Abstract
Introduction
Real-world data with extended-release tacrolimus (ER-T) are lacking in the USA. This study examined clinical outcomes and healthcare resource utilization in kidney transplant patients receiving ER-T in clinical practice.
Methods
This was a retrospective, single-center analysis (February–June 2016) using data from Northwestern University’s Enterprise Data Warehouse. Adult patients receiving a kidney transplant in the preceding 4 years, treated de novo or converted to ER-T from immediate-release tacrolimus (IR-T) within 10 days post-transplantation, and maintained on ER-T (at least 3 months) were included. Patients were matched for demographic and clinical characteristics with IR-T-treated control patients. Endpoints included clinical outcomes and healthcare resource utilization up to 1 year post-transplantation.
Results
A total of 19 ER-T-treated patients were matched with 55 IR-T-treated patients. No ER-T-treated patients experienced biopsy-confirmed acute rejection (BCAR) or graft failure versus 3 (5.5%) and 3 (5.5%) IR-T-treated patients, respectively. Mean estimated glomerular filtration rate (eGFR), the number of all-cause outpatient visits, readmissions, and all-cause hospitalization days were comparable between groups. Tacrolimus trough levels, days to target level (6–10 ng/mL), and number of required dose adjustments were also similar.
Conclusion
Real-world clinical outcomes and healthcare resource utilization were similar with ER-T and IR-T. Larger studies will need to investigate the trend toward fewer BCAR events, and increased graft survival with ER-T.
Funding
Astellas Pharma Global Development, Inc.
Plain Language Summary
Plain language summary available for this article.
Keywords: Calcineurin inhibitor, Glomerular filtration rate (GFR), Graft survival, Immunosuppressant, Kidney (allograft) function/dysfunction, Patient characteristics, Tacrolimus, Urology
Plain Language Summary
Immunosuppressive drugs, such as tacrolimus, are crucial in patients after an organ transplant. Without these drugs, the risk of the body rejecting the new organ is increased, which can leave the patient in a life-threatening condition. Tacrolimus was originally available as a twice-daily, immediate-release formulation (IR-T), with rapid absorption of the drug into the body soon after oral administration. A once-daily, extended-release formulation (ER-T) was subsequently developed to reduce the risk of under- and overexposure to tacrolimus by releasing the drug more slowly over a prolonged period of time. There is a lack of data available from clinical practice in the USA regarding the use of ER-T in kidney transplantation. This study compared clinical outcomes and use of healthcare resources over 1 year in kidney transplant patients receiving IR-T or ER-T at a single US transplant center. There was no statistically significant difference in the incidence of kidney rejection or failure in patients receiving ER-T compared with IR-T, although there was a trend toward a lower incidence with ER-T. There was also no statistical difference between the two groups in the use of healthcare resources; however, there was a trend toward fewer days spent hospitalized during the first year, and fewer outpatient clinic visits during the first 3 months after transplantation with ER-T. This study shows that the use of ER-T in US clinical practice can achieve promising outcomes. However, more studies are now needed to investigate whether prescribing ER-T could lead to increased kidney survival and lower healthcare costs compared with IR-T.
Introduction
Extended-release tacrolimus (ER-T) is an oral, once-daily, immunosuppressive agent that was developed to simplify dosing regimens while retaining the safety and efficacy profile of the immediate-release formulation. In 2013, the US regulatory authority granted approval for ER-T on the basis of data from two large randomized controlled trials (RCTs), conducted in 638 and 667 de novo kidney transplant recipients, respectively [1, 2]. ER-T has, however, been available in the European Union since 2007.
Compared with twice-daily, immediate-release tacrolimus (IR-T), the extended-release formulation has been shown to decrease intrapatient variability in tacrolimus exposure [3, 4] and offer a smoother blood concentration–time profile [3]. ER-T may therefore provide a more consistent area under the concentration–time curve over the long term versus the immediate-release formulation. Additionally, once-daily dosing with ER-T has been shown to improve medication adherence compared with IR-T in both kidney and liver transplantation [5–7]. As both high intrapatient variability in tacrolimus exposure and medication nonadherence are associated with reduced graft survival and donor-specific antibody development [8–13], targeting both risk factors with a formulation of tacrolimus that potentially addresses these concerns could be a relevant mechanism to improve long-term outcomes.
In the USA, the overall 5-year kidney graft survival rate ranges from 81% to approximately 90%, depending on race [14], while the 10-year graft survival rate is between approximately 35% and 50% [15]. Although 1-year kidney graft survival rates have increased in recent years (currently greater than 90%) [14, 15], long-term graft survival in the USA remains 25% lower than in other countries with well-developed kidney transplant systems [16].
