Abstract
Aims
The molecular mechanisms by which muraglitazar (peroxisome proliferator-activated receptor γ/α agonist) improves insulin sensitivity in Type 2 diabetes mellitus are not fully understood. We hypothesized that muraglitazar would increase expression of 5′-monophosphate-activated protein kinase and genes involved in adiponectin signalling, free fatty acid oxidation and mitochondrial function in skeletal muscle.
Methods
Sixteen participants with Type 2 diabetes received muraglitazar, 5 mg/day (n = 12) or placebo (n = 4). Before and after 16 weeks, participants had vastus lateralis muscle biopsy followed by 180 min euglycaemic hyperinsulinaemic clamp.
Results
Muraglitazar increased plasma adiponectin (9.0 ± 1.1 to 17.8 ±1.5 μg/ml, P < 0.05), while no significant change was observed with placebo. After 16 weeks with muraglitazar, fasting plasma glucose declined by 31%, fasting plasma insulin decreased by 44%, insulin-stimulated glucose disposal increased by 81%, HbA1c decreased by 21% and plasma triglyceride decreased by 39% (all P < 0.05). Muraglitazar increased mRNA levels of 5′-monophosphate-activated protein kinase, adiponectin receptor 1, adiponectin receptor 2, peroxisome proliferator-activated receptor gamma coactivator-1 alpha and multiple genes involved in mitochondrial function and fat oxidation. In the placebo group, there were no significant changes in expression of these genes.
Conclusions
Muraglitazar increases plasma adiponectin, stimulates muscle 5′-monophosphate-activated protein kinase expression and increases expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation. These changes represent important cellular mechanisms by which dual peroxisome proliferator-activated receptor agonists improve skeletal muscle insulin sensitivity.
Introduction
Skeletal muscle insulin resistance is an early and characteristic feature of Type 2 diabetes mellitus [1]. Multiple cellular, molecular and biochemical abnormalities have been shown to contribute to the development of insulin resistance, including impaired insulin signalling [2], reduced glucose transport, decreased hexokinase II expression and activity, diminished glycogen synthase activity, decreased pyruvate dehydrogenase activity and impaired mitochondrial function [3]. We have previously demonstrated that nuclear-encoded mitochondrial genes encoding proteins involved in electron transport and oxidative phosphorylation are decreased in insulin-resistant muscle, and many of these changes could be explained by reduced peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) gene expression [4].
Thiazolidinediones represent a class of insulin-sensitizing agents that effectively reduce plasma glucose levels in participants with Type 2 diabetes. Thiazolidinediones are high-affinity ligands for the transcription factor peroxisome proliferator-activated receptor (PPAR). PPARs belong to the nuclear hormone receptor superfamily and there are three isoforms: α, β/δ and γ [5]. PPARα is highly expressed in liver, kidney, small intestine, heart and muscle, whereas PPARβ/δ is ubiquitous [6]. PPARγ is expressed in fat cells, vascular tissue, macrophages, liver, muscle tissue and β-cells [7]. Activation of PPARγ by thiazolidinediones alters the expression of many genes [8], resulting in enhanced insulin sensitivity in liver and muscle [9,10], reduced intracellular lipid content in liver and muscle [11,12], and increased insulin receptor signalling in Type 2 diabetes [13,14]. PPARα agonists, such as fenofibrate, have a potent effect to reduce plasma triglycerides, but they do not augment muscle or liver insulin sensitivity or redistribute fat within the body [15]. PPARβ/δ agonists have been less well studied, but preclinical data suggest that they may improve insulin resistance in Type 2 diabetes [16].
Dual PPARs possess both γ and α activity and may provide broader, more beneficial metabolic effects through combination treatment of hyperglycaemia and dyslipidaemia [6]. Muraglitazar was one of the first dual (γ/α) PPAR agonists developed for the treatment of Type 2 diabetes, and we demonstrated that muraglitazar augments insulin sensitivity, improves β-cell function, mobilises fat out of liver and muscle, increases the ratio of subcutaneous to visceral fat content, improves dyslipidaemia and improves plasma adipocytokine levels [7]. However, the molecular mechanisms by which muraglitazar exert its insulin-sensitizing effect remain unclear. Because the metabolic effects of muraglitazar are very similar to those observed with pioglitazone [7], we hypothesized that the molecular effects also would be similar. We have previously shown that chronic treatment with pioglitazone (PPARγ agonist) augments plasma adiponectin levels, increases expression of both adiponectin receptors, activates adenosine 5′-monophosphate-activated protein kinase (AMPK) in muscle, and increases expression of genes involved in mitochondrial function and fat oxidation [17]. The aim of this study was to evaluate whether muraglitazar activates the AMPK pathway and increases expression of genes involved in adiponectin signalling, fat oxidation and mitochondrial function in human skeletal muscle from patients with Type 2 diabetes.
