Abstract
Population‐level nonalcoholic fatty liver disease (NAFLD) death rate data are sparse. We described death rates for adults with NAFLD in the United States using mortality data from the National Vital Statistics System multiple‐cause mortality data (2007‐2016). Decedents who had NAFLD were identified by International Classification of Diseases (ICD) codes K75.81, K76.0, K74.0, K74.6, and K76.9. Among NAFLD decedents, cause‐specific deaths (e.g., cardiovascular disease [CVD], cirrhosis, hepatocellular carcinoma [HCC], non‐liver cancer, diabetes mellitus [DM]) were identified by underlying cause of death ICD‐10 codes. Trends were evaluated by average annual percentage change (AAPC) in age‐standardized death rate (ASDR) per 100,000 persons. Among the 25,129,960 decedents aged ≥20 years, 353,234 (1.4%) decedents had NAFLD (212,322 men; 260,765 non‐Hispanic whites, 32,868 non‐Hispanic blacks, 46,530 Hispanics, 5,025 non‐Hispanic American Indian or Alaska Natives [AIANs], 7,023 non‐Hispanic Asian or Pacific Islanders [APIs]), with a mean age at death of 64.47 ± 13.17 years. During the study period, the ASDR for NAFLD increased by 15% (12.94 to 14.90; AAPC, 1.98%; P < 0.001]), while women (AAPC, 2.99% vs. 1.16% men; P = 0.003), non‐Hispanic whites (AAPC, 2.48%), non‐Hispanic AIANs (AAPC, 2.31%), and Hispanics (AAPC, 0.74%) experienced the highest annual increases. Stable trends were noted for non‐Hispanic blacks and non‐Hispanic APIs. Among subgroups, Mexican (AAPC, 1.75%) and Asian Indians (AAPC, 6.94%) experienced annual increases. The top six underlying causes of death (155,894 cirrhosis, 38,444 CVD, 19,466 non‐liver cancer, 10,867 HCC, 8,113 DM, and 5,683 lung disease) accounted for 67.5% of NAFLD‐related deaths. For cause‐specific deaths, ASDR increased for HCC (AAPC, 3.82%), DM (AAPC, 2.23%), non‐liver cancer (AAPC, 2.14%), CVD (AAPC, 1.59%), and cirrhosis (AAPC, 0.96%). Conclusion: NAFLD‐related deaths in U.S. adults are increasing. Cirrhosis is the top cause‐specific death, followed by CVD. Women, non‐Hispanic whites, and non‐Hispanic AIANs (subgroups Mexicans and Asian Indians) experienced the highest increases in deaths. Policies addressing the societal burden of NAFLD are needed.
Abbreviations
- AAPC
average annual percent change
- AIAN
American Indian or Alaska Native
- APC
annual percentage change
- API
Asian or Pacific Islander
- ASDR
age‐standardized death rate
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- DM
diabetes mellitus
- HCC
hepatocellular carcinoma
- HTN
hypertension
- ICD
International Classification of Diseases
- JR
joinpoint regression
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NVSS
National Vital Statistics System
- OLD
obstructive lung disease
- PR
prevalence ratio
- WHO
World Health Organization
Nonalcoholic fatty liver disease (NAFLD) is a liver disease associated with components of metabolic syndrome, including insulin resistance, diabetes mellitus (DM), hypertension (HTN), hyperlipidemia, and obesity. In 2016, using specific body mass index (BMI) cutoffs (overweight, BMI ≥25 and obesity, BMI ≥30; Asians with a BMI of 23‐27.5 kg/m2 are overweight, and those with a BMI ≥27.5 kg/m2 are obese), the World Health Organization (WHO) determined that worldwide overweight and obesity rates have nearly tripled since 1975.1 The WHO recently reported that 13% of the world’s adult population is currently obese and 39% are overweight, with the United States leading the way with the highest prevalence of adults with obesity.2 Therefore, NAFLD and obesity are considered significant global health threats, garnering increasing interest among all stakeholders.2
Although most patients with NAFLD do not progress to end‐stage liver disease, patients with nonalcoholic steatohepatitis (NASH), a subtype of NAFLD, can progress to cirrhosis and its complications.3 On the other hand, given the very high prevalence of NAFLD, the projected number of patients with NAFLD facing adverse outcomes may be significant. In fact, a recent report using Markov modeling to forecast NAFLD disease by the year 2030 found the prevalence of NAFLD cases is forecasted to increase 21% and NASH cases will increase 63%, providing an overall NAFLD prevalence rate of 33.5% and a 27% prevalence rate of NASH in adults.4 In addition, the incidence of adverse outcomes will significantly increase, with the incidence of decompensated cirrhosis increasing 168%, hepatocellular carcinoma (HCC) 137%, and liver‐related mortality 178%.4
The magnitude of the burden of NAFLD is also seen in economic modeling and population‐based studies.5, 6, 7, 8, 9 NAFLD‐associated costs over the next 10 years are projected to be an estimated $1.005 trillion in the United States and €334 billion in Europe.8 Patients with NAFLD also report a decrease in their health‐related quality of life, especially in the area of physical functioning, which affects their ability to work and perform activities of daily living.9, 10, 11 Despite the significant burden posed by NAFLD, fully validated noninvasive diagnostic tests are lacking and effective treatment for NASH is limited.12, 13 As a result, there may be an increasing number of deaths due to NAFLD and NASH. However, current NAFLD/NASH‐related death data are lacking.14 Thus, the aim of this study was to describe the NAFLD‐related death rate among adults in the United States, using the National Vital Statistics System (NVSS) database for the years 2007‐2016 to provide a better understanding of NAFLD‐related mortality.
Materials and Methods
All death data were obtained from the NVSS database of the National Center for Health Statistics of the Centers for Disease Control and Prevention, with 2007‐2016 data extracted from the NVSS multiple‐cause of death files.15, 16 The NVSS annually provides public‐use mortality data by multiple‐cause of death, abstracted from death certificates filed in vital statistics offices of 50 states and the District of Columbia. Each record contains a single underlying cause of death, up to 20 contributing causes of death, and demographic characteristics. The underlying cause of death is defined by the WHO as “the disease or injury which initiated the train of events leading directly to death, or the circumstances of the accident or violence which produced the fatal injury.” Underlying cause of death was selected from the conditions entered by the physician on the cause of death section of the death certificate. Cause of death was coded using the International Classification of Diseases (ICD) codes in use at the time of death (ICD‐8 for 1969‐1978, ICD‐9 for 1979‐1998, and ICD‐10 for 1999‐2016).16 More than 99% of deaths in the United States are captured by this database.17
To evaluate trends in death rates among adults with NAFLD, NAFLD was identified from the death certificates using the following ICD‐10 codes and categories: fatty liver not elsewhere classified (K76.0), NASH (K75.81), hepatic fibrosis (K74.0), or cryptogenic liver disease with or without cirrhosis (K76.9 and K74.6, respectively). In addition, NAFLD had to reside by itself; therefore, the absence of other chronic liver diseases, including hepatitis C, hepatitis B, alcoholic liver disease, chronic hepatitis, autoimmune hepatitis, Wilson’s disease, hemochromatosis, iron overload, alpha‐1‐antitrypsin deficiency, or excessive alcohol use, was assessed.18 A flow diagram outlining the inclusion and exclusion of NAFLD‐related deaths is shown in Fig. 1. Among decedents who had NAFLD, cause‐specific deaths (e.g., cardiovascular disease [CVD], cirrhosis, HCC, non‐liver cancer, DM) were identified with the ICD‐10 underlying cause of death codes. The ICD‐10 codes that were used to identify NAFLD and other liver diseases as well as NAFLD‐related extrahepatic manifestations, including CVD, DM, and non‐liver cancer, are shown in Supporting Table S1.19, 20
Figure 1.
