Abstract
Patients harboring germline pathogenic biallelic variants in genes involved in the recognition and repair of DNA damage are known to have a substantially increased cancer risk. Emerging evidence suggests that individuals harboring heterozygous variants in these same genes may also be at heightened, albeit lesser, risk for cancer. Herein, we sought to determine whether heterozygous variants in RECQL4, the gene encoding an essential DNA helicase that is defective in children with the autosomal recessive cancer-predisposing condition Rothmund–Thomson syndrome (RTS), are associated with increased risk for childhood cancer. To address this question, we interrogated germline sequence data from 4435 pediatric cancer patients at St. Jude Children's Research Hospital and 1127 from the National Cancer Institute Therapeutically Applicable Research to Generate Effective Treatment (TARGET) database and identified 24 (0.43%) who harbored loss-of-function (LOF) RECQL4 variants, including five of 249 (2.0%) with osteosarcoma (OS). These RECQL4 variants were significantly overrepresented in children with OS, the cancer most frequently observed in patients with RTS, as compared to 134,187 noncancer controls in the Genome Aggregation Database (gnomAD v2.1; P = 0.00087, odds ratio [OR] = 7.1, 95% CI, 2.9–17). Nine of the 24 (38%) individuals possessed the same c.1573delT (p.Cys525Alafs) variant located in the highly conserved DNA helicase domain, suggesting that disruption of this domain is central to oncogenesis. Altogether these data expand our understanding of the genetic factors predisposing to childhood cancer and reveal a novel association between heterozygous RECQL4 LOF variants and development of pediatric OS.
Keywords: craniopharyngioma, Hodgkin lymphoma, osteosarcoma, pre-B-cell acute lymphoblastic leukemia, T-cell acute lymphoblastic leukemias
INTRODUCTION
The RecQ like helicase 4 (RECQL4) gene encodes a helicase essential for repairing DNA damage and maintaining genomic stability (Lu et al. 2016). Pathogenic homozygous or compound heterozygous variants affecting RECQL4 cause three clinically overlapping autosomal recessive (AR) disorders, namely Rothmund–Thomson syndrome (RTS), Baller–Gerold syndrome (BGS), and RAPADILINO (RAdial ray defect, PAtellar aplasia/arched or cleft PAlate, DIarrhea/Disclosed joints, LIttle size/LImb malformation, NOse slender/NOrmal intelligence) syndrome (Wang and Plon 2019). Rothmund–Thomson syndrome is the most prevalent of these syndromes, with nononcologic features including poikiloderma; sparse hair, eyelashes, and eyebrows; short stature; dental abnormalities; dysplastic or poorly formed nails; and gastrointestinal problems in infancy (Siitonen et al. 2009). BGS is the rarest of these disorders and is characterized by craniosynostosis, radial ray defects, short stature, and malformed or missing patellae (Van Maldergem et al. 2007). Finally, RAPADILINO syndrome is characterized by short stature, radial and patellar aplasia or hypoplasia, absence of thumbs, dislocation of joints, highly arched palate, infantile diarrhea, and pigmentary changes or café au lait macules (Siitonen et al. 2003). A predisposition to cancer has been reported in all three syndromes, with the highest cancer occurrence in RTS. Patients with RTS, and to a lesser degree RAPADILINO, are at greatest risk to develop osteosarcoma (OS) (Siitonen et al. 2003; Cao et al. 2017; Wang and Plon 2019), whereas patients with all three conditions are at risk for lymphoma (Van Maldergem et al. 2007; Siitonen et al. 2009).
Through a clinical research protocol (NCT02530658), we identified a child with OS for whom germline whole-exome and whole-genome sequencing (WGS) and in-depth analysis of 156 cancer predisposition genes revealed a pathogenic alteration and a variant of uncertain significance (VUS) in RECQL4, with the variants present in trans. In light of evolving evidence that heterozygous germline variants affecting other DNA repair genes such as ATM, BRCA2, and PALB2 confer an increased cancer risk (Thompson et al. 2005; Renwick et al. 2006; Antoniou et al. 2014; Helgason et al. 2015; Rebbeck et al. 2015; Esteban-Jurado et al. 2016; Esai Selvan et al. 2019), we questioned whether heterozygous germline RECQL4 variants, such as the one observed in our patient, might be associated with development of pediatric cancer. To address this question, we analyzed 5562 children with cancer and identified a significant enrichment of heterozygous RECQL4 loss-of-function (LOF) variants in patients with OS. Notably, most children carried the same RECQL4 alteration: c.1573delT (p.Cys525Alafs), which is predicted to truncate the RECQL4 protein within its DNA helicase domain. RECQL4 LOF variants were rarely observed in children with other cancer types, in which they were not significantly enriched compared to noncancer controls.
RESULTS
Characteristics of the Index Case
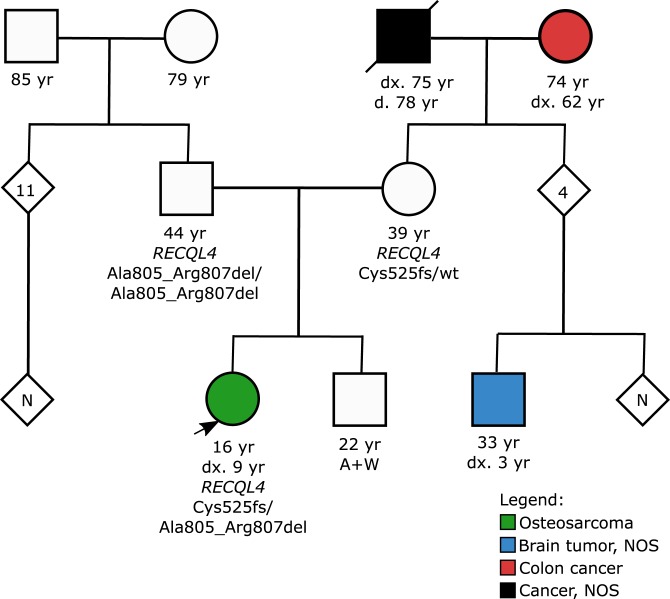
A 16-yr-old girl with previously treated OS of the left tibia developed recurrent OS of the left femur and left wrist, 5 and 8 yr after the initial diagnosis, respectively. Family history revealed a maternal first cousin with an unspecified brain tumor at 3 yr of age (Fig. 1). The maternal grandparents developed cancer after 60 yr of age, but no other family members were reported to have cancer. Germline WGS performed as part of a clinical research protocol (NCT02530658) that comprehensively analyzed 156 cancer predisposition genes (Supplemental Table 1) revealed no pathogenic or likely pathogenic (P/LP) variants in known OS genes such as TP53, RB1, the mismatch repair, and Fanconi anemia genes. However, testing did reveal two rare variants in RECQL4, including a frameshift c.1573delT (p.Cys525Alafs) and an in-frame deletion of nine nucleotides that is predicted to remove three amino acids c.2412_2420del (p.Ala805_Arg807del) (Table 1). These variants were classified by a CLIA-certified laboratory as pathogenic and of uncertain significance, respectively, based on the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) guidelines for sequence variant interpretation (Richards et al. 2015).
Figure 1.