Therefore, there is an urgent need to improve long-term outcomes, a goal that could be accomplished by optimizing immunosuppressive regimens. Tacrolimus has a narrow therapeutic index [17], and so optimizing its exposure is essential, especially as antibody-mediated rejection, now linked to inadequate immunosuppression, is increasingly recognized as a major cause of late graft failure [13, 18].
The use of ER-T is low in clinical practice in the USA due to higher clinician familiarity with IR-T formulations and limited perceived differences in efficacy and safety compared with IR-T formulations. Notably, the pivotal phase III RCTs for ER-T conducted in de novo kidney transplant recipients excluded patients receiving induction with lymphocyte-depleting agents, an induction strategy employed by many US transplant centers today. Real-world database studies can thus provide further insights into treatment patterns and outcomes for a more heterogeneous patient population versus those enrolled in larger, controlled registrational studies. To our knowledge, no studies have been undertaken to date that examine the clinical outcomes and healthcare resource utilization of kidney transplant patients treated with ER-T under real-world conditions in the modern era of immunosuppression. As such, we utilized data from the Northwestern Medicine Enterprise Data Warehouse with the aim of conducting a retrospective, real-world study of clinical outcomes and healthcare resource utilization in kidney transplant patients receiving ER-T versus IR-T at a single US transplant center.
Methods
Study Design
This was a retrospective, single-center, database analysis conducted between February and June 2016. Data for eligible adult patients who had received a kidney transplant during the 4 years preceding the study (January 2012–January 2016) were extracted from the Northwestern Medicine Enterprise Data Warehouse. This database is a single, comprehensive, integrated repository that captures all electronic health records at clinical facilities within the Northwestern Medicine integrated healthcare system (Illinois, USA). Extracted data were de-identified before analysis. This retrospective database analysis study had approval of the Northwestern University Institutional Review Board (IRB#STU0020221) and adhered to the 1964 Helsinki declaration and its later amendments or comparable ethical standards. As a retrospective study, informed consent of individuals was not required.
Study Population
Data were collected for adult patients who had received a kidney transplant at Northwestern Medicine within the study period, who were initiated or switched to routine maintenance immunosuppression with once-daily, ER-T (Astagraf XL®, Astellas Pharma US, Inc., Northbrook, IL; also known outside the USA as Advagraf®, Graceptor®, and Prograf® XL) within 10 days of transplantation, and who continued this regimen for at least 3 months. Per center protocol for tacrolimus initiation, patients received IR-T if therapy was initiated during the initial hospital stay and, subsequently, were switched to ER-T upon hospital discharge. If the first dose of tacrolimus therapy was initiated after hospital discharge, patients were started and maintained on ER-T. Patient cases were matched with control patients who were taking either generic or branded forms of IR-T. Those receiving maintenance therapy with drugs other than tacrolimus (e.g., cyclosporine, belatacept, or sirolimus), or who were participating in a clinical trial during the study period, were excluded.
Endpoints
Clinical outcomes and healthcare resource utilization were assessed at 30, 90, and 365 days post-transplantation. Clinical outcomes included the incidence of biopsy-confirmed acute rejection (BCAR), graft loss (defined as a return to dialysis, primary graft nonfunction, or dialysis for more than 3 months), the need for retransplantation, and mortality. Estimated glomerular filtration rate (eGFR) by the Modification of Diet in Renal Disease-4 formula, and the proportion of patients experiencing delayed graft function (defined as a requirement for renal replacement therapy within 7 days of transplantation) were also analyzed. Healthcare resource utilization was tracked and included the number of all-cause outpatient clinic visits, number of all-cause readmissions, total number of all-cause hospitalization days, and total number of all-cause emergency room visits to Northwestern University facilities. The number of renal biopsies within 365 days of transplantation was also assessed.
The time to reach a tacrolimus trough level of 6–10 ng/mL (therapeutic target) was evaluated, as well as the number of dose adjustments required to reach this target. Tacrolimus trough levels were also compared between patients treated with the IR-T formulation (using trough levels from at least 30 days post-transplantation), and those who received ER-T (from at least 30 days after initiation or switching from the IR-T to the ER-T formulation).
Data Analysis and Statistical Methods
Patients receiving ER-T were matched 1:3 with those receiving IR-T. Patients were propensity score matched for 15 patient demographic and clinical characteristics present at the time of their first transplant procedure. These characteristics included sex, race/ethnicity, payer type, organ donor type (living versus deceased), induction regimen, steroid use, panel reactive antibody score, kidney donor profile index, transplantation status (retransplant), time on dialysis, comorbid conditions (such as obesity, diabetes, hypertension, or focal segmental glomerulosclerosis), number of human leukocyte antigen mismatches, and number of days post-transplantation prior to tacrolimus administration. Among the possible control patients who met these criteria for a given case, the three with the closest propensity score to that of the case were selected. This process was conducted in a randomly assigned order by case; selection of controls was made without replacement.
