Key Points
Question
Is hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin associated with improved outcomes in patients with unresectable intrahepatic cholangiocarcinoma?
Findings
In this single-arm, phase 2 clinical trial, 38 patients with unresectable intrahepatic cholangiocarcinoma were treated with hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin, with 58% of the patients achieving an objective radiographic response and 84% achieving disease control. Median overall survival was 25.0 months, and 4 patients had sufficient response to undergo resection; lymph node status did not appear to be associated with clinical benefit.
Meaning
Combination hepatic arterial infusion floxuridine with systemic chemotherapy appears to be clinically active in patients with unresectable intrahepatic cholangiocarcinoma and should be investigated further.
Abstract
Importance
Unresectable intrahepatic cholangiocarcinoma (IHC) carries a poor prognosis, with a median overall survival (OS) of 11 months. Hepatic arterial infusion (HAI) of high-dose chemotherapy may have potential benefit in these patients.
Objective
To evaluate clinical outcomes when HAI chemotherapy is combined with systemic chemotherapy in patients with unresectable IHC.
Design, Setting, and Participants
A single-institution, phase 2 clinical trial including 38 patients was conducted with HAI floxuridine plus systemic gemcitabine and oxaliplatin in patients with unresectable IHC at Memorial Sloan Kettering Cancer Center between May 20, 2013, and June 27, 2019. A confirmatory phase 1/2 study using the same therapy was conducted during the same time period at Washington University in St Louis. Patients with histologically confirmed, unresectable IHC were eligible. Resectable metastatic disease to regional lymph nodes and prior systemic therapy were permitted. Patients with distant metastatic disease were excluded.
Interventions
Hepatic arterial infusion of floxuridine and systemic administration of gemcitabine and oxaliplatin.
Main Outcomes and Measures
The primary outcome was progression-free survival (PFS) of 80% at 6 months.
Results
For the phase 2 clinical trial at Memorial Sloan Kettering Cancer Center, 42 patients with unresectable IHC were included and, of these, 38 patients were treated (13 [34%] men; median [range] age at diagnosis, 64 [39-81] years). The median follow-up was 30.5 months. Twenty-two patients (58%) achieved a partial radiographic response, and 32 patients (84%) achieved disease control at 6 months. Four patients had sufficient response to undergo resection, and 1 patient had a complete pathologic response. The median PFS was 11.8 months (1-sided 90% CI, 11.1) with a 6-month PFS rate of 84.1% (90% CI, 74.8%-infinity), thereby meeting the primary end point (6-month PFS rate, 80%). The median OS was 25.0 months (95% CI, 20.6-not reached), and the 1-year OS rate was 89.5% (95% CI, 80.2%-99.8%). Patients with resectable regional lymph nodes (18 [47%]) showed no difference in OS compared with patients with node-negative disease (24-month OS: lymph node negative: 60%; 95% CI, 40%-91% vs lymph node positive: 50%; 95% CI, 30%-83%; P = .66). Four patients (11%) had grade 4 toxic effects requiring removal from the study (1 portal hypertension, 2 gastroduodenal artery aneurysms, 1 infection in the pump pocket). Subgroup analysis showed significant improvement in survival in patients with IDH1/2 mutated tumors (2-year OS, 90%; 95% CI, 73%-99%) vs wild-type (2-year OS, 33%; 95% CI, 18%-63%) (P = .01). In the Washington University in St Louis confirmatory cohort, 9 patients (90%) achieved disease control at 6 months; the most common grade 3 toxic effect was elevated results of liver function tests, and median PFS was 12.8 months (1-sided 90% CI, 6.4).
Conclusions and Relevance
Hepatic arterial infusion plus systemic chemotherapy appears to be highly active and tolerable in patients with unresectable IHC; further evaluation is warranted.
This phase 2 clinical trial evaluates the use of hepatic arterial infusion of floxuridine plus systemic administration of gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma.