Three-generation pedigree of a 16-yr-old female with recurrent osteosarcoma (arrowhead). Circles and squares denote female and male family members, respectively, and shaded figures denote persons with cancer. The individuals age in years (yr), age at diagnosis (dx.), and age at death (d.) are indicated on the pedigree where applicable. (NOS) Not otherwise specified.
Table 1.
Germline RECQL4 variants identified in the index case
| Gene | Chr | Location | Exon | HGVS DNA | HGVS protein | Variant type | dbSNP ID | Genotype | Parent of origin | Variant allele frequency | Coverage | Clinical significance in ClinVar | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RECQL4 | 8 | 144,514,983 | 9 | c.1573delT | p.Cys525Alafs*33 | Frameshift | Rs386833845 | Heterozygous | Mother | 0.277 | 90 | P | Mother is heterozygous |
| RECQL4 | 8 | 144,513,269 | 15 | c.2412_ 2420del | p.Ala805_Arg807del | In-frame deletion | Rs766312203 | Heterozygous | Father | 0.308 | 13 | VUS | Father is homozygous |
On physical examination, the patient was well-developed with no dysmorphic facial features. She had relatively thin hair and eyebrows, which by the patient's account predated chemotherapy. Her height was about 2nd percentile for age and in proportion to the calculated midparental height (height = 148 cm, midparental height = 153.5 cm). Although her head circumference was small, it was in proportion to her height and weight. Approximately 12 café au lait macules were observed on the patient's trunk and buttocks ranging in size from 4 mm to 4 cm; scattered hypopigmented areas on her left thigh and trunk were also observed. No axillary freckling was observed, and none of the skin lesions were consistent with poikiloderma. Based on the clinical phenotype, the patient did not meet the diagnostic criteria for RTS (Wang and Plon 2019) by the account of two clinical geneticists who examined the patient on multiple occasions and/or reviewed multiple photographs highlighting close-up views of the face, stature, and pigmented skin lesions. The patient's parents had no clinical features of RTS and no personal history of cancer. Based on parental testing, the two variants found in the patient were determined to be on opposite chromosomes (in trans). This testing revealed that the patient's father was homozygous for the c.2412_2420del (p.Ala805_Arg807del) VUS. The absence of RTS features in the father provided supporting evidence that this VUS was likely benign or that it is a hypomorphic allele that does not have a phenotype when found in the homozygous state but may play some role in this patient's unusual phenotype when opposite a loss-of-function variant. The patient's mother was heterozygous for the pathogenic c.1573delT (p.Cys525Alafs) variant. The patient's brother was not reported to have any clinical features of RTS and no personal history of cancer. He did not opt to pursue genetic testing and as a result, his RECQL4 genetic status remains unknown.
Prevalence of Heterozygous Germline RECQL4 LOF Variants in Pediatric Cancer
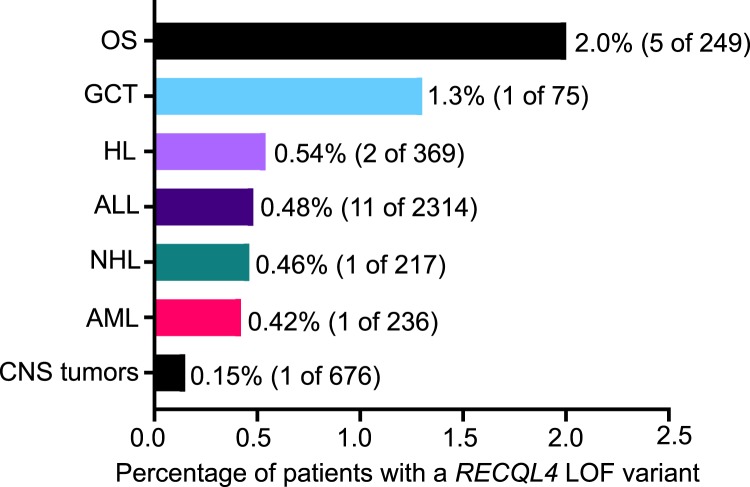
Heterozygous pathogenic variants in DNA damage repair genes (e.g., ATM, BRCA2, PALB2) are associated with a moderate to high cancer risk (Thompson et al. 2005; Renwick et al. 2006; Antoniou et al. 2014; Helgason et al. 2015; Rebbeck et al. 2015; Esteban-Jurado et al. 2016; Esai Selvan et al. 2019), whereas compound heterozygous or homozygous variants confer syndromic presentations such as ataxia-telangiectasia (ATM) (Swift et al. 1987; Ahmed and Rahman 2006) and Fanconi anemia (BRCA2, PALB2) (Howlett et al. 2002; Reid et al. 2007), which confer an even greater cancer risk. Building upon this notion, we sought to determine whether heterozygous RECQL4 LOF variants are more prevalent in pediatric oncology patients as compared to control individuals not selected for cancer. Among 4435 pediatric cancer patients at St. Jude Children's Research Hospital (Zhang et al. 2015; Wang et al. 2018) and 1127 in the National Cancer Institute TARGET database (dbGaP accession phs000218.v20.p7), we identified 24 of 5562 (0.43%) who carried heterozygous RECQL4 LOF variants (Table 2), including five of 249 (2.0%) with OS (Fig. 2).
Table 2.
Heterozygous germline RECQL4 LOF variants detected in pediatric sequencing cohorts
| Subject ID | Cancer diagnosis | Age at diagnosis | Ethnicity of subject | Gene | Chr | Location (hg38) | Exon | HGVS DNA | HGVS protein | Variant class | Variant allele frequency | Coverage | dbSNP ID | Clinical significance in ClinVar | AF in gnomad noncancer v2.1 | Ethnicity-specific AF in gnomAD noncancer v2.1 | Cohort | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WGS variant allele frequency | WES coverage | ||||||||||||||||||
| SJCNS044968 | Craniopharyngioma | 5 | NFE | RECQL4 | 8 | 144,516,696 | 5 | c.423delG | p.Lys141Asnfs*39 | Frameshift | 0.294 | 34 | NA | NA | - | - | NA | NA | SJCRH |
| SJALL016422 | Acute B-lymphoblastic leukemia | NA | NFEb | RECQL4 | 8 | 144,516,254 | 5 | c.865delG | p.Ala289Leufs*4 | Frameshift | NA | NA | 0.687 | 32 | - | - | NA | NA | TARGET |
