Abstract
Cardiovascular disease (CVD) is the leading cause of death in patients with nonalcoholic fatty liver disease (NAFLD). The current analysis expands the knowledge on atherogenic lipid profiles in NAFLD by modeling changes in low‐density lipoprotein cholesterol (LDL‐C) and total cholesterol (TC) in a prospectively enrolling real‐life study cohort to inform physicians on the cardiovascular (CV) event risk based on these changes. A total of 304 patients with histologically confirmed NAFLD were included (mean age, 52 years; equal sex distribution). Of these, 129 (42.4%) patients exhibited a NAFLD activity score ≥4 and 186 (61.2%) had at least intermediate fibrosis ≥F2. The median TC levels were 209 mg/dL (interquartile range [IQR], 183, 239), LDL‐C 131 mg/dL (IQR, 103, 152), and high‐density lipoprotein cholesterol (HDL‐C) 45 mg/dL (IQR, 38, 52). Only 16.9% of patients received lipid‐lowering therapy. According to the LDL/HDL ratio, 69 (23.7%) patients exhibited a high CV risk. The 10‐year CV event risk according to the Framingham risk score (FRS) was low in 91 (41.2%), intermediate in 59 (26.7%), and high in 71 (32.1%) patients and higher in the ≥F2 NAFLD population. A moderate increase in LDL‐C levels by 20 mg/dL led to a transition of 20% of patients into the high‐risk group when assessing the LDL/HDL ratio. According to the FRS, 6 (2.7%) patients moved from low to intermediate and 11 (4.9%) from intermediate to high CV risk. Conclusion: Patients with NAFLD exhibit a substantial CV event risk and are frequently undertreated with lipid‐lowering medication. Moderate increases in LDL‐C would result in worsening of the CV event risk in approximately 7.8% of all patients without a history of CVD.
Abbreviations
- AACE
American Association of Clinical Endocrinologists
- CI
confidence interval
- CV
cardiovascular
- CVD
cardiovascular disease
- F
fibrosis score
- FRS
Framingham risk score
- FXR
farnesoid X receptor
- HDL‐C
high‐density lipoprotein cholesterol
- IQR
interquartile range
- LDL‐C
low‐density lipoprotein cholesterol
- NAFLD
nonalcoholic fatty liver disease
- NAS
nonalcoholic fatty liver disease activity score
- NASH
nonalcoholic steatohepatitis
- OCA
obeticholic acid
- TC
total cholesterol
Globally, nonalcoholic fatty liver disease (NALFD) is the most common liver disease, with an estimated prevalence of 24% worldwide. An increasing incidence has been predicted based on the high prevalence of associated risk factors in Europe.1 NAFLD constitutes a progressive disease spectrum encompassing noninflammatory steatosis (nonalcoholic fatty liver [NAFL]), hepatitis (nonalcoholic steatohepatitis [NASH]), and end‐stage liver disease, with associated complications.2 In addition to the individual disease burden, the large number of patients with NAFLD who are at risk to develop progressive liver disease pose societal and economic challenges for health care systems.3 In 2013, end‐stage liver disease related to NAFLD was the second most common reason for liver transplantation in the United States.4 Beyond liver‐associated mortality, patients with NAFLD exhibit impaired cardiovascular (CV) fitness5 and increased overall mortality, with the primary cause of death being CV events.6 In a recent analysis, NAFLD was associated with incidental nonfatal coronary heart disease and all‐cause mortality events, with a hazard ratio of 1.43 after adjustment for traditional risk factors.7 In observational NAFLD cohorts with long‐term follow‐up, there is an excess of CV events and mortality.8 These clinical data and additional translational studies support the view that NALFD is an inflammatory multisystem disease that affects CV health.9, 10 The mortality risk increases in patients with intermediate and advanced fibrosis, and thus this subgroup has been defined as the target population for liver‐directed therapies that are currently developed in phase 3 trials.11 For this reason, regulatory authorities have accepted histologic surrogates for conditional drug approval.12 One of these endpoints (improvement of fibrosis by at least one point without worsening of steatohepatitis) was recently met in the trial Randomized Global Phase 3 Study to Evaluate the Impact on NASH With Fibrosis of Obeticholic Acid Treatment (REGENERATE), which is the first study to achieve this primary endpoint.13 The REGENERATE study explored the first‐generation steroidal farnesoid X receptor (FXR) agonist obeticholic acid (OCA) in 931 patients over 18 months.13 Although hepatic fibrosis improved in the group receiving 25 mg of OCA in 23.1% of the patients, this also led to an increase in low‐density lipoprotein cholesterol (LDL‐C) by approximately 20%. This was comparable to data observed in the phase 2 Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment Trial (FLINT)14 and a healthy volunteers study.15 The effect on lipids relates back to an FXR‐specific effect leading to decreasing LDL‐C receptor and increasing transfer cholesteryl ester transfer protein, thus reducing high‐density lipoprotein cholesterol (HDL‐C) and increasing LDL‐C levels.16
Although improving hepatic fibrosis could translate into improved overall survival in patients with NAFLD (an endpoint that is currently still under investigation in the ongoing phase 3 trials), the role of increasing LDL‐C can counteract the potential benefits. Ample evidence supports that lowering LDL‐C in patients with CV risk is beneficial.17 Therefore, we analyzed the CV risk profile in patients with histologically confirmed NAFLD and grouped them according to the most commonly used and rapidly available surrogate risk score of cardiovascular disease (CVD) and mortality. Once a medical treatment for NAFLD is approved, safety will be of high priority, and the current analysis provides data on changes in the relative CV event risk in biopsy‐proven NAFLD cases based on a model accounting for LDL‐C and total cholesterol (TC).