Introduction
Intrahepatic cholangiocarcinoma (IHC) is rare but increasing in incidence and mortality largely owing to an increase in the prevalence of risk factors, in particular, nonalcoholic steatohepatitis and hepatitis C.1,2,3,4,5,6 The IHC cure rate is low and generally achievable only after resection of a solitary tumor; even in this case, 60% of patients develop recurrent disease.7,8 Furthermore, most patients present with unresectable or distant metastatic disease, for which the prognosis is poor.7,9,10
Currently, the standard systemic therapy for IHC remains platinum-based chemotherapy in combination with gemcitabine. Cisplatin improves median overall survival (OS) from 8.1 months with gemcitabine alone to 11.7 months with the combination.11 The combination of gemcitabine and oxaliplatin has demonstrated similar efficacy.12 Targeting actionable genomic alterations, specifically IDH1 mutations and FGFR2 fusions,13 is a potential approach for IHC, which remains to be determined by ongoing clinical trials (eg, ivosidenib14).
Most patients with advanced IHC present with disease confined to the liver that is unresectable owing to tumor location and/or multifocal involvement. Liver-directed therapy via a hepatic arterial infusion (HAI) pump enables the delivery of high-dose chemotherapy directly into the liver (eFigure 1 in the Supplement). The liver’s dual blood supply preferentially delivers high doses of chemotherapeutic agents to the hepatic artery, which supplies nearly all of the tumor’s blood flow, while blood delivered by the portal vein maintains the health of the nonneoplastic liver parenchyma.15,16 Because the liver clears the chemotherapy via first-pass metabolism, this approach diminishes systemic toxic effects. Given that advanced disease within the liver accounts for most unresectable cases, continuous HAI chemotherapy is particularly well suited to cancers in the liver. With this approach, floxuridine, a precursor of fluorouracil, is the most active agent, achieving much higher tumor drug levels than systemic administration.17,18
A prior study of HAI floxuridine in patients with advanced IHC19 found a response rate of 45% and an OS of 29 months. A subsequent study found that the addition of bevacizumab to HAI floxuridine increased biliary toxic effects without a meaningful improvement in response or OS compared with HAI floxuridine alone.20 The present study sought to build on liver-directed therapy with the addition of systemic gemcitabine and oxaliplatin chemotherapy. Gemcitabine and oxaliplatin was chosen owing to its efficacy similar to gemcitabine and cisplatin and because the administration schedule coincided best with HAI therapy. Herein, we report the results of HAI floxuridine plus systemic administration of gemcitabine and oxaliplatin in patients with unresectable IHC treated at 2 medical centers.
Methods
Study Design and Participants
From May 20, 2013, to June 27, 2019, we conducted a single-arm phase 2 study of HAI floxuridine and systemic gemcitabine and oxaliplatin in patients with unresectable IHC at Memorial Sloan Kettering Cancer Center and a phase 1/2 study of the same therapy at Washington University in St Louis (NCT01862315). All patients had histologically confirmed, measurable, unresectable IHC. Unresectability was confirmed by hepatobiliary surgeons after multidisciplinary review and inability to achieve an R0 resection owing to extensive vascular and/or biliary involvement, multifocal liver disease, regional lymph node metastases, or a combination of these factors. Prior systemic therapy was permitted. Metastatic disease to regional lymph nodes (porta hepatis, porto-caval, common hepatic artery) was permitted as long as the nodes were resectable at the time of pump placement. Pretreatment evaluation details can be found in the eMethods in the Supplement. The study was approved by the institutional review boards at Memorial Sloan Kettering Cancer Center, New York, New York, and Washington University in St Louis, St Louis, Missouri. Written informed consent was obtained from all participants. Participants did not receive financial compensation.
Surgical HAI Pump Placement
All patients underwent a hepatic computed tomographic angiogram preoperatively to evaluate hepatic arterial blood supply. Guidelines for pump placement have been previously reported.15 Additional details of pump placement can be found in the eMethods in the Supplement. Regional lymph nodes, as defined above, were removed at the time of pump placement.
Treatment
Hepatic arterial infusion chemotherapy was initiated 2 weeks after pump placement on a 4-week cycle (eTable 1 in the Supplement). Additional details of the treatment schema, chemotherapy dosing and administration, and toxic effect grading can be found in the eMethods in the Supplement. Treatment with HAI floxuridine and systemic gemcitabine and oxaliplatin was continued until toxic effects developed; oxaliplatin was discontinued early in most patients to prevent neurotoxic effects. At Washington University in St Louis, because HAI therapy was in the early stages of use, the phase 2 protocol was initiated after an initial phase 1 study confirmed the safety of this treatment approach. The results are reported herein separately as a confirmatory cohort.