| SJALL019720 | Acute lymphoblastic leukemia, NOS | 11 | NFE | RECQL4 | 8 | 144,516,248 | 5 | c.871delG | p.Ala291Leufs*2 | Frameshift | 0.409 | 22 | 0.545 | 33 | rs1389647533 | P | 4.28 × 10−06 | 9.85 × 10−06 | SJCRH |
| SJALL015934 | Acute B-lymphoblastic leukemia | NA | SASb | RECQL4 | 8 | 144,515,890 | 7 | c.1132-2A>G | p.Ala378_E6splice | Splice | NA | NA | 0.510 | 249 | - | - | 4.29 × 10−06 | 3.28 × 10−05 | TARGET |
| SJALL019022 | Acute B-lymphoblastic leukemia | 3 | NFE | RECQL4 | 8 | 144,515,243 | 7 | c.1391-1G>A | p.Glu464_E8splice | Splice | 0.423 | 26 | 0.750 | 16 | rs117642173 | P | 2.89 × 10−05 | 6.59 × 10−05 | SJCRH |
| SJAML007029 | Acute myeloid leukemia | 11 | NFE | RECQL4 | 8 | 144,514,983 | 9 | c.1573delT | p.Cys525Alafs*33 | Frameshift | 0.567 | 30 | 0.550 | 20 | rs386833845 | P | 2.43 × 10−04 | 4.21 × 10−04 | SJCRH |
| SJOS019534 | Osteosarcoma | 6 | NFE | RECQL4 | 8 | 144,514,983 | 9 | c.1573delT | p.Cys525Alafs*33 | Frameshift | 0.355 | 31 | 0.429 | 28 | rs386833845 | P | 2.43 × 10−04 | 4.21 × 10−04 | SJCRH |
| SJCBF029 | Acute myelogeneous leukemia | 11 | NFE | RECQL4 | 8 | 144,514,983 | 9 | c.1573delT | p.Cys525Alafs*33 | Frameshift | NA | NA | 0.571 | 28 | rs386833845 | P | 2.43 × 10−04 | 4.21 × 10−04 | SJCRH |
| SJBALL030048 | Acute B-lymphoblastic leukemia | 3 | NFEc | RECQL4 | 8 | 144,514,983 | 9 | c.1573delT | p.Cys525Alafs*33 | Frameshift | 0.218 | 64 | 0.315 | 200 | rs386833845 | P | 2.43 × 10−04 | 4.21 × 10−04 | SJCRH |
| SJOS030129a | Osteosarcoma | 8 | AMRc | RECQL4 | 8 | 144,514,983 | 9 | c.1573delT | p.Cys525Alafs*33 | Frameshift | NA | NA | 0.277 | 90 | rs386833845 | P | 2.43 × 10−04 | 1.42 × 10−04 | SJCRH |
| SJALL041208 | Acute B-lymphoblastic leukemia | 4 | NFE | RECQL4 | 8 | 144,514,983 | 9 | c.1573delT | p.Cys525Alafs*33 | Frameshift | 0.4 | 30 | 0.470 | 66 | rs386833845 | P | 2.43 × 10−04 | 2.43 × 10−04 | SJCRH |
| SJGCT041514 | Germ cell tumor, testicular | 0.3 | NFE | RECQL4 | 8 | 144,514,983 | 9 | c.1573delT | p.Cys525Alafs*33 | Frameshift | 0.364 | 44 | 0.473 | 55 | rs386833845 | P | 2.43 × 10−04 | 2.43 × 10−04 | SJCRH |
| SJBALL002191 | Acute B-lymphoblastic leukemia | NA | NFEb | RECQL4 | 8 | 144,514,983 | 9 | c.1573delT | p.Cys525Alafs*33 | Frameshift | NA | NA | 0.293 | 58 | rs386833845 | P | 2.43 × 10−04 | 2.43 × 10−04 | TARGET |
| SJTALL022098 | Acute T-lymphoblastic leukemia | 10 | AMRb | RECQL4 | 8 | 144,514,983 | 9 | c.1573delT | p.Cys525Alafs*33 | Frameshift | NA | NA | 0.545 | 11 | rs386833845 | P | 2.43 × 10−04 | 1.44 × 10−04 | TARGET |
| SJHL019300 | Hodgkin lymphoma | 7 | AFR | RECQL4 | 8 | 144,514,363 | 10 | c.1705-1delG | p.Ile569_E11splice | Splice | 0.364 | 44 | 0.500 | 10 | - | - | NA | NA | SJCRH |
| SJST030131 | Spindle cell sarcoma | 16 | AFRc | RECQL4 | 8 | 144,514,350 | 11 | c.1717C>T | p.Gln573* | Nonsense | 0.275 | 58 | 0.25 | 8 | - | - | 3.19 × 10−05 | 1.15 × 10−04 | SJCRH |
| SJTALL065 | Acute T-lymphoblastic leukemia | 8 | AFR | RECQL4 | 8 | 144,513,592 | 13 | c.2178_ 2179insCCTGGGTC | p.Ala727Profs*119 | Frameshift | NA | NA | 0.2 | 73 | - | - | NA | NA | SJCRH |
| SJOS040215 | Osteosarcoma | NA | NFEb | RECQL4 | 8 | 144,513,412 | 14 | c.2269C>T | p.Gln757* | Nonsense | NA | NA | 0.503 | 153 | rs137853229 | P | 1.24 × 10−04 | 1.45 × 10−04 | TARGET |
| SJOS040163 | Osteosarcoma | NA | NFEb | RECQL4 | 8 | 144,513,383 | 15 | c.2296+1C>G | p.Arg766_E15splice | Splice | NA | NA | 0.357 | 26 | rs199605511 | - | NA | NA | TARGET |
| SJNHL019456 | Non-Hodgkin lymphoma | 7 | NFE | RECQL4 | 8 | 144,513,109 | 16 | c.2492_2493delAT | p.His831Argfs*52 | Frameshift | 0.263 | 19 | 0.064 | 47 | rs752729755 | P | 7.02 × 10−05 | 7.01 × 10−05 | SJCRH |
| SJHL042013 | Hodgkin lymphoma | 18 | NFE | RECQL4 | 8 | 144,513,109 | 16 | c.2492_2493delAT | p.His831Argfs*52 | Frameshift | 0.5 | 34 | 0.400 | 5 | rs752729755 | P | 7.02 × 10−05 | 7.01 × 10−05 | SJCRH |
| SJOS040168 | Osteosarcoma | NA | NFEb | RECQL4 | 8 | 144,512,846 | 16 | c.2755+1G>A | p.Ala919_E16splice | Splice | NA | NA | 0.316 | 19 | rs373130543 | - | 3.16 × 10−05 | 2.86 × 10−05 | TARGET |
| SJALL019726 | Acute B-lymphoblastic leukemia | 3 | NFE | RECQL4 | 8 | 144,513,109 | 19 | c.3073_3074delAG | p.Thr1024_Glu1025fs | Frameshift | 0.44 | 25 | 0.385 | 39 | - | - | NA | NA | SJCRH |
| SJRB001130 | Retinoblastoma | NA | OTH | RECQL4 | 8 | 144,511,911 | 20 | c.3393+2T>G | p.Arg1131_E20splice | Splice | 0.6 | 15 | NA | NA | rs557284122 | - | 1.52 × 10−05 | 0.00 × 10+00 | SJCRH |
All ethnicities were computationally predicted unless otherwise noted. All predicted populations were consistent with self-reporting unless otherwise noted.
(LOF) Loss of function, (WGS) whole-genome sequencing, (WES) whole-exome sequencing.
aIndex case.
bSelf-reporting not available.
cSelf-reported ethnicity only.
Figure 2.
Frequency of heterozygous RECQL4 LOF mutations across pediatric cancer types. (OS) osteosarcoma, (GCT) germ cell tumor, (HL) Hodgkin lymphoma, (ALL) acute lymphoblastic leukemia, (NHL) non-Hodgkin lymphoma, (AML) acute myeloid leukemia, (CNS) central nervous system, (LOF) loss-of-function.