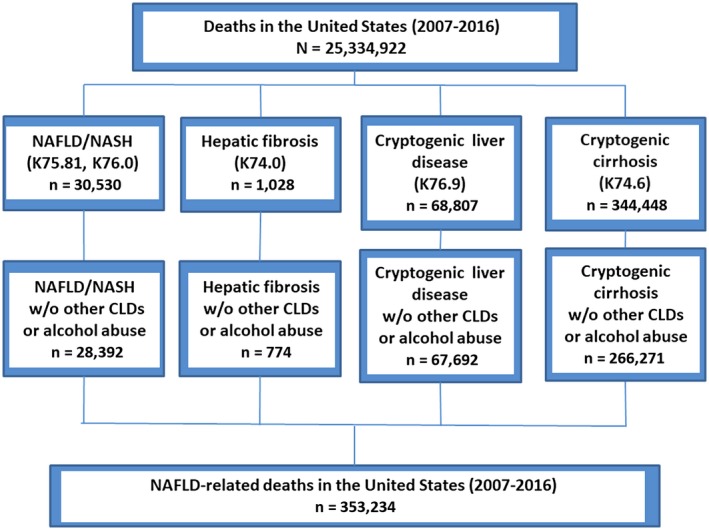
Flow diagram. Abbreviations: CLD, chronic liver disease; w/o, without.
Sociodemographic variables gathered included age at the time of death (20‐44, 45‐54, 55‐64, 65‐74, and ≥75 years), sex, race, Hispanic origin, marital status, and education level. Because race and Hispanic origin are reported independently on the death certificate, race/ethnicity for the non‐Hispanic population (non‐Hispanic white, non‐Hispanic black, Hispanic, non‐Hispanic Asian or Pacific Islander [API], and other, which included American Indian or Alaska Native [AIAN]) was a combination of two questions; the first was answering “no” to the question of whether the decedent was of Hispanic origin and the second was a question of the decedent’s race with the answer selected from a checklist.
Statistical Methods
Age‐specific death rates were calculated by sex and race/ethnicity from 2007 through 2016 based on the corresponding population estimates and adjusted for population shifts due to hurricanes Katrina and Rita, released by the Survey of Epidemiology and End Results (SEER).21, 22 Age‐standardized death rates (ASDRs) per 100,000 with 95% confidence intervals (CIs) were then calculated by using the direct method based on the 2000 Census standard population, using 10‐year age groups starting at 20 years and ending at ≥85 years. Temporal trends in ASDRs were analyzed by the joinpoint regression (JR) model. The JR model identified the best fit for joinpoints at which an apparent change in trend is statistically significant. A maximum number of 2 joinpoints was allowed, and a Monte Carlo permutation method was used for model selection.23 From the selected models, the annual percentage change (APC) for each trend segment and the average annual percentage change (AAPC) for the entire period were reported with a 95% CI. An increasing or decreasing trend was defined if the APC or AAPC was significantly different from 0; otherwise, a stable or level trend was defined. For overall rates, annual death rates were averaged across the entire study period. ASDRs were graphed by sex and race/ethnicity group. Prevalence ratios (PRs) were estimated by modified Poisson regression models with sandwich error variance while adjusting for age, sex, and race/ethnicity. The population counts for Hispanic and Asian American subgroups in non‐Census years were estimated by linear interpolation (2008‐2009), U.S. Census data (2010), and extrapolation (2011‐2016), using the 2000 and 2010 U.S. Census reports.24, 25, 26, 27, 28 Joinpoint trend analysis was run with the JR Program, version 4.5.0.2, from SEER, and all other analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC). Statistical tests were considered significant at P < 0.05 (two‐tailed).
Results
Demographics
Among the 25,129,960 decedents (aged ≥20 years) observed in the United States (2007‐2016), there were 353,234 (1.4%) decedents who had NAFLD listed as a co‐contributory cause of death (212,322 men; 260,765 non‐Hispanic whites, 32,868 non‐Hispanic blacks, 46,530 Hispanics, 5,025 non‐Hispanic AIANs, 7,023 non‐Hispanic APIs; mean age at death 64.47 ± 13.17 years). The age‐specific all‐cause death rate was the highest for decedents aged ≥75 years, followed by age groups 65‐74, 55‐64, 45‐54, and 20‐44 years. The ASDR for NAFLD was the highest among non‐Hispanic AIANs (30.7), followed by Hispanics (20.7), non‐Hispanic whites (13.9), non‐Hispanic blacks (12.2), and non‐Hispanic APIs (6.8) (Table 1; Supporting Table S2). Within the Hispanic subgroup, the ASDR was higher among Mexicans (25.6) and Puerto Ricans (23.4), whereas within the non‐Hispanic API subgroup, the Vietnamese (11.9) had the highest ASDR, followed by the Japanese (8.0) (Tables 2 and 3).
Table 1.
