal muscle using markers for pericytes, or progenitor cells and found a cell type co-expressing CD10, CD34, CD271, and platelet-derived growth factor receptor α omnipresent in the endomysium. The marker profile of these cells changed dynamically in response to muscle damage and atrophy, and they proliferated in response to damage. The cytology and expression profile of the CD10+ cells indicated a capacity to participate in myogenesis. Both morphology and indicated function of these cells matched properties of several previously described interstitial cell types. Our study suggests a limited number of cell types that could embrace many of these described cell types. Our study indicate that the CD10+, CD34+, CD271+, and platelet-derived growth factor receptor α+ cells could have a supportive role in human muscle regeneration, and thus the mechanisms by which they exert their influence could be implemented in stem cell therapy.
Keywords: CD10, fibro adipogenic progenitor cells, fibroblasts, mesenchymal progenitor cells, mesenchymal stem cells, pericytes, skeletal muscle regeneration, telocytes
Introduction
The myogenic stem cell, the satellite cell has been intensively studied since its discovery in 1961.1 However, it becomes increasingly clear that the entire microenvironment supporting the myogenic cells must be taken into account to understand the regeneration process. It has thus been suggested that besides the satellite cell, several interstitial mesenchymal cell types contribute to regeneration by supporting myofiber formation and angiogenesis.2–4
Skeletal muscle interstitial cells are described in many studies, but no consistent nomenclature exists, which challenges comparison of studies. A great deal of studies and reviews are based on animal models that do not necessarily reflect conditions in human muscle. Likewise, observations in in vitro studies may not reflect in vivo conditions. Moreover, many studies have used flow cytometry to characterize cells derived from skeletal muscle, but this does not give topographical information and thus, identification of endomysial interstitial cells in contrast to cells from connective tissue, larger blood vessels, or nerves resident in skeletal muscle tissue, is hampered.
Two larger groups of interstitial cells have been proposed to support muscle regeneration. One group is the pericytes which are supposed to give rise to mesenchymal stem cells (MSC) that are able to support several aspects of regeneration.5 The other group is platelet-derived growth factor receptor α (PDGFRα+) cells that include the mesenchymal progenitor cells and fibro adipogenic progenitor cells (FAPs) that have the potential to develop into fibroblast and adipogenic cells.6,7 Also, the telocyte cell type has been related to this group.8 To which degree these PDGFRα+ cells are different cell types, or different cell states of a single cell type is not clear.
In this study, we addressed which interstitial cell types in human skeletal muscle that are activated in vivo in the context of regeneration and degeneration. By combining information on tissue localization and expression profile in normal skeletal muscle and in pathological conditions we dealt with diversity and dynamic changes of the interstitial cell profile. For this purpose, we applied immunohistochemistry with markers for progenitor cells to identify interstitial cell types in situ (Supplemental Table 1).
Materials and Methods
The use of material from the files of Department of Pathology, Odense University Hospital, was approved by The Regional Ethics Committee of the Region of Southern Denmark S-20070075. The material used can be found in Supplemental Table 2.
Immunohistochemistry
Information on antibodies is shown in Table 1.
Table 1.
Antibodies.
| Antibody | Species | Clone | Manufacturer | Dilution | Epitope retrieval |
|---|---|---|---|---|---|
| αSMA | Mouse | BS66 | Nordic BioSite ApS | 1:1000 | CC1, 32 min 100C |
| CD10 | Mouse | 56C6 | Novocastra | 1:10 (1:50 IF) | CC1, 32 min 100C |
| CD15 | Mouse | MMA | Ventana/Roche | RTU | CC1, 64 min 100C |
| CD34 | Mouse | QBEnd/10 | Novocastra | 1:100 | CC1, 32 min 100C |
| CD45 | Mouse | 2B11 & PD7/26 | Ventana/Roche | RTU | CC1, 32 min 100C |
| CD56 | Rabbit | MRQ-42 | Cell Marque | 1:250 | CC1, 32 min 100C |
| CD73 | Mouse | 1D7 | Abcam | 1:4000 | CC1, 32 min 100C |
| CD90 | Rabbit | EPR3132 | Abcam | 1:100 | CC1, 32 min 100C |
| CD105 | Mouse | 4G11 | Novocastra | 1:50 | CC1, 32 min 100C |
| CD146 | Mouse | N1238 | Novocastra | 1:50 | CC1, 32 min 100C |
| CD271 | Mouse | MRQ-21 | Cell Marque | 1:500 | TRS-L, 30 min 97C |
| HLA-ABC | Mouse | EMR8-5 | Abcam | 1:1000 | CC1, 32 min 100C |
| Ki67 | Rabbit | 30-9 | Ventana/Roche | RTU | CC1, 48 min 100C |
| NG2 | Rabbit | EPR9195 | Abcam | (1:50 IF) | |
| PDGFRα | Goat | AF-307-NA | R&D Systems | 1:200 (1:100 IF) | CC1, 32 min 100C |
| TCF7L2 | Rabbit | EP2033Y | Abcam | 1:1000 | CC1, 32 min 100C |
| Vimentin | Mouse | V9 | Dako/Agilent | 1:1000 | CC1, 32 min 100C |
Abbreviations: IF, immunofluorescence; CC1, cell conditioning 1 buffer (Ventana/Roche); TRS-L, target retrieval solution buffer, low pH (Dako/Agilent); RTU, ready to use.
Formalin-fixed paraffin embedded human skeletal muscle was sectioned at 2 µm and deparaffinized followed by blocking of endogenous peroxidase activity with H2O2 before epitope retrieval. For staining with one antibody, the sections were stained on a BenchMark Ultra immunostainer (Ventana Medical Systems, Tucson, AZ) using the OptiView-DAB detection system (Ventana Medical Systems).
For cryosections, the tissue was embedded in Tissue-Tek OCT (Sakura Finetek, Torrance, CA) and frozen in isopentane (-80C) and 5 µm cryosections were cut at -18C. The sections on slides were fixed in acetone for 10 min and stained on a BenchMark Ultra immunostainer (Ventana Medical Systems) with the OptiView-DAB detection system (Ventana Medical Systems). More than 50 biopsies were stained for CD10 and additional antigens. Different strategies for double staining were used.
Sequential Staining
For detection of two antigens with different subcellular localizations, a sequential double staining protocol based on OptiView-DAB (Ventana Medical Systems) and UltraView-RED (Ventana Medical Systems) was performed on a Benchmark Ultra immunostainer (Ventana Medical Systems). Heat-based elution/denaturation was performed after the first sequence. Biopsies from 15 persons were used.
SIMPLE
For antigens with possible co-localization a sequential immunoperoxidase labeling and erasing method (SIMPLE) was used, modified from Glass et al.9 Briefly, sections were deparaffinized, followed by blocking of endogenous peroxidase activity, before epitope retrieval of the anti-CD10 antibody. CD10 was stained on an Omnis immunostainer (Dako/Agilent, Glostrup, Denmark) with aminoethylcarbazole (AEC) ImmPACT horse radish peroxidase substrate chromogen solution (Vector Laboratories, Burlingame, CA, USA) used for detection. After immunostaining, the slides were counterstained with Mayer’s hematoxylin and coverslipped with Aquatex mounting medium. Slides were scanned on a NDP-scanner (Hamamatsu Photonics, Herrsching, Germany), and subsequently, the coverslips were removed by placing the slides in 60C Tris buffered saline for 60 min. To remove AEC, the demounted slides were placed in 99% ethanol for 5 min and then rehydrated. Remaining CD10 antibody was removed by denaturation in a 100 mL SDS/Tris-HCl/(2-carboxyethyl) phosphine hydrochloride (TCEP) buffer, pH 6.8, containing 200 µl Bond-Breaker (ThermoFisher Scientific, Waltham, MA) for 10 min at 95C. Biopsies from 20 people were used.
Controls
Parallel sections with single staining for the second primary antibody served as controls for the quality of the staining and to ensure that the type of structures labeled in the SIMPLE procedure resembled what was stained in the single stain. A control without the second primary antibody was made to ensure that the first primary antibody and detection system had been completely erased.
Image Processing
To produce a merged image, NDP view (Hamamatsu Photonics) was used to find identical regions in the scanned slides from the two steps of SIMPLE. The images were processed and merged using Photoshop C6 (Adobe Systems, San Jose, CA) and Fiji.10
Simultaneous Dual Immunofluorescence
For antigens which could not be retrieved after the SIMPLE erase procedure or didn’t work on formalin fixed paraffin embedded sections we used immunofluorescence double staining. Cryosections were fixed in acetone for 10 min. The two primary antibodies CD10 and PDGFRα, or NG2 and alpha-smooth muscle actin (αSMA), were mixed in S2022 antibody diluent (Dako/Agilent) and incubated on the slides for 60 min at room temperature. The secondary antibodies used were donkey-anti-goat conjugated Alexa Fluor 594 (ThermoFisher Scientific) for detection of PDGFRα, donkey-anti-mouse conjugated Alexa Fluor 488 for CD10 and αSMA, and donkey-anti-rabbit conjugated Alexa Fluor 594 for NG2, all diluted 1:200. The two secondary antibodies were mixed and incubated on slides for 45 min. Slides were coverslipped using Vectashield mounting media with DAPI (Vector Laboratories).