Methods
Participants
Participants with Type 2 diabetes were randomized to receive muraglitazar, 5 mg/day, or placebo for 16 weeks. The metabolic data for these participants were included in a previous publication. Briefly, in that paper we provide the metabolic effects of muraglitazar and placebo treatment in 20 and 7 participants, respectively [7]. Only participants that received a muscle biopsy before and after treatment were included in the present study (n = 12 with muraglitazar and n = 4 with placebo). The discrepancy in participants between the original article and the present study was due to insufficient material to perform the studies outlined here. Participants were free of organ system disease as determined by medical history, physical examination, screening chemistries, complete blood cell count, urinalysis and electrocardiogram. Glucose tolerance was established by 75 g oral glucose tolerance test (OGTT) [7]. All participants demonstrated stable body weight for at least 3 months prior to the study. No participants participated in any heavy exercise. Participants either were drug naïve or taking anti-diabetic medications (metformin alone, sulfonylureas alone or metformin plus sulfonylureas) that were stable for at least 6 months. No participant ever had taken insulin or a thiazolidinedione. All participants gave informed written consent prior to their participation in the study. The study protocol was approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio.
Study design
Following a 10–12 h overnight fast, participants reported to the clinical research centre at 0800 h for a screening visit and OGTT. Within 3–7 days, participants returned to the clinical research centre at 0800 h after an overnight fast for vastus lateralis muscle biopsy [2], followed by a 180 min euglycaemic-hyperinsulinaemic (60 mU/m2/min) clamp performed in combination with [6–6,2H2]-glucose [7,18]. Following completion of these studies, participants were randomized to receive muraglitazar, 5 mg/day (n = 12) or placebo (n = 4) for 16 weeks. Prior to the start of therapy, all participants received 10 h of diabetes management in an American Diabetes Association-certified educational programme, received one-on-one nutritional advice for 2 h and met with a dietician for 30 min on each follow-up visit, which occurred monthly. All participants consumed a weight-maintaining diet containing 50% carbohydrate, 30% fat and 20% protein, and were instructed not to engage in any new physical activity programme during the study period. After 16 weeks of the muraglitazar or placebo treatment, participants returned to the clinical research centre for repeat OGTT and euglycaemic-hyperinsulinaemic clamp with muscle biopsy.
Substrate and hormone determinations
Plasma glucose concentration was determined by the glucose oxidase method on a Beckman Glucose Analyser (Beckman Instruments, Fullerton, CA, USA). Plasma insulin was determined by radioimmunoassay (Diagnostic Products, Los Angeles, CA, USA). HbA1c was measured by affinity chromatography (Biochemical Methodology, Drower 4350; Isolab, Akron, OH, USA) and plasma free fatty acids by enzymatic colorimetric quantification (Wako Chemicals, Neuss, Germany). Plasma total cholesterol and triglyceride were measured enzymatically (Boehringer-Mannheim, Indianapolis, IN, USA) on a Hitachi 704 autoanalyser. Plasma HDL cholesterol was measured enzymatically on Hitachi 704 autoanalyser after precipitation of chylomicron, and VLDL and LDL cholesterol by phosphotungstic acid. LDL cholesterol was calculated from the Friedewald equation. Plasma adiponectin was measured by radioimmunoassay (Linco Research, St Charles, MO, USA).
Muscle biopsy processing
RNA extraction was performed on frozen muscle biopsy specimens that were homogenized directly in RNAStat solution (Tel-Test, Friendswood, TX, USA), using a Polytron homogenizer (Brinkmann Instruments, Westbury, NY, USA). RNA pellets were stored in 75% (w/v) ethanol at −80 °C. Prior to use, total RNA was purified with RNeasy and Dnase I treatment (Qiagen, Chatsworth, CA, USA). For immunoblot analysis, muscle samples were weighed while still frozen and homogenized in ice-cold lysis buffer (1:10, w/v) containing 50 mmol/l Hepes (pH 7.6), 150 mmol/l NaCl, 20 mmol/l sodium pyrophosphate, 20 mmol/l β-glycerophosphate, 10 mmol/l sodium fluoride, 2 mmol/l sodium orthovanadate, 2 mmol/l EDTA (pH 8.0), 1% (v/v) Nonidet P-40, 10% (v/v) glycerol, 1 mmol/l phenylmethylsulfonylfluoride, 1 mmol/l MgCl2, 1 mmol/l CaCl2, 10 μg/ml leupeptin and 10 μg/ml aprotinin. Homogenates were incubated on ice for 20 min and then centrifuged at 15 000 g for 20 min at 4 °C. Supernatant fractions were collected, and protein concentrations measured by the Lowry method. Supernatant fractions were stored at −80 °C until used.
Quantitative real-time PCR
Muscle gene expression was determined using one-step quantitative real-time PCR (Q-RT-PCR) from total RNA. Q-RT-PCR was performed on an ABI PRISM 7900HT sequence detection system (Life Technologies, Carlsbad, CA, USA) using TaqMan One Step RT-PCR Master Mix reagents and Assay On Demand gene expression primer pairs and probes, as described previously [17]. Expression data were normalized by dividing the amount of the gene of interest by the amount of ribosomal 18S gene used as the internal control.
