Abstract
Background
This study aimed to investigate the effects of dimethyl fumarate (DMF) on thoracic aortic atherosclerosis in the apolipoprotein E (apo-E)-deficient mouse model with streptozotocin (STZ)-induced hyperglycemia, and the signaling pathways involved.
Material/Methods
Eight-week-old ApoE−/− male mice (n=30) were randomly divided into three groups: the Control group (ApoE−/−) (n=10); the diabetic model (STZ) group (n=10); and the DMF-treated (25 mg/kg) diabetic model (DMF+STZ) group (n=10). The area of the thoracic aortic atherosclerosis was determined by histology. Reactive oxygen species (ROS) levels in mouse serum and homogenates of the thoracic aorta were determined by colorimetry. Levels of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase subunit gp91phox were detected by immunological hybridization, and levels of heme oxygenase-1 (HO-1) were measured by enzyme-linked immunosorbent assay (ELISA).
Results
Compared with the Control group, in the STZ group, the area of aortic atherosclerosis was significantly increased, the levels of serum and aortic ROS, HO-1, nuclear factor-κB (NF-κB), intercellular adhesion molecule 1 (ICAM-1), and gp91phox were increased, and nuclear factor erythroid 2-related factor 2 (Nrf2), endothelial nitric oxide synthase (eNOS), and phosphorylated eNOS (p-eNOS) were significantly reduced. Compared with the STZ group, in the DMF+STZ group, the area of aortic atherosclerosis was significantly reduced, the levels of serum and aortic ROS, HO-1, NF-κB, ICAM-1, and gp91phox were significantly reduced, and Nrf2, eNOS, and p-eNOS were significantly increased.
Conclusions
In the apo-E-deficient mouse model with STZ-induced hyperglycemia, DMF reduced the development of atherosclerosis of the thoracic aorta through the nuclear factor erythroid 2-related factor 2/antioxidant response element (Nrf2/ARE) signaling pathway.
MeSH Keywords: Atherosclerosis, Diabetes Mellitus, NF-E2 Transcription Factor
Background
Atherosclerosis and its clinical consequences result in high levels of patient morbidity and mortality, and the incidence of atherosclerosis is significantly higher in patients with diabetes mellitus. Compared with nondiabetic individuals, coronary heart disease (CHD) has been estimated to be four times higher in men and eight times higher in women with diabetes [1]. Diabetes affects the prognosis of patients with CHD, increasing morbidity and rates of hospitalization, and increasing rates of mortality [1]. Among the many factors associated with the etiology of atherosclerosis, oxidative stress and inflammatory responses are two important factors that lead to atherosclerosis in diabetes. Reactive oxygen species (ROS) are produced directly by oxidative stress or indirectly by damage to the physiological functions of macromolecules that include cell proteins, lipids, and nucleic acids.
Reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is the main source of reactive oxygen species (ROS) in vascular endothelial cells and participates in the pathophysiological processes involved in atherogenesis [2,3]. The activation of NADPH oxidase requires the participation of the gp91phox subunit [2,3]. Normally, there is a dynamic balance of ROS production and elimination in the body. However, in diabetic patients, there is a sharp increase in the production of ROS and thus causes vascular endothelial cells injury, B cell dysfunction, insulin resistance, and inflammatory responses including increased expression of nuclear factor kappa-B (NF-κB) and intercellular cell adhesion molecule-1 (ICAM-1) [2,3]. The activation of NADPH oxidase is involved in atherogenesis [2,3]. Therefore, reducing the level of oxidative stress in the body to protect vascular endothelial cells from oxidative damage, inhibiting insulin resistance, and inhibiting inflammatory responses may have roles in the prevention of atherosclerosis associated with diabetes mellitus.
The human body has a complex response to oxidative stress to deal with damage from free radicals and toxic substances when exposed to electrophilic agents or ROS, and the body can activate a series of protective proteins to reduce cell damage [4]. This coordinated response is regulated by the antioxidant response element (ARE), an upstream regulatory sequence that activates gene transcription in cells following exposure to oxidative stress [4]. The transcription factor NF-E2 related factor 2 (Nrf2) also regulates oxidative stress in vivo. In normal conditions, the binding of the Nrf2 protein and its chaperone protein Kelch-like ECH2 associated protein 1 (Keap1) are inhibited [5–8]. When exposed to electrophilic compounds and ROS, upstream signaling pathways such as MAPK, PKC, PI3K, Keapl, and Nrf2 are dissociated [5–8]. Activated Nrf2 enters the cell nucleus and binds to muscle aponeurotic fibrosarcoma protein (Mafp) in the nucleus in the form of a heterodimer, and then binds to ARE to promote the synthesis of peroxidase [5–8]. As the most important endogenous antioxidant stress pathway currently discovered, the Nrf2/ARE pathway has a close relationship with the occurrence and development of atherosclerosis in patients with diabetes [9–12].
Dimethyl fumarate (DMF) (BG-12) is an oral drug that is used in the treatment of multiple sclerosis [13–15]. The mechanisms of action of DMF include the inhibition of NF-κB, the promotion of apoptosis of activated T lymphocytes, the increase in expression of cytokines IL-4 and IL-5, and the activation of Nrf2 to increase the levels of NAD(P)H: quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), and glutathione [13–15]. DMF stimulates the Nrf2/ARE pathway of stellate cells and increases the expression of glutathione, to confer protection for glial cells and their neighboring neurons [16]. However, the role of DMF in atherosclerosis associated with diabetes mellitus requires further study.