Patients and Methods
Patient Cohort and Ethical Considerations
A total of 304 adult patients with biopsy‐proven NAFLD were included in this prospectively enrolling protocol, which is part of the European NAFLD registry, between December 2013 and January 2019. Prior to inclusion, informed consent was obtained at the outpatient clinic of the University Medical Center Mainz, Germany. Alcohol use was assessed on clinical grounds and random ethyl glucuronide measurements in the urine. Patients with coexisting or other liver disease, including chronic or acute viral hepatitis, cholestasis, and autoimmune liver disease, were excluded as were causes of secondary steatohepatitis, including steatogenic medications. Diabetes mellitus, hypertension, hyperlipidemia, and metabolic syndrome were defined according to the definitions of the Joint Scientific Statement for Harmonizing the Metabolic Syndrome.18 Laboratory test results were obtained within 30 days of liver biopsy. Samples for analyses of lipids were obtained in patients after overnight fasting. Liver biopsies were performed by laparoscopy or transcutaneously and scored by a liver histopathologist experienced in NAFLD (B.K.S.). Patients with liver biopsies < 10 mm in length were excluded. NASH was diagnosed and subsequently scored according to the NASH Clinical Research Network criteria.19
Patient Consent and Ethical Consideration
The study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008). The study protocol was approved by the ethics committee of the regional medical association of Rhineland‐Palatinate (ethical proposal no. 873.199.10 [7208]).
Surrogate Scores of CV Risk
The Framingham risk score (FRS) was used to predict incident CVD. FRS combines sex, age, TC, HDL‐C, presence of diabetes, systolic blood pressure, treatment for hypertension, and current smoking status, specifying the 10‐year CVD risk in patients without a known CVD.20 We assigned the risk estimate of a 30‐year‐old to all individuals between the age of 18 and 29 years. In addition, the LDL/HDL ratio21 and TC, LDL‐C, triglycerides, and low HDL‐C concentrations were analyzed according to established cutoffs.22
Statistical Analysis
Quantitative data are expressed as median with interquartile range (IQR). Pairwise comparisons for quantitative variables were performed with an unpaired t test or the Mann‐Whitney U test. Categorical variables are given as frequencies and percentages, respectively, and for the comparison of two or more patient groups, a chi‐square test was applied. Data analysis was exploratory, and no adjustments for multiple testing were performed. For all tests, a 0.05 level to define statistically relevant deviations from the respective null hypothesis was applied. However, due to the large number of tests, P values should be interpreted with caution and in connection with effect estimates. Data were analyzed using IBM SPSS Statistics, version 23.0 (IBM Corp., Armonk, NY).
Results
Clinical Baseline Characteristics
A total of 304 patients with histologically confirmed NAFLD were included in this analysis. Half of the patients were men, with a median age of 52 years (IQR, 39, 59). Metabolic risk factors were highly prevalent in the population, with 60.9% exhibiting arterial hypertension and 36.2% diabetes mellitus type 2. Median body mass index (BMI) was 31.4 kg/m2 (IQR, 27.8, 35.5), and only 26 (8.6%) patients were not overweight (BMI, 19‐25 kg/m2). Of the patients, 12.8% were current smokers, and an additional 18.8% reported a history of smoking. Standard laboratory assessment is summarized in Table 1. Median LDL‐C levels were 131 mg/dL (IQR, 103, 152) in the entire cohort, with 67 (22%) patients exhibiting elevations >155 mg/dL. The distribution of the lipid profile in relation to the underlying disease activity and histologic stage of liver disease is shown in Table 2. Importantly, only 16.1% of the entire cohort received lipid‐lowering drugs at the time of referral, and a total of 16 (5.3%) patients had a history of coronary heart disease. CV events in relatives before the age of 60 were present in 40 (13.2%) patients (Table 1). Consumption of low degrees of alcohol, compatible with NAFLD, was reported by 45.7% of patients, whereas 13.5% reported no alcohol and 40.8% prior alcohol consumption. There was no significant influence of alcohol consumption on lipid levels when compared to abstainers (P value not significant).
Table 1.