Age‐Standardized Death Rate (per 100,000) For NAFLD Among U.S. Adults Aged ≥20 Years by Age, Sex, and Race, United States, 2007‐2016
| All‐Cause Deaths (Rate per 100,000) | AAPC*, § (95% CI) | |||
|---|---|---|---|---|
| 2007‐2016† | 2007 | 2016 | ||
| Total | 353,234 (14.05) | 29,339 (12.94) | 40,895 (14.90) | 1.98 (1.57‐2.39)*, § |
| Age group, years‡ | ||||
| 20‐44 | 20,317 (1.08) | 2,069 (1.11) | 2,023 (1.07) | −0.14 (−0.92‐0.64) |
| 45‐54 | 61,259 (13.90) | 6,200 (14.11) | 5,259 (12.29) | −1.21 (−1.97 to −0.45)*, § |
| 55‐64 | 100,686 (26.49) | 7,443 (22.47) | 11,687 (28.19) | 2.57 (2.11‐3.04)*, § |
| 65‐74 | 84,752 (35.31) | 6,300 (31.98) | 11,393 (39.79) | 2.67 (1.47‐3.89)*, § |
| ≥75 | 86,220 (44.85) | 7,327 (40.42) | 10,533 (51.10) | 2.78 (1.60‐3.96)*, § |
| Sex | ||||
| Female | 140,912 (10.41) | 11,374 (9.24) | 16,970 (11.54) | 2.99 (2.50‐3.49)*, § |
| Male | 212,322 (18.21) | 17,965 (17.19) | 23,925 (18.76) | 1.16 (0.56‐1.77)*, § |
| Race | ||||
| Non‐Hispanic white | 260,765 (13.87) | 21,825 (12.51) | 30,147 (15.04) | 2.48 (2.07‐2.88)*, § |
| Non‐Hispanic black | 32,868 (12.24) | 2,942 (12.38) | 3,456 (11.61) | −0.01 (−0.73‐0.71) |
| Hispanic | 46,530 (20.65) | 3,546 (19.7) | 5,668 (20.81) | 0.74 (0.26‐1.22)*, § |
| Non‐Hispanic American Indian or Alaska Native | 5,025 (30.68) | 415 (28.71) | 640 (35.19) | 2.31 (1.24‐3.38)*, § |
| Non‐Hispanic Asian or Pacific Islander | 7,023 (6.77) | 564 (6.81) | 865 (6.66) | −0.02 (−0.98‐0.95) |
AAPC is a weighted average of the APCs (a maximum number of 2 joinpoints was allowed). Age standardization is based on the direct method to the Census 2000 population by 10‐year age groups. NAFLD‐related deaths are defined by ICD‐10 codes using underlying or contributing cause of death certificates.
Age‐standardized death rates were averaged over the period 2007‐2016.
Age‐specific death rates.
Significantly different from 0 (P < 0.05).
Table 2.
Age‐Standardized Cause‐Specific Death Rate (per 100,000) For NAFLD Among U.S. Adults Aged ≥20 Years, 2007‐2016
| Cause‐Specific Deaths (Rate per 100,000) | AAPC* (95% CI) | |||
|---|---|---|---|---|
| 2007‐2016† | 2007 | 2016 | ||
| Cause‐specific death‡ | ||||
| Cardiovascular disease | 38,444 (1.54) | 3,353 (1.48) | 4,627 (1.7) | 1.59 (0.85‐2.34)|| |
| Cirrhosis | 155,894 (6.21) | 13,401 (5.91) | 17,174 (6.27) | 0.96 (0.18‐1.74)|| |
| Diabetes | 8,113 (0.32) | 704 (0.31) | 1,027 (0.37) | 2.23 (0.01‐4.49)|| |
| Non‐liver cancer | 19,466 (0.76) | 1,644 (0.72) | 2,440 (0.86) | 2.14 (0.25‐4.06)|| |
| HCC | 10,867 (0.42) | 798 (0.35) | 1,408 (0.49) | 3.82 (3.20‐4.44)|| |
| Lung disease | 5,683 (0.22) | 463 (0.20) | 703 (0.25) | 2.13 (1.08‐3.20)|| |
| Contributory causes of death§ | ||||
| Diabetes | 31,132 (1.24) | 2,461 (1.10) | 4,045 (1.46) | 2.80 (2.03‐3.58)|| |
| Hypertension | 28,222 (1.12) | 2,040 (0.90) | 3,876 (1.41) | 5.21 (4.58‐5.85)|| |
| Cardiovascular disease | 80,080 (3.20) | 6,573 (2.92) | 9,802 (3.59) | 2.27 (1.66‐2.89)|| |
| Lung disease | 21,116 (0.83) | 1,608 (0.71) | 2,647 (0.94) | 3.10 (2.65‐3.56)|| |
| Renal disease | 36,726 (1.46) | 3,219 (1.42) | 4,169 (1.52) | 1.19 (−1.00‐3.43) |
| Neurological disorder | 8,613 (0.34) | 704 (0.31) | 1,156 (0.42) | 3.56 (−0.04‐7.29) |
| Non‐liver cancer | 8,414 (0.33) | 703 (0.31) | 1,003 (0.36) | 1.46 (0.75‐2.17)|| |
| Dementia | 5,035 (0.21) | 390 (0.18) | 667 (0.25) | 4.26 (2.94‐5.61)|| |
| Thyroid | 2,327 (0.09) | 207 (0.09) | 332 (0.12) | 2.78 (0.14‐5.50)|| |
| Obstructive sleep apnea | 1,315 (0.05) | 44 (0.02) | 211 (0.07) | 16.78 (6.66‐27.86)|| |
| Depression | 1,033 (0.04) | 65 (0.03) | 137 (0.05) | 5.55 (2.27‐8.93)|| |
| Osteoporosis | 461 (0.02) | 39 (0.02) | 46 (0.02) | −3.71 (−7.29‐0.00) |
AAPC is a weighted average of the APCs (a maximum number of 2 joinpoints was allowed).
Age‐standardized death rates were averaged over the period 2007‐2016.
Defined by using underlying cause of death.
Defined by using contributory causes of death; because a decedent can have multiple contributory causes of death, a decedent could be counted multiple times.
Significantly different from 0 (P < 0.05).
Table 3.