Therefore, this study aimed to investigate the effects of DMF on thoracic aortic atherosclerosis in the apolipoprotein E (apo-E)-deficient mouse model with streptozotocin (STZ)-induced hyperglycemia and the role of the Nrf2/ARE pathway.
Material and Methods
Materials and reagents
Streptozotocin (STZ), 2′,7′-dichlorodihydro-fluorescein diacetate (H2-DCFDA), and dihydroethidium (DHE) were purchased from Sigma (St. Louis, MO, USA). The reactive oxygen species (ROS) test kit and the heme oxygenase-1 (HO-1) test kit were purchased from Nanjing Jiancheng Bioengineering Research Institute (Nanjing, China). The small interfering RNA (siRNA) transfection kit was purchased from New England BioLab (Beverley, MA, USA). The protease inhibitor cocktail kit was purchased from Pierce (Rockford, IL, USA). Antibodies to β-actin, gp91phox, nuclear factor erythroid 2-related factor 2 (Nrf2), endothelial nitric oxide synthase (eNOS), phosphorylated eNOS (p-eNOS), nuclear factor-κB (NF-κB), and intercellular adhesion molecule 1 (ICAM-1) were obtained from Abcam (Cambridge, MA, USA).
Animals
Specific pathogen-free (SPF) apolipoprotein E knockout (ApoE−/−) male mice aged 8 weeks and weighing 23±2 g, with normal nutritional and behavioral status, were purchased from the Model Animal Research Center of Nanjing Medical University. This study was approved by the Animal Ethics Committee of Nanjing Medical University Animal Center. All the mice were housed under the same conditions, with a constant temperature of 22±2°C, a constant humidity of 55±5%, a 12-hour light and dark cycle, and free access to food and water. The formula of the high-fat diet included 1% cholesterol, 5% fat, 5% egg yolk powder, and 0.1% sodium cholate. All the diets were provided by Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China).
The apolipoprotein E (apo-E)-deficient and hyperglycemic mouse model
Thirty ApoE−/− mice were fed with the high-fat diet for six weeks and then randomly divided into three groups, two of which were injected intraperitoneally with streptozocin (STZ) (100 mg/kg). STZ was prepared for use with 100 mg of STZ dissolved in 10 mL of citric acid buffer solution (pH 4.4) to a concentration of 10 g/L. Before intraperitoneal injection of STZ, the animals fasted for 12 h, and the injection dose was 0.1 mL/10 g body weight. After injection, the mice could drink water and eat food freely. Three days later, the fasting blood glucose of the mice was measured. Mice with a blood glucose level ≥11.2 mmol/L were used for the subsequent experiments. The laboratory animals were divided into three groups: the Control group (ApoE−/−) (n=10); the diabetic model (STZ) group (n=10); and the DMF-treated (25 mg/kg) diabetic model (DMF+STZ) group (n=10). The drugs were dissolved in distilled water and administered by gavage once a day for seven days. The mice in the non-treated Control group received an equal volume of distilled water by gavage once a day.
Histology
The heart and aorta of the mice in the three study groups were removed under aseptic conditions. The tissue was fixed and then embedded in paraffin wax and sectioned at 4 μm. The tissue sections were dewaxed with xylene and cleared in graded ethanol to water with xylene for 5 min, xylene for 5 min, 100% ethanol for 2 min, 95% ethanol for 1 min, 80% ethanol for 1 min, and 75% ethanol for 1 min. The tissue sections were washed in distilled water for 2 min and then underwent hematoxylin staining for 5 min and rinsed with tap water before staining with eosin and washing again. The tissue sections stained with hematoxylin and eosin (H&E) were dehydrated, mounted with glass coverslips, and observed using a light microscope.
Measurement of reactive oxygen species (ROS) level in serum and thoracic aorta homogenate
Retro-orbital blood was collected from the mice in each group. Blood samples were centrifuged at 3,500 r/min for 10 min. The serum was sampled into Eppendorf (EP) tubes and stored at −80°C with a 5-fold dilution in normal saline. The thoracic aorta (0.5 cm long) was added to 0.5 mL of normal saline and homogenized for 2 min, followed by centrifugation at 500 rpm for 5 min. The supernatant was stored in EP tubes at −80°C. The level of reactive oxygen species (ROS) in the serum and thoracic aorta homogenates were measured with a colorimetric enzyme immunoassay using the Fenton reaction and Griess solution. One unit (U) of ROS was defined as 1 mmol/L decrease in H2O2 concentration after the reaction of every 1 mL of serum or homogenate supernatant at 37°C for 1 min.
Measurement of the activity of the antioxidant enzyme heme oxygenase-1 (HO-1) using immunoassay
Fresh thoracic aorta tissue was prepared from each group with 5% (w/v) tissue homogenates using normal saline and centrifuged at 1,000×g for 15 min. The supernatant was diluted to 1% for further study.