To determine whether these LOF variants are enriched in children with cancer, we examined their prevalence in the Genome Aggregation Database (gnomAD v2.1, noncancer; Lek et al. 2016), which spans 118,479 whole-exome and 15,708 whole-genome sequences from individuals who were not ascertained for having cancer. There were a total of 385 RECQL4 LOF alleles in the gnomAD noncancer population after removal of the founder variant, c.1390+2delT (p.Ala420_Ala463del), which is commonly encountered in the Finnish population at a minor allele frequency of 0.4% (Lek et al. 2016) but was absent from our cohort. Low-confidence LOF variants and variants of dubious quality were also removed as detailed in the Methods. Compared to the noncancer cohort in gnomAD, we observed a significant association between heterozygous RECQL4 LOF variants and OS (P = 0.00087; OR = 7.1; 95% CI, 2.9–17.0) but no association for other tumor types. One patient with retinoblastoma was noted to harbor an additional pathogenic variant in RB1 and was excluded from subsequent analysis. Available tumor data from 12 germline RECQL4 LOF variant positive cases did not show loss of heterozygosity for RECQL4 or any pathogenic or likely pathogenic variants in the remaining RECQL4 allele.
Evaluation of RECQL4 Genotype and Association with Clinical Phenotype
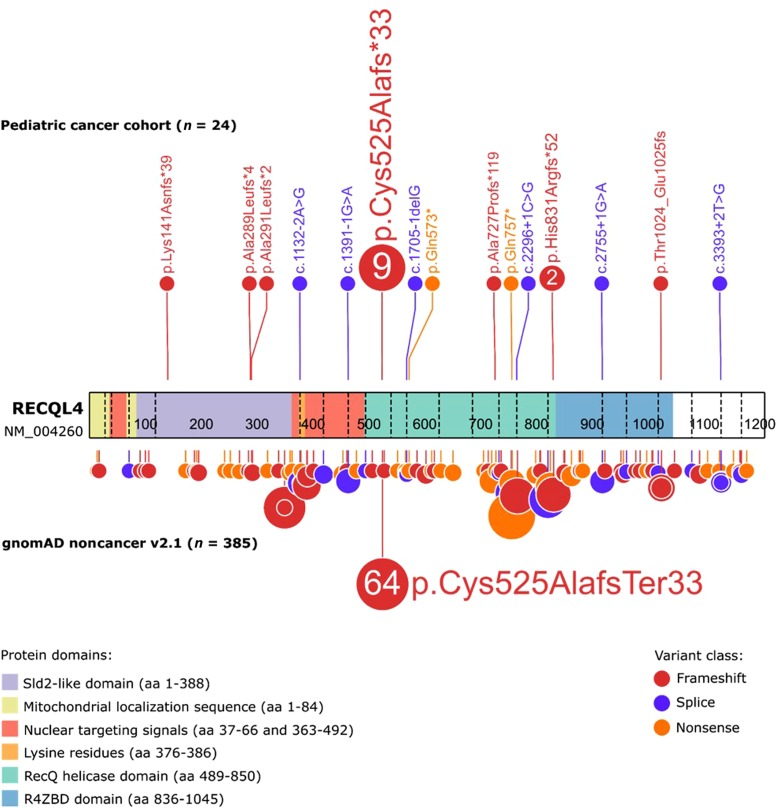
To understand how germline RECQL4 LOF variants impacted the encoded protein, we evaluated their location within the gene. Among the 24 variants identified, 17 (71%) resided within the helicase domain (residues 489–850; Fig. 3), which is critical to maintaining genome stability, specifically in the case of DNA repair (Chu and Hickson 2009; Croteau et al. 2012). All variants in the helicase domain are predicted to result in NMD or a disrupted protein product. Four of 24 (17%) patients harbored germline RECQL4 variants amino-terminal to the helicase domain, all of which are predicted to cause nonsense-mediated decay (NMD). Of these, three were located in a region having sequence similarity to yeast Sld2 (the Sld2-like domain; residues 1–388), which is essential for the initiation of DNA replication, and the fourth was located in a nuclear targeting signal (residues 363–492) (Colombo et al. 2018). Three of 24 patients had variants located carboxy-terminal to the helicase domain, in exons 16 and 19, which are also predicted to cause NMD.
Figure 3.
Distribution of the germline heterozygous RECQL4 LOF mutations in the pediatric cancer cohort examined in this study and in gnomAD noncancer v2.1. Known domains of the RECQL4 protein are as shown.
Notably, nine (38%) of the RECQL4 LOF variant–positive individuals were noted to carry the same alteration, c.1573delT (p.Cys525Alafs), which was also present in our index case (Fig. 3). Patients with this variant carried diagnoses of acute lymphoblastic leukemia (ALL; n = 4), OS (n = 2), acute myeloid leukemia (AML; n = 2), and germ cell tumor (GCT; n = 1) and were of different racial and ethnic backgrounds (Table 2). Our analysis revealed that this particular variant is significantly enriched in the pediatric cancer population (P = 0.0024; OR = 3.3; 95% CI, 1.7–6.7) compared to the gnomAD noncancer cohort in which this variant accounts for 64 of 385 (17%) RECQL4 LOF variants with a global allele frequency of 2.43 × 10−4. This supports the notion that the c.1573delT (p.Cys525Alafs) alteration contributes to the pathogenesis of pediatric cancer; however, its presence in a presumably healthy population suggests that it may be a lower penetrance allele.
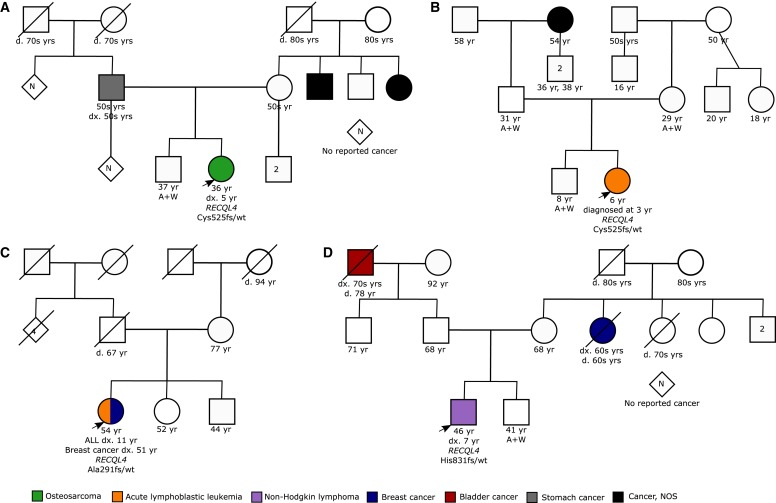
To investigate the possible penetrance of germline RECQL4 LOF variants, we examined the family histories of four patients from the St. Jude cohort for whom such information was available. None of these patients had first- or second-degree relatives with early onset of cancer (defined here as cancer before 50 yr of age; Fig. 4). This further supports the idea that heterozygous RECQL4 LOF variants function as lower penetrance alleles. These data are consistent with the literature in which there are only rare reports of relatives of RTS patients who have developed OS (Siitonen et al. 2009).
Figure 4.
Pedigrees of families with RECQL4 LOF mutations.