Outcomes
The primary outcome was 6-month PFS. Secondary analyses included response rate, OS, and safety and toxic effects, as well as genomic analyses, dynamic contrast–enhanced magnetic resonance imaging, diffusion-weighted imaging, and circulating tumor DNA as a potential biomarker. The results of the imaging and biomarker studies will be reported separately.
Tumor Genomic Analyses
Tumor DNA and matched germline DNA from prospectively collected blood samples were analyzed using the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) deep sequencing assay.21 Additional details can be found in the eMethods in the Supplement.
Statistical Analysis
Previous data report a median PFS of 6 to 8 months for patients treated with systemic chemotherapy.11 The single-stage study design was built around a null rate of 60% PFS at 6 months and required 36 patients for detection of an absolute difference of 20% for a target rate of 80% PFS at 6 months. The trial was designed to have a 10% type 1 error (1-sided) and 90% power. Progression-free survival was calculated from the date of pump placement until the date of progression or death, whichever occurred first. Further details can be found in the eMethods in the Supplement. Progression-free survival was estimated using Kaplan-Meier methods and per protocol design, the 1-sided 90% CI is presented for overall median PFS and 6-month PFS. A sensitivity analysis of PFS was also conducted separately in both cohorts in which patients for whom participation ended for reasons other than progression of disease (eg, toxic effects) but continued to receive systemic treatment and had routine scans were followed up until the first documented progression of disease or death, whichever occurred first. In the sensitivity PFS analysis, patients who were able to undergo resection were censored at the time of surgery.
Secondary objectives included OS, overall best response rate, and genomic analysis. Overall survival was calculated from the date of pump placement until the date of last follow-up and estimated using Kaplan-Meier methods along with the 95% CI. For PFS and OS, patients alive and without an event of interest were censored at the date of the last follow-up or at the cutoff date of June 27, 2019. Overall best response rate was defined as partial response and stable disease. Response rates were calculated based on Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1,22 and estimated using binomial distribution along with the 95% CI.
For the genomic analysis, common recurrent mutations were defined as those occurring in 4 or more patients. Univariate Cox regression was used to test for associations between outcomes and genomic alterations, clinical risk factors (age at diagnosis, largest tumor size, tumor differentiation, lymph node status, sex, and multifocal disease), and baseline biomarkers (aspartate aminotransferase, alanine aminotransferase, bilirubin, alkaline phosphatase, carcinoembryonic antigen, and cancer antigen 19-9 levels). Baseline biomarkers were transformed using natural logarithm transformation.
All P values were 2-sided with type 1 error of 5%; P < .05 was considered statistically significant. All statistical analyses were performed using SAS, version 9.3 (SAS Institute Inc) or R, version 3.3.2 (R Foundation).
Results
Patient Characteristics
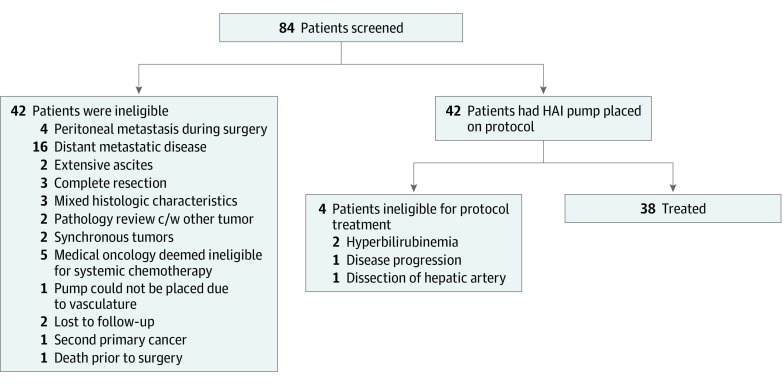
A total of 42 patients were enrolled. Thirty-eight patients (13 [34%] men; median [range] age at diagnosis, 64 [39-81] years) with unresectable IHC were treated with HAI floxuridine and systemic gemcitabine and oxaliplatin at Memorial Sloan Kettering Cancer Center (Figure 1). No patients were lost to follow-up. Patient characteristics are presented in Table 1. In the treated cohort, the reasons for unresectability were multifocal disease (21 [55%]), histologically proven lymph node involvement (18 [47%]), and/or anatomic location of the tumor with involvement of multiple major inflow pedicles or hepatic veins vessels (12 [32%]). Three patients (8%) required biliary stent placement prior to HAI pump placement. The median time from diagnosis to HAI pump placement was 1.5 months (range, 0.5-10.2). The median time from pump placement to start of therapy was 0.57 months (range, 0.26-1.0).