For the immunoassay to detect HO-1, the supernatant containing the tissue homogenate was used to coat a microtiter plate. The antibody to HO-1 was added, followed by a biotinylated anti-mouse HO-1 antibody and horseradish peroxidase (HRP)-labeled streptavidin. The microtiter plate was washed and underwent color development (blue) with the 3,3,5,5′-tetramethylbenzidine (TMB) substrate. 1 mg of protein was added to 1 mL of solution, and the HO-1 amount corresponding to the inhibition rate of HO-1 of 50% was regarded as one superoxide dismutase (SOD) activity unit (U).
Western blot for gp91phox protein expression in mouse thoracic aorta homogenates
The total protein in the thoracic aorta from the mice in each group was extracted. Then, 15 μg of protein extract was separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a membrane, followed by incubation with 5% dried skimmed milk powder in Tris-buffered saline-Tween 20 (TBST) (10 mmol/L Tris·HCl, 150 mmol/L NaCl, and 0.1% Tween, pH 7.2) at room temperature for 30 min. The antibody to gp91phox (1: 500) was incubated on the membrane at 4°C overnight. The membrane was rinsed three times for 5 min with TBST. The horseradish peroxidase (HRP)-labeled secondary antibody (1: 2000) was incubated with the membrane at 37°C for 1.5 h and rinsed three times for 5 min with TBST. The absorbance ratio of gp91phox to the β-actin standard protein was taken as the relative content of gp91phox.
Chemiluminescent Western blot for protein expression in mouse thoracic aorta homogenates
The stored thoracic aorta homogenates were used for chemiluminescent Western blot to measure the expression of gp91phox, Nrf2, eNOS, p-eNOS, NF-κB, and ICAM-1. Frozen homogenates of aortic tissue were added to 0.8 ml of cold lysis buffer per 100 mg of the ground tissue, and the cells were disrupted by ultrasound using an ice bath, centrifuged at 7,000×g for 5 min at 4°C. The protein concentration in the supernatant was measured. Then, 50 μg of total protein underwent SDS-PAGE gel electrophoresis (80 V/120 V voltage), and were transferred to nitrocellulose membranes under 200 mA constant current. The membranes were incubated with 5% dried skimmed milk powder for 2 h. After blocking, the primary rabbit antibodies were added (1: 1000) and incubated overnight at 4°C. The membranes were washed three times with PBST, and the HRP-conjugated goat anti-rabbit secondary antibody (1: 5000) was incubated for 2 h at room temperature. The membranes were washed, and the chemiluminescent scanning results were analyzed by Quantity One analysis software (Bio-Rad, Hercules, CA, USA). The optical density (OD) value of the target band was compared with the corresponding OD value of the internal reference, β-actin, to obtain a ratio that represented the relative content of the target protein.
Transfection of human umbilical vein endothelial cells (HUVECs) with nuclear factor erythroid 2-related factor 2 (Nrf2) small interfering RNA (siRNA)
The synthesized retinoid X receptor α (RXRα) siRNA (sense strand: 5′-AGCCCAGCACAUCCAGUCA-3′, anti-sense strand: 5′-AGCCCAGCACAUCCAGUCA-3′) was diluted with sterile double-distilled water to a final concentration of 50 μmol/L. Then, 2.5 μL of transfection solution A was added into 1.2 mL of Opti-MEM reduced serum media (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and mixed, followed by the addition of 5 μL of solution B, and mixed. Then, 0.6 μL of RXR-alpha siRNA was aspirated into the diluent, mixed, and incubated at room temperature for 20 min.
Before transfection, human umbilical vein endothelial cells (HUVECs) were seeded into six-well plates and cultured in serum-free Opti-MEM until they reached 40–60% confluence. The culture medium was changed, followed by rinsing twice, the transfection mixture was added and mixed. The cells were cultured at 37°C for 2–4 h, and 1 mL of Opti-MEM containing 10% fetal bovine serum (FBS) was added into each well, and the culture medium was replaced with Opti-MEM containing 20% FBS 24 h later. The Nrf2 expression level in transfected cells was detected using Western blot.
Measurement of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase activity by lucigenin chemiluminescence
Cells were washed twice with preheated phosphate-buffered saline (PBS), and 1mL of PBS was added to each well of the six-well plate. The cells were scraped, and a cell suspension was prepared in an EP tube, followed by centrifugation at 4°C and 4000×g for 5 min. After the supernatant was discarded, 100 μL of precooled PBS containing a protease inhibitor cocktail was added into each tube. The cells were frozen and thawed rapidly four times and centrifuged at 4°C and 12000×g for 10 min. The supernatant was taken for protein detection. A light-impermeable 96-well microtiter plate containing PBS with 1 mmol/L of EDTA, 150 mmol/L of sucrose, 5 pmol/L of lucigenin electron acceptor, and 100 μmol/L of NADPH reaction substrate were added to each well. Then, 50 μL of supernatant containing the proteins was added into each well, and a cold light instrument was used for measurement. The photon emission of the samples was measured once every 30 s for 30 min, and the mean value was calculated. The NADPH oxidase activity was expressed as relative light units (RLUs) as counts per min per ng of protein. Each measurement was performed in triplicate using double tubes.