Prevalence of Germline LOF Variants in Other Genes of the RecQ Helicase Family
Because the RecQ helicase genes are highly conserved, it remained possible that other family members might also be associated with childhood cancer development. Therefore, we examined the St. Jude and TARGET cohorts for heterozygous LOF variants in RECQL, BLM, WRN, and RECQL5. Through these studies, we identified 51 children with heterozygous LOF variants in one of these genes, including 13 with variants in RECQL, 13 with variants in BLM, 15 with variants in WRN, and 10 with variants in RECQL5 (Supplemental Table 2). Pan-cancer analyses did not reveal any significant associations between the presence of heterozygous LOF variants in other RecQ helicases and pediatric cancer (Table 3). However, cancer-specific analyses identified a significant association in which RECQL variants were present in 1.2% (3 out of 249) of OS cases (P = 0.037; OR = 4.2; 95% CI, 1.3–13.1). Additional nonsignificant associations of potential interest include the identification of RECQL variants in 1.3% (2 out of 150) of rhabdomyosarcoma cases (P = 0.071; OR = 4.6; 95% CI, 1.2–18.7) and WRN variants in 0.39% (9 out of 2314) of pediatric ALL patients (P = 0.061; OR = 1.9; 95% CI, 0.98–3.7).
Table 3.
Comparison of heterozygous RECQL4 LOF variants in pediatric cancer patients and the gnomAD noncancer cohort
| Cancer diagnosis | Pediatric cancer patients | gnomAD noncancer cohort | Cancer risk | |||
|---|---|---|---|---|---|---|
| Carriers | Noncarriers | Carriers | Noncarriers | Odds ratio (95% CI) | P-value | |
| RECQL | ||||||
| Pan-cancer (all diagnoses) | 13 | 5549 | 388 | 133,799 | 0.81 (0.46, 1.4) | 0.52 |
| Osteosarcoma | 3 | 246 | 388 | 133,799 | 4.2 (1.3, 13.1) | 0.037* |
| BLM | ||||||
| Pan-cancer (all diagnoses) | 13 | 5549 | 279 | 133,908 | 1.1 (0.64, 2.0) | 0.65 |
| Osteosarcoma | 0 | 249 | 279 | 133,908 | 0 | 1 |
| WRN | ||||||
| Pan-cancer (all diagnoses) | 15 | 5547 | 273 | 133,914 | 1.3 (0.79, 2.2) | 0.29 |
| Osteosarcoma | 0 | 249 | 273 | 133,914 | 0 | 1 |
| RECQL4 | ||||||
| Pan-cancer (all diagnosesa) | 23 | 5539 | 385 | 133,802 | 1.4 (0.95, 2.2) | 0.098 |
| Osteosarcoma | 5 | 244 | 385 | 133,802 | 7.1 (2.9, 17.0) | 0.00087** |
| RECQL5 | ||||||
| Pan-cancer (all diagnoses) | 10 | 5552 | 284 | 133,903 | 0.85 (0.45, 1.6) | 0.76 |
| Osteosarcoma | 0 | 249 | 284 | 133,903 | 0 | 1 |
*P < 0.05.
**P < 0.005.
aPatient with pathogenic RB1 variant was excluded from statistical analysis.
Population Admixture of the Childhood Cancer Cohort Compared to the gnomAD Cohort
To determine whether the enrichment of germline RECQL4 or RECQL variants might be due to differences in the ethnic composition of pediatric cancer versus gnomAD noncancer cohorts, we compared the population admixtures of these two groups. Here, the ethnicity of individuals in the pediatric cancer cohort was computationally predicted (Supplemental Fig. 1). As shown in Supplemental Table 3, the population admixture in the pediatric cancer cohort is not equivalent to that found in gnomAD noncancer version 2.1. Nevertheless, all RECQL4 and RECQL LOF variants are extremely rare with respect to both global and ethnicity-specific allele frequencies (Table 2; Supplemental Table 2). Therefore, it is unlikely that the differences in the composition of the two populations significantly impacts our results. To further examine this possibility, we evaluated for enrichment of germline heterozygous LOF variants in RECQL4 using only non-Finnish Europeans who comprise the majority of individuals in the cancer and noncancer cohorts (Supplemental Table 3). Through this analysis, significant enrichment in pediatric OS was once again observed for variants affecting RECQL4 (P = 0.0012, OR = 6.77 [2.79, 16.45]) and RECQL (P = 0.024, OR = 5.04 [1.60, 15.86]).
DISCUSSION
As genetic testing is increasingly applied to patients with cancer, our knowledge of the germline contributions to cancer risk is dramatically expanding. Through such efforts, it has become clear that heterozygous pathogenic variants in DNA damage repair genes, such as ATM, BRCA2, and PALB2, are associated with a significant increase in risk to develop certain cancers. Here, we examined whether heterozygous LOF variants in RECQL4, the gene encoding an important DNA helicase that is mutated in individuals with RTS, are more prevalent in children with cancer.
After screening 5562 pediatric cases, we identified a significant enrichment of heterozygous germline RECQL4 LOF variants in patients with OS, the most common cancer in RTS, which is caused by homozygous or compound heterozygous variants in RECQL4. Although not significantly overrepresented, such variants were also identified in children with other tumor types, such as ALL, AML, craniopharyngioma, GCT, HL, and NHL. In 12 cases for whom tumor data were available, no deletions or mutations within the remaining RECQL4 allele were detected. Although other mechanisms might account for a second hit (e.g., changes in methylation) (Mazor et al. 2015; Di Ruscio et al. 2016), it remains possible that RECQL4 haploinsufficiency, perhaps in combination with other oncogenic events, is enough to promote malignant transformation.
Our findings are consistent with recent studies documenting heterozygous germline RECQL4 variants in adults with a variety of malignancies (Schrader et al. 2016; Jalkh et al. 2017; Mandelker et al. 2017; Tedaldi et al. 2017; AlDubayan et al. 2018; Bonache et al. 2018; Lowery et al. 2018; Na et al. 2018; Paulo et al. 2018; Penkert et al. 2018; Quezada Urban et al. 2018; Slavin et al. 2018). In addition to these studies are reports of an increased prevalence of RECQL4 variants in patients with bladder (Na et al. 2018) or colorectal cancer (AlDubayan et al. 2018) compared to the general population. Moreover, a heterozygous germline truncating variant in RECQL4 has been implicated as a possible cancer risk factor in an individual with prostate cancer (Paulo et al. 2018). Curiously, a previous investigation did not find enrichment of RECQL4 mutations in sporadic cases of OS compared to the general population (Nishijo et al. 2004); however, this prior study examined a much smaller cohort including only 71 patients with OS. Thus, this association could potentially have been missed.
Among the 24 patients identified as harboring a single germline RECQL4 LOF variant, 17 (71%) of these variants reside in the highly conserved helicase domain, with nine of these carrying the same c.1573delT (p.Cys525Alafs) alteration. Most RTS patients also harbor variants that result in a truncated protein lacking some or all of the helicase domain. Correspondingly, it has been reported that many RTS patients who developed OS harbor at least one truncating variant in RECQL4, whereas individuals with a clinical diagnosis of RTS and either a missense variant or no identified variant in RECQL4 were not reported to develop OS (Wang et al. 2003). In contrast to these deleterious truncating variants, the hallmark variant in RAPADILINO syndrome, a splice site variant that causes in-frame skipping of exon 7 (Van Maldergem et al. 2006), leaves the helicase domain largely intact (Kitao et al. 1999; Siitonen et al. 2003), which may correspond with the lower incidence of cancer.