Figure 1. Patient Screening and Enrollment Flowchart.
Abbreviation: c/w indicates consistent with; HAI, hepatic arterial infusion.
Table 1. Patient Characteristics (n = 38).
| Characteristic | No. (%) |
|---|---|
| Age at diagnosis, median (range), y | 64 (39-81) |
| Sex, No. (%) | |
| Men | 13 (34) |
| Women | 25 (66) |
| Karnofsky performance status, median (range) | 80 (70-100) |
| Prior chemotherapy, No. (%) | |
| No prior | 35 (92) |
| Gemcitabine/cisplatin | 2 (5) |
| Folfirinox | 1 (3) |
| Histologic grade, No. (%) | |
| Differentiated | |
| Well | 3 (8) |
| Moderately | 19 (50) |
| Poorly | 14 (37) |
| Unknown | 2 (5) |
| Tumor size prior to treatment, median (range), cm | 8.3 (1.7-24.8) |
| Disease, No. (%) | |
| Bilobar | 25 (66) |
| Multifocal | 21 (55) |
| Lymph node disease, No. (%) | |
| Positive | 18 (47) |
| Negative | 16 (42) |
Radiographic Response and Survival
The median follow-up at the time of data cutoff (June 27, 2019) among survivors was 30.5 months (range, 17.2-56.5). As of that date, 4 patients (11%) continued to receive study treatment. Reasons for treatment discontinuation included disease progression (25 [66%]), toxic effects (4 [11%]), resection due to response (4 [11%]), and withdrawal of consent (1 [3%]).
Overall, 32 patients (84%) achieved disease control at 6 months, with 22 patients (58%) demonstrating a partial radiographic response. Four patients had sufficient response to undergo resection and 1 had a complete pathologic response (Figure 2A). At the time of analysis, 25 of the 38 patients (66%) had progression of disease. Most patients (18 [47%]) had progression outside of the dominant tumor, with the most common sites of progression being satellite liver lesions (8 [21%]), lungs (5 [13%]), peritoneum (3 [8%]), and lymph nodes (3 [8%]).
Figure 2. Hepatic Arterial Infusion Chemotherapy With Floxuridine Plus Systemic Gemcitabine and Oxaliplatin.
A, Percent change from baseline in target lesion as assessed per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria in all patients (n = 38). Prior chemotherapy and resection status are noted. The squares represent patients; black indicates that the patient underwent resection or had prior chemotherapy. B, Progression-free survival. C, Overall survival.
The study met its primary end point of 6-month PFS of 80%; clinical response data are summarized in eTable 3 in the Supplement. The median PFS was 11.8 months (1-sided 90% CI, 11.1) with a 6-month PFS of 84.1% (90% CI, 74.8%-infinity) (Figure 2B). Sensitivity analysis yielded a similar estimate for median PFS. Three patients received chemotherapy prior to pump insertion; 1 of these patients died due to disease 3.3 months after pump placement, and the other 2 patients were alive without progression at 5.2 and 16.0 months. A total of 3 patients were pretreated and 35 patients were chemotherapy naive. The 6-month PFS was 67% (95% CI, 30.0%-99.9%) for pretreated patients and 89% (95% CI, 78.6%-99.7%) for chemotherapy-naive patients (P = .70). In addition, median PFS did not differ between node-positive and node-negative patients (11.5 vs 14.6 months, P = .37) (eFigure 2 in the Supplement).
At the time of analysis, we observed 21 deaths. The median OS was 25.0 months (95% CI, 20.6-not reached) (Figure 2C). The 1-year OS rate was 89.5% (95% CI, 80.2%-99.8%). Patients with node-negative disease showed no difference in survival from those with histologically proven nodal disease (24-month OS: lymph node negative: 60%; 95% CI, 40%-91% vs lymph node positive: 50%; 95% CI, 30%-83%; P = .66) (eFigure 3 in the Supplement).