Demographics and Clinical Characteristics
| Variable* | All Patients | Patients With NAS ≥4 | Patients With F ≥2 | |
|---|---|---|---|---|
| N = 304 | n = 129 | n = 186 | ||
| Age, years | 52 (39, 59) | 52 (38, 59) | 54 (39, 60) | |
| Male sex, n | 156 (51.3) | 62 (48.1) | 97 (52.2) | |
| Current smoker, n† | 39 (12.8) | 21 (16.3) | 30 (16.1) | |
| History of smoking, n† | 57 (18.8) | 24 (18.6) | 37 (19.9) | |
| ALT, U/L | 72 (48, 109) | 87 (57, 122) | 80 (52, 120) | |
| AST, U/L | 52 (38, 75) | 63 (47, 87) | 62 (42, 85) | |
| CVD | Total, n | 30 (9.9) | 11 (8.5) | 20 (10.8) |
| Coronary heart disease, n | 16 (5.3) | 6 (4.7) | 11 (5.9) | |
| History of stroke, n | 14 (4.6) | 5 (3.9) | 9 (4.8) | |
| CVD in family history | 40 (13.2) | 17 (13.2) | 24 (12.9) | |
| Diabetes type 2, n | 110 (36.2) | 59 (45.7) | 87 (46.8) | |
| Arterial hypertension, n | 185 (60.9) | 82 (63.6) | 129 (69.4) | |
| Cholesterol,‡ mg/dL | 209 (183, 239) | 205 (172, 236) | 205 (177, 236) | |
| Triglycerides,§ mg/dL | 154 (115, 215) | 168 (119, 230) | 164 (120, 214) | |
| HDL‐C, mg/dL | 45 (38, 52) | 45 (38, 52) | 44 (36, 52) | |
| LDL‐C, mg/dL | Total | 131 (103, 152) | 126 (99, 149) | 128 (99, 148) |
| Lipid‐lowering drugs | Total, n | 49 (16.1) | 24 (18.6) | 37 (19.9) |
| Statins, n | 43 (14.1) | |||
| Other, n | 6 (2.0) | |||
| NASH | 151 (49.7) | 113 (87.6) | 124 (66.7) | |
| Cirrhosis | 40 (13.2) | 18 (14.0) | 40 (21.5) | |
| NAS | Median | 3 (2, 4) | 4 (3, 5) | |
| ≥4, n | 129 (42.4) | 129 (100) | 109 (58.6) | |
| Fibrosis ≥2 | 186 (61.2) | 109 (84.5) | 186 (100) | |
Data are expressed as medians and IQRs or as frequencies and percentages.
Data were available in 234 patients.
Data were available in 289 patients.
Data were available in 288 patients.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Table 2.
Lipid Profiles in Patients With Different Histologic Grades
| Variable* | NAS <4 | NAS ≥4 | F ≥2 | F <2 | NAS ≥4 and F ≥2 |
|---|---|---|---|---|---|
| Cholesterol | 212 (186, 243) | 205 (172, 236) | 205 (177, 236) | 215 (187, 248) | 205 (174, 236) |
| >240 mg/dL | 42 (25.6%) | 27 (21.6%) | 36 (20.2%) | 33 (30.0%) | 23 (21.7%) |
| <165 mg/dL | 23 (14.0%) | 24 (19.2%) | 32 (18.0%) | 15 (13.6%) | 19 (17.9%) |
| LDL‐C | 135 (108, 156) | 126 (99, 149) | 128 (99, 148) | 136 (111, 163) | 126 (98, 149) |
| >155 mg/dL | 44 (25.1%) | 23 (17.8%) | 33 (17.7%) | 33 (28.2%) | 20 (18.3%) |
| <90 mg/dL | 23 (13.1%) | 22 (17.1%) | 32 (17.2%) | 13 (11.1%) | 20 (18.3%) |
| HDL‐C | 44 (38, 52) | 45 (38, 52) | 44 (36, 52) | 46 (41, 52) | 45 (38, 52) |
| >70 mg/dL | 12 (6.9%) | 3 (2.3%) | 10 (5.4%) | 5 (4.3%) | 3 (2.8%) |
| <40 mg/dL | 50 (28.6%) | 42 (32.6%) | 67 (36.0%) | 25 (21.4%) | 35 (32.1%) |
| Triglycerides | 149 (111, 210) | 168 (119, 230) | 164 (120, 214) | 149 (106, 225) | 159 (118, 229) |
| Cholesterol >240 mg/dL + LDL‐C >155 mg/dL + HDL‐C <40 mg/dL | 3 (1.8%) | 6 (4.8%) | 6 (1.7%) | 3 (2.7%) | 4 (3.8%) |
Data are expressed as medians and IQRs or as frequencies and percentages.