In addition to the new association of heterozygous germline RECQL4 LOF variants with pediatric OS, our data also showed evidence of an association linking RECQL variants with OS, which deserves further investigation. Germline RECQL variants have been reported in BRCA1/2-negative breast cancers (Cybulski et al. 2015; Sun et al. 2015; Kwong et al. 2016); however, this association is disputed (Kwong et al. 2016; Li et al. 2018). To date, RECQL variants have not been reported in pediatric cancer. No strong associations were found between pediatric cancer and germline heterozygous LOF variants in the other RecQ helicase genes (i.e., BLM, WRN, RECQL5). Although all RecQ helicase genes contain the highly conserved helicase domain (Hickson 2003), they each play unique roles in genome maintenance and stability (Croteau et al. 2014). Toward this end, previous work has demonstrated that only two of the five human RecQ helicases, RECQL and RECQL4, bind specifically to three well-defined DNA replication origins under native conditions (Thangavel et al. 2010), whereas the other helicases (i.e., BLM, WRN, RECQL5) can only be found at replication origins following treatment with replication inhibitors (Thangavel et al. 2010). The similar localization and putative roles of RECQL and RECQL4 may underlie how disruption of these helicases promotes the development of OS and possibly other cancers.
This study has several limitations. First, the prevalence of germline RECQL4 variants identified in this study might not reflect the prevalence in newly diagnosed pediatric cancer patients because 50% of the patients studied were long-term survivors of childhood cancer. If the presence of heterozygous germline LOF variants is associated with poorer (or better) outcomes, it is possible that their prevalence in our cohort will be lower (or higher) than in newly diagnosed patients. A second limitation is that the population admixture of the pediatric cancer cohort examined in this study is not equivalent to that found in the gnomAD noncancer cohort (Supplemental Data). We suspect that this is due to differences in outcomes or referral patterns among different populations. Regardless, all of the LOF alleles reported in this study are extremely rare with respect to both global and ethnicity-specific allele frequencies. Last, this study is limited by the small number of individuals who harbor germline LOF variants. This factor makes it difficult to define the true spectrum of cancers associated with these germline variants. Continued evaluation of patients with cancer for the presence of heterozygous germline RECQL4 LOF variants, and LOF variants in the other RecQ helicase genes, is warranted to validate and refine this new association.
METHODS
Study Participants
Study participants were from the Pediatric Cancer Genome Project (n = 1120), Genomes for Kids protocol (n = 309), St. Jude Lifetime Cohort (SJLIFE) Study (n = 3006), and Therapeutically Applicable Research to Generate Effective Treatment (TARGET) program (n = 1127). The index case consented to participate in an IRB-approved study at St. Jude Children's Research Hospital that specifically allowed for germline interrogation and reporting.
Variant Detection and Classification
Single-nucleotide variants, small insertions and deletions, and copy-number variations were detected from whole-exome and/or genome sequencing (WGS) of the germline as previously described (Zhang et al. 2015; Rusch et al. 2018). Genetic variants were annotated, and LOF variants were further analyzed.
Curation of LOF Variants in gnomAD
Variants in RecQ helicase genes (RECQL, BLM, WRN, RECQL4, RECQL5) in the noncancer subset of the Genome Aggregation Database (gnomAD v2.1) were queried (Supplemental Tables 4–8). The putative LOF variants in gnomAD noncancer set v2.1 were downloaded first, and subsequently all variants of dubious quality via the tags “Flag” = “lc_lof” (low confidence-loss-of-function) were excluded. Variants with the “Flag” = ”lcr” or “Flag” = ”segdup” were reviewed and retained if they passed the quality control and manual curation steps. In the case of RECQL4, the founder variant, c.1390+2delT (p.Ala420_Ala463del), was removed as it is commonly encountered in the Finnish population at a minor allele frequency of 0.4% (Lek et al. 2016) but was absent from our cohort.
Statistical Analysis
Statistical analysis of population enrichment was calculated via a 2 × 2 Fisher's exact test, and estimates of the OR were performed using the RStudio R statistical computing environment with the epiR package (https://CRAN.R-project.org/package=epiR). Statistical significance was defined by a two-sided P = 0.05.
Computational Prediction of Ethnicity
Data from our pediatric cancer cohort (comprised of 4435 pediatric cancer patients at St. Jude Children's Research Hospital and 1127 from the National Cancer Institute TARGET database) was combined with data from 1000 Genomes, and the principal components (PCs) were extracted. Ethnicity data from 1000 Genomes were then used to train a random forest using the top 10 PCs which was used to predict the ethnicity of individuals in the pediatric cancer cohort. A visualization of the unsupervised ethnicity clustering is shown in Supplemental Figure 1.
ADDITIONAL INFORMATION
Data Deposition and Access
Genomic sequence data from St. Jude Children's Research Hospital is available for request on the St. Jude Cloud Platform. The variants were submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers SCV000288218.6 and SCV000890968.1.
Ethics Statement
Patients reported here provided written informed consent permitting genomic analysis. Family histories have been illustrated in a de-identified manner. Participants at St. Jude Children's Research Hospital were consented to at least one of the following protocols: institutional banking protocol for Collecting, Banking and Distributing Human Tissue Samples at St. Jude Children's Research Hospital (TBANK; NCT01354002), Genomes for Kids (G4K; NCT02530658), and/or Establishment of a Lifetime Cohort of Adults Surviving Childhood Cancer (SJLIFE; NCT00760656).
Acknowledgments
We thank the staff of the Genomes for Kids protocol, the St. Jude Lifetime Cohort Study, the Pediatric Cancer Genome Project, and the Molecular Pathology Laboratory at St. Jude Children's Research Hospital for next-generation sequencing and validation of identified pathogenic variants. We also thank the patients and families who participated in the research studies that enabled this investigation.
Author Contributions
J.L.M., K.V.H., K.E.N., and C.A.K. conceived the study and wrote the initial draft of the manuscript. K.V.H., R.B.M., R.N., R.M., and C.A.K. provided genetic counseling. A.S.P. and V.S. were and are involved with the clinical management of the index case. K.V.H., R.B.M., R.N., R.M., S.H.D., L.H., L.T., E.L.G., and A.O. provided participant recruitment, consent, and support. N.O., W.C., M.N.E., A.P., S.N., J.Z., Z.W., and G.W. developed software and pipelines for sequence data analysis and variant interpretation. N.O., W.C., Z.W., J.N., E.M.A., S.A.S., S.N., and G.W. analyzed sequence data and interpreted variants. J.L.M., N.O., G.W., and C.A.K. reviewed population databases and performed statistical analyses. D.W.E., J.R.D., M.M.H., L.L.R., S.N., J.Z., G.W., K.E.N., and C.A.K. provided project oversight. All coauthors reviewed the manuscript.
Funding
This work was supported by funding from the American Lebanese Syrian Associated Charities to St. Jude Children's Research Hospital.
Competing Interest Statement
The authors have declared no competing interest.
Supplementary Material
Footnotes
[Supplemental material is available for this article.]