Immunoblot analysis
Proteins were transferred to nitrocellulose membranes, and the membranes were probed with antibody against phospho-AMPK (Thr172) (Cell Signaling, Beverly, MA, USA). Bound antibodies were detected with anti-(rabbit immunoglobulin)-horseradish peroxidase-linked whole antibody and by using enhanced chemiluminescent reagents (PerkinElmer Life Science, Boston, MA, USA). The concentration used for primary and secondary antibodies was 1:1000 and 1:2000, respectively. The amount of protein loaded was 40 μg. The membranes were exposed to film, and band intensity was quantified using Image Tool Software (University of Texas Health Science Center at San Antonio, San Antonio, TX, USA).
Statistical analysis
Statistical analyses were performed with SPSS v. 20.0. All values before and after treatment within each group were analysed using paired Student’s t-test. Comparison between groups was performed using analysis of variance (ANOVA) with Bonferroni/Dunn post hoc testing. Data are presented as mean ± se. For all analyses, P < 0.05 was considered to be statistically significant. Q-RT-PCR statistical analysis is described above.
Results
Participant characteristics and in vivo data
Table 1 shows the participant characteristics pre and post 16 weeks of muraglitazar/placebo therapy. Participants were well matched for age and metabolic parameters; BMI was significantly lower in the placebo-treated group than in the muraglitazar-treated group (Table 1). After 16 weeks of muraglitazar, fasting plasma glucose and HbA1c declined by 31% and 21%, respectively (both P < 0.05 vs. baseline) with a 3% increase in BMI from 33.3 ± 0.8 to 34.3 ± 0.8 (P < 0.05). Insulin-stimulated glucose disposal increased by 81% in the muraglitazar group (P < 0.01 vs. baseline and placebo) and by 7% in the placebo group (P = NS). Fasting plasma triglyceride decreased by 39% following muraglitazar treatment (both P < 0.05 vs. baseline and placebo). LDL cholesterol declined modestly but not significantly in both groups.
Table 1.
Clinical and metabolic characteristics before and after treatment with muraglitazar (5 mg/day) or placebo for 16 weeks
| Placebo | Muraglitazar | P† | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Change (%) | Pre | Post | Change (%) | ||
| Number | 4 | 12 | |||||
| Sex | 2F/2M | 8F/4M | |||||
| Age (years) | 54 ± 6 | 50 ± 3 | |||||
| BMI (kg/m2) | 30.1 ± 2.4 | 29.1 ± 2.3 | −3.3 | 33.3 ± 0.8 | 34.3 ± 0.8* | +3 | 0.024 |
| Fasting plasma glucose (mmol/1) | 11.1 ± 1.4 | 9.8 ± 1.9 | −11.7 | 10.5 ± 0.7 | 7.3 ± 0.4* | −30.5 | 0.010 |
| Fasting plasma insulin (pmol/1) | 77.4 ± 45 | 57.0 ± 22.2 | −26.4 | 87.0 ± 21.6 | 48.6 ± 9.0* | −44.1 | NS |
| HbA1c (mmol/mol) | 67 | 61 | 72 | 52 | |||
| HbAlc (%) | 8.3 ± 0.8 | 7.7 ± 1.1 | −7.2 | 8.7 ± 0.6 | 6.9 ± 0.5* | −20.7 | NS |
| Total glucose disposal (μmo1/kg/min) | 14 ± 4 | 15 ± 2 | +7.1 | 16 ± 2 | 29 ± 3* | +81.3 | 0.0023 |
| Triglycerides (mmol/1) | 1.80 ± 0.32 | 1.91 ± 0.15 | +6.1 | 1.74 ± 0.16 | 1.06 ± 0.10* | −39.1 | < 0.0001 |
| Total cholesterol (mmol/1) | 4.82 ± 0.44 | 4.25 ± 0.60 | −11.8 | 4.40 ± 0.16 | 4.12 ± 0.16* | −6.4 | NS |
| HDL cholesterol (mmol/1) | 1.35 ± 0.21 | 1.09 ± 0.21 | −19.3 | 1.01 ± 0.08 | 1.14 ± 0.08* | +12.9 | NS |
| LDL cholesterol (mmol/1) | 3.00 ± 0.41 | 2.64 ± 0.57 | −12.0 | 2.56 ± 0.18 | 2.31 ± 0.21 | −9.8 | NS |
Data represent mean ± SE.
P < 0.05 versus pre-treatment.
P-value (ANOVA) for the comparison between muraglitazar and placebo therapy-treated groups (change from baseline).