Surrogates of CVD and All‐Cause Mortality in Patients With Histologically Defined NAFLD
Decreasing LDL‐C and increasing HDL‐C are associated with regression of coronary atherosclerosis.17 To assess the CV risk, the number of patients exceeding an LDL/HDL ratio of >3.5 was determined. Although LDL‐C thresholds have been abandoned, the LDL/HDL ratio has historically been defined for primary and secondary prevention of CV events.23 A total of 235 (76.3%) patients exhibited a low risk (cutoff, ≤3.5), whereas 69 (23.7%) of the patients had a high risk for a CV event (cutoff, >3.5) (Table 3). According to the LDL/HDL ratio, significantly fewer patients with NASH were grouped as high risk compared to NAFL (18.5% vs. 26.8%; P = 0.040). There was no significant difference in the number of patients with an LDL/HDL ratio >3.5 when comparing NAFLD activity score (NAS) <4 with NAS ≥4 (26.3% vs. 17.8%; P = 0.082) (Table 3).
Table 3.
CV Event Risk According to the FRS and LDL/HDL Ratio in Patients With Different Histologic Grades
| Variable | n | 10‐year CVD Risk According to FRS* | P Value | n | High Risk According to LDL/HDL* | P Value |
|---|---|---|---|---|---|---|
| Total cohort (N) | 221 | 13.7 (6.3, 25.3) | 304 | 69 (23.7%) | ||
| Advanced fibrosis (≥F2) | 143 | 15.9 (7.3, 28.5) | 0.002 | 186 | 41 (22.0%) | 0.703 |
| Early fibrosis (<F2) | 78 | 10.0 (4.4, 18.5) | 118 | 28 (23.7%) | ||
| NAS ≥4 | 103 | 13.7 (5.3, 25.3) | 0.711 | 129 | 23 (17.8%) | 0.082 |
| NAS <4 | 118 | 13.7 (6.7, 24.8) | 175 | 46 (26.3%) | ||
| NASH | 125 | 15.6 (6.3, 28.5) | 0.099 | 151 | 28 (18.5%) | 0.040 |
| NAFLD | 96 | 12.5 (5.6, 21.5) | 153 | 41 (26.8%) | ||
| Advanced fibrosis + NAS ≥4 | 88 | 15.6 (5.4, 27.7) | 0.660 | 109 | 20 (18.3%) | 0.176 |
| Others | 133 | 13.7 (6.3, 23.2) | 195 | 49 (25.1%) |
Data are expressed as medians and IQRs or as frequencies and percentages.
Absolute cutoffs for cholesterol are frequently used in clinical routine and practice. Within the Framingham Heart Study, a >2.25‐fold risk of atherosclerotic CVD and mortality over 35 years was observed for TC (>240 vs. <165 mg/dL), LDL‐C (>155 vs. <90 mg/dL), and HDL‐C (<40 vs. >70 mg/dL).22 Patients with significant liver disease, defined as NAS ≥4 and fibrosis (F) score ≥F2, exhibited TC >240 mg/dL in 21.7%, LDL‐C >155 mg/dL in 18.3%, and HDL < 40 mg/dL in 32.1% (Table 2).
The FRS is most commonly used to estimate the incident 10‐year risk of nonfatal and fatal arterial CV events or CVD mortality.24 The FRS was calculated in 221 patients without prior CVD.20 A total of 51 patients were excluded for incomplete data, mostly missing systolic blood pressure. According to the FRS, 32.1% of the patients exhibited a high risk, with a predicted CV risk >20% in the next 10 years. At the other end of the CV risk spectrum, 41.2% of the patients were assigned to the low‐risk category (Table 4). When comparing patients with at least intermediate fibrosis on liver biopsy (≥F2) to patients with absent or early fibrosis (≤F1), the ≥F2 group had a significantly higher 10‐year CVD event risk according to the FRS (Table 3). When analyzing patients with relevant liver disease on biopsy defined by an NAS ≥4 and ≥F2 (n = 88, 39%), the following CV risk categories were observed: 15.6% (95% confidence interval [CI], 13.6, 18.0) 10‐year CVD according to the FRS (Table 3), 18.3% high risk according to the LDL/HDL ratio (Table 3), and >2.25‐fold risk of atherosclerotic CV and mortality over 35 years according to either TC, LDL‐C, or HCL‐C in 33.2%. Interestingly, when analyzing the group with less severe liver disease, defined by NAS ≤ 3 and F1 or F0, CVD risk surrogates according to the FRS and the LDL/HDL ratio were comparable to patients with advanced disease (Table 3).
Table 4.
FRS Groups in the Total Cohort
| n = 221 | Low Risk (≤10% 10‐Year CVD Risk) | Intermediate Risk (10%‐20% 10‐Year CVD Risk) | High Risk (>20% 10‐Year CVD Risk) |
|---|---|---|---|
| Male | 50 (22.6%) | 25 (11.3%) | 39 (17.6%) |
| Female | 41 (18.6%) | 34 (15.4%) | 32 (14.5%) |
| Total cohort | 91 (41.2%) | 59 (26.7%) | 71 (32.1%) |
Data are expressed as frequencies and percentages.