Transmission Electron Microscopy
Muscle specimens approximately 2 mm x 2 mm were fixed in 2% buffered glutaraldehyde and washed in 0.1% phosphate buffer. Following postfixation in OsO₄, the specimens were dehydrated in graded alcohols and propylene oxide and infiltrated with embedding epoxy resin 812 (TAAB, Reading,UK) on a Leica EM TP instrument (Leica Microsystems, Wetzlar, Germany). The epoxy resin was polymerized at 60C. Ultra-thin sections were cut on a Leica ultracut UCT (Leica Microsystems) and contrasted with uranyl acetate and lead citrate. The sections were analyzed with a JEM-1400 Plus (JEOL USA Inc., MA, USA) and images obtained with a Quemesa TEM CCD camera (Olympus Europa, Hamburg, Germany).
Immuno-electron Microscopy
For immuno-electron microscopy approx. 3 mm x 3 mm muscle specimens were fixed in buffered formaldehyde for 2 hr and afterwards placed in Holt’s solution overnight. The tissue was frozen in isopentane (-80C) and 50 µm cryosections were cut at -18C and stored in 4% horse serum in TBS/Triton X-100 buffer. The sections were incubated in TBS for 30 min at 60C and then transferred back to 4% horse serum in TBS/Triton X-100 buffer at room temperature. Subsequent steps were performed in a 96-well plate vacuum manifold on a shaker, with TBS washes in between steps. The sections were incubated with a 1:10 dilution of anti-CD10 antibody in 2% horse serum in TBS/Triton X-100 overnight followed by incubation with ImmPACT SG Peroxidase (HRP) Substrate Kit (Vector Laboratories) for 12 min. Endogenous peroxidase activity was blocked by H2O2 in a 33% methanol TBS solution before incubation with the ImmPACT SG Peroxidase (HRP) Substrate Kit for 60 min. The immunostained 50 µm sections were afterwards processed according to the standard EM procedure.
Alkaline Phosphatase Enzyme Histochemistry
Cryosections were fixed in 4% neutral buffered formalin with 7.5% sucrose for 5 min, washed in water and stained with a mixture of Fast Red TR and Naphthol AS-TR phosphate disodium salt in 0.05 M Tris buffer.
Morphometry
Estimation of volume fraction was performed by point counting.11 Images from quadriceps from 3 normal (56, 61, and 68 years) and 3 inclusion body myositis patients (62, 70, and 76, years) were analyzed by applying a grid over the images and counting cross points over endothelial cells, pericytes, and mesenchymal interstitial cells. The mesenchymal interstitial cells were defined as cell with angular or fusiform cell bodies, the extensions from these as cell processes with rough endoplasmic reticulum12 and more over very slender processes without organelles were included. Fifteen EM images at 1000x magnification were used from each patient. To count TCF7L2 marked nuclei the QuPath13 software was used. The parameters used were background radius 0, Sigma 1.5 µm, minimum area 15 µm², maximum area 400 µm², threshold 0.7, and cell expansion 2 µm. An un-paired t-test based on equal variances was used to compare the means of the volume fractions.
Results
An Endomysial Mesenchymal Cell Type Expresses PDGFRα, CD10, CD34 and CD271
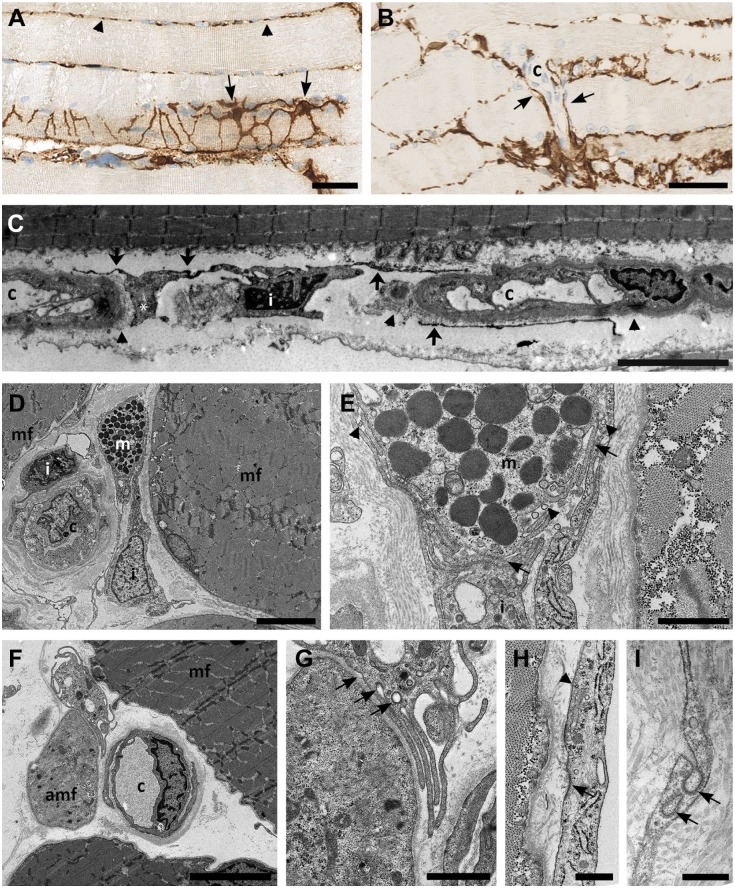
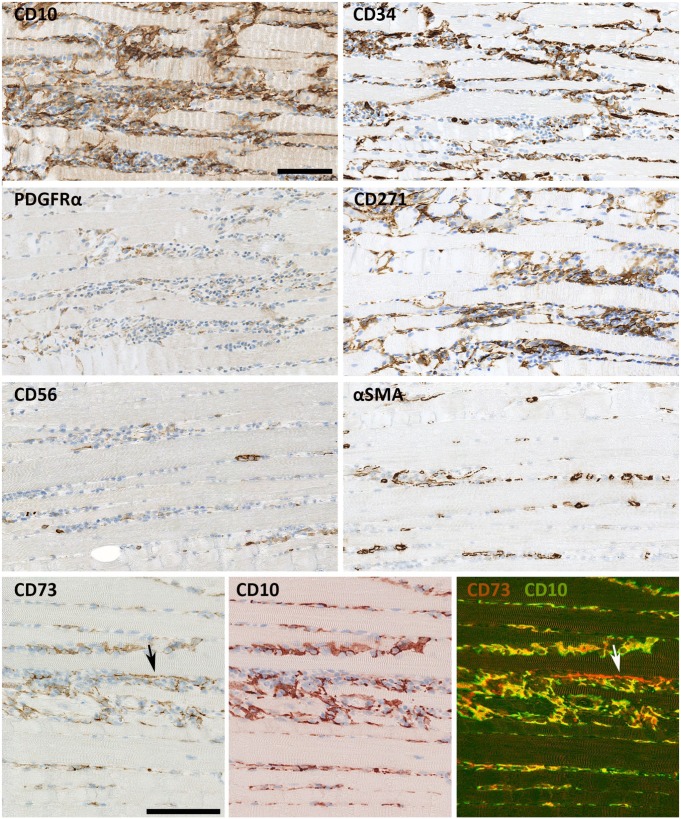
In the interstitial space of human skeletal muscle, a vimentin+ mesenchymal cell expressing CD10, CD34, CD271, and PDGFRα was found (Figs. 1 and 2). CD10 was the most robust and extensive marker for this cell. The cells had long cytoplasmic, branched processes that created a network connecting with neighboring CD10+ cells and surrounding the myofibers (Fig. 1A). In cross sections, the network presented as dots or thin processes between fibers. The cells were closely related to the periphery of small vessels (Figs. 1B and 2, CD34). Most fibers and capillaries were in contact with CD10+ cells implying the density and distribution of these cells. Morphometric analyses revealed that they occupied 49% (45–52%) of the cell volume in normal endomysium. In immuno-electron microscopy CD10 positivity was detected as small electron dense granules in the cell membranes consistent with CD10 being a membrane protein. Positive cell processes could be seen around capillaries just outside the basement membrane (Fig. 1C). The dimensions of the processes were irregular with both thin and dilated segments (Fig. 1A and C). Conventional electron microscopy moreover demonstrated that the branches of these cells could be in close apposition to a diversity of cell types including myofibers (Fig. 1). The presence of caveolae and the formation of coated vesicles in the plasma membrane, as well as vesicles of exosome size in the close extra cellular space further suggested capacity for communication (Fig. 1E, G, H, and I).
Figure 1.
CD10+ interstitial cells. (A) CD10+ interstitial cells (arrows) at the surface of a muscle fiber forming an interconnected network of processes of varying sizes. Transected CD10+ processes (arrow heads) are seen between other muscle fibers. (B) CD10+ interstitial cells (arrows) surrounding capillaries. (C) Immuno transmission electron microscopy shows CD10 as small dark grains on the surface (arrows) of an interstitial cell (i). Local widening of a process (*). CD10+ processes are localized outside the capillary (c). The capillary basement membrane is shown with arrow heads. (D) Interstitial cell (i) in close apposition to a mast cell (m). Muscle fibers (mf). (E) A larger magnification of D shows the close contact between the interstitial cells and mast cell (m) (arrows). Vesicles the size of exosomes are seen in the space between the mast cell and the interstitial cells (arrow heads). (F): Interstitial cell (i) in apposition to both normal (mf) and atrophic (amf) muscle fiber, (c) capillary. (G): larger magnification of F shows the formation of coated vacuoles (arrows) in the membrane of an interstitial cell facing the atrophic fiber. (H and I) Formation of coated vesicles in the slender processes of the interstitial cells (arrows). Caveolae are mainly seen near the membrane facing the muscle fiber (arrow head). Biopsy from normal skeletal muscle. Scale bars: A = 100 µm, B = 50 µm, C = 10 µm, D and E = 5 µm, F and G = 1 µm, H = 500 nm, I = 200 nm.