Effect of muraglitazar on AMPK signalling and adiponectin receptor gene expression
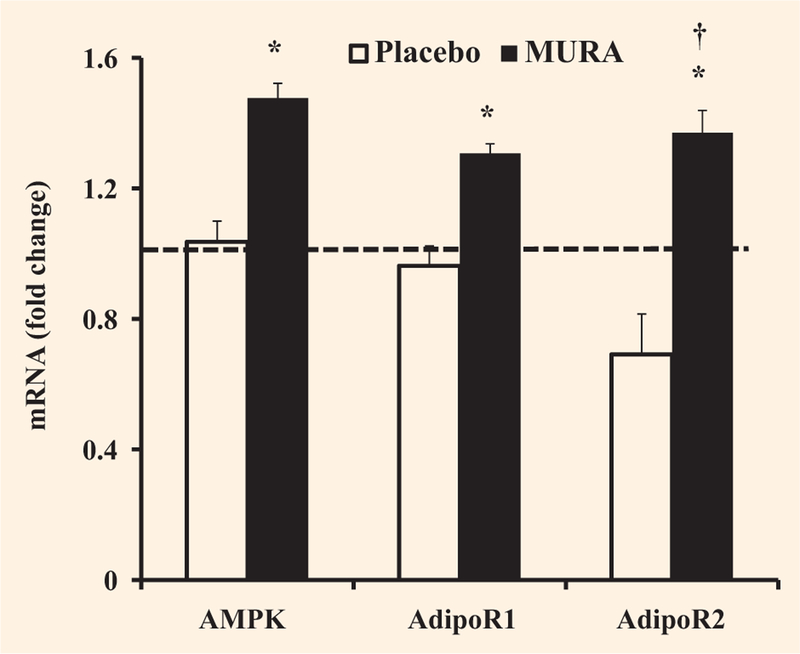
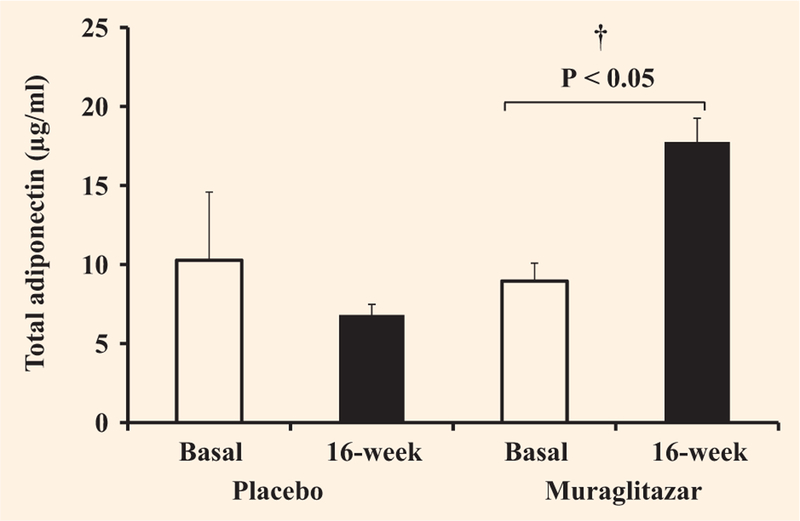
Muraglitazar treatment increased AMPK gene expression in skeletal muscle by 1.5-fold (P < 0.05 versus baseline), while there was no change in the placebo group (Fig. 1). Following muraglitazar treatment, AMPK phosphorylation in skeletal muscle increased slightly although not significantly from 1.4 ± 0.2 to 1.8 ± 0.4 a.u. There was no change in AMPK phosphorylation in the placebo treated group (1.1 ± 0.2 before vs. 1.3 ± 0.2 a.u. after). Plasma adiponectin concentration increased significantly from 9.0 ± 1.1 to 17.8 ± 1.5 μg/ml following muraglitazar (P < 0.01 vs. baseline and placebo) and declined from 10.3 ± 4.3 to 6.8 ± 0.7 μg/ml in the placebo group (Fig. 2). The increase in plasma adiponectin concentration and muscle AMPK1 mRNA expression after muraglitazar treatment was accompanied by elevated adiponectin receptor 1 (AdipoR1) by 1.3-fold (P < 0.05 vs. baseline) and adiponectin receptor 2 (AdipoR2) gene expression by 1.4-fold, respectively (P < 0.05 vs. baseline and placebo) (Fig. 1). AdipoR1 and AdipoR2 expression did not change significantly in the placebo group (Fig. 1).
FIGURE 1.
Effect of treatment with placebo or muraglitazar on mRNA expression of AMPK, AdipoR1 and AdipoR2. The dashed line indicates no change in gene expression. *P < 0.05 versus pre-treatment. †P < 0.05 for the comparison between muraglitazar and placebo therapy-treated groups. Data are shown as means ± se.
FIGURE 2.
Effect of treatment with placebo or muraglitazar on plasma total adiponectin. †P < 0.01 for the comparison between muraglitazar and placebo therapy-treated groups. Data are shown as means ± se.