Somatic mutation data from targeted next-generation sequencing were available for 33 patients (eFigure 4 in the Supplement). The most commonly mutated loci were IDH1/2 (147700/147650 OMIM) (30.3%), BAP1 (603089 OMIM) (27.2%), CDKN2A (600160 OMIM) (18.1%), TP53 (191170 OMIM) (12.1%), ARID1A (603024 OMIM) (12.1%), FGFR2 (176943 OMIM) (12.1%), and PBRM1 (606083 OMIM) (12.1%). IDH1/2 genomic alterations were significantly associated with a benefit in OS (P = .01), with a 2-year OS of 90% (95% CI, 73%-99%) in the IDH1/2 mutant group vs 33% (95% CI, 18%-63%) in the IDH1/2 wild-type group. The median PFS in the IDH1/2 mutant group was 14.1 months (95% CI, 11.5-not reached) vs 10.9 months (95% CI, 7.8-14.8) in the wild-type group (P = .42). None of the other patient/tumor characteristics examined were associated with a significant survival benefit (eFigure 5 in the Supplement).
Toxic Effects and Chemotherapy Dose Modifications
The most common grade 3 and 4 adverse events are reported in Table 2 and were commonly related to elevated levels shown in liver function tests. Four patients (11%) required biliary stents: 2 owing to chemotherapy-induced biliary sclerosis and 2 owing to progression of the primary tumor following discontinuation of protocol therapy. Four patients (11%) had grade 4 toxic effects requiring removal from the study, including 1 for portal hypertension, 2 for gastroduodenal artery aneurysm, and 1 for infection in the pump pocket.
Table 2. Adverse Events (n = 38).
| Toxic Effects | No. (%) | |
|---|---|---|
| Grade 3 | Grade 4 | |
| Elevated levels | ||
| Alanine aminotransferase | 16 (42) | 2 (5) |
| Aspartate aminotransferase | 13 (34) | 2 (5) |
| Bilirubin | 7 (18) | 2 (5) |
| Alkaline phosphatase | 5 (13) | 0 |
| Lipase | 0 | 2 (5) |
| Serum amylase | 0 | 1 (3) |
| Blood glucose | 2 (5) | 1 (3) |
| Decreased levels | ||
| Hemoglobin | 6 (16) | 0 |
| Platelet count | 1 (3) | 0 |
| Lymphocytes | 2 (5) | 1 (3) |
| Phosphate | 5 (13) | 0 |
| Sodium | 3 (8) | 0 |
| Potassium | 1 (3) | 0 |
| Albumin | 1 (3) | 0 |
| Ascites | 1 (3) | 0 |
| Nausea | 1 (3) | 0 |
| Abdominal pain | 4 (11) | 0 |
In the initial 3 months of therapy, 27 patients (71%) received the expected dose of floxuridine. This number decreased to 18 patients (47%) receiving the expected dose over the next 6 months (eTable 2 in the Supplement) consistent with prior HAI floxuridine studies.19,20 The most common reasons for discontinuation and dose reduction of floxuridine included elevated levels shown on liver function tests and abdominal pain. Gemcitabine was most commonly discontinued due to thrombocytopenia (3 [8%]), fatigue (4 [11%), abdominal pain (4 [11%]), and poor tolerance (3 [8%]). Oxaliplatin was discontinued after 8 cycles in most patients (30 [80%]) for prevention of neurotoxic effects. Of the patients who continued to receive treatment, gemcitabine was most often continued (8 [21%]) even after stopping floxuridine and oxaliplatin.
Confirmatory Cohort
Ten patients with unresectable IHC were enrolled and treated with HAI floxuridine plus systemic gemcitabine and oxaliplatin at Washington University in St Louis. The median age was 63 years (range, 45-80). Most patients (9 [90%]) had moderately to poorly differentiated tumors, 7 patients (70%) had multifocal disease, and 2 patients (20%) had lymph node involvement. Two patients (20%) received prior chemotherapy: 1 patient received gemcitabine and cisplatin and the other received capecitabine and oxaliplatin.
The most common grade 3 toxic effects included elevated levels noted in liver function test results, including alanine aminotransferase (7 [70%]), aspartate aminotransferase (3 [30%]), and bilirubin (1 [10%]); anemia (1 [10%]); and abdominal pain (1 [10%]). Grade 4 toxic effects included elevated alanine aminotransferase levels (2 [20%]) and hyperbilirubinemia (1 [10%]). Three patients (30%) had grade 4 toxic effects requiring removal from the study: 1 for gastroduodenal artery aneurysm, 1 for extravasation related to the HAI catheter, and 1 for hyperbilirubinemia.