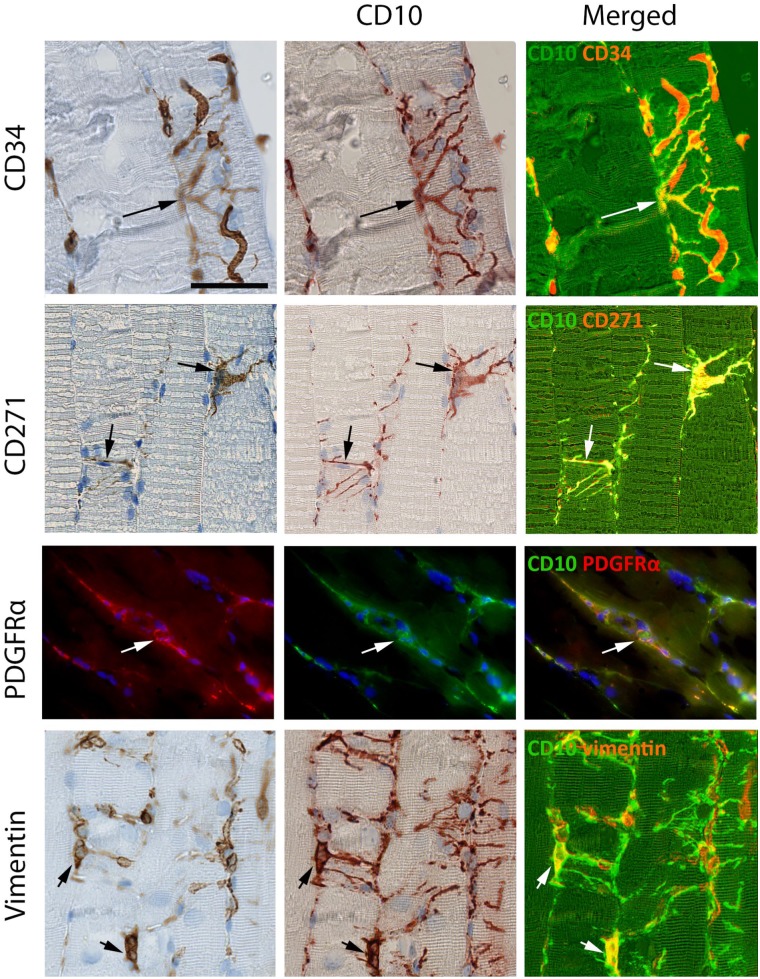
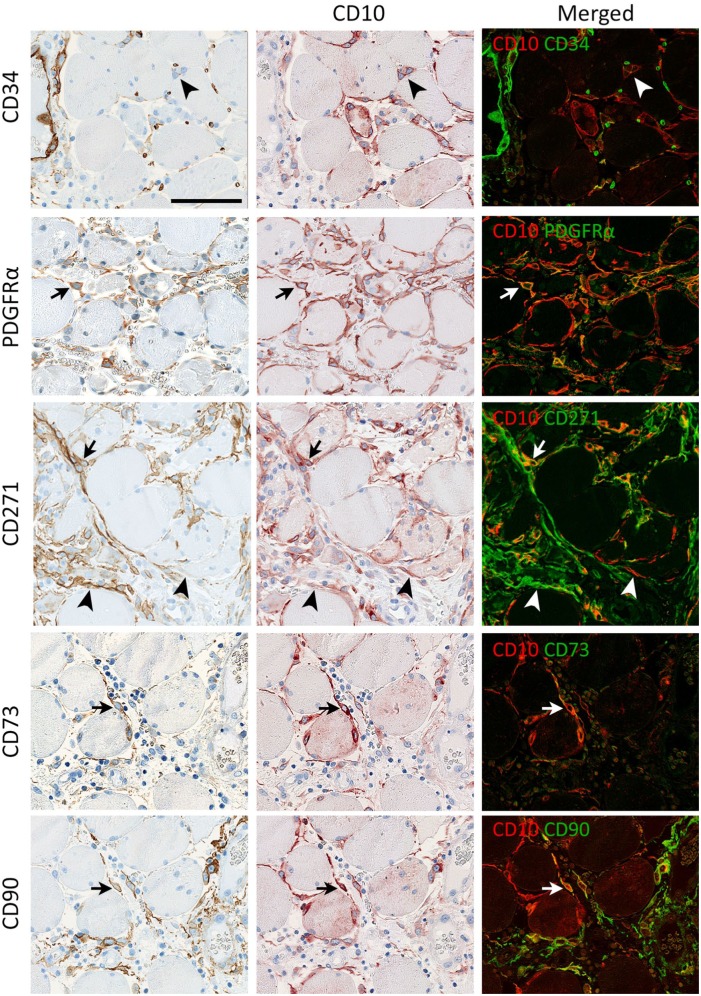
Figure 2.
Co-localization of CD10 with CD34, CD271, PDGFRα and vimentin. CD34 is co-expressed with CD10 in interstitial cells at the surface of a muscle fiber (arrow). In addition CD34 is expressed in capillaries (red on the merged image) and the close connection between capillaries and interstitial cells is seen. CD271 and PDGFRα co-localize with CD10+ interstitial cells (arrows). CD10+ cells express vimentin in the perinuclear cytoplasm (arrows) but not in the processes. Co-localization of CD10 with CD34, CD271 and vimentin was shown by SIMPLE, while co-localization of CD10 with PDGFRα was shown by immunofluorescence. Biopsy from normal skeletal muscle. Scale bar = 50 µm. Abbreviation: PDGFRα, platelet-derived growth factor receptor α.
Skeletal muscles from several locations including extremities, eyes, tongue, truncus, and diaphragm were stained for CD10 and all presented with positive interstitial cells. CD10 positive cells were even present between ectopic muscle fibers (hamartoma S1).
Double staining employing the SIMPLE technique showed that interstitial cells could co-express CD10 with CD34, CD271 and vimentin respectively (Fig. 2). For demonstration of co-localization of PDGFRα with CD10, immunofluorescent staining was employed (Fig. 2). CD34, CD271 and PDGFRα were expressed both in the cell body and cytoplasmic processes while anti-vimentin only stained the cell body. The most extensive staining was obtained with CD10, and it appeared that all CD271, PDGFRα, and CD34 expression was co-localized with CD10 except for CD34 positivity in endothelial cells.
Macrophages, Dendritic Cells, Satellite Cells and Fibroblasts
Other cell types with a branched morphology that have been described in the endomysium include macrophages, dendritic cells and fibroblasts; therefore, we applied markers to determine a possible relationship between these cells and the CD10+ cells. The marker, CD45 is expressed in dendritic cells and macrophages, and CD15 is expressed in dendritic cells but has also been found in cultured mesenchymal cells co-expressing PDGFRα.14 In our study, CD45 did not co-localize with CD10 in interstitial cells (S2), indicating that the interstitial CD10+ cells were not bone marrow derived and we did not detect any CD15+ cells apart from granulocytes (not shown).
In normal muscle no co-localization between the satellite cell marker CD56 and CD10 was found (not shown).
The marker TCF7L2 has been used to identify fibroblast in skeletal muscle tissue.15 In healthy muscle tissue, we found that most nuclei stained positive for TCF7L2, and many cell types expressed this antigen including some of the CD10+ cells and some of the myonuclei (S2). Among cells with a high TCF7L2 expression were the endothelial cells. We reduced the antibody concentration in order only to detect cells with a high expression of TCF7L2. This resulted in a smaller proportion of positive nuclei but still many cells types were represented among the positive cells.
Pericytes in Endomysial Vessels Express αSMA, CD146, CD90 and NG2
Applying the markers αSMA, CD146, CD90, and NG2, we could label cells in the wall of small interstitial vessels abluminal to the endothelium (S3) showing that the stained cells are bona fide pericytes. In cryosections, NG2 was observable in pericytes, where it could be shown to co-localize with αSMA. Nestin was found in a subpopulation of pericytes (Fig. S3). None of these pericyte markers co-localized with CD10 in interstitial cells. Alkaline phosphatase has in some studies been employed for pericyte identification.16,17 However, in normal muscle, we found that the majority of alkaline phosphatase was expressed in endothelial cells (S2).