Effect of muraglitazar on expression of PGC-1α and genes involved in mitochondrial function
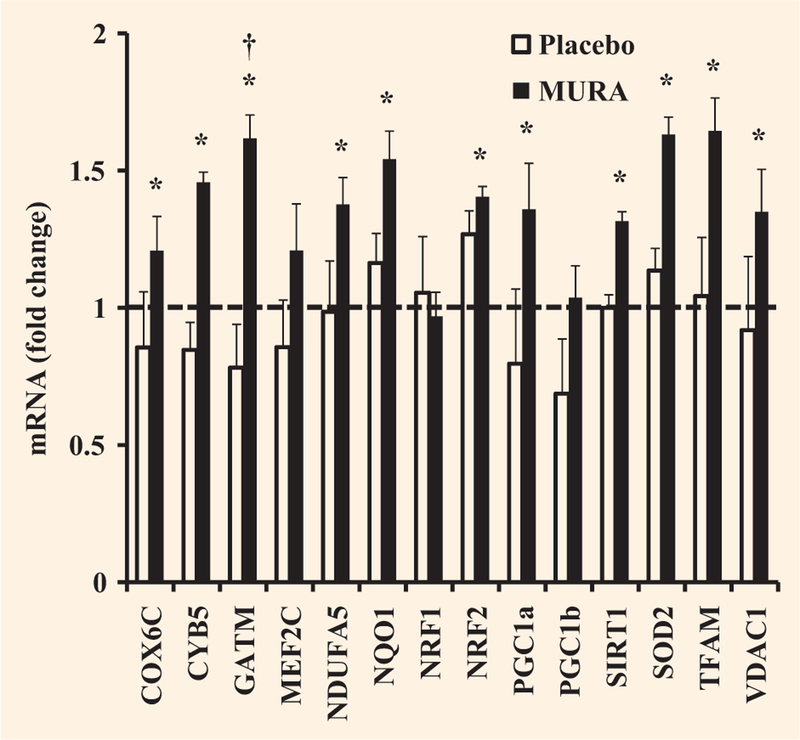
The PGC-1α mRNA level increased significantly by 1.4-fold following muraglitazar treatment (P < 0.05 vs. baseline), whereas there was no significant change in the placebo group (Fig. 3). Muraglitazar significantly increased the expression of mRNAs for a number of other nuclear-encoded mitochondrial genes and genes encoding for scavenging enzymes that are important in protecting the mitochondria against the effects of reactive oxygen species: cytochrome c oxidase subunit Vic (1.2-fold); cytochrome b5 type B (1.5-fold); NADH dehydrogenase (ubiquinone) 1-alpha, subcomplex 5 (1.4-fold), NAD(P)H dehydrogenase, quinine 1 (1.5-fold); nuclear respiratory factor 2 (1.4-fold); sirtuin 1 (1.3-fold); superoxide dismutase 2 (1.6-fold); transcription factor A, mitochondrial (1.6-fold); and voltage-dependent anion channel 1 (1.4-fold) (Fig. 3). Glycine amidinotransferase was increased by 1.6-fold (P < 0.05 vs. baseline and placebo, Fig. 3). Peroxisome proliferator-activated receptor gamma coactivator-1 beta (PGC-lβ), nuclear respiratory factor 1 and myocyte enhancer factor 2C mRNA was unchanged in the muraglitazar treated group. The mitochondrial genes did not change significantly in the placebo group (Fig. 3).
FIGURE 3.
Effect of treatment with placebo or muraglitazar on expression of peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α and genes involved in mitochondrial function. The dashed line indicates no change in gene expression. *P < 0.05 versus pre-treatment. †P < 0.05 for the comparison between muraglitazar and placebo therapy-treated groups. Data are shown as means ± se. COX6C, cytochrome c oxidase subunit VIc; CYB5, cytochrome b5 type B; GATM, glycine amidinotransferase; MEF2C, myocyte enhancer factor 2C; NDUFA5, NADH dehydrogenase (ubiquinone) 1-alpha subcomplex, 5; NQO1, NAD(P)H dehydrogenase, quinine 1; NRF1, nuclear respiratory factor 1; NRF2, nuclear respiratory factor 2; PGC-1b, peroxisome proliferator-activated receptor gamma coactivator-1 beta; SIRT1, sirtuin 1; SOD2, superoxide dismutase 2; TFAM, transcription factor A, mitochondrial; VDAC1, voltage-dependent anion channel 1.