Statistical analysis
Data were expressed as the mean±standard deviation (SD) and analyzed using a paired or unpaired t-test. The t-test was used to analyze the measurement data. Differences between two groups were analyzed using the Student’s t-test. Comparison between multiple groups was performed using one-way analysis of variance (ANOVA) followed by a post hoc least significant difference (LSD) test. Data were analyzed using Statistical Product and Service Solutions (SPSS) version 20.0 software (IBM, Armonk, NY, USA), and data plots were performed using GraphPad software (GraphPad, La Jolla, CA, USA). A P-value <0.05 was considered to be statistically significant.
Results
Dimethyl fumarate (DMF) reduced the formation of streptozotocin (STZ)-induced thoracic aortic atheroma in ApoE−/− mice
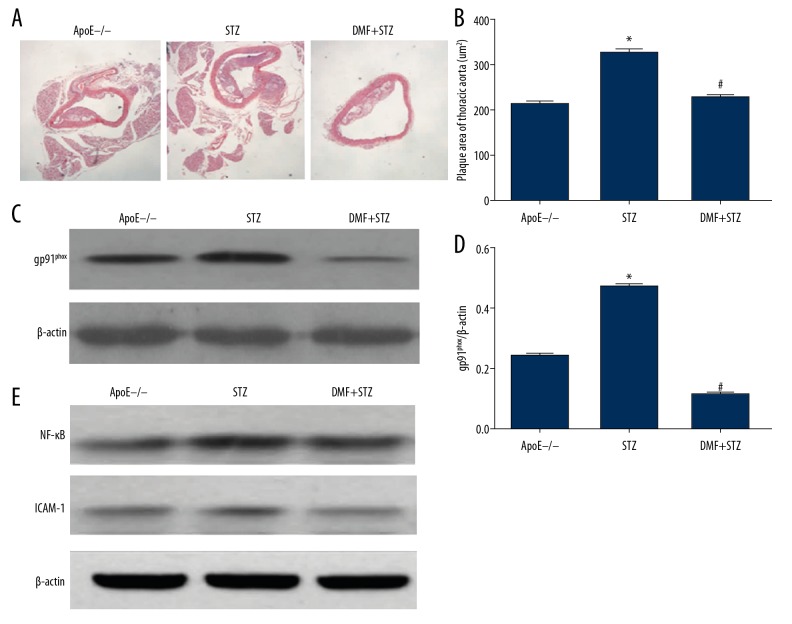
This study included three groups of ApoE−/− mice: the untreated Control group (ApoE−/−), the diabetic model streptozotocin (STZ) group, and the dimethyl fumarate (DMF)-treated diabetic model (DMF+STZ) group. In the STZ group, the histology of the aortic intima was irregular, and large intimal atheromatous plaques were present, with thin fibrous caps and lipid vacuoles within the plaques. The area of the thoracic aortic atheromatous plaque in the STZ group was significantly greater than that in the ApoE−/− group (P<0.01). In the DMF+STZ group, only small fatty plaques were formed in the thoracic aortic intima, and the area of the plaques was small, with a small amount of lipid, and a thick fibrous surface layer. The plaque area was significantly less in the DMF+STZ group compared with the STZ group (P<0.05) (Figure 1A, 1B).
Figure 1.
Comparison of the findings in the three groups of apolipoprotein E-deficient (ApoE−/−) mice, the untreated Control group, the streptozotocin-treated diabetic model (the STZ group), and the dimethyl fumarate-treated diabetic model (the DMF+STZ group). (A) Photomicrograph of the histology of the thoracic aorta in the Control group, the STZ group, and the DMF+STZ group. Hematoxylin and eosin (H&E). Magnification ×40. (B) The plaque area of the thoracic aorta (μm2) in the Control group, the STZ group, and the DMF+STZ group, expressed as the mean±standard deviation (SD). * P<0.05 compared with the Control group. # P<0.05 compared with the STZ group. (C) The gp91phox protein in the thoracic aorta in the Control group, the STZ group, and the DMF+STZ group, detected by Western blot. (D) Quantitative analysis results of gp91phox protein expression, expressed as the mean ± standard deviation (SD). * P<0.05 compared with the Control group. # P<0.05 compared with the STZ group. (E) Nuclear factor-κB (NF-κB) and intercellular adhesion molecule 1 (ICAM-1) protein expression in the thoracic aorta in the Control group, the STZ group, and the DMF+STZ group, detected by Western blot.
DMF reduced the oxidation level and improved endothelial function in the thoracic aorta of ApoE−/− mice with STZ-induced hyperglycemia
Following STZ-induced hyperglycemia, the levels of reactive oxygen species (ROS) in the serum and thoracic aorta and the heme oxygenase-1 (HO-1) level in the thoracic aorta in STZ group were increased compared with the Control ApoE−/− group. Compared with the STZ group, the DMF+STZ group had significantly lower ROS levels in the serum and thoracic aorta and HO-1 levels in the thoracic aorta (P<0.05) (Tables 1, 2).
Table 1.
The levels of reactive oxygen species (ROS) in the serum and thoracic aorta homogenates in the study groups.
| Group | n | Serum (103 U/L) (mean±SD) | Thoracic aorta homogenates 103 U/L) (mean±SD) |
|---|---|---|---|
| ApoE−/− | 9 | 858.69±114.08 | 458.13±41.67 |
| STZ | 8 | 1538.5±380.74 | 918.61±73.85 |
| DMF+STZ | 10 | 828.43±76.31 | 563.19±108.64 |
Three groups of ApoE−/− mice: the untreated Control group (ApoE−/−); the diabetic model streptozotocin (STZ) group; and the dimethyl fumarate (DMF)-treated diabetic model (DMF+STZ) group.