Recipient age and the Charlson Comorbidity Index were excluded from the propensity score model and exact-matched instead. Specifically, an exact match was performed on tertiles of age and Charlson Comorbidity Index, and quintiles of propensity score, in order to limit selection bias.
For the analysis of eGFR, values greater than 60 mL/min/1.73 m2 were truncated, with values reported only as high as 60 mL/min/1.73 m2 given that values above this threshold can be unreliable [19].
The comparisons between groups were made using generalized estimating equations to account for the match. Comparisons were considered statistically significant at the p < 0.05 level. All analyses were performed using SAS® version 9.4, and R version 3.2.2 using the R Studio interface.
Results
Patient Demographics and Clinical Characteristics
During the observation period, 19 patients were initiated on treatment with ER-T, or were converted to treatment from IR-T to ER-T within 10 days post-transplantation, and continued therapy for a period of at least 3 months. Of these 19 patients, 17 (89.5%) received ER-T as part of their planned discharge immunosuppression regimen, one (5.3%) received ER-T because of loss of insurance, and one (5.3%) received ER-T because of concern over deteriorating renal function (no further details available). The 19 patients were subsequently matched with 55 patients treated with IR-T (Table 1). The exact proportions of patients receiving generic versus branded forms of IR-T were unavailable; however, use of generic preparations increased during the study period.
Table 1.
Patient demographics and clinical characteristics
| Parameter | Extended-release tacrolimus (N = 19) | Immediate-release tacrolimus (N = 55) | p value |
|---|---|---|---|
| Sex | 0.90 | ||
| Female | 7 (36.8) | 21 (38.2) | |
| Male | 12 (63.2) | 34 (61.8) | |
| Race/ethnicity | 0.45 | ||
| Asian | 2 (10.5) | 5 (9.1) | |
| Hispanic | 6 (31.6) | 15 (27.3) | |
| Non-Hispanic black | 1 (5.3) | 14 (25.5) | |
| Non-Hispanic white | 10 (52.6) | 21 (38.2) | |
| Insurance | 0.39 | ||
| Medicaid | 1 (5.3) | 6 (10.9) | |
| Medicare | 7 (36.8) | 26 (47.3) | |
| Private | 11 (57.9) | 23 (41.8) | |
| Education | 0.39 | ||
| Attended some college | 1 (5.3) | 18 (32.7) | |
| Bachelors/associate degree | 5 (26.3) | 11 (20.0) | |
| Grade school | 2 (10.5) | 4 (7.3) | |
| High school | 10 (52.6) | 19 (34.5) | |
| Postgraduate degree | 1 (5.3) | 3 (5.5) | |
| Primary language | 0.42 | ||
| English | 15 (78.9) | 47 (85.5) | |
| Spanish | 4 (21.1) | 6 (10.9) | |
| Other | 0 (0.0) | 2 (3.6) | |
| Obesity | 0.19 | ||
| Normal weight | 5 (26.3) | 16 (29.1) | |
| Obese | 6 (31.6) | 18 (32.7) | |
| Overweight | 5 (26.3) | 21 (38.2) | |
| Underweight | 3 (15.8) | 0 (0.0) | |
| PRA | 0.68 | ||
| Highly sensitized [81+] | 2 (10.5) | 4 (7.3) | |
| Sensitized [21–80] | 3 (15.8) | 11 (20.0) | |
| Unsensitized [0–20] | 14 (73.7) | 40 (72.7) | |
| Induction regimen | 0.13 | ||
| Alemtuzumab | 11 (57.9) | 47 (85.5) | |
| Basiliximab | 7 (36.8) | 8 (14.5) | |
| Neither | 1 (5.3) | 0 (0.0) | |
| Steroid use after 3 months post-transplantation | 0.11 | ||
| Yes | 9 (47.4) | 17 (30.9) | |
| No | 10 (52.6) | 37 (67.3) | |
| Unknown | 0 (0.0) | 1 (1.8) | |
| Prior transplant | 0.79 | ||
| No | 19 (100.0) | 52 (94.5) | |
| Yes | 0 (0.0) | 3 (5.5) | |
| Number of HLA-A mismatches | 0.34 | ||
| 0 | 5 (26.3) | 19 (34.5) | |
| 1 | 12 (63.2) | 31 (56.4) | |
| 2 | 2 (10.5) | 5 (9.1) | |
| Number of HLA-B mismatches | 0.49 | ||
| 0 | 6 (31.6) | 13 (23.6) | |
| 1 | 11 (57.9) | 41 (74.5) | |
| 2 | 2 (10.5) | 1 (1.8) | |
| Number of HLA-DR mismatches | 0.41 | ||
| 0 | 8 (42.1) | 20 (36.4) | |
| 1 | 9 (47.4) | 29 (52.7) | |