Effect of muraglitazar on expression of genes involved in free fatty acid oxidation
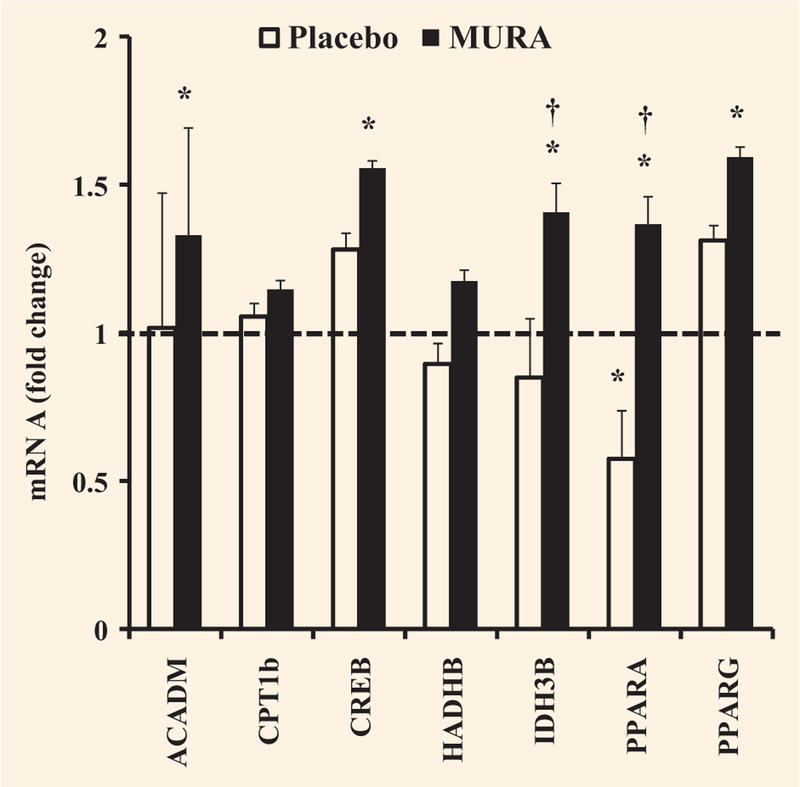
Muraglitazar significantly increased the mRNA expression of PPARα and PPARγ by 1.4- and 1.6-fold, respectively (both P < 0.05 vs. baseline) (Fig. 4). PPARγ mRNA expression increased slightly in the placebo group but the change was not significant. PPARα mRNA expression was significantly decreased in the placebo group (P < 0.05, Fig. 4), and the comparison between muraglitazar- and placebo-treated groups was significant (P < 0.05). Other mRNAs that were significantly increased following muraglitazar treatment, but remained unchanged in the placebo group, included acyl-CoA dehydrogenase (1.3-fold) and cAMP-responsive element binding protein 1 (1.6-fold) (Fig. 4). Isocitrate dehydrogenase 3 (NAD+) beta was increased by 1.4-fold (P < 0.05 vs. baseline and placebo, Fig. 4). Carnitine palmitoyltransferase 1B and hydroxyacyl-CoA dehydrogenase were increased slightly, but not significantly following muraglitazar treatment.
FIGURE 4.
Effect of treatment with placebo or muraglitazar on expression of genes involved in free fatty acid oxidation. The dashed line indicates no change in gene expression. *P < 0.05 versus pretreatment. †P < 0.05 for the comparison between muraglitazar and placebo therapy-treated groups. Data as means ± se. ACADM, acyl-CoA dehydrogenase; CPT1b, carnitine palmitoyltransferase 1B; CREB1, cAMP-responsive element binding protein 1; HADH, hydroxyacyl-CoA dehydrogenase; IDH3B, isocitrate dehydrogenase 3 (NAD+) beta.
Discussion
Muraglitazar treatment for 16 weeks significantly improved glycaemic control (fasting plasma glucose and HbA1c) and insulin-stimulated whole-body (primarily reflects muscle) glucose disposal in participants with diabetes, as described previously [7]. Muraglitazar also increased the mRNA expression of AMPK, suggesting that activation of AMPK signalling may be involved in the insulin-sensitizing effect of the dual PPARγ/α agonist, muraglitazar. However, the increase in AMPK phosphorylation with muraglitazar did not reach statistical significance. Poor correlation between changes in mRNA and protein expression have been reported by Vogel and Marcotte, who demonstrated that ~ 40% of the variation in protein concentration can be explained by knowing mRNA abundances [19]. They suggest that ~ 60% of the variation in protein expression can be explained by post-transcriptional regulation and measurements [19].
In the present study, enhanced insulin sensitivity was accompanied by a slight increase in BMI following muraglitazar treatment. Although both groups were instructed to keep their calorie intake and body weight constant, the placebo-treated group BMI decreased by 3%, whereas a significant increase of 3% was observed in the muraglitazar group. Thiazolidinediones, including pioglitazone and muraglitazar, have been associated with weight gain, which correlates strongly with improvements in glycaemic control [20]. Other factors have been shown to contribute to this weight gain, including fluid retention. As discussed elsewhere, about 30% of the weight gain in our muraglitazar group could be accounted for by fluid retention. Importantly, we observed no peripheral oedema in our participants [7].