REFERENCES
- Ahmed M, Rahman N. 2006. ATM and breast cancer susceptibility. Oncogene 25: 5906–5911. 10.1038/sj.onc.1209873 [DOI] [PubMed] [Google Scholar]
- AlDubayan SH, Giannakis M, Moore ND, Han GC, Reardon B, Hamada T, Mu XJ, Nishihara R, Qian Z, Liu L, et al. 2018. Inherited DNA-repair defects in colorectal cancer. Am J Hum Genet 102: 401–414. 10.1016/j.ajhg.2018.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou AC, Casadei S, Heikkinen T, Barrowdale D, Pylkäs K, Roberts J, Lee A, Subramanian D, De Leeneer K, Fostira F, et al. 2014. Breast-cancer risk in families with mutations in PALB2. N Engl J Med 371: 497–506. 10.1056/NEJMoa1400382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonache S, Esteban I, Moles-Fernández A, Tenés A, Duran-Lozano L, Montalban G, Bach V, Carrasco E, Gadea N, López-Fernández A, et al. 2018. Multigene panel testing beyond BRCA1/2 in breast/ovarian cancer Spanish families and clinical actionability of findings. J Cancer Res Clin Oncol 144: 2495–2513. 10.1007/s00432-018-2763-9 [DOI] [PubMed] [Google Scholar]
- Cao F, Lu L, Abrams SA, Hawthorne KM, Tam A, Jin W, Dawson B, Shypailo R, Liu H, Lee B, et al. 2017. Generalized metabolic bone disease and fracture risk in Rothmund–Thomson syndrome. Hum Mol Genet 26: 3046–3055. 10.1093/hmg/ddx178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu WK, Hickson ID. 2009. RecQ helicases: multifunctional genome caretakers. Nat Rev Cancer 9: 644–654. 10.1038/nrc2682 [DOI] [PubMed] [Google Scholar]
- Colombo EA, Locatelli A, Cubells Sánchez L, Romeo S, Elcioglu NH, Maystadt I, Esteve Martínez A, Sironi A, Fontana L, Finelli P, et al. 2018. Rothmund–Thomson syndrome: insights from new patients on the genetic variability underpinning clinical presentation and cancer outcome. Int J Mol Sci 19: 1103 10.3390/ijms19041103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau DL, Rossi ML, Canugovi C, Tian J, Sykora P, Ramamoorthy M, Wang Z, Singh DK, Akbari M, Kasiviswanathan R, et al. 2012. RECQL4 localizes to mitochondria and preserves mitochondrial DNA integrity. Aging Cell 11: 456–466. 10.1111/j.1474-9726.2012.00803.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau DL, Popuri V, Opresko PL, Bohr VA. 2014. Human RecQ helicases in DNA repair, recombination, and replication. Annu Rev Biochem 83: 519–552. 10.1146/annurev-biochem-060713-035428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulski C, Carrot-Zhang J, Kluźniak W, Rivera B, Kashyap A, Wokołorczyk D, Giroux S, Nadaf J, Hamel N, Zhang S, et al. 2015. Germline RECQL mutations are associated with breast cancer susceptibility. Nat Genet 47: 643–646. 10.1038/ng.3284 [DOI] [PubMed] [Google Scholar]
- Di Ruscio A, Welner RS, Tenen DG, Amabile G. 2016. The second hit of DNA methylation. Mol Cell Oncol 3: e1093690 10.1080/23723556.2015.1093690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esai Selvan M, Klein RJ, Gümüş ZH. 2019. Rare, pathogenic germline variants in Fanconi anemia genes increase risk for squamous lung cancer. Clin Cancer Res 25: 1517–1525. 10.1158/1078-0432.CCR-18-2660 [DOI] [PubMed] [Google Scholar]
- Esteban-Jurado C, Franch-Expósito S, Muñoz J, Ocaña T, Carballal S, López-Cerón M, Cuatrecasas M, Vila-Casadesús M, Lozano JJ, Serra E, et al. 2016. The Fanconi anemia DNA damage repair pathway in the spotlight for germline predisposition to colorectal cancer. Eur J Hum Genet 24: 1501–1505. 10.1038/ejhg.2016.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason H, Rafnar T, Olafsdottir HS, Jonasson JG, Sigurdsson A, Stacey SN, Jonasdottir A, Tryggvadottir L, Alexiusdottir K, Haraldsson A, et al. 2015. Loss-of-function variants in ATM confer risk of gastric cancer. Nat Genet 47: 906–910. 10.1038/ng.3342 [DOI] [PubMed] [Google Scholar]
- Hickson ID. 2003. RecQ helicases: caretakers of the genome. Nat Rev Cancer 3: 169–178. 10.1038/nrc1012 [DOI] [PubMed] [Google Scholar]
- Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, de Die-Smulders C, Persky N, Grompe M, Joenje H, Pals G, et al. 2002. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297: 606–609. 10.1126/science.1073834 [DOI] [PubMed] [Google Scholar]
- Jalkh N, Chouery E, Haidar Z, Khater C, Atallah D, Ali H, Marafie MJ, Al-Mulla MR, Al-Mulla F, Megarbane A. 2017. Next-generation sequencing in familial breast cancer patients from Lebanon. BMC Med Genomics 10: 8 10.1186/s12920-017-0244-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitao S, Shimamoto A, Goto M, Miller RW, Smithson WA, Lindor NM, Furuichi Y. 1999. Mutations in RECQL4 cause a subset of cases of Rothmund–Thomson syndrome. Nat Genet 22: 82–84. 10.1038/8788 [DOI] [PubMed] [Google Scholar]
- Kwong A, Shin VY, Cheuk IW, Chen J, Au CH, Ho DN, Chan TL, Ma ES, Akbari MR, Narod SA. 2016. Germline RECQL mutations in high risk Chinese breast cancer patients. Breast Cancer Res Treat 157: 211–215. 10.1007/s10549-016-3784-1 [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O'Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, et al. 2016. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536: 285–291. 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Rowley SM, Goode DL, Amarasinghe KC, McInerny S, Devereux L, Wong-Brown MW, Lupat R, Lee JEA, Hughes S, et al. 2018. Mutations in RECQL are not associated with breast cancer risk in an Australian population. Nat Genet 50: 1346–1348. 10.1038/s41588-018-0206-9 [DOI] [PubMed] [Google Scholar]
- Lowery MA, Wong W, Jordan EJ, Lee JW, Kemel Y, Vijai J, Mandelker D, Zehir A, Capanu M, Salo-Mullen E, et al. 2018. Prospective evaluation of germline alterations in patients with exocrine pancreatic neoplasms. J Natl Cancer Inst 110: 1067–1074. 10.1093/jnci/djy024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Shamanna RA, Keijzers G, Anand R, Rasmussen LJ, Cejka P, Croteau DL, Bohr VA. 2016. RECQL4 promotes DNA end resection in repair of DNA double-strand breaks. Cell Rep 16: 161–173. 10.1016/j.celrep.2016.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelker D, Zhang L, Kemel Y, Stadler ZK, Joseph V, Zehir A, Pradhan N, Arnold A, Walsh MF, Li Y, et al. 2017. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA 318: 825–835. 10.1001/jama.2017.11137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazor T, Pankov A, Johnson BE, Hong C, Hamilton EG, Bell RJ, Smirnov IV, Reis GF, Phillips JJ, Barnes MJ, et al. 2015. DNA methylation and somatic mutations converge on the cell cycle and define similar evolutionary histories in brain tumors. Cancer Cell 28: 307–317. 10.1016/j.ccell.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na R, Wu Y, Jiang G, Yu H, Lin X, Wang M, Conran CA, Fantus RJ, Zhang N, Liu S, et al. 2018. Germline mutations in DNA repair genes are associated with bladder cancer risk and unfavourable prognosis. BJU Int 122: 808–813. 10.1111/bju.14370 [DOI] [PubMed] [Google Scholar]