| 2 | 2 (10.5) | 6 (10.9) | |
| Number of HLA-A, -B, -DR mismatches | 0.57 | ||
| 0 | 1 (5.3) | 1 (1.8) | |
| 1 | 1 (5.3) | 1 (1.8) | |
| 2 | 0 (0.0) | 1 (1.8) | |
| 3 | 3 (15.8) | 7 (12.7) | |
| 4 | 2 (10.5) | 11 (20.0) | |
| 5 | 7 (36.8) | 18 (32.7) | |
| 6 | 5 (26.3) | 16 (29.1) | |
| Diabetes | 0.94 | ||
| No | 15 (78.9) | 44 (80.0) | |
| Yes | 4 (21.1) | 11 (20.0) | |
| Hypertension | 0.30 | ||
| No | 10 (52.6) | 32 (58.2) | |
| Yes | 9 (47.4) | 23 (41.8) | |
| Polycystic kidney disease | 0.63 | ||
| No | 16 (84.2) | 48 (87.3) | |
| Yes | 3 (15.8) | 7 (12.7) | |
| Glomerulonephritis | 0.62 | ||
| No | 18 (94.7) | 51 (92.7) | |
| Yes | 1 (5.3) | 4 (7.3) | |
| IgA nephropathy | 0.27 | ||
| No | 18 (94.7) | 49 (89.1) | |
| Yes | 1 (5.3) | 6 (10.9) | |
| Focal segmental glomerulosclerosis | 0.40 | ||
| No | 18 (94.7) | 51 (92.7) | |
| Yes | 1 (5.3) | 4 (7.3) | |
| Systemic lupus erythematosus | 0.99 | ||
| No | 18 (94.7) | 52 (94.5) | |
| Yes | 1 (5.3) | 3 (5.5) | |
| Other diagnosis | 0.99 | ||
| No | 16 (84.2) | 49 (89.1) | |
| Yes | 3 (15.8) | 6 (10.9) | |
HLA human leukocyte antigen, IgA immunoglobulin A, PRA panel reactive antibody
Data are n (%)
During the study period, four patients (21.1%) switched from ER-T to IR-T. One of these patients showed borderline changes on a protocol biopsy at 3 months, with new onset BK viruria; one had changes suggestive of borderline cellular infiltrates with subtherapeutic tacrolimus levels at 3 months; and one patient was highly sensitized and experienced supratherapeutic tacrolimus levels on the extended-release preparation. The fourth patient switched to IR-T because of cost differences between generic IR-T and the branded ER-T formulation.
As anticipated, there were no significant differences in baseline covariates between the ER-T and IR-T groups after propensity score and exact matching (Table 1). Most patients receiving ER-T were male (63.2%), with a mean ± standard deviation (SD) age of 49.5 ± 16.3 years (range 23.0–74.0 years) at transplantation. Patients taking ER-T had received dialysis for a mean ± SD of 3.1 ± 3.7 years at the time of transplantation, had a mean kidney donor profile index of 38.1 ± 23.3%, and a mean Charlson Comorbidity Index score of 3.7 ± 1.4 points. Most patients received induction therapy with alemtuzumab (57.9%) or basiliximab (36.8%). The median time between transplantation and initiation of ER-T treatment was 1.5 days (interquartile range 1.3–3.0 days), with patients receiving ER-T for a median of 490.0 days (interquartile range 111.0–632.0 days).
Mortality and Graft Loss
No patients (0/19) in the ER-T group experienced a BCAR event compared with 3/55 (5.5%) patients in the IR-T group. As previously noted, one patient on ER-T showed borderline changes on a protocol biopsy at 3 months with new onset BK viruria and one patient had changes suggestive of borderline cellular infiltrates at 3 months with subtherapeutic tacrolimus levels. Neither of these patients met Banff diagnostic criteria for BCAR. Graft failure did not occur in any patients in the ER-T group (0/19) compared with 3/55 (5.5%) patients in the IR-T group. No patients required retransplantation or died during the study, in either cohort.
Renal Function
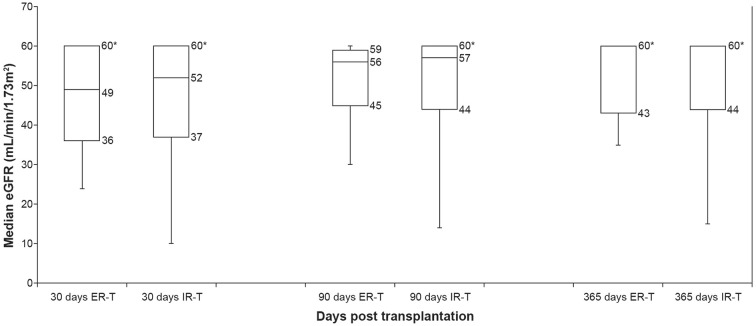
No patients in either treatment group experienced delayed graft function post-transplantation. Mean eGFR ranged between 46.2 and 52.6 mL/min/1.73 m2 across time points and treatment groups. The values were similar in both treatment groups at 30, 90, and 365 days post-transplantation (p = 0.71, p = 0.51, and p = 0.47, respectively), the caveat being that data were capped when patients achieved the maximum eGFR of 60 mL/min/1.73 m2 (Fig. 1).