Distribution of CD10 and CD34 Change During Fetal Development
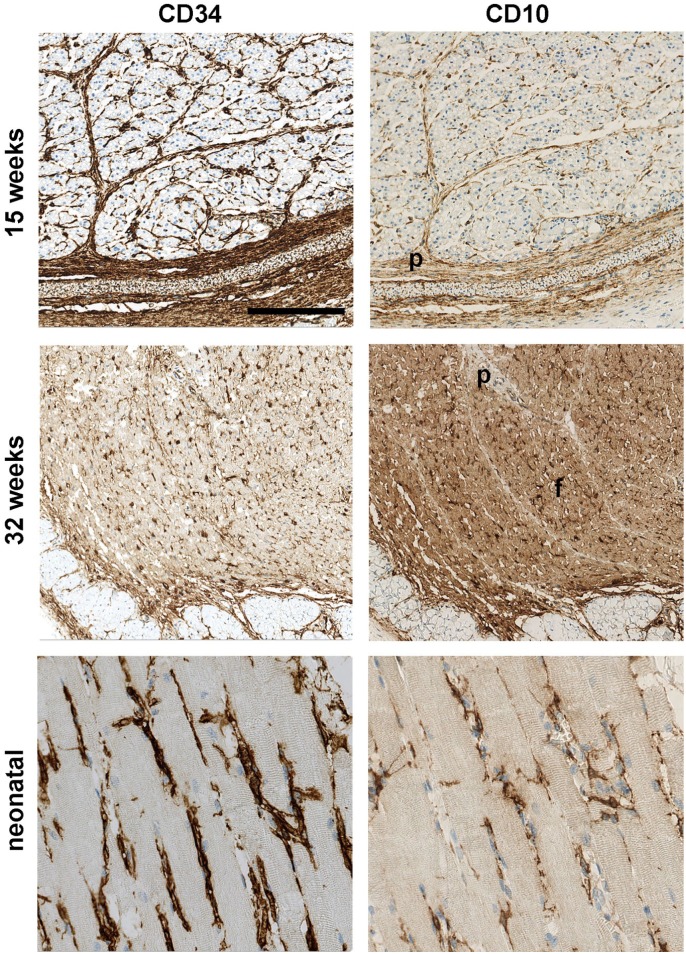
In 15-week-old fetuses, CD10 and CD34 positive cells were found delineating groups of myotubes. At 32 weeks, CD34+ and CD10+ were present in the endomysium surrounding individual cells. In the neonatal, the cells were organized like in adult tissue, having slender processes with relation to fibers and capillaries (Fig. 3). Thus, a reorganization of the endomysial cells takes place during muscle maturation
Figure 3.
CD10 and CD34 expression during development. In 15-week-old fetuses CD10 and CD34 are expressed in fascia and perimysium (p) and in connective tissue surrounding groups of fibers. At 32 weeks, the fascial and perimysial (p) in staining is reduced or lost and CD10 and CD34 are localized between the individual fibers the muscle fascicle (f). At birth, the adult pattern of CD10 and CD34 is established. Scale bar = 250 µm.
PDGFRα+, CD10+, CD34+, CD271+ Cells but Not Pericytes Respond to Pathological Conditions
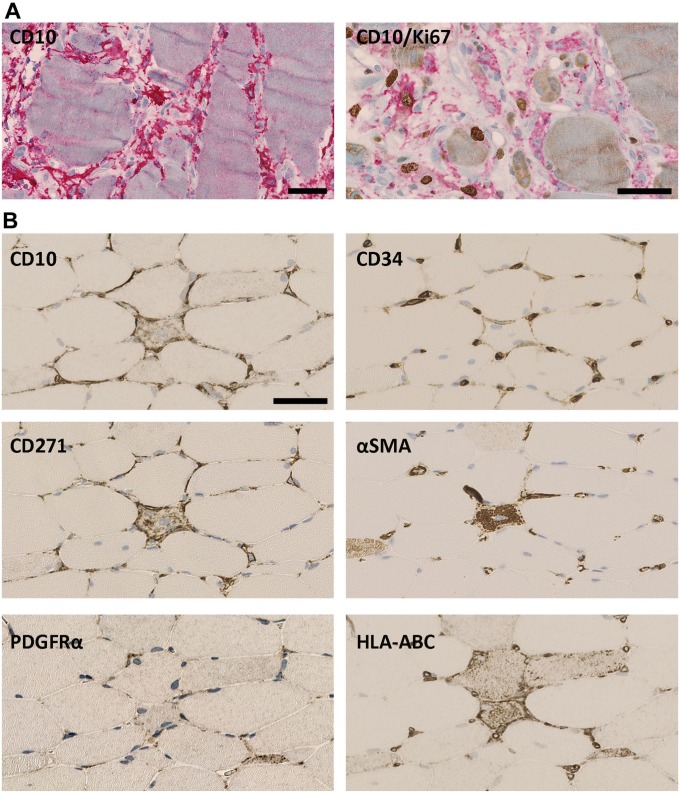
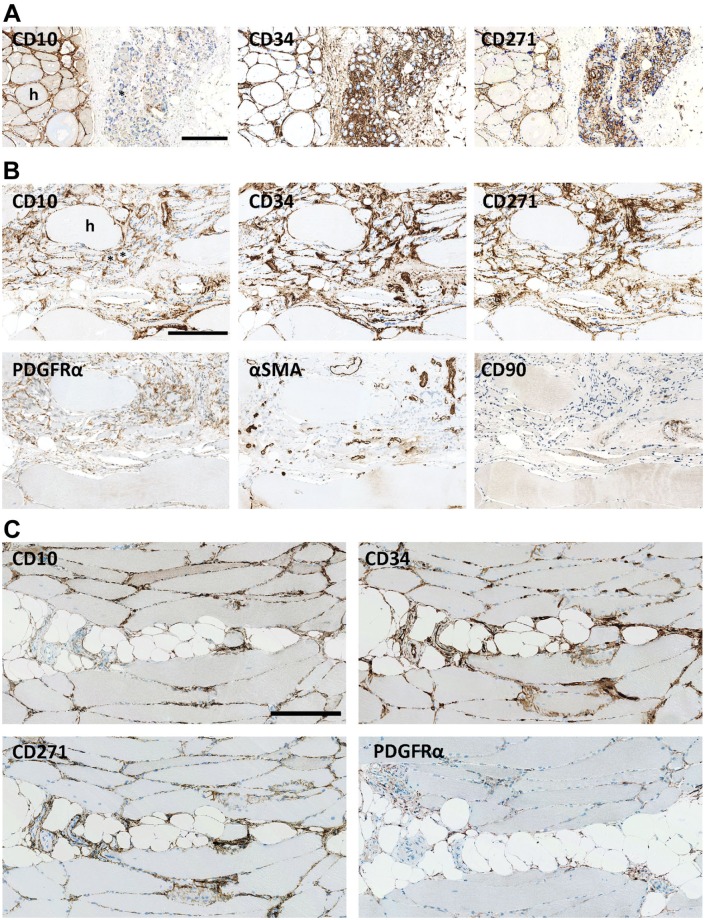
In muscle lesions, the number of CD10+ interstitial cells in the endomysium increased (Fig. 4A). Ultrastructural analysis comparing 3 normal muscles with 3 muscles with inclusion body myositis all from quadriceps showed that the volume fraction of interstitial cells increased significantly (p=0.002) from 49% of normal muscle endomysial cells (range: 45–52%) to 64% in inclusion body myositis (range: 63–66%), and the ratio between endothelial cells and interstitial cells changed from 1.2 to 2.9, respectively (p=0.0007). Correspondingly, some CD10+ cells expressed the proliferation marker Ki67 (Fig. 4A). CD10+/Ki67+ were not found in normal muscle. No significant increase in cells expressing the pericyte marker αSMA could be found in the interstitial space (Figs. 4B and 5) and no significant changes could be seen in the estimated volume fraction of pericytes (p=0.651). Thus, the major responders to muscle damage were the CD10+ cells.
Figure 4.
Muscle damage. (A) In response to muscle damage CD10+ cells (red) become hyperplastic and increase in number. CD10/Ki67: CD10+ cells (red) express Ki67 (brown). (B): In the endomysium surrounding damaged single fibers, CD10, CD34, and CD271 are upregulated while only a slight increase is seen of PDGFRα. No αSMA+ cells are seen outside capillaries. HLA-ABC marks fiber damage. (A) Immune mediated necrotizing myopathy, (B) Inclusion body myositis. Scale bars = 50 µm. Abbreviations: PDGFRα, platelet-derived growth factor receptor α; αSMA, alpha-smooth muscle actin.
Figure 5.
Focal damage, inflammatory infiltrate. CD10, CD34, PDGFRα, and CD271 are upregulated in relation to inflammatory infiltrates. αSMA is distinctly expressed in the small vessels but no increase in the number of αSMA+ cells is seen. CD56 visualizes myogenic activity and NK cells. CD73 is found in endothelium (red, arrow) and co-expresses with CD10+ cells (yellow on the merged image), shown by SIMPLE technique. Biopsy from inclusion body myositis. Scale bars = 100 µm. Abbreviations: PDGFRα, platelet-derived growth factor receptor α; αSMA, alpha-smooth muscle actin; NK: natural killer.
The phenotype of interstitial cells changed in response to damage. In skeletal muscle with focal single fiber damages (Fig. 4B), CD10 was upregulated along with CD34 and CD271, and to a lesser extent PDGFRα. In larger focal lesions (Fig. 5), also CD73 was expressed. No change was observed for CD90 (not shown) or αSMA in the interstitial space. Likewise, CD105, a marker for MSCs, was not observed in interstitial cells but was present in endothelial cells (not shown).