An increase in plasma adiponectin following muraglitazar treatment was observed in this study. Consistent with this, we and others have previously shown that thiazolidinediones increase plasma adiponectin in insulin-resistant rodents and in insulin-resistant humans with and without Type 2 diabetes [17,21–23]. Previous studies in animals have indicated that AMPK plays a central role in mediating the metabolic effects of adiponectin [24,25]. In the present study, the muraglitazar-stimulated increase in plasma adiponectin was associated with an increase in AMPK gene expression; although a significant increase in AMPK phosphorylation was not observed. Therefore, it is unclear what role, if any, AMPK plays in mediating the effect of adiponectin
The present study also allowed us to examine the in vivo effect of muraglitazar on the expression of genes involved in adiponectin signalling in skeletal muscle from participants with Type 2 diabetes. In previous studies, we documented that insulin-resistant participants with Type 2 diabetes are characterized by reduced adiponectin signalling [26]. Adiponectin is a hormone that is expressed only by adipocytes and it acts through its receptors, AdipoR1 and AdipoR2. Because we observed an increase in plasma adiponectin concentration and improvements in insulin-mediated glucose disposal in the muraglitazar-treated group, we expected to observe changes in both AdipoR1 and AdipoR2 gene expression. It was initially thought that AdipoR1 was primarily expressed in skeletal muscle, whereas AdipoR2 was predominantly expressed in liver [27]. However, we previously demonstrated that AdipoR2 is highly expressed in human muscle and may be the predominant isoform through which adiponectin exerts its insulin-sensitizing effects in skeletal muscle [26]. In the present study, both AdipoR1 and AdipoR2 mRNA increased following muraglitazar treatment in participants with Type 2 diabetes. This was similar to our previous finding in individuals with Type 2 diabetes treated with pioglitazone for 6 months [17]. These results suggest that muraglitazar works through a similar mechanism to pioglitazone with regard to adiponectin signalling.
Insulin-resistant muscle exhibits decreased expression of oxidative phosphorylation genes, including PGC-lα [4], which co-ordinately regulate the expression of multiple genes involved in mitochondrial bioenergetics [4,28]. Previously, we demonstrated that the pioglitazone-induced increase in peripheral tissue (muscle) glucose disposal was associated with increased expression of PGC-lα and genes that code for mitochondrial and free fatty acid oxidation in human skeletal muscle [17]. In the present study, we observed increases in multiple mitochondrial genes in the muraglitazar-treated group. To our knowledge, no other study has investigated the molecular actions of muraglitazar in human skeletal muscle. We also documented that intramyocellular fat content is increased in participants with Type 2 diabetes and is reduced following treatment with muraglitazar [7] and pioglitazone [29,30]. The increased expression of multiple genes involved in free fatty acid oxidation in the present study provides a potential explanation via which muraglitazar reduces muscle fat content. We did not observe significant changes in AMPK or the expression of AdipoR1, AdipoR2, PGC-1α and genes involved in mitochondrial function and fat oxidation in the placebo-treated group, consistent with findings in a previous study [17]. However, a limitation of the present study was the small sample size, particularly for the placebo group, and additional studies with more subjects would be beneficial. Nuclear respiratory factor 1 mRNA content was unchanged following treatment with muraglitazar, a finding similar to that observed with pioglitazone [17]. Although we observed increases in mRNAs coding for genes involved in adiponectin signalling, mitochondrial function and fatty acid metabolism following muraglitazar treatment, these results may not reflect changes in the abundance of the proteins associated with these genes, and further studies are warranted to examine this issue.
In summary, we demonstrate that in participants with Type 2 diabetes, chronic treatment with muraglitazar increases plasma adiponectin levels and mRNA expression of both AdipoR1 and AdipoR2 and AMPK in muscle. Muraglitazar also increases expression of genes involved in mitochondrial function and fat oxidation. We hypothesize that increases in the expression of genes involved in mitochondrial function and fat oxidation lead to a decrease in toxic intracellular lipid metabolites (fatty acyl CoA, diacylglycerol, ceramides), which in turn leads to improvements in insulin signalling and insulin sensitivity in muscle.
What’s new?
Skeletal muscle insulin resistance is a characteristic feature of Type 2 diabetes mellitus.
Muraglitazar, a peroxisome proliferator-activated receptor β/δ agonist augments insulin sensitivity, improves β-cell function, mobilizes fat out of the liver and muscle, and improves dyslipidaemia.
However, the molecular mechanisms by which muraglitazar exert its insulin-sensitizing effect remain unclear.
This study demonstrates that muraglitazar increases plasma adiponectin levels and mRNA muscle expression of adiponectin receptors, 5′-monophosphate-activated protein kinase and genes involved in mitochondrial function and fat oxidation.
To our knowledge, no other study has investigated the molecular actions of muraglitazar in human skeletal muscle.
Acknowledgments
We thank the nurses at the CRC for their excellent care of our participants.
Funding sources
This work was supported by a research grant from Bristol Myers Squibb. Ralph A. DeFronzo’s salary is supported by the Veterans Administration Service.
Footnotes
Competing interests
Ralph A. DeFronzo has received investigational grants from Takeda Pharmaceuticals North America, Bristol Myers Squibb and Amylin. Ralph A. DeFronzo is a member of the Advisory Board for Takeda, BMS, Boehringer-Ingelheim, Janssen, Lexicon, Novo Nordisk and is a member of the Speakers Bureau for Novo-Nordisk, Takeda, Janssen and BMS. Eugenio Cersosimo serves as a speaker for Takeda, Amylin and Eli Lilly. Amalia Gastaldelli has received an investigational grant from Amylin and is a consultant for Roche and Eli Lilly. Dawn K. Coletta, Marianella Fernandez and Nicolas Musi have no conflict of interest regarding this manuscript.