Fig. 1.
Median estimated glomerular filtration rate at 30, 90, and 365 days post-transplantation for patients who received extended-release (N = 19) or immediate-release tacrolimus (N = 55). Whiskers represent range. *eGFR was capped at 60 mL/min/1.73 m2
Tacrolimus Dose and Trough Levels
There were no significant differences between the ER-T and IR-T groups with regard to the number of days (mean ± SD) taken to reach the target trough level of 6–10 ng/mL (9.2 ± 8.3 versus 7.8 ± 7.9 days, respectively; p = 0.18), or the number of dose adjustments required to reach the target trough level (1.3 ± 1.3 versus 0.9 ± 1.3 adjustments; p = 0.13). The number of tacrolimus trough levels drawn was similar in both treatment groups at 30, 90, and 365 days post-transplantation (Table 2). There was also no significant difference in tacrolimus trough levels (mean ± SD) beyond 30 days post-transplant with ER-T compared with IR-T (8.5 ± 1.2 versus 7.7 ± 1.3 ng/mL, respectively; p = 0.51) (Table 3). The dose-unadjusted SDs of tacrolimus trough concentrations were also similar in both treatment groups after 30 days post-transplant.
Table 2.
Number of tacrolimus trough concentration measurements
| Time point post-transplant (days) | Extended-release tacrolimus (N = 19) | Immediate-release tacrolimus (N = 55) | p value | |
|---|---|---|---|---|
| Tacrolimus measurements, mean (SD) | 30 | 11.8 (1.8) | 11.9 (2.6) | 0.58 |
| 90 | 25.6 (3.6) | 26.1 (7.1) | 0.41 | |
| 365 | 47.0 (14.5) | 46.9 (18.1) | 0.51 |
SD standard deviation
Table 3.
Tacrolimus trough levels beyond 30 days post-transplant
| Extended-release tacrolimus (N = 19) | Immediate-release tacrolimus (N = 55) | p value | |
|---|---|---|---|
| Tacrolimus trough levels, ng/mL, mean (SD) | 8.5 (1.2) | 7.7 (1.3) | 0.51 |
| n | 19 | 52 |
SD standard deviation
Clinic Visits and Hospitalization
The mean number of all-cause outpatient clinic visits by 365 days post-transplantation was 4.8 in the ER-T group and 4.4 in the IR-T group (p = 0.72). The mean number of all-cause outpatient clinic visits within 30 days was lower with ER-T, but this difference reached only borderline statistical significance (1.0 ± 0.3 versus 1.5 ± 1.2; p = 0.05). No statistically significant differences were seen between the two treatment groups regarding the number of outpatient clinic visits within 90 days post-transplantation (p = 0.13) and the mean number of readmissions at 30, 90, and 365 days post-transplantation (p = 0.38, p = 0.41, and p = 0.52, respectively) (Table 4). The total number of all-cause hospitalization days within 30 and 90 days post-transplantation was also similar in the ER-T group versus the IR-T group (p = 0.42 and p = 0.77, respectively) (Table 4). By 365 days post-transplantation, the number of all-cause hospitalization days was numerically greater with IR-T versus ER-T; however, this difference was not statistically significant (3.4 ± 7.3 versus 1.4 ± 2.7 days; p = 0.86).
Table 4.