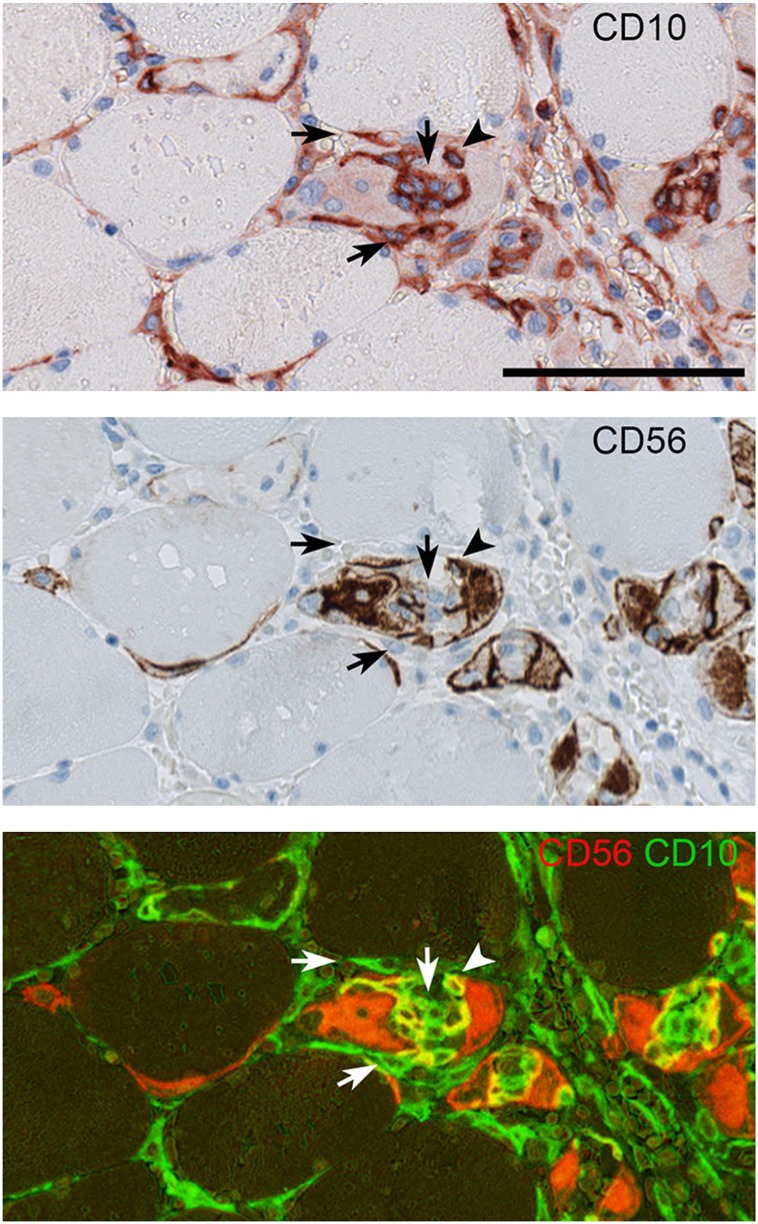
In severe muscle lesions defined as lesions with widespread coherent damage (Fig. 6), the CD10+ cell in the endomysium could lose their CD34 expression but gained a more intense PDGFRα positivity. Likewise, CD271 could be seen co-expressed with CD10, but the expression varied, thus some cells were strongly positive for CD10 and weakly positive for CD271 and vice versa, while other cells were strongly positive for both markers. The pericyte marker CD146 (not shown) was only present in vessel walls, but in contrast to healthy muscle, CD73 and CD90 were found co-localizing with CD10 in interstitial cells, though not all CD10+ cells expressed these markers. In actively regenerating fibers, CD10+ cells could be found not only surrounding the regenerating fiber, but also inside muscle fibers together with CD56+/CD10+ myoblasts (Fig. 7). No CD56+/CD10+ co-expression was found outside regenerating fibers.
Figure 6.
Marker expressions in severely damaged muscle. CD34 expression is down regulated in CD10+ endomysial cells (arrow head) but not in the endothelial cells. PDGFRα expression is increased and co-localizes with CD10. An example is shown by arrow. CD271 is up regulated and co-localizes with CD10 (arrow) but the intensity of both CD10 and CD271 varies (arrow heads).. In severely damaged muscle, CD73 and CD90 are found in some interstitial cells co-localized with CD10 (arrows). Biopsy from compartment syndrome. SIMPLE technique was used. Scale bar = 100 µm. Abbreviation: PDGFRα, platelet-derived growth factor receptor.
Figure 7.
CD10 and CD56 within regenerating muscle fibers. In regenerating muscle fibers, CD10+/CD56+ cells (arrow head) can be seen. In contrast CD10+/CD56- cells (arrows) are seen both in the fiber and at its surface. Biopsy from compartment syndrome. SIMPLE technique was used. Scale bar = 100 µm.
Severe damages with actively regenerating muscle fibers were seen in compartment syndrome, newer necrotic parts of ischemic amputated legs and in the borders of an abscess.
In the endomysium, present in larger groups of neurogenic atrophic muscle fibers, CD34 and CD271 were upregulated while CD10 was downregulated (Fig. 8A), although in the endomysium of the neighboring groups of hypertrophic fibers, CD10 as well as CD34, PDGFRα and CD271 were abundantly expressed. However, where neurogenic atrophic fibers were mixed with hypertrophic fibers, CD10 appeared to be preserved (Fig. 8B). Expression of αSMA and CD90 was unchanged (Fig. 8B). In atrophy with fatty replacement, it appeared that CD10 expression was downregulated in the interface between fat and muscle, while CD34 and CD271 were still present (Fig. 8C). A similar tendency was found at the interface between muscle and connective tissue in ectopic muscle (S1).
Figure 8.
Atrophy. (A): In long standing denervation, CD10 is upregulated between hypertrophic fibers (h) but almost lost in groups of atrophic fibers (*). CD34 and CD271 are intensely upregulated in relation to both atrophic and hypertrophic fibers. Biopsy from Kennedy’s disease. (B): In denervation atrophy where atrophic fibers(*) are mixed with hypertrophic fibers (h) CD34, PDGFRα, CD271, and CD10, are upregulated in the atrophic area while no increase in αSMA or CD90 is seen. (C) CD10 is seen between muscle fibers but is scarcely represented in the interface between adipocytes and muscle fibers, while cells positive for CD34, CD272, and PDGFRα are present in this interface. Biopsy from inclusion body myositis. Scale bars = 250 µm. Abbreviations: PDGFRα, platelet-derived growth factor receptor α; αSMA, alpha-smooth muscle actin.
TCF7L2 Is Upregulated in Damaged Muscle
Along with the increase of endomysial cells in muscle lesions, also the number of cells with TCF7L2+ nuclei increased. Comparing normal and lesioned muscle (Fig. S2, TCF7L2 B and E), the number of stained nuclei increased from 290/mm² to 2900/mm². A large number of endomysial cells in damaged muscle expressed TCF7L2 including CD10 positive, although not all CD10 positive cells had TCF7L2+ nuclei (Fig. S2).
CD10+ Cells Associated With Vessels and Peripheral Nerves
In addition to the CD10+ cells found between muscle fibers, a population of CD10+ cells was found in the inner adventitia of larger vessels (S4). These cells shared antigen expression with the interstitial CD10+ cells in varying degree. Moreover, myelinating Schwann cells and perineurial cells were CD10+ (S5).
Discussion
In the present study, we have focused on histology to be able to identify cells and distinguish cell profiles and reaction patterns in the endomysium. This approach makes detection of focal changes possible in contrast to studies on isolated or cultured cells. Moreover, cultured cells may change expression profile over time,18 changes that not necessarily are reflections of the processes that take place in muscle tissue. More fundamentally, the properties of cells obtained during in vitro culturing and differentiation may not, though interesting in the aspect of regenerative medicine, be a recapitulation of the in vivo functions of the corresponding resident cells. We have furthermore focused on human muscle because although animal models have provided valuable information on muscle interstitial cells, species differences are observed, hampering a direct translation to clinical use. Examples of species differences include Sca-1 which in mice is an important discriminating cell marker,19 but is not present in humans. Also CD34, required for efficient myogenic progression in murine satellite cells20 is not present in human satellite cells but as here shown in interstitial cells. Finally, we have almost exclusively studied muscle from patients without genetic disorders.
Based on location and expression of the applied panel of markers, we were able to demonstrate that the endomysial mesenchymal cell had a dynamic profile and responded in different ways to various pathological conditions.
Pericytes
Divergent definitions of pericytes can be found in the literature. By the histological definitions, pericytes and pericyte-like cells are cells located abluminal to endothelial cells in capillaries, venules, and arterioles.21–23 In some studies, cells separated from the endothelium by smooth muscle cells are also referred to as pericytes, but these have different phenotypes and are elsewhere referred to as adventitial cells.