Mortality Ratios Among Decedents With NAFLD, United States, 2007‐2016
| Mortality Ratio* (95% CI) | |||||
|---|---|---|---|---|---|
| Male to Female | Non‐Hispanic Black to Non‐Hispanic White | Hispanicto Non‐Hispanic White | Non‐Hispanic American Indian or Alaska Native to Non‐Hispanic White | Non‐Hispanic Asian to Non‐Hispanic White | |
| Cause‐specific death† | |||||
| Cardiovascular disease | 1.34 (1.31‐1.36) | 1.23 (1.19‐1.26) | 0.91 (0.88‐0.94) | 0.87 (0.79‐0.95) | 1.21 (1.14‐1.29) |
| Cirrhosis | 0.95 (0.94‐0.96) | 0.89 (0.88‐0.90) | 0.99 (0.98‐1.00) | 0.90 (0.87‐0.93) | 0.83 (0.81‐0.86) |
| Diabetes | 0.83 (0.80‐0.87) | 1.11 (1.03‐1.20) | 1.40 (1.32‐1.48) | 1.76 (1.52‐2.04) | 1.15 (0.99‐1.33) |
| Non‐liver cancer | 1.11 (1.08‐1.14) | 0.96 (0.92‐1.01) | 0.87 (0.83‐0.91) | 0.80 (0.69‐0.91) | 1.11 (1.02‐1.22) |
| HCC | 2.39 (2.28‐2.50) | 1.39 (1.30‐1.47) | 1.67 (1.59‐1.76) | 1.39 (1.19‐1.63) | 2.53 (2.31‐2.77) |
| Lung disease | 1.12 (1.06‐1.18) | 0.70 (0.63‐0.77) | 0.41 (0.36‐0.45) | 0.71 (0.54‐0.92) | 0.42 (0.32‐0.54) |
| Contributory causes of death‡ | |||||
| Diabetes | 0.84 (0.83‐0.86) | 0.85 (0.82‐0.89) | 1.34 (1.30‐1.38) | 1.34 (1.24‐1.46) | 1.20 (1.12‐1.29) |
| Hypertension | 0.96 (0.94‐0.99) | 1.39 (1.34‐1.44) | 1.23 (1.19‐1.27) | 1.09 (0.99‐1.20) | 1.23 (1.15‐1.32) |
| Cardiovascular disease | 1.08 (1.06‐1.09) | 1.09 (1.07‐1.12) | 1.16 (1.14‐1.18) | 1.08 (1.03‐1.14) | 1.22 (1.17‐1.26) |
| Lung disease | 1.04 (1.01‐1.07) | 0.77 (0.73‐0.81) | 0.52 (0.49‐0.54) | 0.72 (0.63‐0.82) | 0.61 (0.54‐0.68) |
| Renal disease | 1.05 (1.03‐1.07) | 1.52 (1.48‐1.57) | 1.29 (1.26‐1.33) | 1.33 (1.23‐1.44) | 1.30 (1.22‐1.38) |
| Neurological disorder | 0.90 (0.86‐0.94) | 1.19 (1.11‐1.27) | 1.03 (0.97‐1.10) | 1.29 (1.11‐1.51) | 0.97 (0.83‐1.13) |
| Non‐liver cancer | 1.06 (1.01‐1.10) | 0.97 (0.89‐1.05) | 0.83 (0.77‐0.89) | 0.77 (0.61‐0.96) | 0.95 (0.82‐1.10) |
| Dementia | 0.75 (0.71‐0.79) | 1.46 (1.32‐1.61) | 1.02 (0.94‐1.11) | 1.18 (0.90‐1.55) | 0.82 (0.68‐0.99) |
| Thyroid | 0.40 (0.36‐0.43) | 0.61 (0.51‐0.74) | 0.94 (0.83‐1.06) | 0.94 (0.66‐1.35) | 0.96 (0.73‐1.25) |
| Obstructive sleep disorder | 1.04 (0.93‐1.17) | 0.52 (0.41‐0.66) | 0.60 (0.50‐0.73) | 0.57 (0.33‐0.99) | 0.74 (0.48‐1.13) |
| Depression | 0.52 (0.46‐0.58) | 0.47 (0.35‐0.62) | 0.65 (0.53‐0.79) | 0.43 (0.21‐0.85) | 0.73 (0.45‐1.18) |
| Osteoporosis | 0.19 (0.15‐0.24) | 0.29 (0.16‐0.55) | 0.53 (0.38‐0.75) | 1.00 (0.45‐2.24) | 0.86 (0.48‐1.52) |
Robust Poisson models were used while adjusting for age, sex, and race.
Defined by using underlying cause of death.
Defined by using contributory causes of death; because a decedent can have multiple contributory causes of death, a decedent could be counted multiple times.
The top six underlying causes (155,894 cirrhosis, 38,444 CVD, 19,466 non‐liver cancer, 10,867 HCC, 8,113 DM, and 5,683 lung disease) accounted for 67.5% of the deaths among adults with NAFLD. CVD (n = 80,080) was the largest contributory cause of death, followed by renal disease (n = 36,726), DM (n = 31,132), and HTN (n = 28,222) (Table 4; Supporting Table S2).
Table 4.
Age‐Standardized Death Rates for NAFLD Among U.S. Hispanic Adults Aged ≥20 Years by Age, Sex, and Ethnicity, United States, 2007‐2016
| All‐Cause Deaths (Rate per 100,000) | AAPC* (95% CI) | |||
|---|---|---|---|---|
| 2007‐2016† | 2007 | 2016 | ||
| Total | 46,530 (20.65) | 3,546 (19.7) | 5,668 (20.81) | 0.74 (0.20 ‐1.22)§ |
| Age group, years‡ | ||||
| 20‐44 | 3,717 (0.94) | 374 (1.03) | 392 (0.93) | −1.35 (−2.69‐0.02) |
| 45‐54 | 9,033 (15.74) | 805 (16.89) | 873 (13.06) | −2.28 (−3.93 to −0.60)§ |
| 55‐64 | 12,512 (34.93) | 831 (30.69) | 1,565 (35.01) | 1.14 (−0.49‐2.81) |
| 65‐74 | 10,838 (57.89) | 768 (53.64) | 1,419 (59.09) | 1.33 (0.43‐2.25)§ |
| ≥75 | 10,430 (82.31) | 768 (77.49) | 1,419 (90.53) | 1.80 (0.79‐2.82)§ |
| Sex | ||||
| Female | 19,030 (16.4) | 1,356 (14.89) | 2,477 (17.52) | 1.81 (1.03‐2.60)§ |
| Male | 27,500 (25.25) | 2,190 (25.04) | 3,191 (24.29) | −0.04 (−0.73‐0.64) |
| Race | ||||
| Mexican | 3,0242 (25.61) | 2,269 (23.38) | 3,664 (26.92) | 1.75 (1.14‐2.37)§ |
| Puerto Rican | 5,565 (23.36) | 449 (21.65) | 621 (23.23) | 0.62 (−0.37‐1.62) |
| Cuban | 1,993 (11.84) | 173 (11.60) | 222 (11.81) | −0.38 (−2.01‐1.29) |
AAPC is a weighted average of the APCs (a maximum number of 2 joinpoints was allowed). Age standardization is based on the direct method to the Census 2000 population by 10‐year age groups. NAFLD‐related deaths are defined by ICD‐10 codes using underlying or contributing cause of death certificates.
Age‐standardized death rates were averaged over the period 2007‐2016.
Age‐specific death rates.
Significantly different from 0 (P < 0.05).
Time Trends in ASDR Between 2007 and 2016
Between 2007 and 2016, the ASDR for NAFLD increased by 15%, from 12.94 to 14.90 (AAPC, 1.98%; P < 0.001), with women experiencing the most significant annual increase (AAPC, 2.99% in women vs. 1.16% in men; P = 0.003) (Fig. 2A). The increase in the ASDR (per 100,000) for adults with NAFLD was most pronounced in those aged ≥75 years (AAPC, 2.78%; 95% CI, 1.60%‐3.96%), followed by those aged 65‐74 years (AAPC, 2.67%; 95% CI, 1.47%‐3.89%) and those aged 55‐64 years (AAPC, 2.57%; 95% CI, 2.11%‐3.04%). On the other hand, the death rate decreased for those aged 45‐54 years (AAPC, −1.21%; 95% CI, −1.97% to −0.45%) and remained stable for those aged 20‐44 years. By ethnicity, the highest increase in the ASDR for NAFLD was seen in non‐Hispanic whites (AAPC, 2.48%; 95% CI, 2.07%‐2.88%), followed by non‐Hispanic AIANs (AAPC, 2.31%; 95% CI, 1.24%‐3.38%) and Hispanics (AAPC, 0.74%; 95% CI, 0.26%‐1.22%), whereas the ASDR remained stable in non‐Hispanic blacks and non‐Hispanic APIs (Fig. 2B). However, there was substantial heterogeneity in the ASDR for NAFLD among the Hispanic and non‐Hispanic API subgroups. The pattern of change for ASDR between 2007 and 2016 shows a substantial increase among Mexicans (AAPC, 1.8%; 95% CI, 1.1%‐2.4%) and Asian Indians (AAPC, 6.9%; 95% CI, 4.7%‐9.3%), whereas the ASDR remained stable among other subgroups (Tables 2 and 3; Fig. 3).