Modeling of the CV Risk in Patients With NAFLD and Increasing LDL‐C
Recently, the REGENERATE study investigated the effect of the FXR agonist OCA in NASH and reported positive results meeting its primary endpoint. While OCA improved hepatic fibrosis in 23.1% of patients, this treatment also increased LDL‐C by approximately 20 mg/dL. To explore the impact of this increase in LDL‐C and consequently in TC in patients with histologically confirmed NAFLD, we modeled a rise in LDL‐C by 20 mg/dL in our patients and used the FRS to assess changes in the CV risk profile (Fig. 1). A moderate increase of LDL‐C by 20 mg/dL resulted in 20.7% (n = 63) of patients to move from a low to a high CV risk category based on the LDL/HDL ratio (Table 5). When modeling the FRS and using an increase of TC by 20 mg/dL, this resulted in a median increase of 1.9% for the 10‐year CVD event risk in the entire group and the number needed to harm of 53 (no increase, 13.7% [95% CI, 14.0, 16.6] vs. TC increase, 15.6% [95% CI, 14.7, 17.4]). In patients with significant liver disease, defined by NAS ≥4 and ≥F2, this effect did not differ from the entire cohort. According to the FRS, 6 (2.7%) patients moved from low to intermediate and 11 (4.9%) from intermediate to high CV risk (Table 6). When modeling an increase of TC and LDL‐C by 20 mg/dL, 41.5% of patients exhibited a TC >240 mg/dL and 45.1% an LDL‐C >155 mg/dL.
Figure 1.
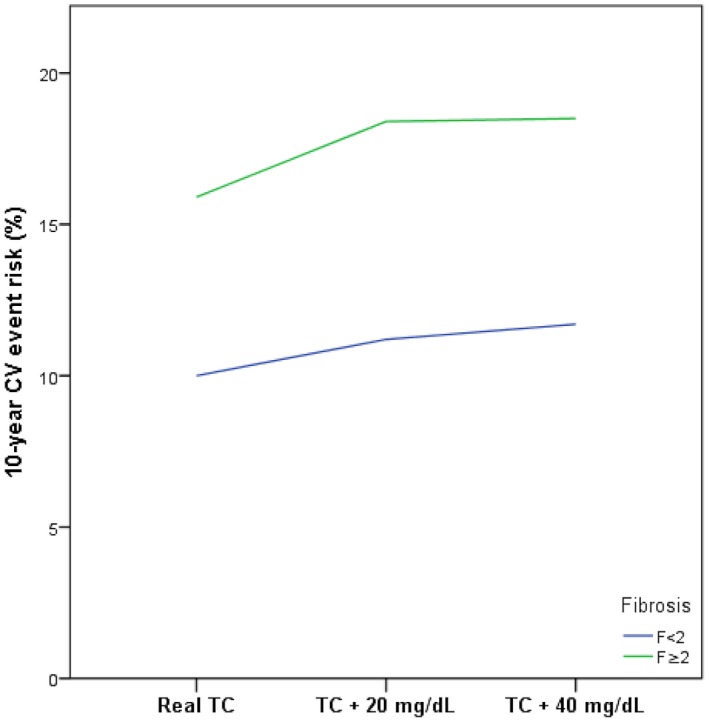
Modulation of the incident 10‐year CV risk using the FRS in patients with histologically confirmed NAFLD and early (<F2) versus significant (≥F2) fibrosis.
Table 5.
Shift Table for LDL/HDL Ratio in the Total Cohort
| Baseline risk | Risk From Treatment (LDL + 20 mg/dL) | |
|---|---|---|
| Low risk | High risk | |
| Low risk | 172 (56.6%) | 63 (20.7%) |
| High risk | 0 | 69 (22.7%) |
Data are expressed as frequencies and percentages.
Table 6.
Shift Table for the FRS
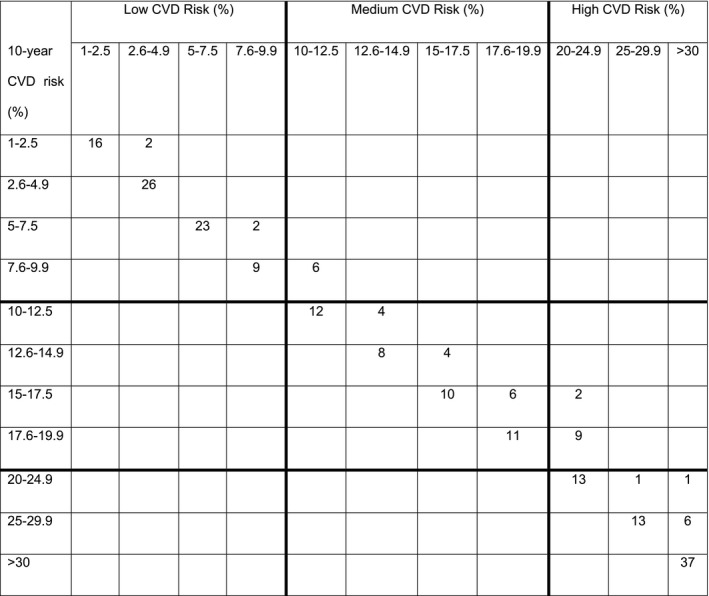
A total of 221 patients were analyzed according to the FRS and classified as low (1%‐9.9%), medium (10%‐19.9%), and high (≥20%) risk. By modeling a 20‐mg/dL increase of TC, 2.7% (6/221) of patients went from a low to a medium and 4.9% (11/211) from a medium to a high CVD risk according to the FRS. Left column shows baseline CVD risk category; top column modeled CVD risk category.