In the context of regeneration, the pericyte itself is of interest. In vitro, promotion of muscle fiber growth24 and capacity for pericyte-derived cells to be structurally included in muscle fibers25 have been reported. The pericyte has also been connected to the MSC, a cell type which has been shown to have a wide range of effects supporting regeneration.26 The idea of the pericyte as a MSC precursor resident in all tissue types27 has been challenged by the demonstration of tissue-specific differences in the phenotype of pericytes,28 but the observation that cultured pericytes can develop MSC properties could support that pericytes could be MSC progenitors.29 Vice versa, MSCs have also been reported to be able to develop into pericytes.30
CD73 and CD105 are among the markers included in the minimal criteria for defining MSC,31 but in normal muscle, we only found CD73 and CD105 in endothelial cells. Corresponding to our findings, CD73 and CD105 have previously been described as endothelial markers.32,33
In previous immunohistochemical studies, CD146, NG2, alkaline phosphatase, αSMA and CD9016,17,29,34 have been described in pericytes in human skeletal muscle. We were likewise able to show these markers in pericytes, except for alkaline phosphatase which in our study predominantly was expressed in endothelial cells as also previously shown.35 In addition, our demonstration in human skeletal muscle of a nestin+ subpopulation of pericytes corresponds with previous reports on skeletal muscle pericytes in mice.36
Studies have described an increase in the number of pericytes in neuromuscular disorders16 and after exercise.17 However, equivalent to observations in mice37 we saw no upregulation of pericytes in relation to damaged fibers compared to normal regions. Likewise, we found no evidence of transition of pericytes into MSCs. In conclusion, there was no histological support for the participation of pericytes or MSC in muscle regeneration in vivo.
CD10, CD34, PDGFRα, and CD271 Positive Endomysial Cells
The morphology of this cell and the localization in the interstitial space outside the vascular basal lamina, in addition to the PDGFRα and CD34 expression, suggest that it is identical to the fibro adipogenic progenitors,7 mesenchymal progenitor cells,6 and skeletal muscle telocytes.12 Moreover, it could belong to the family of CD34+ stromal cells/fibroblasts/fibrocytes/telocytes8 which are found in various tissues.
Telocytes are a recently identified cell type characterized by distinctive prolongations named telopodes. They are described in multiple tissue types including skeletal muscle.3,12,38,39 CD34 is among the markers that have been used to identify telocytes immunohistochemically. Other markers include c-kit and PDGFRβ. Electron microscopy demonstrating telopodes with caveolae and rough endoplasmic reticulum also has been significant for the identification. The interstitial CD10+ cells in our study share this ultrastructure and CD34 positivity.
Fibro adipogenic progenitors were initially identified in situ in mice as cells positive for the stem cell antigen-1 (Sca1) and PDGFRα, and negative for the endothelial marker CD31.7 These cells have been described as equivalent to human skeletal muscle resident mesenchymal progenitor cells6 both characterized by PDGFRα expression. Cultured human CD15+/PDGFRα+CD56− cells have also been assigned the name fibro adipogenic progenitors,14 but in our study, cells with this expression profile were not present in vivo.
Fibroblasts have been suggested to belong to a family of interstitial cells including the telocytes.8 The definition of a fibroblast is an extracellular matrix producing cell residing in connective tissue. Because this broad definition includes several cell types, specific fibroblast markers have been suggested. Recent studies have employed TCF7L2.15,40,41 However, the use of TCF7L2 as a marker for fibroblast in skeletal muscle requires caution because it is based on the assumption that fibroblast express the antigen at a high concentration, and that cells expressing lower concentrations belongs to other cell types.15 We show that the signal intensity is very dependent on the staining protocol used and that also endothelial cells also often express TCF7L2 at a high intensity. Moreover, we found that the intensity and number of positive nuclei increased in pathological skeletal muscle tissue in accordance with observations in a human regeneration study.40 Many but not all of the nuclei seen in CD10+ cells expressed TCF7L2 and not all TCF7L2+ cells were CD10+. This indicates that TCF7L2 in humans is present in several cell types but also that it does not label all cells of a specific type. Human TCF7L2+ fibroblasts40 have in vitro been described to support myoblast proliferation and differentiation which could place them in the group of interstitial cells supporting muscle regeneration.
The transcription factor TCF7L2 is involved in many biological processes including regulation of the canonical wnt/beta catenin signaling pathway participating in myogenesis and fibrosis,42 and a study using genetically modified mice demonstrates that TCF7L2 itself is important for myogenesis.15
The Expression Profile Is Dynamic in CD10+ Cells
In pathological conditions, the CD10+ cells, in contrast to pericytes, prolifrerated, and showed changes in marker profile. During pathophysiological activation, we found that CD34 could be lost, while CD10, PDGFRα, and CD271 were upregulated. In addition, a subset of CD10+ cells also expressed CD73 and CD90. The activated cells thus obtained an immunophenotype with similarities to pericytes and MSCs.
CD34 loss has previously been reported in telocytes in granulation tissue43 and isolated CD34+ stromal cells lose CD34 in culture.8 In focal damages, however, CD34 was upregulated. These findings indicate a differentiated response to damage and regeneration.
In contrast, CD10 could be seen downregulated between atrophic fibers in neurogenic myopathy and in conditions with fatty replacement at the interface between adipocytes and muscle. Here, however, CD34 and CD271 were upregulated. This suggests that CD10 expression depends on the interaction with functional muscle.
Structure and Expression Profile of CD10+ Cells Indicate Capacity for Communication and Support of Regeneration
Muscle-derived stromal cells broadly have been shown to have a range of properties which can support muscle regeneration.7,24,44–47 Many of the observations, however, are based on studies in animal or in vitro.
Structural findings indicate that interstitial CD10+ cells have the potential to interact with a range of other cells including other CD10+ cells, muscle fibers and bone marrow derived cells, due to close contact, communication through caveolae, exosomes, and receptor mediated uptake.
CD10 and CD34 have previously been shown in mid-term fetuses.48 We showed that the distribution of CD10 changed from mid-term to term. During the third trimester it obtained the same distribution as in mature muscle. This re-organization could support the presumption that the cells are involved in muscle formation. Likewise this could be indicated by the presence of CD10+ cells within regenerating fibers.
The demonstrated expression of CD10, CD34, CD73, CD90, CD271 and PDGFRα can indicate specific functions, where these markers are involved. A previous study shows that CD10 expression was associated with muscle fibers and was upregulated in muscle disorders and that the expression corresponded with muscle fiber regeneration.49 We, however, found CD10 in interstitial cells. CD10 is a zinc-dependent metalloprotease with several peptide hormone substrates including oxytocin,50 enkephalin51 and bradykinin52 all of which have regulatory effects in skeletal muscle. The myokine IL-6 has been shown to induce CD10 activity.53 Considering that IL-6 is upregulated during exercise54 and muscle damage55 IL-6 could be part of the background for upregulation of CD10 in damaged muscle and decreased CD10 expression during muscle atrophy.
The function of CD34 in skeletal muscle interstitial cells is not known, but in other cell types, it has been connected with cell migration56 and regulation of cell adhesion.57
The tyrosine kinase receptor PDGFRα is involved in skeletal muscle fibrosis58,59 and tyrosine kinase receptor inhibitors have been shown to attenuate skeletal muscle dystrophy in mice60,61 an effect also seen by intronic polyadenylation of PDGFRα.62 Considering that PDGFR can also interact with VEGF63 opens for an influence on the PDGFR presenting cells by the change in VEGF signaling of the muscle fibers.64
CD271 has been demonstrated in human muscle, both in muscle fibers and in the perimysium, and is upregulated during myogenesis and myopathy.65,66 We could show a similar response to muscle damage and a more exact cellular localization of CD271 in the endomysium. A ligand for CD271, brain-derived nerve growth factor (BNDF), is produced in muscle fibers and is upregulated during myogenesis and muscle tissue regeneration.67,68 Thus, muscle fibers can influence the interstitial cells through BNDF.
The function of CD73 and CD90 in muscle has not yet been determined. We found an induction of CD73 and CD90 in endomysial cells in response to muscle damage. Considering that the functions ascribed to CD73 and CD90 in general include angiogenesis, anti-apoptosis, and anti-inflammation,69,70 the damage induced expression could support muscle regeneration.
Several lines of evidence thus indicate that these cells expressing CD10, CD34, PDGFRα, CD271, CD73 and CD90 could be active participants in the creation of the micro environment supporting muscle regeneration. Considering the suggested potentials of the fibroadipogenic cells,45 these cells could also counteract muscle restoration.
In this study, we have presented an endomysial cell that in normal muscle expressed CD10, CD34, PDGFRα, CD271, and vimentin. In response to muscle lesions, it increased in number and the marker profile was dynamic and changed in response to both acute damage and atrophy. The histological approach allowed detection of focal responses. Peripheral nerves and in the adventitia of blood vessels, moreover, contained cells with an overlapping expression profile, underlining the significance of combining expression and topography.
The CD10+ cells are omnipresent in the muscle interstitium, they respond to muscle activity and damage, and have morphology and expression profile that indicate interactivity. Thereby, they are candidates for a supportive role in muscle regeneration.