References
- 1.DeFronzo RA. Lilly Lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988; 37: 667–687. [DOI] [PubMed] [Google Scholar]
- 2.Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 2000; 105: 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajaj M, Defronzo RA. Metabolic and molecular basis of insulin resistance. J Nucl Cardiol 2003; 10: 311–323. [DOI] [PubMed] [Google Scholar]
- 4.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 2003; 100: 8466–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott BD, Wolf CJ, Das KP, Zehr RD, Schmid JE, Lindstrom AB et al. Developmental toxicity of perfluorooctane sulfonate (PFOS) is not dependent on expression of peroxisome proliferator activated receptor-alpha (PPAR alpha) in the mouse. Reprod Toxicol 2009; 27: 258–265. [DOI] [PubMed] [Google Scholar]
- 6.Rogue A, Spire C, Brun M, Claude N, Guillouzo A. Gene expression changes induced by PPAR gamma agonists in animal and human liver. PPAR Res 2010; 2010: 325183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez M, Gastaldelli A, Triplitt C, Hardies J, Casolaro A, Petz R et al. Metabolic effects of muraglitazar in type 2 diabetic subjects. Diabetes Obes Metab 2011; 13: 893–902. [DOI] [PubMed] [Google Scholar]
- 8.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 1998; 47: 507–514. [DOI] [PubMed] [Google Scholar]
- 9.Maggs DG, Buchanan TA, Burant CF, Cline G, Gumbiner B, Hsueh WA et al. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1998; 128: 176–185. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki Y, Mahankali A, Matsuda M, Glass L, Mahankali S, Ferrannini E et al. Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care 2001; 24: 710–719. [DOI] [PubMed] [Google Scholar]
- 11.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. New Engl J Med 2006; 355: 2297–2307. [DOI] [PubMed] [Google Scholar]
- 12.Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 2002; 51: 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YB, Ciaraldi TP, Kong A, Kim D, Chu N, Mohideen P et al. Troglitazone but not metformin restores insulin-stimulated phosphoinositide 3-kinase activity and increases p110beta protein levels in skeletal muscle of type 2 diabetic subjects. Diabetes 2002; 51: 443–148. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki Y, He H, Mandarino LJ, DeFronzo RA. Rosiglitazone improves downstream insulin receptor signaling in type 2 diabetic patients. Diabetes 2003; 52: 1943–1950. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj M, Suraamornkul S, Hardies LJ, Glass L, Musi N, DeFronzo RA. Effects of peroxisome proliferator-activated receptor (PPAR)-alpha and PPAR-gamma agonists on glucose and lipid metabolism in patients with type 2 diabetes mellitus. Diabetologia 2007; 50: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 16.Salvado L, Serrano-Marco L, Barroso E, Palomer X, Vazquez-Carrera M. Targeting PPARbeta/delta for the treatment of type 2 diabetes mellitus. Expert Opin Ther Targets 2012; 16: 209–223. [DOI] [PubMed] [Google Scholar]
- 17.Coletta DK, Sriwijitkamol A, Wajcberg E, Tantiwong P, Li M, Prentki M et al. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia 2009; 52: 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–E223. [DOI] [PubMed] [Google Scholar]
- 19.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 2012; 13: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kendall DM, Rubin CJ, Mohideen P, Ledeine JM, Belder R, Gross J et al. Improvement of glycemic control, triglycerides, and HDL cholesterol levels with muraglitazar, a dual (alpha/gamma) peroxisome proliferator-activated receptor activator, in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a double-blind, randomized, pioglitazone-comparative study. Diabetes Care 2006; 29: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 21.Tan GD, Fielding BA, Currie JM, Humphreys SM, Desage M, Frayn KN et al. The effects of rosiglitazone on fatty acid and triglyceride metabolism in type 2 diabetes. Diabetologia 2005; 48: 83–95. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki Y, Mahankali A, Wajcberg E, Bajaj M, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2004; 89: 4312–4319. [DOI] [PubMed] [Google Scholar]
- 23.Gastaldelli A, Miyazaki Y, Mahankali A, Berria R, Pettiti M, Buzzigoli E et al. The effect of pioglitazone on the liver: role of adiponectin. Diabetes Care 2006; 29: 2275–2281. [DOI] [PubMed] [Google Scholar]
- 24.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 2002; 99: 16309–16313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002; 8: 1288–1295. [DOI] [PubMed] [Google Scholar]
- 26.Civitarese AE, Jenkinson CP, Richardson D, Bajaj M, Cusi K, Kashyap S et al. Adiponectin receptors gene expression and insulin sensitivity in non-diabetic Mexican Americans with or without a family history of Type 2 diabetes. Diabetologia 2004; 47: 816–820. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003; 423: 762–769. [DOI] [PubMed] [Google Scholar]
- 28.Richardson DK, Kashyap S, Bajaj M, Cusi K, Mandarino SJ, Finlayson J et al. Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J Biol Chem 2005; 280: 10290–10297. [DOI] [PubMed] [Google Scholar]
- 29.Bajaj M, Baig R, Suraamornkul S, Hardies LJ, Coletta DK, Cline GW et al. Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2010; 95: 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2002; 87: 2784–2791. [DOI] [PubMed] [Google Scholar]