Table 2.
The levels of heme oxygenase-1 (HO-1) in the thoracic aorta homogenates in the study groups.
| Group | n | Thoracic aorta homogenates (U/min·mg) (mean±SD) |
|---|---|---|
| ApoE−/− | 9 | 23.2±1.5 |
| STZ | 8 | 28.4±0.7 |
| DMF+STZ | 10 | 22.9±1.3 |
Three groups of ApoE−/− mice: the untreated Control group (ApoE−/−); the diabetic model streptozotocin (STZ) group; and the dimethyl fumarate (DMF)-treated diabetic model (DMF+STZ) group.
The levels of gp91phox protein, nuclear factor-κB (NF-κB), and intercellular adhesion molecule 1 (ICAM-1) in the thoracic aorta in the STZ group were significantly higher than in the Control ApoE−/− group (P<0.05). DMF significantly reduced the expression of gp91phox, NF-κB, and ICAM-1 protein in the thoracic aorta in the STZ-ApoE−/− mice (P<0.05) (Figure 1C–1E). The DMF+STZ group showed significantly increased expression levels of nuclear factor erythroid 2-related factor 2 (Nrf2), endothelial nitric oxide synthase (eNOS), and phosphorylated eNOS (p-eNOS) in the thoracic aorta compared with the STZ group (P<0.05) (Figure 2A–2D).
Figure 2.
Comparison of the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and nitric oxide synthases (NOS) in the three groups of apolipoprotein E-deficient (ApoE−/−) mice, the untreated Control group, the streptozotocin-treated diabetic model (the STZ group), and the dimethyl fumarate-treated diabetic model (the DMF+STZ group). (A) Nuclear factor erythroid 2-related factor 2 (Nrf2) protein in the thoracic aorta of the Control group, the STZ group, and the DMF+STZ group detected using Western blot. (B) Quantitative analysis of Nrf2 protein expression in the Control group, the STZ group, and the DMF+STZ group, expressed as the mean±standard deviation (SD). # P<0.05 compared with the STZ group. (C) Endothelial nitric oxide synthase (eNOS) and phosphorylated eNOS (p-eNOS) proteins in the thoracic aorta in the Control group, the STZ group, and the DMF+STZ group, detected using Western blot. (D) Quantitative analysis of the p-eNOS and eNOS, expressed as the mean±standard deviation (SD). * P<0.05 compared with the Control group. # P<0.05 compared with the STZ group.
DMF inhibited NADPH oxidase activity and gp91phox protein levels in human umbilical vein endothelial cells (HUVECs) under high glucose (HG) conditions and improved endothelial function
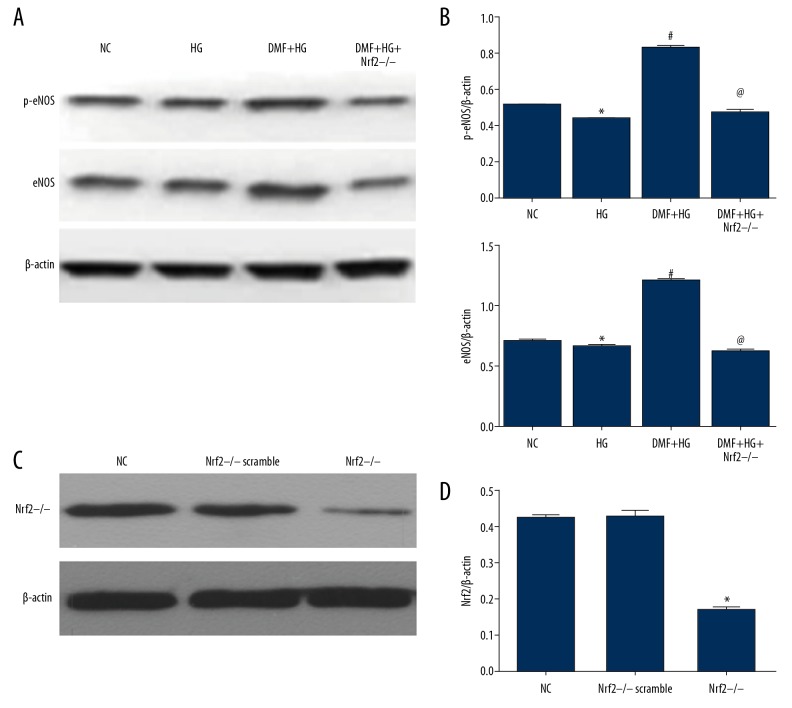
Compared with the Control group, NADPH oxidase activity was significantly increased in the high glucose (HG) group (P<0.05). DMF reduced the increase in the NADPH oxidase activity in HG-induced HUVECs (Figure 3A). Compared with the Control group, the HG group had a significantly increased expression level of the NADPH oxidase subunit gp91phox protein (P<0.05), and DMF inhibited the expression of gp91phox protein in the HG-induced endothelial cells. After treatment with DMF in the HG group, the expression of gp91phox protein significantly declined compared with the control group (P<0.05) (Figure 3B–3C). DMF promoted the protein expression of p-eNOS and eNOS in HUVECs in the HG group (Figure 4A, 4B).