The current American Association of Clinical Endocrinologists (AACE) categorizes patients into extreme, very high, high, moderate, and low risk of a subsequent CV event and defines LDL‐C target corridors.25 In the entire cohort, 33.2% (101/304) were very high risk, mainly based on established CVD and diabetes with one additional risk factor, and 37.5% (114/304) were high risk, mainly defined through two risk factors, or the presence of diabetes.25 When analysing all patients according to FRS categories, only 13/101 in the very high‐risk and 34/114 in the high‐risk groups were below the recommended LDL‐C of <70 mg/dL, or <100 mg/dL, respectively. The remaining 88/101 patients in the very high and 80/114 in the high‐risk groups that were above the recommended LDL‐C level had a low rate of statin prescription, with 24 of the 88 and 21 of the 80 patients being on statins.
Discussion
CV risk factors are highly prevalent in patients with NAFLD, and CV mortality contributes significantly to their overall loss of life time.26 It has been estimated that 5% to 10% of patients with NAFLD die from CVD, and patients with NAFLD exhibit a 2‐fold increased risk of CVD.27, 28 A meta‐analysis exploring more than 17,000 patient‐years observed CVD to be the leading cause of mortality in patients with NAFLD.6 In a recent exploration of the National Health and Nutrition Examination Survey III, a 42% higher overall mortality rate and doubling of the risk of CV mortality for patients with NAFLD were observed.29 This risk was even higher in a robust meta‐analysis that included data of 16 observational studies with a total of 34,043 patients. Here, the presence of NAFLD related to an increase of 64% for fatal or nonfatal CVD, and this risk was higher in patients with more advanced fibrosis or NASH inflammation.30 The number of patients with a history of CVD in the current study was relatively low (5.3%) compared to other cohorts that ranged from 18% in a population‐based cohort31 and 9.3% in a cohort with liver biopsy.27 Additionally, by exploring a referral cohort at an outpatient hepatology center, patients with recent CV events were likely not to be included because treatment of their underlying CVD was a priority at that time. This is also reflected by the absence of patients in the extreme atherosclerotic CVD risk categories according to the AACE in our study cohort.25 Nonetheless, 32.2% of all patients had a high 10‐year risk for development of CVD events according to the FRS, and we report these figures for the first time for a German cohort. When interpreting these data, the descriptive nature must be taken into account and the causality remains to be proven. Nonetheless, there is increasing evidence indicating a link between NAFLD, and especially NASH, and deterioration of atherogenic dyslipidemia, systemic inflammation, insulin resistance, and thrombogenic factors, which can all increase the risk for incident CVD.32, 33
Analysis of Mortality
Currently, several phase 3 trials are underway to investigate the benefit of liver‐directed therapy to induce the resolution of steatohepatitis or regression of fibrosis defined by liver histology. There is ample evidence that advanced fibrosis leads to excess mortality related to CVD and nonhepatic malignancy.6 Additionally, data from the literature suggest that resolution of steatohepatitis improves endothelial dysfunction and lowers CVD risk.32 However, the long‐term benefit and, in particular, effects on total mortality remain to be shown. Any pharmacologic agent used for the treatment of NAFLD is likely to be a long‐term treatment, and therefore safety profiles will have to be beneficial in the overall assessment. In this context, the increase of LDL‐C and TC that is observed from FXR agonists is worrisome if these translate into an increased CV risk. Although an atherogenic potential is of less concern in most patients with primary biliary cholangitis (an indication for which there are approved FXR agonists available), the relevance of LDL‐C and TC increase in NAFLD can only be assessed through long‐term follow‐up. The recently reported phase 3 trial using OCA in NAFLD showed a 11.2% greater benefit from OCA in achieving fibrosis regression of ≥1 stage at 18 months compared to placebo.13 Importantly, the treatment group experienced an increase of TC and LDL‐C by 20 mg/dL on average within the first weeks.13 The increase was transient and counterbalanced by the protocol‐initiated use of statins.