Figure 2.
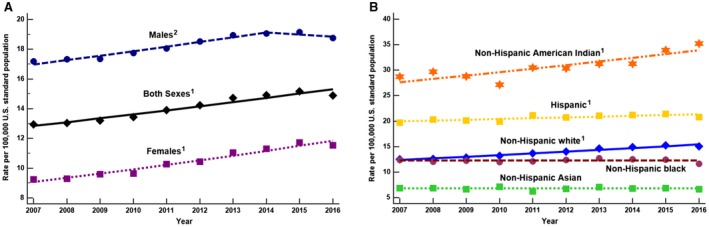
Age‐standardized death rates for NAFLD, United States, 2007‐2016. (A) By sex; (B) by race/ethnicity. 1Significant increasing trend from 2007 to 2016; 2significant increasing trend from 2007 to 2014 and stable trend from 2014 to 2016. Data markers denote observed rates; lines are fitted based on joinpoint analysis. Age standardization is based on the direct method to the Census 2000 population by 10‐year age groups. (From the National Center for Health Statistics, 2007‐2016.)
Figure 3.
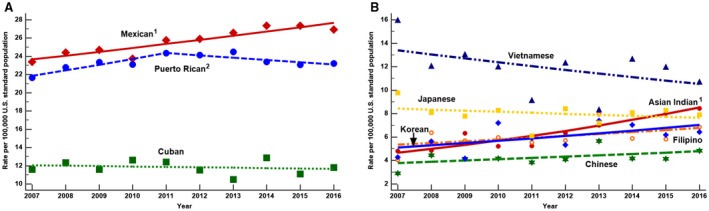
Age‐standardized death rates for NAFLD, United States, 2007‐2016. (A) Hispanic subgroup; (B) Asian American subgroup. 1Significant increasing trend from 2007 to 2016; 2significant increasing trend from 2007 to 2011 and stable trend from 2011 to 2016. Data markers denote observed rates; lines are fitted based on joinpoint analysis. Age standardization is based on the direct method to the Census 2000 population by 10‐year age groups. NAFLD‐related deaths are defined by ICD‐10 codes, using underlying or contributing cause of death noted on the death certificates. Because of small sample size and livability, an increasing or decreasing trend among Asian American subgroup are not statistically significant but among Asian Indians. See Table 5 AAPC column.
For cause‐specific deaths among adults with NAFLD, ASDRs increased in 2016 from 2007 for HCC (AAPC, 3.82%; 95% CI, 3.20%‐4.44%), DM (AAPC, 2.23%; 95% CI, 0.01%‐4.49%), non‐liver cancer (AAPC, 2.14%; 95% CI, 0.25%‐4.06%), CVD (AAPC, 1.59%; 95% CI, 0.85%‐2.34%), and cirrhosis (AAPC, 0.96%; 95% CI, 0.18%‐1.74%). Among the top six contributory causes of death, the ASDR increased for four: CVD (AAPC, 2.27%), DM (AAPC, 2.80%), HTN (AAPC, 5.21%), and lung disease (AAPC, 3.10%) (all P < 0.001). The remaining two contributory causes of death (renal disease and neurological disorders) remained stable. In a sensitivity test, we evaluated trends in ASDR for decedents coded for NAFLD/NASH compared to those coded with cryptogenic liver disease/cirrhosis to demonstrate homogeneity of our NAFLD group. The ASDR trends for death significantly increased for both groups (Supporting Table S3).
Sex and Race/Ethnicity Differences
The age–sex–race‐adjusted mortality ratios for cause‐specific deaths among decedents with NAFLD are described in Table 5. Among decedents with NAFLD, there were 139% more deaths due to HCC in men than in women, whereas women experienced 5% more deaths due to cirrhosis than men (PR, 0.95; 95% CI, 0.80%‐0.96%).
Table 5.
Age‐Standardized Death Rates for NAFLD Among U.S. Non‐Hispanic Asian or Pacific Islander Adults Aged ≥20 Years by Age, Sex, and Ethnicity, United States, 2007‐2016
| All‐Cause Deaths (Rate per 100,000) | AAPC* (95% CI) | |||
|---|---|---|---|---|
| 2007‐2016† | 2007 | 2016 | ||
| Total | 7,023 (6.77) | 564 (6.81) | 865 (6.66) | −0.02 (−0.98‐0.95) |
| Age group, years‡ | ||||
| 20‐44 | 470 (0.42) | 47 (0.46) | 45 (0.36) | −2.70 (−6.29‐1.02) |
| 45‐54 | 854 (3.73) | 97 (4.78) | 80 (3.05) | −5.34 (−7.97 to −2.64)§ |
| 55‐64 | 1,439 (8.1) | 111 (7.95) | 187 (8.93) | 1.36 (−0.36‐3.11) |
| 65‐74 | 1,721 (16.83) | 127 (16.65) | 228 (16.93) | 0.88 (−1.93‐3.77) |
| ≥75 | 2,539 (37.39) | 182 (35.54) | 325 (36.73) | 0.62 (−0.65‐1.90) |
| Sex | ||||
| Female | 3,130 (5.59) | 250 (5.73) | 392 (5.5) | −0.19 (−1.46‐1.10) |
| Male | 3,893 (8.17) | 314 (8.04) | 473 (7.99) | 0.18 (−2.01‐2.41) |
| Race | ||||
| Asian Indian | 159 (6.44) | 12 (4.82) | 23 (8.44) | 6.94 (4.6 ‐9.27)§ |
| Chinese | 167 (4.25) | 12 (2.92) | 21 (4.84) | 2.66 (−1.25‐6.73) |
| Filipino | 264 (5.99) | 20 (4.08) | 42 (6.85) | 2.67 (−1.24‐6.74) |
| Korean | 84 (5.96) | 7 (4.28) | 9 (6.45) | 3.60 (−0.74‐8.13) |
| Japanese | 155 (7.99) | 14 (9.76) | 22 (7.9) | −1.09 (−3.86‐1.77) |
| Vietnamese | 99 (11.85) | 7 (16) | 15 (10.74) | −2.64 (−6.50‐1.38) |
AAPC is a weighted average of the APCs (a maximum number of 2 joinpoints was allowed). Age standardization is based on the direct method to the Census 2000 population by 10‐year age groups. NAFLD‐related deaths are defined by ICD‐10 codes using underlying or contributing cause of death certificates.