Clinic visits and hospitalization post-transplantation
| Parameter | Extended-release tacrolimus (N = 19) | Immediate-release tacrolimus (N = 55) | p value |
|---|---|---|---|
| Number of all-cause outpatient clinic visits (days) | |||
| Within 30 |
1.0 ± 0.3 [0.0–2.0] |
1.5 ± 1.2 [0.0–8.0] |
0.05 |
| Within 90 |
2.0 ± 0.7 [0.0–3.0] |
2.5 ± 1.8 [1.0–10.0] |
0.13 |
| Within 365 |
4.8 ± 1.3 [2.0–8.0] |
4.4 ± 2.1 [1.0–11.0] |
0.72 |
| Number of all-cause readmissions (days) | |||
| Within 30 |
0.3 ± 0.6 [0.0–2.0] |
0.3 ± 0.5 [0.0–2.0] |
0.38 |
| Within 90 |
0.5 ± 1.0 [0.0–4.0] |
0.6 ± 1.0 [0.0–4.0] |
0.41 |
| Within 365 |
0.9 ± 1.2 [0.0–4.0] |
1.1 ± 2.1 [0.0–11.0] |
0.52 |
| Total number of all-cause hospitalizations (days) | |||
| Within 30 |
0.7 ± 1.6 [0.0–5.0] |
0.7 ± 2.3 [0.0–14.4] |
0.42 |
| Within 90 |
0.7 ± 1.6 [0.0–5.0] |
1.8 ± 4.5 [0.0–25.2] |
0.77 |
| Within 365 |
1.4 ± 2.7 [0.0–10.0] |
3.4 ± 7.3 [0.0–32.2] |
0.86 |
| Total number of all-cause emergency room visits (days) | |||
| Within 30 |
0.0 ± 0.0 [0.0–0.0] |
0.1 ± 0.3 [0.0–1.0] |
|
| Within 90 |
0.0 ± 0.0 [0.0–0.0] |
0.2 ± 0.4 [0.0–2.0] |
|
| Within 365 |
0.0 ± 0.0 [0.0–0.0] |
0.3 ± 0.9 [0.0–6.0] |
|
| Number of renal biopsies within 365 days post-transplantation |
0.9 ± 0.3 [0.0–1.0] |
1.6 ± 0.8 [0.0–4.0] |
0.01 |
SD standard deviation
Data are mean ± SD [range]
There were no emergency room visits for patients receiving ER-T compared with 0.3 ± 0.9 visits for patients receiving IR-T within 365 days post-transplantation (Table 4). Significantly fewer renal biopsies were performed within 365 days post-transplantation in the ER-T versus the IR-T group (0.9 ± 0.3 versus 1.6 ± 0.8 biopsies; p = 0.01) (Table 4).
Discussion
The aim of this 1-year retrospective analysis exploring clinical outcomes and healthcare resource utilization in matched patients who received ER-T versus IR-T-based maintenance immunosuppression was to further characterize the use of ER-T in a real-world setting. To our knowledge, there is a paucity of data describing outcomes for ER-T under alemtuzumab induction. This is the first study to report the use of ER-T following induction with alemtuzumab in a real-world setting. In addition, while numerous studies have confirmed the safety and efficacy of converting stable adult kidney transplant recipients from IR-T to ER-T [20–23], none of these studies has included patients converted within 10 days after transplantation [24].
This is the first study reporting outcomes using a novel approach of early conversion from IR-T to ER-T for de novo kidney transplant recipients. Overall, no patients receiving the extended-release formulation experienced BCAR or graft failure, required retransplantation, or died. Renal function was also similar over 1 year of follow-up in both groups.
In this study, most patients received induction therapy with alemtuzumab (57.9%) or basiliximab (36.8%). The regimen was associated with good clinical efficacy, which is in line with low rates of BCAR reported in patients treated with alemtuzumab induction therapy followed by IR-T-based maintenance immunosuppression [25].
Although 1-year graft and patient survival rates were high with both tacrolimus formulations, no patients receiving ER-T experienced graft failure compared with 5.5% of patients receiving IR-T. Similar 1-year graft and patient survival rates with ER-T and IR-T have been reported previously. For example, in a phase III study of de novo kidney transplant patients, 1-year graft survival rates were 91.5% and 92.8%, with patient survival rates of 96.9% and 97.5% for ER-T and IR-T, respectively [2]. Another phase III study showed that 1-year graft survival rates were 96.7% and 92.9%, while patient survival rates were 98.6% and 95.7% for ER-T and IR-T, respectively [26].
In line with the high graft survival rate, the proportion of patients experiencing a BCAR event was low in both treatment groups and consistent with previous publications. For example, results from a phase II study extension showed a high (93.3%) BCAR-free survival rate in de novo kidney transplant patients receiving ER-T at 1 year [27], which remained greater than 90% at 4 years post-transplantation [23]. Furthermore, our study supports the similar rate of BCAR between the extended- and immediate-release formulations, as reported by Silva et al. (10.3% versus 7.5%, respectively) [26]. Indeed, it is encouraging that no patients receiving ER-T in our study experienced BCAR, and outcomes appear to be similar in a real-world setting under alemtuzumab induction and an early conversion strategy from IR-T to ER-T.