The lack of an accepted nomenclature based on common criteria hampers communication in the field of skeletal muscle interstitial cells, but the cell type, which we have dealt with by its marker expression, including PDGFRα, and ultrastructure resembled FAPs/mesenchymal progenitor cells and telocytes. A relevant discussion is whether we deal with a few cell types with dynamic profiles adapting to different conditions, or with various cell types with specific functions. Our study could indicate the former. Further IHC multiplexing and flow cytometry analyses of freshly isolated muscle derived cells in normal and pathological conditions applying our antibody panel could help elucidate the heterogeneity of the interstitial cell population. The specific functions that could be ascribed to the endomysial cells include maintenance of ECM under normal conditions,71 and support of regeneration in acute damage. Moreover, they could be the source of cells participating in pathologic reactions such as fibrosis. In this context, it would be of interest to know the fate of the activated cells, whether they return to the pre-activated state or if they retain their acquired state, and what happens to the surplus of these cells after regeneration. As we primarily have examined non-genetic disorders, it would also be relevant to see if our observations extend to diseases as dystrophies and congenital myopathies. Since age influences muscle regeneration,72 it might also be worth studying the expression of these cells at different ages.
We also examined the role of pericytes during muscle damage but found no histological evidence for their participation. Likewise, in our study, we did not find cells in vivo that expressed CD105, CD73 and CD90 and thus matched the minimum criteria of MSCs defined by The International Society for Cellular Therapy.31 Although pericytes/MSCs have been considered as major supporting cells in regeneration, our study rather points to the CD10+ cells for this role in skeletal muscle.
Cellular therapy for regeneration of skeletal muscle by transplantation of myoblast has had limited success, though some positive effect on sphincter function has been reported73 and similar results have been reported when less well defined muscle derived cell populations have been used.74 A possibility suggested by our findings would be to test CD10+ cells together with myogenic cells to improve cell therapy by taking advantage of factors provided by the CD10+ cells. Furthermore, characterization of their secreted compounds also could allow construction of enriched scaffolds for myoblast transplantation and development of cell-free functionalized scaffolds able to recruit resident cells involved in myogenesis.
Supplemental Material
Supplemental material, DS_10.1369_0022155419871033 for Marker Expression of Interstitial Cells in Human Skeletal Muscle: An Immunohistochemical Study by Eva K. Hejbøl, Mohammad A. Hajjaj, Ole Nielsen and Henrik D. Schrøder in Journal of Histochemistry & Cytochemistry
Acknowledgments
We thank Julie Rex, Karin Trampedach Siemonsen, Terese Bjerrum, Lone Christiansen, and Lisbet Mortensen from the Department of Pathology, Odense University Hospital, for their technical support.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: EKH contributed to the conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing. MAH contributed to the collection and/or assembly of data, data analysis and interpretation. ON contributed to the conception and design. HDS contributed to the conception and design, financial support, data analysis and interpretation, and manuscript writing. All authors have read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Innovation Fund Denmark 5166-00002B and grants from Odense University Hospital.
Contributor Information
Eva K. Hejbøl, Department of Pathology, Odense University Hospital, Odense, Denmark; Department of Clinical Research, Faculty of Health, University of Southern Denmark, Odense, Denmark.
Mohammad A. Hajjaj, Department of Pathology, Odense University Hospital, Odense, Denmark
Ole Nielsen, Department of Pathology, Odense University Hospital, Odense, Denmark.
Henrik D. Schrøder, Department of Pathology, Odense University Hospital, Odense, Denmark Department of Clinical Research, Faculty of Health, University of Southern Denmark, Odense, Denmark.
Literature Cited
- 1. Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ceafalan LC, Popescu BO, Hinescu ME. Cellular players in skeletal muscle regeneration. Biomed Res Int. 2014;2014:957014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suciu LC, Popescu BO, Kostin S, Popescu LM. Platelet-derived growth factor receptor-beta-positive telocytes in skeletal muscle interstitium. J Cell Mol Med. 2012;16(4):701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tedesco FS, Moyle LA, Perdiguero E. Muscle interstitial cells: a brief field guide to non-satellite cell populations in skeletal muscle. Methods Mol Biol. 2017;1556:129–47. [DOI] [PubMed] [Google Scholar]
- 5. Crisan M, Corselli M, Chen WC, Peault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16(12):2851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uezumi A, Fukada S, Yamamoto N, Ikemoto-Uezumi M, Nakatani M, Morita M, Yamaguchi A, Yamada H, Nishino I, Hamada Y, Tsuchida K. Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5:e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diaz-Flores L, Gutierrez R, Garcia MP, Saez FJ, Diaz-Flores L, Jr, Valladares F, Madrid JF. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol Histopathol. 2014;29(7):831–70. [DOI] [PubMed] [Google Scholar]
- 9. Glass G, Papin JA, Mandell JW. SIMPLE: a sequential immunoperoxidase labeling and erasing method. J Histochem Cytochem. 2009;57(10):899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96(5):379–94. [DOI] [PubMed] [Google Scholar]
- 12. Popescu LM, Manole E, Serboiu CS, Manole CG, Suciu LC, Gherghiceanu M, Popescu BO. Identification of telocytes in skeletal muscle interstitium: implication for muscle regeneration. J Cell Mol Med. 2011;15(6):1379–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bankhead P, Loughrey MB, Fernandez JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arrighi N, Moratal C, Clement N, Giorgetti-Peraldi S, Peraldi P, Loubat A, Kurzenne JY, Dani C, Chopard A, Dechesne CA. Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis. 2015;6:e1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development (Cambridge, England). 2011;138(2):371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diaz-Manera J, Gallardo E, de Luna N, Navas M, Soria L, Garibaldi M, Rojas-Garcia R, Tonlorenzi R, Cossu G, Illa I. The increase of pericyte population in human neuromuscular disorders supports their role in muscle regeneration in vivo. J Pathol. 2012;228(4):544–53. [DOI] [PubMed] [Google Scholar]
- 17. Farup J, De Lisio M, Rahbek SK, Bjerre J, Vendelbo MH, Boppart MD, Vissing K. Pericyte response to contraction mode-specific resistance exercise training in human skeletal muscle. J Appl Physiol (1985). 2015;119(10):1053–63. [DOI] [PubMed] [Google Scholar]
- 18. Lecourt S, Marolleau JP, Fromigue O, Vauchez K, Andriamanalijaona R, Ternaux B, Lacassagne MN, Robert I, Boumediene K, Chereau F, Marie P, Larghero J, Fiszman M, Vilquin JT. Characterization of distinct mesenchymal-like cell populations from human skeletal muscle in situ and in vitro. Exp Cell Res. 2010;316(15):2513–26. [DOI] [PubMed] [Google Scholar]
- 19. Cottle BJ, Lewis FC, Shone V, Ellison-Hughes GM. Skeletal muscle-derived interstitial progenitor cells (PICs) display stem cell properties, being clonogenic, self-renewing, and multi-potent in vitro and in vivo. Stem Cell Res Ther. 2017;8(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alfaro LA, Dick SA, Siegel AL, Anonuevo AS, McNagny KM, Megeney LA, Cornelison DD, Rossi FM. CD34 promotes satellite cell motility and entry into proliferation to facilitate efficient skeletal muscle regeneration. Stem Cells (Dayton, Ohio). 2011;29(12):2030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. [DOI] [PubMed] [Google Scholar]
- 22. Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58(1):1–10. [DOI] [PubMed] [Google Scholar]
- 23. Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol. 2013;305(11):C1098–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kostallari E, Baba-Amer Y, Alonso-Martin S, Ngoh P, Relaix F, Lafuste P, Gherardi RK. Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence. Development (Cambridge, England). 2015;142(7):1242–53. [DOI] [PubMed] [Google Scholar]
- 25. Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255–67. [DOI] [PubMed] [Google Scholar]
- 26. Caplan AI. New MSC: MSCs as pericytes are Sentinels and gatekeepers. J Orthop Res. 2017;35(6):1151–9. [DOI] [PubMed] [Google Scholar]
- 27. Crisan M, Chen CW, Corselli M, Andriolo G, Lazzari L, Peault B. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009;1176:118–23. [DOI] [PubMed] [Google Scholar]
- 28. Pierantozzi E, Vezzani B, Badin M, Curina C, Severi FM, Petraglia F, Randazzo D, Rossi D, Sorrentino V. Tissue-specific cultured human pericytes: perivascular cells from smooth muscle tissue have restricted mesodermal differentiation ability. Stem Cells Dev. 2016;25(9):674–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13. [DOI] [PubMed] [Google Scholar]
- 30. Joensuu K, Uusitalo-Kylmala L, Hentunen TA, Heino TJ. Angiogenic potential of human mesenchymal stromal cell and circulating mononuclear cell cocultures is reflected in the expression profiles of proangiogenic factors leading to endothelial cell and pericyte differentiation. J Tissue Eng Regen Med. 2018;12(3):775–83. [DOI] [PubMed] [Google Scholar]