The current study highlights that 32.1% of all patients with biopsy‐proven NAFLD in this German cohort exhibit a high (>20%) risk for developing a CV event in the next 10 years according to the FRS. In the subgroup of patients with significant liver disease, defined as NAS ≥4 and ≥F2, 35.2% exhibit a high risk. When modeling a TC or LDL‐C increase of 20 mg/dL from a hypothetical drug, this led to a 1.9% increase of the median CV event risk. In total, 7.6% of patients changed their FRS risk category from low to medium or medium to high. Although these numbers seem to be low, a total of 32.5% of patients were considered at high risk of developing a CV event in the next 10 years, and the inability to lower their LDL‐C with statins into the target range according to AACE guidelines could translate into increased mortality. Therefore, once an FXR agonist for the treatment of NASH has been approved, the individual assessment of the CV risk before the initiation of liver‐directed therapy could become important. If the FXR‐mediated effect occurs transiently and LDL‐C target levels can be reached, the addition of a liver‐directed drug will potentially further add to the benefit for the patient. Importantly, second‐generation nonsteroidal FXR agonists that are currently explored in phase 2 trials could overcome these limitations because their safety profile and effects on lipids seem to be less pronounced. The limitations of FXRs in patients with high‐risk CVD could also be potentially overcome by using combination therapies of metabolically active anti‐inflammatory and antifibrotic compounds, allowing for adjusting the dose of the steroidal FXR or counterbalancing the metabolic adverse effects.
One major finding in the current study is that only a minority of patients received statin therapy. This also held true for patients in the very high and high CVD risk categories who did not reach their AACE‐recommended LDL‐C goals. This is troublesome because there is excellent evidence supporting the use of statins to decrease overall mortality in patients without prior CV events.34 The underlying causes are likely multicausal. Idiosyncratic hepatotoxicity is among the main concerns, and it can be severe,35 although it occurs in very few cases. Thus, elevated liver function tests can be a trigger leading to the discontinuation or posing a barrier to initiating treatment with statins in the examined cohort. Importantly, most idiosyncratic drug injury patterns were associated with a cholestatic laboratory profile, in particular when using highly potent statins, for example, atorvastatin.35 Thus, close monitoring and education of prescribers can help to distinguish liver injury from underlying NAFLD and idiosyncratic drug‐induced liver injury. Beyond the CVD risk, statins have been attributed to a favorable effect on hepatocellular carcinoma incidence in a case‐control study,36 and thus their use can be beneficial from both a liver and CVD perspective. Additional measures to improve the CV risk could be the addition of omega n‐3 fatty acid supplements that were recently shown to decrease CV risk.37
Our study has limitations. First, the current analysis enrolled patients who were referred to a tertiary outpatient clinic for evaluation of liver disease, thus introducing a selection bias with weight on more advanced patients. Still, the cohort was fairly balanced, with 49.7% of the patients exhibiting NASH and an equal distribution of fibrosis stages.38 Additionally, the implementation of a prospective study protocol within the scope of the European NAFLD registry ensured standardized workup and comparability to published cohorts.38 A second limitation is the inability to predict the CVD event risk using the FRS in 51 patients, mostly due to missing data.
In conclusion, our study explores a biopsy‐proven German NAFLD cohort and classifies them according to their CV event risk. The overall CV risk is high; however, only a minority of patients received statin therapy. Additionally, the used model suggested that a small but potentially relevant risk increase in the FRS occurs if FXR‐based therapy leads to increases in LDL‐C. Therefore, management of CV risk is likely to be center stage in the complex management of patients with NAFLD over the upcoming years, especially when liver‐directed drugs become available.
Acknowledgment
We thank the patients who participated in the study.
Supported in part by the University Medical Center Mainz (intramural funds to J.M.S.).