Importantly, ER-T and IR-T both supported long-term renal function, as evidenced by comparable eGFR levels between formulations over 1 year of follow-up. These observations agree with Silva et al. who reported a comparable mean eGFR with ER-T and IR-T at 1-year post-transplantation (58.6 mL/min/1.73 m2 versus 59.7 mL/min/1.73 m2, respectively) [26]. Our findings are also consistent with the 1-year results of a long-term follow-up study by Van Hooff et al., in which mean serum creatinine and creatinine clearance rates remained stable over 4 years in de novo kidney transplant patients receiving ER-T [23]. Likewise, good long-term renal function has been reported in kidney transplant recipients converted from IR-T to ER-T. For example, in the R-EVOLUTION study—a 3-year follow-up of 1798 patients who were receiving ER-T from the EVOLUTION trial [22, 28]—the mean eGFR by the Modification of Diet in Renal Disease-4 remained stable over 3 years after conversion from IR-T (56.7 mL/min/1.73 m2 versus 58.1 mL/min/1.73 m2 at baseline and year 3, respectively) [28].
In this study, the time taken to achieve target tacrolimus trough levels was not significantly different between ER-T and IR-T (9.2 versus 7.8 days). Both the number of dose adjustments required to attain target levels and the tacrolimus trough levels on or after 30 days of treatment were also similar between both formulations. Furthermore, these data are consistent with a study of de novo kidney transplant patients in which systemic tacrolimus exposure was similar for both formulations at steady state (following dose adjustment), as evidenced by a comparable area under the concentration–time curve at day 14 and at week 6 [27]. These data suggest that initiating ER-T in a real-world setting is no more complex than it is with IR-T, and that early conversion from IR-T to ER-T post-transplant yields similar outcomes to that of traditional, continuous treatment with IR-T. Importantly, ER-T provides patients with the option of once-daily dosing of tacrolimus.
Given the reported positive clinical outcomes with ER-T-based immunosuppression, several studies have assessed the economic impact of treating kidney transplant patients with this formulation versus IR-T [29–31]. Such studies have predicted that ER-T would be associated with a range of cost savings due to improved rejection and graft survival rates [29–31]. For example, using cost data from Medicare and the US Renal Data System, Abecassis et al. showed that ER-T was associated with a 5-year cost saving of US$9411 [31]. This was driven largely by a reduction in the costs associated with graft loss and dialysis compared with IR-T [31]. While our study did not directly assess cost savings, we did not find statistically significant differences between treatment groups for the number of outpatient clinic visits, readmissions, hospitalization days, and emergency room visits. However, nonsignificant differences in outpatient clinic visits during the first 90 days, and total hospitalization days during the first 365 days, may have reached statistical significance with a larger number of patients.
A number of limitations in the design of this study should be noted. As this was a retrospective study, a number of study biases are likely to be inherent. First, the number of patients who were available for this study based on the inclusion criteria was small. The study was also undertaken shortly after the introduction of ER-T into the US market when a paucity of real-world data were available characterizing the use of ER-T. The small sample size may have affected the power to detect differences between the two treatment groups. In addition, the center-specific practice of capping the measured eGFR values at 60 mL/min/1.73 m2 may have limited the ability to fully assess differences in renal function over time between the two groups. As this study explored the impact of ER-T versus IR-T on clinical and healthcare resource utilization parameters over 1 year only, longer-term differences between the formulations could not be detected. This is noteworthy in that nonadherence to treatment, which has a substantial impact on antibody-mediated rejection, graft survival, and costs [11, 13, 32, 33], is known to increase over time [34].
There are several strengths of this study that should be noted. For instance, propensity score matching against a number of characteristics helped control for bias in patient selection between treatment groups. Furthermore, we used a large patient database, which provided real-world data outside of the controlled environment of an RCT. As such, we anticipate that our findings may be more generalizable to other transplant populations receiving ER-T or IR-T in routine clinical settings than data generated from RCTs.
Conclusion
While ER-T has been available in Europe for almost a decade, to our knowledge this is the first study examining clinical outcomes and healthcare resource utilization of kidney transplant patients treated with the extended-release formulation under US real-world practice conditions. Exploring a real-world data set also enabled us to characterize a unique treatment approach: early conversion from IR-T to ER-T under alemtuzumab induction in de novo kidney transplant recipients. As such, this study affirms that ER-T can be reliably employed in this practice setting, while achieving promising outcomes on a par with those seen in several large European trials. Healthcare resource utilization was generally similar between treatments; however, there were fewer outpatient visits with ER-T at borderline statistical significance (p = 0.05). Further investigation in larger real-world studies is warranted to fully characterize the real-world clinical, economic, and resource utilization outcomes with the extended- versus immediate-release formulation employing the use of novel treatment approaches not captured in controlled clinical trials.
Acknowledgements
Assistance with chart reviews was provided by Clinical Research Core, Comprehensive Transplant Center, Northwestern University Feinberg School of Medicine.