- 31. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–7. [DOI] [PubMed] [Google Scholar]
- 32. Zukowska P, Kutryb-Zajac B, Toczek M, Smolenski RT, Slominska EM. The role of ecto-5’-nucleotidase in endothelial dysfunction and vascular pathologies. Pharmacol Rep. 2015;67(4):675–81. [DOI] [PubMed] [Google Scholar]
- 33. Wong SH, Hamel L, Chevalier S, Philip A. Endoglin expression on human microvascular endothelial cells association with betaglycan and formation of higher order complexes with TGF-beta signalling receptors. Eur J Biochem. 2000;267(17):5550–60. [DOI] [PubMed] [Google Scholar]
- 34. Murray IR, Baily JE, Chen WC, Dar A, Gonzalez ZN, Jensen AR, Petrigliano FA, Deb A, Henderson NC. Skeletal and cardiac muscle pericytes: functions and therapeutic potential. Pharmacol Ther. 2017;171:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schultz-Hector S, Balz K, Bohm M, Ikehara Y, Rieke L. Cellular localization of endothelial alkaline phosphatase reaction product and enzyme protein in the myocardium. J Histochem Cytochem. 1993;41(12):1813–21. [DOI] [PubMed] [Google Scholar]
- 36. Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res. 2013;10(1):67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans SM. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017;20(3):345–59.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marini M, Rosa I, Ibba-Manneschi L, Manetti M. Telocytes in skeletal, cardiac and smooth muscle interstitium: morphological and functional aspects. Histol Histopathol. 2018;33:1151–65. [DOI] [PubMed] [Google Scholar]
- 39. Bojin FM, Gavriliuc OI, Cristea MI, Tanasie G, Tatu CS, Panaitescu C, Paunescu V. Telocytes within human skeletal muscle stem cell niche. J Cell Mol Med. 2011;15(10):2269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mackey AL, Magnan M, Chazaud B, Kjaer M. Human skeletal muscle fibroblasts stimulate in vitro myogenesis and in vivo muscle regeneration. J Physiol. 2017;595(15):5115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development (Cambridge, England). 2011;138(17):3625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cisternas P, Henriquez JP, Brandan E, Inestrosa NC. Wnt signaling in skeletal muscle dynamics: myogenesis, neuromuscular synapse and fibrosis. Mol Neurobiol. 2014;49(1):574–89. [DOI] [PubMed] [Google Scholar]
- 43. Diaz-Flores L, Gutierrez R, Diaz-Flores L, Jr, Gomez MG, Saez FJ, Madrid JF. Behaviour of telocytes during physiopathological activation. Semin Cell Dev Biol. 2016;55:50–61. [DOI] [PubMed] [Google Scholar]
- 44. Sugg KB, Korn MA, Sarver DC, Markworth JF, Mendias CL. Inhibition of platelet-derived growth factor signaling prevents muscle fiber growth during skeletal muscle hypertrophy. FEBS Lett. 2017;591:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Contreras O, Rebolledo DL, Oyarzun JE, Olguin HC, Brandan E. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res. 2016;364(3):647–60. [DOI] [PubMed] [Google Scholar]
- 46. Pumberger M, Qazi TH, Ehrentraut MC, Textor M, Kueper J, Stoltenburg-Didinger G, Winkler T, von Roth P, Reinke S, Borselli C, Perka C, Mooney DJ, Duda GN, Geissler S. Synthetic niche to modulate regenerative potential of MSCs and enhance skeletal muscle regeneration. Biomaterials. 2016;99:95–108. [DOI] [PubMed] [Google Scholar]
- 47. Kulesza A, Burdzinska A, Szczepanska I, Zarychta-Wisniewska W, Pajak B, Bojarczuk K, Dybowski B, Paczek L. The mutual interactions between mesenchymal stem cells and myoblasts in an autologous co-culture model. PLoS ONE. 2016;11(8):e0161693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim JH, Hwang SE, Yu HC, Hwang HP, Katori Y, Murakami G, Cho BH. Distribution of CD10-positive epithelial and mesenchymal cells in human mid-term fetuses: a comparison with CD34 expression. Anat Cell Biol. 2014;47(1):28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Broccolini A, Gidaro T, Morosetti R, Gliubizzi C, Servidei T, Pescatori M, Tonali PA, Ricci E, Mirabella M. Neprilysin participates in skeletal muscle regeneration and is accumulated in abnormal muscle fibres of inclusion body myositis. J Neurochem. 2006;96(3):777–89. [DOI] [PubMed] [Google Scholar]
- 50. Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Denning GM, Ackermann LW, Barna TJ, Armstrong JG, Stoll LL, Weintraub NL, Dickson EW. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides. 2008;29(1):83–92. [DOI] [PubMed] [Google Scholar]
- 52. Langberg H, Bjorn C, Boushel R, Hellsten Y, Kjaer M. Exercise-induced increase in interstitial bradykinin and adenosine concentrations in skeletal muscle and peritendinous tissue in humans. J Physiol. 2002;542(Pt 3):977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morisaki N, Moriwaki S, Sugiyama-Nakagiri Y, Haketa K, Takema Y, Imokawa G. Neprilysin is identical to skin fibroblast elastase: its role in skin aging and UV responses. J Biol Chem. 2010;285(51):39819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529(Pt 1):237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cruz-Guzman Odel R, Rodriguez-Cruz M, Escobar Cedillo RE. Systemic inflammation in duchenne muscular dystrophy: association with muscle function and nutritional status. Biomed Res Int. 2015;2015:891972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci. 2008;121(Pt 22):3683–92. [DOI] [PubMed] [Google Scholar]
- 57. Healy L, May G, Gale K, Grosveld F, Greaves M, Enver T. The stem cell antigen CD34 functions as a regulator of hemopoietic cell adhesion. Proc Natl Acad Sci U S A. 1995;92(26):12240–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ieronimakis N, Hays A, Prasad A, Janebodin K, Duffield JS, Reyes M. PDGFRalpha signalling promotes fibrogenic responses in collagen-producing cells in Duchenne muscular dystrophy. J Pathol. 2016;240(4):410–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Olson LE, Soriano P. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16(2):303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huang P, Zhao XS, Fields M, Ransohoff RM, Zhou L. Imatinib attenuates skeletal muscle dystrophy in mdx mice. FASEB J. 2009;23(8):2539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ito T, Ogawa R, Uezumi A, Ohtani T, Watanabe Y, Tsujikawa K, Miyagoe-Suzuki Y, Takeda S, Yamamoto H, Fukada S. Imatinib attenuates severe mouse dystrophy and inhibits proliferation and fibrosis-marker expression in muscle mesenchymal progenitors. Neuromuscul Disord. 2013;23(4):349–56. [DOI] [PubMed] [Google Scholar]
- 62. Mueller AA, van Velthoven CT, Fukumoto KD, Cheung TH, Rando TA. Intronic polyadenylation of PDGFRalpha in resident stem cells attenuates muscle fibrosis. Nature. 2016;540(7632):276–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol. 2007;177(3):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Richardson RS, Wagner H, Mudaliar SR, Henry R, Noyszewski EA, Wagner PD. Human VEGF gene expression in skeletal muscle: effect of acute normoxic and hypoxic exercise. Am J Physiol. 1999;277(6, Pt 2):H2247–52. [DOI] [PubMed] [Google Scholar]
- 65. Baron P, Scarpini E, Meola G, Santilli I, Conti G, Pleasure D, Scarlato G. Expression of the low-affinity NGF receptor during human muscle development, regeneration, and in tissue culture. Muscle Nerve. 1994;17(3):276–84. [DOI] [PubMed] [Google Scholar]
- 66. Heuss D. Light microscopy study of low-affinity nerve growth factor receptor and phosphoprotein B-50/neuromodulin in inflammatory myopathies. Acta Neuropathol. 1996;91(4):409–15. [DOI] [PubMed] [Google Scholar]
- 67. Mousavi K, Jasmin BJ. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J Neurosci. 2006;26(21):5739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Colombo E, Bedogni F, Lorenzetti I, Landsberger N, Previtali SC, Farina C. Autocrine and immune cell-derived BDNF in human skeletal muscle: implications for myogenesis and tissue regeneration. J Pathol. 2013;231(2):190–8. [DOI] [PubMed] [Google Scholar]
- 69. Kumar A, Bhanja A, Bhattacharyya J, Jaganathan BG. Multiple roles of CD90 in cancer. Tumour Biol. 2016;37(9):11611–22. [DOI] [PubMed] [Google Scholar]
- 70. Gao ZW, Dong K, Zhang HZ. The roles of CD73 in cancer. Biomed Res Int. 2014;2014:460654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44(3):318–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Suetta C, Frandsen U, Mackey AL, Jensen L, Hvid LG, Bayer ML, Petersson SJ, Schroder HD, Andersen JL, Aagaard P, Schjerling P, Kjaer M. Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J Physiol. 2013;591(15):3789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Blaganje M, Lukanovic A. Ultrasound-guided autologous myoblast injections into the extrinsic urethral sphincter: tissue engineering for the treatment of stress urinary incontinence. Int Urogynecol J. 2013;24(4):533–5. [DOI] [PubMed] [Google Scholar]
- 74. Sharifiaghdas F, Tajalli F, Taheri M, Naji M, Moghadasali R, Aghdami N, Baharvand H, Azimian V, Jaroughi N. Effect of autologous muscle-derived cells in the treatment of urinary incontinence in female patients with intrinsic sphincter deficiency and epispadias: a prospective study. Int J Urol. 2016;23(7):581–6. [DOI] [PubMed] [Google Scholar]
- 75. Alvarez-Viejo M, Menendez-Menendez Y, Otero-Hernandez J. CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World J Stem Cells. 2015;7(2):470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol (1985);2001;91(2):534–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1369_0022155419871033 for Marker Expression of Interstitial Cells in Human Skeletal Muscle: An Immunohistochemical Study by Eva K. Hejbøl, Mohammad A. Hajjaj, Ole Nielsen and Henrik D. Schrøder in Journal of Histochemistry & Cytochemistry