Nine patients (90%) achieved disease control at 6 months, with 5 patients (50%) demonstrating a partial radiographic response; 2 patients (20%) underwent resection. One patient had progression of disease as the best response. This patient received 1 dose of HAI floxuridine and systemic gemcitabine and oxaliplatin and was then removed from the study owing to clinical decline. By sensitivity analysis, the median PFS was 12.8 months (1-sided 90% CI, 6.4), the 1-year OS rate was 70% (95% CI, 46%-100%), and the 2-year OS rate was 40% (95% CI, 19%-85%).
Discussion
The combination of HAI floxuridine plus systemic gemcitabine and oxaliplatin in patients with unresectable IHC was associated with clinical response in comparison with published data on systemic chemotherapy alone.11,12 This finding is consistent with prior studies of HAI therapy in unresectable IHC.19,20 Herein, we observed a response rate of 58% and excellent disease control of 84% in the primary tumor at 6 months. These results are likely related to the high dose of floxuridine delivered continuously into the liver via the HAI pump. With the HAI approach, diffuse distribution also permits perfusion of multifocal lesions. Unlike other locoregional approaches, such as radioembolization (y90), radiation, or chemoembolization, HAI chemotherapy is not limited by tumor size or location.23,24,25
All patients experienced response or disease control in the primary tumor, and the responses were durable in most. More than 70% of the patients developed progression of disease outside of the dominant liver mass. It is conceivable that this initial robust response in the dominant mass helped to prevent liver-related complications, such as biliary obstruction and portal vein occlusion, which are associated with clinical sequelae that can lead to interruption of systemic therapy. Furthermore, the sustained response of the primary tumor likely led to longer survival. A recent study26 reported that use of systemic gemcitabine, cisplatin, and nab-paclitaxel in patients with advanced biliary tumors, including 38 patients with IHC, led to a comparable PFS of 12.9 months and a median OS of 19.0 months in the subgroup with liver-only disease. A post hoc analysis of the ABC-01, ABC-02, and ABC-03 clinical trials evaluated survival and PFS in patients with locally advanced IHC.27 Sixty-six patients (60.6%) received gemcitabine and cisplatin, and PFS was 8.4 months and OS was 16.7 months. In both of these studies, the median OS was shorter than the median OS of 25.0 months achieved in our study. This difference is also supported by retrospective data suggesting that control of hepatic disease is associated with better survival in patients with unresectable IHC.28
In our cohort, subgroup analysis showed that patients with node-positive disease had similar survival outcomes as patients with node-negative disease. Prior retrospective analyses reported worse survival in node-positive patients treated with systemic chemotherapy alone vs the combination of HAI therapy and systemic platinum and gemcitabine chemotherapy.29 In patients with node-positive disease, the microscopic tumor burden is expected to be higher and outcomes worse; however, in the present study, there was no significant difference at 2 years, suggesting that the addition of systemic chemotherapy benefited node-positive patients. It is known that HAI-delivered floxuridine perfuses the porta hepatis tissue; whether this offers some additional advantage in node-positive patients is unclear. In addition, this finding suggests that adequate and early treatment of the primary tumor portends a longer survival owing to maintenance of adequate liver function and less tumor burden, even with metastatic disease. Although most patients experienced response, only 4 underwent resection, reflecting that many tumors remain involved with major vascular structures despite significant disease regression.
Consistent with published data, the most common somatic genomic alterations in our cohort were hotspot mutations in IDH1/2, BAP1, and TP53. Published data do not indicate that IDH1 mutations are prognostic in cholangiocarcinoma.13 Our data suggest that the presence of IDH1/2 alterations was protective and associated with a statistically significant improvement in OS. Four of the 10 patients with IDH1 mutations later received investigational IDH1-targeted therapy (ivosidenib; NCT02989857, NCT02073994),30 and 2 patients underwent resection of all visible disease. Because of the small sample size, it is possible that the survival advantage seen in our analyses without PFS benefit may be due to resection and subsequent IDH1-directed therapy. However, given that 2 of the 4 patients who underwent resection had IDH1-mutated tumors, it is also possible that IDH1/2 mutation conferred increased sensitivity to HAI floxuridine therapy. Studies in other tumor types, particularly glioma and acute myeloid leukemia, have shown differences in therapeutic responsiveness and prognosis between IDH wild-type and mutant tumors.31,32 Studies in larger cohorts are necessary to confirm the associations between IDH mutations and treatment response.