Potential conflict of interest: Dr. Galle advises and consults for Novartis. Dr. Schattenberg received grants from and consults for Gilead Sciences; he is on the speakers’ bureau for Falk Foundation, Takeda, and MSD and received grants from Yakult Europe B.V.; he consults for AbbVie, BMS, BBN Cardio, Boehringer Ingelheim, Galamed, Genfit, Intercept Pharmaceuticals, IQVIA, MedImmune, Novartis, and Pfizer. The other authors have nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Estes C, Anstee QM, Arias‐Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol 2018;69:896‐904. [DOI] [PubMed] [Google Scholar]
- 2. Schattenberg JM, Schuppan D. Nonalcoholic steatohepatitis: the therapeutic challenge of a global epidemic. Curr Opin Lipidol 2011;22:479‐488. [DOI] [PubMed] [Google Scholar]
- 3. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 4. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 5. Huber Y, Pfirrmann D, Gebhardt I, Labenz C, Gehrke N, Straub BK, et al. Improvement of non‐invasive markers of NAFLD from an individualised, web‐based exercise program. Aliment Pharmacol Ther 2019; 10.1111/apt.15427. [DOI] [PubMed] [Google Scholar]
- 6. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology 2017;65:1557‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeb I, Li D, Budoff MJ, Katz R, Lloyd‐Jones D, Agatston A, et al. Nonalcoholic fatty liver disease and incident cardiac events: the multi‐ethnic study of atherosclerosis. J Am Coll Cardiol 2016;67:1965‐1966. [DOI] [PubMed] [Google Scholar]
- 8. Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year‐community study. Hepatology 2018;67:1726‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. VanWagner LB, Wilcox JE, Colangelo LA, Lloyd‐Jones DM, Carr JJ, Lima JA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: a population‐based study. Hepatology 2015;62:773‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2018;15:425‐439. [DOI] [PubMed] [Google Scholar]
- 11. Ratziu V. A critical review of endpoints for non‐cirrhotic NASH therapeutic trials. J Hepatol 2018;68:353‐361. [DOI] [PubMed] [Google Scholar]
- 12. Center for Drug Evaluation and Research (CDER) . Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment Guidance for Industry. Rockville, MD: U.S. Department of Health and Human Services Food and Drug Administration; 2018, downloaded July 2019 from https://www.fda.gov/media/119044. [Google Scholar]
- 13. Younossi Z, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al.; REGENERATE Study Investigators . GS‐06‐positive results from REGENERATE: a phase 3 international, randomized, placebo‐controlled study evaluating obeticholic acid treatment for NASH. J Hepatol 2019;70(Suppl.):e5. [Google Scholar]
- 14. Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al.; NASH Clinical Research Network . Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 2015;385:956‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pencek R, Marmon T, Roth JD, Liberman A, Hooshmand‐Rad R, Young MA. Effects of obeticholic acid on lipoprotein metabolism in healthy volunteers. Diabetes Obes Metab 2016;18:936‐940. [DOI] [PubMed] [Google Scholar]
- 16. Halilbasic E, Claudel T, Trauner M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J Hepatol 2013;58:155‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, et al. Statins, high‐density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007;297:499‐508. [DOI] [PubMed] [Google Scholar]
- 18. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al.; International Diabetes Federation Task Force on Epidemiology and Prevention ; Hational Heart, Lung, and Blood Institute ; American Heart Association ; World Heart Federation ; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640‐1645. [DOI] [PubMed] [Google Scholar]
- 19. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 20. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743‐753. [DOI] [PubMed] [Google Scholar]
- 21. Kastelein JJ, van der Steeg WA, Holme I, Gaffney M, Cater NB, Barter P, et al.; TNT Study Group ; IDEAL Study Group . Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation 2008;117:3002‐3009. [DOI] [PubMed] [Google Scholar]
- 22. Duncan MS, Vasan RS, Xanthakis V. Trajectories of blood lipid concentrations over the adult life course and risk of cardiovascular disease and all‐cause mortality: observations from the Framingham study over 35 years. J Am Heart Assoc 2019;8:e011433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Millan J, Pinto X, Munoz A, Zuniga M, Rubies‐Prat J, Pallardo LF, et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 2009;5:757‐765. [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837‐1847. [DOI] [PubMed] [Google Scholar]
- 25. Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr Pract 2017;23(Suppl. 2):1‐87. [DOI] [PubMed] [Google Scholar]
- 26. Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol 2012;10:646‐650. [DOI] [PubMed] [Google Scholar]
- 27. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 28. Spahillari A, Mukamal KJ, DeFilippi C, Kizer JR, Gottdiener JS, Djousse L, et al. The association of lean and fat mass with all‐cause mortality in older adults: The Cardiovascular Health Study. Nutr Metab Cardiovasc Dis 2016;26:1039‐1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Golabi P, Paik J, Fukui N, Locklear CT, de Avilla L, Younossi ZM. Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher risk for mortality. Clin Diabetes 2019;37:65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non‐alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta‐analysis. J Hepatol 2016;65:589‐600. [DOI] [PubMed] [Google Scholar]
- 31. Adams LA, Anstee QM, Tilg H, Targher G. Non‐alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138‐1153. [DOI] [PubMed] [Google Scholar]
- 32. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330‐344. [DOI] [PubMed] [Google Scholar]
- 33. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015;62(Suppl):S47‐S64. [DOI] [PubMed] [Google Scholar]
- 34. Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;316:2008‐2024. [DOI] [PubMed] [Google Scholar]
- 35. Bjornsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post‐marketing. J Hepatol 2012;56:374‐380. [DOI] [PubMed] [Google Scholar]
- 36. Kim G, Jang SY, Nam CM, Kang ES. Statin use and the risk of hepatocellular carcinoma in patients at high risk: a nationwide nested case‐control study. J Hepatol 2018;68:476‐484. [DOI] [PubMed] [Google Scholar]
- 37. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al.; VITAL Research Group. Marine n‐3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Labenz C, Huber Y, Kalliga E, Nagel M, Ruckes C, Straub BK, et al. Predictors of advanced fibrosis in non‐cirrhotic non‐alcoholic fatty liver disease in Germany. Aliment Pharmacol Ther 2018;48:1109‐1116. [DOI] [PubMed] [Google Scholar]