Age‐standardized death rates were averaged over the period 2007‐2016.
Age‐specific death rates.
Significantly different from 0 (P < 0.05).
Compared to non‐Hispanic whites, every ethnicity experienced more deaths due to HCC, with non‐Hispanic Asians experiencing the highest ratio of 2.53 (PR, 2.53; 95% CI, 2.31‐2.77). On the other hand, non‐Hispanic whites experienced more deaths due to cirrhosis and lung disease than all other ethnicities. Non‐Hispanic AIANs experienced 76% more deaths due to DM (PR, 1.76; 95% CI, 1.52‐2.04), which is similar for Hispanics, who had 40% more deaths due to DM (PR, 1.40; 95% CI, 1.32‐1.48) compared to non‐Hispanic whites. Non‐Hispanic blacks and non‐Hispanic APIs were more likely to die due to CVD (PR, 1.23; 95% CI, 1.19‐1.26 and PR, 1.21; 95% CI, 1.14‐1.29, respectively) than non‐Hispanic whites. In contrast, the number of deaths due to CVD was lower among Hispanics (PR, 0.91; 95% CI, 0.88‐0.94) and non‐Hispanic AIANs (PR, 0.87; 95% CI, 0.79‐0.95) than non‐Hispanic whites (Table 5).
Among the contributory causes of death, men experienced more deaths from CVD, lung disease, renal disease, non‐liver cancers, and obstructive sleep disorder but fewer deaths from DM, HTN, neurological disorders, dementia, thyroid, depression, and osteoporosis compared to women (Table 5). By ethnicity, non‐Hispanic blacks incurred more deaths from HTN, renal disease, neurological disorders, and dementia compared to non‐Hispanic whites, whereas Hispanics, non‐Hispanic AIANs, and non‐Hispanic APIs incurred more deaths from DM, HTN, renal disease, and CVD compared to non‐Hispanic whites (Table 5). However, overall, CVD, renal disease, and DM were the top three contributory causes of death (Supporting Table S3).
Discussion
In this study, we categorized deaths among adults with NAFLD or NASH in the United States, using data from the NVSS multiple‐cause of death files. Using these data, we found that NAFLD‐related deaths in the United States significantly increased, with an average of almost 2% a year, from 2007 to 2016 (15% overall). The majority of all‐cause deaths occurred in those aged ≥55 years (the highest ASDR was in those aged ≥75 years), non‐Hispanic whites (the highest ASDR was for non‐Hispanic AIANs), and men. Interestingly, although men accounted for the largest percentage of deaths, they only experienced a significant increasing trend from 2007 to 2014, followed by a steady trend from 2014 to 2016. In addition, non‐Hispanic AIANs, non‐Hispanic whites, and Hispanics experienced the most significant increases in deaths from 2007 to 2016, whereas the death rates for all other ethnicities remained stable.
Cirrhosis was the leading cause of death among adults with NAFLD (2007‐2016), with a significant increasing trend. CVD remained the second leading specific cause of death but was fifth among the six leading causes of death for an increase in its trend. CVD was the main contributing cause of death, followed by DM and HTN; however, HTN had the most significant increasing trend. Combined, these findings point to a potential change in the main cause of death between 2007 and 2016. Earlier studies reported that the major cause of death among adults with NAFLD was CVD, but in this study, the major cause was liver related.29, 30 However, further research is warranted to corroborate our findings because we realize that our definition of NAFLD may have captured patients with cirrhosis as a result of other liver diseases rather than NAFLD, even though we tried to exclude all those with other known causes of liver disease. In addition, the difference in our findings may also be a result of how we obtained the cause of death. For this study, we took the underlying cause of death from the death certificate as the cause‐specific death, and all other categories on the death certificate used were considered contributory causes of death.
Of note, HCC among adults with NAFLD was the fourth leading cause of death; however, HCC was found to have experienced the most significant increasing trend over the study time from 2007 through 2016. In fact, men and all ethnicities when compared to non‐Hispanic whites experienced a higher ratio of deaths attributed to HCC; this was especially the case among non‐Hispanic Asians. These findings are important, first, because they confirm findings from recent studies that the stage of fibrosis is the independent predictor for death among those with NAFLD/NASH.29, 30 Second, these findings further explain why NAFLD is now the second most common reason for liver transplant and that the rate of listing for NASH‐related HCC is the highest in the United States.31, 32, 33, 34, 35 Third, patients with NASH‐related HCC have a higher rate of mortality following their HCC diagnosis,36, 37, 38, 39 which portends the necessity of developing screening and surveillance guidelines dedicated to patients with NAFLD and fibrosis in hopes of reversing this dismal trend. However, we recognize that it is plausible that the current screening modalities for HCC fail to detect small‐tumor HCC due to the presence of central obesity in many patients with NASH. As a result, patients with NASH with HCC may present with more invasive and complicated tumors, which are not conducive to treatment or liver transplant. Furthermore, we recognize that, due to the presence of many other metabolic diseases associated with NAFLD, patients may be too sick for transplant and/or die from CVD or their liver disease while awaiting transplant.39 Yet, these reasons are also why earlier screening and surveillance for cirrhosis and HCC may need to be advocated for, especially as newer technology and serum biomarkers with better precision are being investigated.12
A somewhat surprising finding was the significant increasing trend of the underlying cause of death as a result of lung disease, especially in non‐Hispanic whites, which is the group that comprises the largest number of deaths among adults with NAFLD. Lung disease incurred the second highest average APC as the underlying cause of death among adults with NAFLD, increasing 2% a year from 2007 to 2016. In fact, studies are now beginning to investigate the possible relationship between lung disease and NAFLD. One such study among a Korean population found that those with NAFLD were 33% more likely to have obstructive lung disease (OLD) and those with OLD were 55% more likely to have NAFLD after adjusting for age, sex, and smoking history.40 Another study found similar results that, despite the presence or nonpresence of smoking, the incidence of NAFLD in those with OLD increased.41 A recent study conducted in the United States also found that those with moderate or severe hepatic steatosis were 65% more likely to have a restrictive lung disease than those without steatosis. However, caution is needed in interpreting these findings because there are many environmental issues that may affect lung function. Nonetheless, combined with our results, further investigation is warranted into the link between NAFLD and lung disease; some have suggested that decreased lung function is related to an increase in low‐grade inflammation associated with DM, CVD, and metabolic syndrome (all components also associated with NAFLD), suggesting that there is a pathobiological pathway between all disease entities.42
Our analysis found that across all ethnicities, CVD was the number one contributory cause of death among patients with NAFLD.43, 44, 45 Among the top six contributory causes (CVD, DM, HTN, renal disease, lung disease, and neurological disorders), however, HTN had the highest AAPC (5.21%). This is an interesting finding and may help to decipher which components of metabolic syndrome have the most impact on NAFLD‐related mortality and suggests that further study is needed, especially the impact of treating HTN and outcomes in patients with NAFLD.45 In addition, many of the contributory causes of death are components of metabolic syndrome, which helps to confirm our prior work that reported an association between higher mortality and an increasing number of components of metabolic syndrome.46
Also of importance is that the death rate for adults with NAFLD is increasing significantly more in women than men.3 Such a finding confirms another report that also found the mortality rate is increasing among women with NAFLD.47 However, our study furthers this knowledge and notes that women encounter more deaths from cirrhosis when compared with men, suggestive that many women may have NASH rather than just NAFLD.47, 48, 49 Furthermore, our work highlights that there is possibly a sex bias when diagnosing NAFLD and that attention should be directed toward educating the public and health care workers that women are at risk for NAFLD and its negative outcomes, including patient‐reported outcomes and NAFLD‐related mortality.