Figure 3.
Comparison of the expression of gp91phox and nuclear factor erythroid 2-related factor 2 (Nrf2) in human umbilical vein endothelial cells (HUVECs) in the normal control (NC) group, the high glucose (HG) group, the dimethyl fumarate and high glucose (DMF+HG) group, and the DMF+HG+Nrf2−/− group. (A) Quantitative results of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase subunit gp91phox activity in HUVECs in the NC group, the HG group, the DMF+HG group, and the DMF+HG+Nrf2−/− group, expressed as the mean ± standard deviation (SD). * P<0.05 compared with the NC group. # P<0.05 compared with the HG group. @ P<0.05 compared with the DMF+HG group. (B) Nuclear factor erythroid 2-related factor 2 (Nrf2) protein expression in HUVECs in the NC group, the HG group, the DMF+HG group, and the DMF+HG+Nrf2−/− group, detected using Western blot. (C) Quantitative analysis of Nrf2 protein expression in the NC group, the HG group, the DMF+HG group, and the DMF+HG+Nrf2−/− group, expressed as the mean ± standard deviation (SD). * P<0.05 compared with the NC group. # P<0.05 compared with the HG group. @ P<0.05 compared with the DMF+HG group.
Figure 4.
Comparison of the expression of nitric oxide synthases (NOS) and nuclear factor erythroid 2-related factor 2 (Nrf2) in human umbilical vein endothelial cells (HUVECs) in the normal control (NC) group, the high glucose (HG) group, the dimethyl fumarate and high glucose (DMF+HG) group, the Nrf2−/− scramble group, and the Nrf2−/− group. (A) Endothelial nitric oxide synthase (eNOS) and phosphorylated eNOS (p-eNOS) protein expression in HUVECs in the NC group, the HG group, the DMF+HG group, and the DMF+HG+Nrf2−/− group, detected using Western blot. (B) Quantitative analysis results of p-eNOS and eNOS protein expression in the NC group, the HG group, the DMF+HG group, and the DMF+HG+Nrf2−/− group, expressed as the mean ± standard deviation (SD). * P<0.05 compared with the NC group. # P<0.05 compared with the HG group. @ P<0.05 compared with the DMF+HG group. (C) Nuclear factor erythroid 2-related factor 2 (Nrf2) protein expression in HUVECs in the NC group, Nrf2−/− scramble group and Nrf2−/− group detected using Western blot. (D) Quantitative analysis of the expression of Nrf2 protein in the NC group, the Nrf2−/− scramble group, and the Nrf2−/− group, expressed as the mean±standard deviation (SD). * P<0.05 compared with the NC group.
DMF exerted antioxidant effects in HUVECs under high glucose (HG) conditions through the Nrf2/ARE signaling pathway
After the HUVECs were transfected with specific Nrf2 small interfering RNA (siRNA), the expression of Nrf2 protein in the HUVECs significantly decreased in both the control group and the HG group (Figure 4C). Also, the inhibitory effect of DMF on NADPH oxidase activity under HG conditions was significantly reduced (P<0.05) (Figure 3A), and the inhibitory effect of DMF on the expression of gp91phox protein in the HG-induced HUVECs was also reduced (P<0.05) (Figure 3B, 3C). After transfection with Nrf2 siRNA, the levels of p-eNOS and eNOS proteins expressed by the HUVECs in the HG group treated with DMF were also reduced (Figure 4A, 4B).
Discussion
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor containing a leucine zipper as the basic structure [17]. The protein has a molecular weight of 66 kDa, and belongs to the Cap’n’collar (Cnc) regulatory protein family, which share a highly conserved leucine zipper structure [17]. After Nrf2 is activated, it dissociates from Keap1, enters the nucleus, and binds to the antioxidant response element (ARE) sequence in the nucleus to initiate transcription of ARE-regulated genes to initiate the expression of antioxidant enzyme genes, protecting the normal function of cells and tissues [18].
Heme oxygenase 1 (HO-1) is the rate-limiting enzyme for heme degradation, catalyzing the production of equimolar amounts of bilirubin, carbon monoxide (CO), and iron [19,20]. HO-1 has antioxidant functions that prevent free heme from involvement in the oxidation reaction and has important anti-oxidation and anti-inflammatory roles, as well as inhibiting apoptosis, and vasodilation, to improve tissue microcirculation and protect the heart, and other major organs from oxidative damage [19,20]. Studies have also shown that increased expression of HO-1 is mediated by the activation of Nrf2 [19,20].
NADPH quinone oxidoreductase (NQO1) is a flavin enzyme that regulates the redox state of intracellular substances [21]. NQO1 uses NADPH as a receptor to catalyze quinones and their derivatives, losing two electrons to undergo a reduction reaction [21]. The catalytic properties of NQO1 ensure that no single-electron reduction products are formed, such as semiquinone and free radicals, and result in a protective effect on metabolically induced oxidative stress reactions [21]. Chromosome immunoprecipitation analysis has confirmed that Nrf2 binds to the ARE sequence of the NQO1 gene and activates the expression of NQO1 proteins [21].