- Nishijo K, Nakayama T, Aoyama T, Okamoto T, Ishibe T, Yasura K, Shima Y, Shibata KR, Tsuboyama T, Nakamura T, et al. 2004. Mutation analysis of the RECQL4 gene in sporadic osteosarcomas. Int J Cancer 111: 367–372. 10.1002/ijc.20269 [DOI] [PubMed] [Google Scholar]
- Paulo P, Maia S, Pinto C, Pinto P, Monteiro A, Peixoto A, Teixeira MR. 2018. Targeted next generation sequencing identifies functionally deleterious germline mutations in novel genes in early-onset/familial prostate cancer. PLoS Genet 14: e1007355 10.1371/journal.pgen.1007355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkert J, Schmidt G, Hofmann W, Schubert S, Schieck M, Auber B, Ripperger T, Hackmann K, Sturm M, Prokisch H, et al. 2018. Breast cancer patients suggestive of Li–Fraumeni syndrome: mutational spectrum, candidate genes, and unexplained heredity. Breast Cancer Res 20: 87 10.1186/s13058-018-1011-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada Urban R, Díaz Velásquez C, Gitler R, Rojo Castillo M, Sirota Toporek M, Figueroa Morales A, Moreno García O, García Esquivel L, Torres Mejía G, Dean M, et al. 2018. Comprehensive analysis of germline variants in Mexican patients with hereditary breast and ovarian cancer susceptibility. Cancers (Basel) 10: 361 10.3390/cancers10100361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck TR, Mitra N, Wan F, Sinilnikova OM, Healey S, McGuffog L, Mazoyer S, Chenevix-Trench G, Easton DF, Antoniou AC, et al. 2015. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA 313: 1347–1361. 10.1001/jama.2014.5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid S, Schindler D, Hanenberg H, Barker K, Hanks S, Kalb R, Neveling K, Kelly P, Seal S, Freund M, et al. 2007. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet 39: 162–164. 10.1038/ng1947 [DOI] [PubMed] [Google Scholar]
- Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, Ahmed M, North B, Jayatilake H, Barfoot R, Spanova K, et al. 2006. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet 38: 873–875. 10.1038/ng1837 [DOI] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. 2015. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17: 405–423. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch M, Nakitandwe J, Shurtleff S, Newman S, Zhang Z, Edmonson MN, Parker M, Jiao Y, Ma X, Liu Y, et al. 2018. Clinical cancer genomic profiling by three-platform sequencing of whole genome, whole exome and transcriptome. Nat Commun 9: 3962 10.1038/s41467-018-06485-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader KA, Cheng DT, Joseph V, Prasad M, Walsh M, Zehir A, Ni A, Thomas T, Benayed R, Ashraf A, et al. 2016. Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol 2: 104–111. 10.1001/jamaoncol.2015.5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siitonen HA, Kopra O, Kääriäinen H, Haravuori H, Winter RM, Säämänen A-M, Peltonen L, Kestilä M. 2003. Molecular defect of RAPADILINO syndrome expands the phenotype spectrum of RECQL diseases. Hum Mol Genet 12: 2837–2844. 10.1093/hmg/ddg306 [DOI] [PubMed] [Google Scholar]
- Siitonen HA, Sotkasiira J, Biervliet M, Benmansour A, Capri Y, Cormier-Daire V, Crandall B, Hannula-Jouppi K, Hennekam R, Herzog D, et al. 2009. The mutation spectrum in RECQL4 diseases. Eur J Hum Genet 17: 151–158. 10.1038/ejhg.2008.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin TP, Neuhausen SL, Nehoray B, Niell-Swiller M, Solomon I, Rybak C, Blazer K, Adamson A, Yang K, Sand S, et al. 2018. The spectrum of genetic variants in hereditary pancreatic cancer includes Fanconi anemia genes. Fam Cancer 17: 235–245. 10.1007/s10689-017-0019-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Wang Y, Xia Y, Xu Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, et al. 2015. Mutations in RECQL gene are associated with predisposition to breast cancer. PLoS Genet 11: e1005228 10.1371/journal.pgen.1005228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift M, Reitnauer PJ, Morrell D, Chase CL. 1987. Breast and other cancers in families with ataxia-telangiectasia. N Engl J Med 316: 1289–1294. 10.1056/NEJM198705213162101 [DOI] [PubMed] [Google Scholar]
- Tedaldi G, Tebaldi M, Zampiga V, Danesi R, Arcangeli V, Ravegnani M, Cangini I, Pirini F, Petracci E, Rocca A, et al. 2017. Multiple-gene panel analysis in a case series of 255 women with hereditary breast and ovarian cancer. Oncotarget 8: 47064 10.18632/oncotarget.16791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel S, Mendoza-Maldonado R, Tissino E, Sidorova JM, Yin J, Wang W, Monnat RJ, Falaschi A, Vindigni A. 2010. Human RECQ1 and RECQ4 helicases play distinct roles in DNA replication initiation. Mol Cell Biol 30: 1382–1396. 10.1128/MCB.01290-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Duedal S, Kirner J, McGuffog L, Last J, Reiman A, Byrd P, Taylor M, Easton DF. 2005. Cancer risks and mortality in heterozygous ATM mutation carriers. J Natl Cancer Inst 97: 813–822. 10.1093/jnci/dji141 [DOI] [PubMed] [Google Scholar]
- Van Maldergem L, Siitonen HA, Jalkh N, Chouery E, De Roy M, Delague V, Muenke M, Jabs EW, Cai J, Wang LL, et al. 2006. Revisiting the craniosynostosis-radial ray hypoplasia association: Baller–Gerold syndrome caused by mutations in the RECQL4 gene. J Med Genet 43: 148–152. 10.1136/jmg.2005.031781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maldergem L, Piard J, Larizza L, Wang L. 2007. Baller–Gerold syndrome. Internet, University of Washington, Seattle. [Google Scholar]
- Wang LL, Plon SE. 2019. Rothmund–Thomson syndrome. GeneReviews® Retrieved Feb 11, 2019, from https://www.ncbi.nlm.nih.gov/books/NBK1237/.
- Wang LL, Gannavarapu A, Kozinetz CA, Levy ML, Lewis RA, Chintagumpala MM, Ruiz-Maldanado R, Contreras-Ruiz J, Cunniff C, Erickson RP, et al. 2003. Association between osteosarcoma and deleterious mutations in the RECQL4 gene in Rothmund–Thomson syndrome. J Natl Cancer Inst 95: 669–674. 10.1093/jnci/95.9.669 [DOI] [PubMed] [Google Scholar]
- Wang Z, Wilson CL, Easton J, Thrasher A, Mulder H, Liu Q, Hedges DJ, Wang S, Rusch MC, Edmonson MN, et al. 2018. Genetic risk for subsequent neoplasms among long-term survivors of childhood cancer. J Clin Oncol 36: 2078–2087. 10.1200/JCO.2018.77.8589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Walsh MF, Wu G, Edmonson MN, Gruber TA, Easton J, Hedges D, Ma X, Zhou X, Yergeau DA, et al. 2015. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med 373: 2336–2346. 10.1056/NEJMoa1508054 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genomic sequence data from St. Jude Children's Research Hospital is available for request on the St. Jude Cloud Platform. The variants were submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers SCV000288218.6 and SCV000890968.1.