Limitations
This study has several limitations. As with all single-institution phase 2 trials, there is the possibility of selection bias owing to small sample size. In addition, our small confirmation cohort suggests that HAI therapy can be administered at other institutions with comparable safety and efficacy, supporting broader applicability. However, a larger number of institutions is needed to validate this.
Conclusions
Liver-directed floxuridine in combination with systemic gemcitabine and oxaliplatin chemotherapy appears to show good clinical activity and appears to be safe and tolerable in patients with unresectable IHC in this prospective study compared with contemporary results in this population.11 Given these results, a multicenter randomized study of HAI pump-based therapy is warranted to confirm our results and determine whether liver-directed therapy should be incorporated into first-line treatment for patients with unresectable IHC.
eMethods. Detailed Methodology
eReferences.
eTable 1. Treatment Schema
eTable 2. Chemotherapy and Hepatic Arterial Infusions Floxuridine Dosing
eTable 3. Clinical Response in Phase II Cohort (n = 38)
eFigure 1. Hepatic Arterial Infusion Pump Chemotherapy for Intrahepatic Cholangiocarcinoma
eFigure 2. Progression-Free Survival in the MSK Cohort by Lymph Node Status
eFigure 3. Overall Survival in the MSK Cohort by Lymph Node
eFigure 4. The Oncoprint of Mutations Found in 33 Patients Enrolled in the Study
eFigure 5. Forest Plot Showing the Relative Hazard Ratio (HR) for Overall Survival According to Patient Characteristics at Baseline
References
- 1.Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37(6):806-813. doi: 10.1016/S0168-8278(02)00297-0 [DOI] [PubMed] [Google Scholar]
- 2.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the US: intrahepatic disease on the rise. Oncologist. 2016;21(5):594-599. doi: 10.1634/theoncologist.2015-0446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor-Robinson SD, Toledano MB, Arora S, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968-1998. Gut. 2001;48(6):816-820. doi: 10.1136/gut.48.6.816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1198-1203. doi: 10.1158/1055-9965.EPI-05-0811 [DOI] [PubMed] [Google Scholar]
- 5.Welzel TM, McGlynn KA, Hsing AW, O’Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98(12):873-875. doi: 10.1093/jnci/djj234 [DOI] [PubMed] [Google Scholar]
- 6.Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149(6):565-574. doi: 10.1001/jamasurg.2013.5137 [DOI] [PubMed] [Google Scholar]
- 7.Spolverato G, Kim Y, Alexandrescu S, et al. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann Surg Oncol. 2016;23(1):235-243. doi: 10.1245/s10434-015-4642-9 [DOI] [PubMed] [Google Scholar]
- 8.Doussot A, Gönen M, Wiggers JK, et al. Recurrence patterns and disease-free survival after resection of intrahepatic cholangiocarcinoma: preoperative and postoperative prognostic models. J Am Coll Surg. 2016;223(3):493-505.e2. doi: 10.1016/j.jamcollsurg.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193(4):384-391. doi: 10.1016/S1072-7515(01)01016-X [DOI] [PubMed] [Google Scholar]
- 10.Endo I, Gönen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84-96. doi: 10.1097/SLA.0b013e318176c4d3 [DOI] [PubMed] [Google Scholar]
- 11.Valle J, Wasan H, Palmer DH, et al. ; ABC-02 Trial Investigators . Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281. doi: 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 12.André T, Tournigand C, Rosmorduc O, et al. ; GERCOR Group . Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15(9):1339-1343. doi: 10.1093/annonc/mdh351 [DOI] [PubMed] [Google Scholar]
- 13.Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018;24(17):4154-4161. doi: 10.1158/1078-0432.CCR-18-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ClinicalTrials.gov Study of AG-120 in Previously Treated Advanced Cholangiocarcinoma With IDH1 Mutations (ClarIDHy). NCT02989857. https://clinicaltrials.gov/ct2/show/NCT02989857. Accessed October 1, 2019.