This study aimed to investigate the effects of dimethyl fumarate (DMF) on thoracic aortic atherosclerosis in the apolipoprotein E (apo-E)-deficient mouse model with streptozotocin (STZ)-induced hyperglycemia. Three groups of ApoE−/− mice were investigated, including the untreated Control group (ApoE−/−), the diabetic model streptozotocin (STZ) group, and the DMF-treated diabetic model (DMF+STZ) group. The findings from the present study showed that in STZ-induced diabetic ApoE−/− mice and in human umbilical vein endothelial cells (HUVECs), a high glucose environment promoted the production of reactive oxygen species (ROS), which is a factor involved in the development of acute and chronic clinical complications in diabetes. In oxidative stress-induced reactions, the Nrf2/ARE signaling pathway has been confirmed to be related to diseases that include diabetic nephropathy [22]. Both in vitro and in vivo studies have shown that the activity of Nrf2 can be down-regulated by extracellular signal-regulated kinase (ERK) in diabetes, and inhibition of Nrf2 resulted in increased oxidative stress, which then induced insulin resistance and glucose utilization in the myocardium of patients with diabetes mellitus [22]. This clinical finding was supported by the findings from the present animal model and the in vitro study, as in the STZ-induced diabetic ApoE−/− mouse, the protein expression of Nrf2 in the thoracic aorta was inhibited while the production of gp91phox and ROS was increased. Also, heme oxygenase-1 (HO-1), endothelial nitric oxide synthase (eNOS), and phosphorylated eNOS (p-eNOS) expression in the thoracic aorta of the STZ-induced diabetic ApoE−/− mouse were down-regulated following the inhibition of Nrf2.
In atherosclerosis associated with diabetes mellitus, oxidative stress caused by hyperglycemia leads to vascular endothelial injury, increased production of advanced glycation end products (AGEs), and an increased inflammatory response, which are important factors in the occurrence and development of atherosclerosis. Nrf2 is a key factor that regulates oxidative stress in vivo, and activation of the Nrf2/ARE pathway protects the vascular endothelium from oxidative stress [23], reduces the production of ROS, and reduces the inflammatory responses [24]. Activation of the Nrf2/ARE pathway further protects endothelial cells from oxidative damage, inhibits vascular smooth muscle cell proliferation and migration [25], reduces the development of oxidized low-density lipoprotein-induced foam cells [26], reduce the production of AGEs [27], which are properties that may halt the progression of atherosclerosis.
As a first-line treatment for multiple sclerosis, DMF can inhibit lipid peroxidation, and regulate free radical metabolism [28]. The findings from the present study showed that in STZ-induced hyperglycemic mice with experimental aortic atherosclerosis, DMF increased the expression of Nrf2, reduced aortic oxidation, and improved thoracic aortic endothelial function. With the use of DMF in the STZ-induced diabetic ApoE−/− mouse, the expression of Nrf2 was associated with upregulated expression of HO-1, p-eNOS, and eNOS and down-regulated expression of gp91phox and ROS. In HUVECs cultured in high glucose (HG) conditions, DMF reduced cell oxidation, reduced the expression of the oxidation-related protein gp91phox, and increased the expression of p-eNOS and eNOS. After Nrf2 siRNA knockdown, these effects were reduced, which indicated that DMF has an antioxidant effect on endothelial cells through the Nrf2/ARE pathway. However, whether DMF also protects endothelial function through other mechanisms, such as the control of inflammation, requires further study.
Conclusions
This study aimed to investigate the effects of dimethyl fumarate (DMF) on thoracic aortic atherosclerosis in the apolipoprotein E (apo-E)-deficient mouse model with streptozotocin (STZ)-induced hyperglycemia, and the signaling pathways involved. In this animal model, DMF reduced the development of atherosclerosis through the nuclear factor erythroid 2-related factor 2/antioxidant response element (Nrf2/ARE) signaling pathway. Further studies on the role of DMF are required to determine its potential role as a targeted drug for Nrf2 expression and its potential role in the control of atherosclerosis associated with diabetes mellitus.
Footnotes
Source of support: This study was funded by the School Foundation of Nanjing Medical University (No. 2017NJMU092)
Conflict of interest
None.