Finally, the results of our analysis for Hispanics and Asians represent another finding that may provide further insight into awareness of patients who may be at the highest risk for NAFLD‐related mortality. Among Hispanics, Mexicans followed closely by Puerto Ricans accounted for the group with the most deaths among Hispanic adults with NAFLD. However, those aged ≥65 years, women, and Mexicans experienced a significantly increased mortality trend over the study period.49 Therefore, public health outreach to educate these groups on their risk of this chronic liver disease as well as educating health care providers who deliver care to these populations must continue and may need to be further expanded to include a focus on these Hispanic subgroups.
Among non‐Hispanic Asians with NAFLD, we found Vietnamese had the highest ASDR followed by Japanese. However, Asian Indians experienced a significant increase in their death rate trend, whereas overall, Asians aged 45‐54 years experienced a decreasing trend in their death rate.
We acknowledge limitations to this study. First, this study was conducted with a national database abstracted from death certification, which may not be accurate.50 To our knowledge, the concordance between the underlying cause of death from death certificates and the leading cause of death among subjects with NAFLD had not been studied. Regardless of this inherent limitation of the NVSS database, this study provided us with the ability to understand nationally representative trends in mortality among NAFLD. Second, we restricted our analysis to NAFLD listed as the ICD‐10 underlying or contributory cause of death codes on the death certification; therefore, true mortality rates for NAFLD might be underestimated. Because the coding of the underlying or contributory cause of death was not changed during the study period, we assumed something of a systematic underestimation of NAFLD mortality constant over time. Third, we relied on ICD codes to identify NAFLD and other diseases, but they can be miscoded, leading to either an undercoding or overcoding of the data we used. However, given the possibility for these coding errors in either direction, we feel that over time the miscoding balanced out so that there was neither a net gain nor a loss of data. Also, our extended definition of NAFLD including the ICD codes for cryptogenic cirrhosis may have led to an overestimation. However, NAFLD defined as the ICD‐10 codes of K76.0 and K75.81 certainly underestimates the true prevalence of NAFLD. Our sensitivity analyses showed that trends in all‐cause mortality due to NAFLD/NASH only and cryptogenic liver disease and cirrhosis both experienced a similar increasing trend. Therefore, we believe that our estimates of NAFLD in the United States reflect the true burden of this liver disease.
In summary, NAFLD‐related deaths are increasing in the United States, especially among men, those aged ≥55 years, and non‐Hispanic whites and Hispanics. However, attention must be drawn to the increasing rate of NAFLD‐related deaths among women, non‐Hispanic AIANs, Mexicans, and Asian Indians. Cirrhosis accounted for the majority of deaths, but CVD, DM, HCC, renal disease, lung disease, and HTN were all associated with the increase in deaths among adults with NAFLD, with HCC having the most significant increase in the trend for cause of death. Research efforts must continue to determine the best education, diagnostic, and treatment interventions as well as screening and surveillance guidelines in order to reverse this disease trajectory in addition to continuing endeavors investigating the mortality trends among adults with NAFLD.
Supporting information
Supported in part by the Beatty Research Fund, Gilead Sciences, and the Center for Outcomes Research in Liver Diseases.
Potential conflict of interest: Dr. Younossi has received research funds from or served as a consultant to Gilead Sciences, Intercept, Novo Nordisk, BMS, AbbVie, Bristol‐Myers Squibb , Terns, and Viking. The other authors have nothing to report.
References
- 1. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157‐163. [DOI] [PubMed] [Google Scholar]
- 2. World Health Assembly . Obesity and Overweight. http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Published February 16, 2018. Accessed February 2019.
- 3. Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non‐alcoholic fatty liver disease. Metabolism 2016;65:1017‐1025. [DOI] [PubMed] [Google Scholar]
- 4. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stepanova M, De Avila L, Afendy M, Younossi I, Pham H, Cable R, et al. Direct and indirect economic burden of chronic liver disease in the United States. Clin Gastroenterol Hepatol 2017;15:759‐766.e5. [DOI] [PubMed] [Google Scholar]
- 6. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577‐1586. [DOI] [PubMed] [Google Scholar]
- 7. Allen AM, VanHouten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real‐world data from a large U.S. claims database. Hepatology 2018;68:2230‐2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Younossi ZM, Tampi R, Priyadarshini M, Nader F, Younossi IM, Racila A. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology 2019;69:564‐572. [DOI] [PubMed] [Google Scholar]
- 9. Younossi ZM. Patient‐reported outcomes and the economic effects of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis: the value proposition. Hepatology 2018;68:2405‐2412. [DOI] [PubMed] [Google Scholar]
- 10. Golabi P, Otgonsuren M, Cable R, Felix S, Koenig A, Sayiner M, et al. Non‐alcoholic fatty liver disease (NAFLD) is associated with impairment of health related quality of life (HRQOL). Health Qual Life Outcomes 2016;14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dan AA, Kallman JB, Wheeler A, Younoszai Z, Collantes R, Bondini S, et al. Health‐related quality of life in patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2007;26:815‐820. [DOI] [PubMed] [Google Scholar]
- 12. Younossi ZM, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander‐Tetri BA, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2018;68:361‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 14. Ofosu A, Ramai D, Reddy M. Non‐alcoholic fatty liver disease: controlling an emerging epidemic, challenges, and future directions. Ann Gastroenterol 2018;31:288‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention, National Center for Health Statistics . National vital statistics system. https://www.cdc.gov/nchs/nvss/index.htm. Published August 2018. Accessed December 26, 2018.
- 16. Centers for Disease Control and Prevention, National Center for Health Statistics . Mortality data. https://www.cdc.gov/nchs/nvss/deaths.htm. Published August 2018. Accessed December 26, 2018.
- 17. Centers for Disease Control and Prevention, National Center for Health Statistics . Datasets and related documentation. https://www.cdc.gov/nchs/nvss/mortality_methods.htm. Published August 2018. Accessed December 26, 2018.