- 15.Kemeny N, Daly J, Oderman P, et al. Hepatic artery pump infusion: toxicity and results in patients with metastatic colorectal carcinoma. J Clin Oncol. 1984;2(6):595-600. doi: 10.1200/JCO.1984.2.6.595 [DOI] [PubMed] [Google Scholar]
- 16.McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol. 2007;16(1):3-5. doi: 10.1016/j.suronc.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 17.Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol. 1983;10(2):176-182. [PubMed] [Google Scholar]
- 18.Cohen AD, Kemeny NE. An update on hepatic arterial infusion chemotherapy for colorectal cancer. Oncologist. 2003;8(6):553-566. doi: 10.1634/theoncologist.8-6-553 [DOI] [PubMed] [Google Scholar]
- 19.Jarnagin WR, Schwartz LH, Gultekin DH, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20(9):1589-1595. doi: 10.1093/annonc/mdp029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemeny NE, Schwartz L, Gönen M, et al. Treating primary liver cancer with hepatic arterial infusion of floxuridine and dexamethasone: does the addition of systemic bevacizumab improve results? Oncology. 2011;80(3-4):153-159. doi: 10.1159/000324704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251-264. doi: 10.1016/j.jmoldx.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 23.Akinwande O, Dendy M, Ludwig JM, Kim HS. Hepatic intra-arterial injection of irinotecan drug eluting beads (DEBIRI) for patients with unresectable colorectal liver metastases: a systematic review. Surg Oncol. 2017;26(3):268-275. doi: 10.1016/j.suronc.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 24.Elganainy D, Holliday EB, Taniguchi CM, et al. Dose escalation of radiotherapy in unresectable extrahepatic cholangiocarcinoma. Cancer Med. 2018;7(10):4880-4892. doi: 10.1002/cam4.1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong TS, Wo JY, Yeap BY, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016;34(5):460-468. doi: 10.1200/JCO.2015.64.2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019;5(6):824-830. doi: 10.1001/jamaoncol.2019.0270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamarca A, Ross P, Wasan HS, et al. Advanced intrahepatic cholangiocarcinoma: post-hoc analysis of the ABC-01, -02 and -03 clinical trials. J Natl Cancer Inst. 2019;djz071. doi: 10.1093/jnci/djz071 [DOI] [PubMed] [Google Scholar]
- 28.Yamashita S, Koay EJ, Passot G, et al. Local therapy reduces the risk of liver failure and improves survival in patients with intrahepatic cholangiocarcinoma: a comprehensive analysis of 362 consecutive patients. Cancer. 2017;123(8):1354-1362. doi: 10.1002/cncr.30488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konstantinidis IT, Groot Koerkamp B, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122(5):758-765. doi: 10.1002/cncr.29824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowery MA, Abou-Alfa GK, Valle JW, et al. ClarIDHy: a phase 3, multicenter, randomized, double-blind study of AG-120 vs placebo in patients with an advanced cholangiocarcinoma with an IDH1 mutation [abstract]. J Clin Oncol. 2017;35(15)(suppl):TPS4142. doi: 10.1200/JCO.2017.35.15_suppl.TPS4142 [DOI] [Google Scholar]
- 31.Miller JJ, Shih HA, Andronesi OC, Cahill DP. Isocitrate dehydrogenase–mutant glioma: evolving clinical and therapeutic implications. Cancer. 2017;123(23):4535-4546. doi: 10.1002/cncr.31039 [DOI] [PubMed] [Google Scholar]
- 32.DiNardo CD, De Botton S, Stein EM, et al. Ivosidenib (AG-120) in mutant IDH1 AML and advanced hematologic malignancies: results of a phase 1 dose escalation and expansion study. Blood. 2017;130(suppl 1):725-725. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Methodology
eReferences.
eTable 1. Treatment Schema
eTable 2. Chemotherapy and Hepatic Arterial Infusions Floxuridine Dosing
eTable 3. Clinical Response in Phase II Cohort (n = 38)
eFigure 1. Hepatic Arterial Infusion Pump Chemotherapy for Intrahepatic Cholangiocarcinoma
eFigure 2. Progression-Free Survival in the MSK Cohort by Lymph Node Status
eFigure 3. Overall Survival in the MSK Cohort by Lymph Node
eFigure 4. The Oncoprint of Mutations Found in 33 Patients Enrolled in the Study
eFigure 5. Forest Plot Showing the Relative Hazard Ratio (HR) for Overall Survival According to Patient Characteristics at Baseline