References
- 1.Bao SL, Pan J, Sun HX, Liu WT. Valsartan improves cardiac function in mice with diabetes mellitus by CaMKII/AngII. Eur Rev Med Pharmacol Sci. 2018;22:5327–34. doi: 10.26355/eurrev_201808_15733. [DOI] [PubMed] [Google Scholar]
- 2.Amirbekian V, Lipinski MJ, Briley-Saebo KC, et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci USA. 2007;104:961–66. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–97. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talalay P, Dinkova-Kostova AT, Holtzclaw WD. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv Enzyme Regul. 2003;43:121–34. doi: 10.1016/s0065-2571(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 5.Xiao H, Lv F, Xu W, et al. Deprenyl prevents MPP(+)-induced oxidative damage in PC12 cells by the upregulation of Nrf2-mediated NQO1 expression through the activation of PI3K/Akt and Erk. Toxicology. 2011;290:286–94. doi: 10.1016/j.tox.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Park SH, Jang JH, Chen CY, et al. A formulated red ginseng extract rescues PC12 cells from PCB-induced oxidative cell death through Nrf2-mediated upregulation of heme oxygenase-1 and glutamate cysteine ligase. Toxicology. 2010;278:131–39. doi: 10.1016/j.tox.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min KJ, Kim JH, Jou I, Joe EH. Adenosine induces hemeoxygenase-1 expression in microglia through the activation of phosphatidylinositol 3-kinase and nuclear factor E2-related factor 2. Glia. 2008;56:1028–37. doi: 10.1002/glia.20676. [DOI] [PubMed] [Google Scholar]
- 9.Williams K, Sobol RW. Mutation research/fundamental and molecular mechanisms of mutagenesis: special issue: DNA repair and genetic instability. Mutat Res. 2013;743–44:1–3. doi: 10.1016/j.mrfmmm.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayachandran M, Chandrasekaran B, Namasivayam N. Geraniol attenuates oxidative stress by Nrf2 activation in diet-induced experimental atherosclerosis. J Basic Clin Physiol Pharmacol. 2015;26:335–46. doi: 10.1515/jbcpp-2014-0057. [DOI] [PubMed] [Google Scholar]
- 11.Donovan EL, McCord JM, Reuland DJ, et al. Phytochemical activation of Nrf2 protects human coronary artery endothelial cells against an oxidative challenge. Oxid Med Cell Longev. 2012;2012 doi: 10.1155/2012/132931. 132931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HJ, Seo M, Lee EJ. Salvianolic acid B inhibits atherogenesis of vascular cells through induction of Nrf2-dependent heme oxygenase-1. Curr Med Chem. 2014;21:3095–106. doi: 10.2174/0929867321666140601195940. [DOI] [PubMed] [Google Scholar]
- 13.Vandermeeren M, Janssens S, Wouters H, et al. Dimethylfumarate is an inhibitor of cytokine-induced nuclear translocation of NF-kappa B1, but not RelA in normal human dermal fibroblast cells. J Invest Dermatol. 2001;116:124–30. doi: 10.1046/j.1523-1747.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 14.Treumer F, Zhu K, Glaser R, Mrowietz U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol. 2003;121:1383–88. doi: 10.1111/j.1523-1747.2003.12605.x. [DOI] [PubMed] [Google Scholar]
- 15.Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–92. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 16.Lin SX, Lisi L, Dello RC, et al. The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase-1. ASN Neuro. 2011;3:75–84. doi: 10.1042/AN20100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- 18.Kim SK, Yang JW, Kim MR, et al. Increased expression of Nrf2/ARE-dependent anti-oxidant proteins in tamoxifen-resistant breast cancer cells. Free Radic Biol Med. 2008;45:537–46. doi: 10.1016/j.freeradbiomed.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Zhang L, Wang F, et al. Attenuation of glomerular injury in diabetic mice with tert-butylhydroquinone through nuclear factor erythroid 2-related factor 2-dependent antioxidant gene activation. Am J Nephrol. 2011;33:289–97. doi: 10.1159/000324694. [DOI] [PubMed] [Google Scholar]
- 20.Ye Z, Guo Q, Xia P, et al. Sevoflurane postconditioning involves an up-regulation of HIF-1alpha and HO-1 expression via PI3K/Akt pathway in a rat model of focal cerebral ischemia. Brain Res. 2012;1463:63–74. doi: 10.1016/j.brainres.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 21.Kaspar JW, Jaiswal AK. Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. J Biol Chem. 2010;285:153–62. doi: 10.1074/jbc.M109.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan Y, Ichikawa T, Li J, et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60:625–33. doi: 10.2337/db10-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HJ, Seo M, Lee EJ. Salvianolic acid B inhibits atherogenesis of vascular cells through induction of Nrf2-dependent heme oxygenase-1. Curr Med Chem. 2014;21:3095–106. doi: 10.2174/0929867321666140601195940. [DOI] [PubMed] [Google Scholar]
- 24.Lee BH, Hsu WH, Hsu YW, Pan TM. Dimerumic acid attenuates receptor for advanced glycation endproducts signal to inhibit inflammation and diabetes mediated by Nrf2 activation and promotes methylglyoxal metabolism into d-lactic acid. Free Radic Biol Med. 2013;60:7–16. doi: 10.1016/j.freeradbiomed.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 25.Donovan EL, McCord JM, Reuland DJ, et al. Phytochemical activation of Nrf2 protects human coronary artery endothelial cells against an oxidative challenge. Oxid Med Cell Longev. 2012;2012 doi: 10.1155/2012/132931. 132931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazaro I, Ferre R, Masana L, Cabre A. Akt and ERK/Nrf2 activation by PUFA oxidation-derived aldehydes upregulates FABP4 expression in human macrophages. Atherosclerosis. 2013;230:216–22. doi: 10.1016/j.atherosclerosis.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 27.Huang K, Huang J, Xie X, et al. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-beta1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med. 2013;65:528–40. doi: 10.1016/j.freeradbiomed.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Kume T, Suenaga A, Izumi Y, Akaike A. Protective effect of dimethyl fumarate on an oxidative stress model induced by sodium nitroprusside in mice. Biol Pharm Bull. 2016;39:1055–59. doi: 10.1248/bpb.b16-00134. [DOI] [PubMed] [Google Scholar]