- 18. Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med 2012;156:271‐278. [DOI] [PubMed] [Google Scholar]
- 19. Peng TC, Kao TW, Wu LW, Chen YJ, Chang YW, Wang CC, et al. Association between pulmonary function and nonalcoholic fatty liver disease in the NHANES III study. Medicine (Baltimore) 2015;94:e907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 2014;59:1174‐1197. [DOI] [PubMed] [Google Scholar]
- 21. Royall RM. Model robust confidence intervals using maximum likelihood estimators. Int Stat Rev 1986;54:221‐226. [Google Scholar]
- 22. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702‐706. [DOI] [PubMed] [Google Scholar]
- 23. Barnes JS, Bennett CE. The Asian population: 2000. Census 2000 brief. Washington, DC: U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau; https://www2.census.gov/library/publications/decennial/2000/briefs/c2kbr01-16.pdf. Published February 2002. Accessed August 8, 2019. [Google Scholar]
- 24. Guzman B. The Hispanic population. Census 2000 brief. Washington, DC: U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau; https://www.census.gov/prod/2001pubs/c2kbr01-3.pdf. Published May 2001. Accessed August 8, 2019. [Google Scholar]
- 25. Hoeffel EM, Rastogi S, Kim MO, Shahid H. The Asian population: 2010. 2010 Census briefs. Washington, DC: U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau; https://www.census.gov/prod/cen2010/briefs/c2010br-11.pdf. Published March 2012. Accessed August 8, 2019. [Google Scholar]
- 26. Ennis SR, Ríos‐Vargas M, Albert NG. The Hispanic population: 2010. 2010 Census briefs. Washington, DC: U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau; https://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. Published May 2011. Accessed August 8, 2019. [Google Scholar]
- 27. Kim D, Li AA, Perumpail RB, Cholankeril G, Gonzalez SA, Kim W, et al. Disparate trends in mortality of etiology‐specific chronic liver disease among Hispanic sub‐populations. Clin Gastroenterol Hepatol 2019;17:1607‐1615.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li AA, Kim D, Kim W, Dibba P, Wong K, Cholankeril G, et al. Disparities in mortality for chronic liver disease among Asian subpopulations in the United States from 2007 to 2016. J Viral Hepat 2018;25:1608‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 30. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Younossi ZM. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Liver Transpl 2018. Feb;24:166‐170. [DOI] [PubMed] [Google Scholar]
- 32. Cholankeril G, Wong RJ, Hu M, Perumpail RB, Yoo ER, Puri P, et al. Liver transplantation for nonalcoholic steatohepatitis in the US: temporal trends and outcomes. Dig Dis Sci 2017;62:2915‐2922. [DOI] [PubMed] [Google Scholar]
- 33. Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al.; Global Nonalcoholic Steatohepatitis Council . Non‐alcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17: 748‐755.e3. [DOI] [PubMed] [Google Scholar]
- 34. Tavakoli H, Robinson A, Liu B, Bhuket T, Younossi Z, Saab S, et al. Cirrhosis patients with nonalcoholic steatohepatitis are significantly less likely to receive surveillance for hepatocellular carcinoma. Dig Dis Sci 2017;62:2174‐2181. [DOI] [PubMed] [Google Scholar]
- 35. Young K, Aguilar M, Gish R, Younossi Z, Saab S, Bhuket T, et al. Lower rates of receiving model for end‐stage liver disease exception and longer time to transplant among nonalcoholic steatohepatitis hepatocellular carcinoma. Liver Transpl 2016;22:1356‐1366. [DOI] [PubMed] [Google Scholar]
- 36. Hester D, Golabi P, Paik J, Younossi I, Mishra A, Younossi ZM. Among Medicare patients with hepatocellular carcinoma, non‐alcoholic fatty liver disease is the most common etiology and cause of mortality. J Clin Gastroenterol 2019; 10.1097/MCG.0000000000001172. [DOI] [PubMed] [Google Scholar]
- 37. Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62:1723‐1730. [DOI] [PubMed] [Google Scholar]
- 38. Kim D, Li AA, Perumpail BJ, Gadiparthi C, Kim W, Cholankeril G, et al. Changing trends in etiology‐based and ethnicity‐based annual mortality rates of cirrhosis and hepatocellular carcinoma in the United States. Hepatology 2019;69:1064‐1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Golabi P, Bush H, Stepanova M, Locklear CT, Jacobson IM, Mishra A, et al. Liver transplantation (LT) for cryptogenic cirrhosis (CC) and nonalcoholic steatohepatitis (NASH) cirrhosis: data from the Scientific Registry of Transplant Recipients (SRTR): 1994 to 2016. Medicine (Baltimore) 2018;97:e11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moon SW, Kim SY, Jung JY, Kang YA, Park MS, Kim YS, et al. Relationship between obstructive lung disease and non‐alcoholic fatty liver disease in the Korean population: Korea National Health and Nutrition Examination Survey, 2007‐2010. Int J Chron Obstruct Pulmon Dis 2018;13:2603‐2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Song JU, Jang Y, Lim SY, Ryu S, Song WJ, Byrne C, et al. Decreased lung function is associated with risk of developing non‐alcoholic fatty liver disease: a longitudinal cohort study. PLoS ONE 2019;14:e0208736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Engstrom G, Hedblad B, Nilsson P, Wollmer P, Berglund G, Janzon L. Lung function, insulin resistance and incidence of cardiovascular disease: a longitudinal cohort study. J Intern Med 2003;253:574‐581. [DOI] [PubMed] [Google Scholar]
- 43. Stepanova M, Younossi Z. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol 2012;10:646‐650. [DOI] [PubMed] [Google Scholar]
- 44. Anstee QM, Targher G, Day CP. Progression of NAFLD to DM mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330‐344. [DOI] [PubMed] [Google Scholar]
- 45. Toshikuni N, Tsuchishima M, Fukumura A, Arisawa T, Tsutsumi M. Associations of fatty liver disease with hypertension, diabetes, and dyslipidemia: comparison between alcoholic and nonalcoholic steatohepatitis. Gastroenterol Res Pract 2017;2017:9127847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Golabi P, Otgonsuren M, de Avila L, Sayiner M, Rafiq N, Younossi ZM. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine (Baltimore) 2018;97:e0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arshad T, Golabi P, Paik J, Mishra A, Younossi ZM. Prevalence of nonalcoholic fatty liver disease in the female population. Hepatol Commun 2018;3:74‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Udelsman BV, Corey KE, Lindvall C, Gee DW, Meireles OR, Hutter MM, et al. Risk factors and prevalence of liver disease in review of 2557 routine liver biopsies performed during bariatric surgery. Surg Obes Relat Dis 2019;15:843‐849. [DOI] [PubMed] [Google Scholar]
- 49. Kalia HS, Gaglio PJ. The prevalence and pathobiology of nonalcoholic fatty liver disease in patients of different races or ethnicities. Clin Liver Dis 2016;20:215‐224. [DOI] [PubMed] [Google Scholar]
- 50. German RR, Fink AK, Heron M, Stewart SL, Johnson CJ, Finch JL, et al.; Accuracy of Cancer Mortality Study Group . The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol 2011;35:126‐131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