Funding
This study, the journal’s article processing charges, and open access fee were funded by Astellas Pharma Global Development, Inc. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Medical Writing and Editorial Assistance
James Wallis, MRes, from Cello Health MedErgy assisted in drafting the initial version of the manuscript under the direction of the authors, and provided editorial support throughout its development. Editorial support was funded by Astellas Pharma, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors contributed to drafting the article and data analysis. BH, SEM, LZ, AIS, and DPL contributed to research design and performance of the research. HB, EL, RK, and JJS contributed to research design. KA contributed to performance of the research.
Disclosures
Bing Ho reports a contract with Astellas to conduct the study research. Hardik Bhagat is employed by Astellas Pharma US, Inc. and has received a salary from Astellas Pharma US, Inc. Edward Lee is a former employee of Astellas, and has received a salary from Astellas. Kofi Atiemo reports a contract with Astellas to conduct the study research. Amna Daud reports a contract with Astellas to conduct the study research. Raymond Kang reports a contract with Astellas to conduct the study research. Samantha E. Montag reports a contract with Astellas to conduct the study research. Lihui Zhao reports a contract with Astellas to conduct the study research. Jason J. Schwartz is employed by Astellas and has received a salary from Astellas. Anton I. Skaro reports a contract with Astellas to conduct the study research. Daniela P. Ladner reports a contract with Astellas to conduct the study research.
Compliance with Ethics Guidelines
This retrospective database analysis study had approval of the Northwestern University Institutional Review Board (IRB#STU0020221) and adhered to the 1964 Helsinki declaration and its later amendments or comparable ethical standards. As a retrospective study, informed consent of individuals was not required.
Data Availability
Access to anonymized individual patient-level data will not be provided for this study as it meets one or more of the exceptions described under the Sponsor Specific Information for Astellas on http://www.clinicalstudydatarequest.com.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7687397.
References
- 1.Silva HT, Jr, Yang HC, Meier-Kriesche HU, et al. Long-term follow-up of a phase III clinical trial comparing tacrolimus extended-release/MMF, tacrolimus/MMF, and cyclosporine/MMF in de novo kidney transplant recipients. Transplantation. 2014;97:636–641. doi: 10.1097/01.TP.0000437669.93963.8E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krämer BK, Charpentier B, Bäckman L, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant. 2010;10:2362–2643. doi: 10.1111/j.1600-6143.2010.03256.x. [DOI] [PubMed] [Google Scholar]
- 3.Stifft F, Stolk LM, Undre N, van Hooff JP, Christiaans MH. Lower variability in 24-hour exposure during once-daily compared to twice-daily tacrolimus formulation in kidney transplantation. Transplantation. 2014;97:775–780. doi: 10.1097/01.TP.0000437561.31212.0e. [DOI] [PubMed] [Google Scholar]
- 4.Wu MJ, Cheng CY, Chen CH, et al. Lower variability of tacrolimus trough concentration after conversion from Prograf to Advagraf in stable kidney transplant recipients. Transplantation. 2011;92:648–652. doi: 10.1097/TP.0b013e3182292426. [DOI] [PubMed] [Google Scholar]
- 5.Kuypers DR, Peeters PC, Sennesael JJ, et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013;95:333–340. doi: 10.1097/TP.0b013e3182725532. [DOI] [PubMed] [Google Scholar]
- 6.Eberlin M, Otto G, Krämer I. Increased medication compliance of liver transplant patients switched from a twice-daily to a once-daily tacrolimus-based immunosuppressive regimen. Transplant Proc. 2013;45:2314–2320. doi: 10.1016/j.transproceed.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Beckebaum S, Iacob S, Sweid D, et al. Efficacy, safety, and immunosuppressant adherence in stable liver transplant patients converted from a twice-daily tacrolimus-based regimen to once-daily tacrolimus extended-release formulation. Transpl Int. 2011;24:666–675. doi: 10.1111/j.1432-2277.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 8.Sapir-Pichhadze R, Wang Y, Famure O, Li Y, Kim SJ. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int. 2014;85:1404–1411. doi: 10.1038/ki.2013.465. [DOI] [PubMed] [Google Scholar]
- 9.Pollock-BarZiv SMS, Finkelstein Y, Manlhiot C, et al. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant. 2010;14:968–975. doi: 10.1111/j.1399-3046.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 10.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–67. [DOI] [PubMed]
- 11.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant. 2015;15:2921–2930. doi: 10.1111/ajt.13347. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigo E, Segundo DS, Fernández-Fresnedo G, et al. Within-patient variability in tacrolimus blood levels predicts kidney graft loss and donor-specific antibody development. Transplantation. 2016;100:2479–2485. doi: 10.1097/TP.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 13.Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 14.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 annual data report: kidney. Am J Transplant. 2017;17(Suppl 1):21–116. doi: 10.1111/ajt.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gondos A, Döhler B, Brenner H, Opelz G. Kidney graft survival in Europe and the United States: strikingly different long-term outcomes. Transplant J. 2013;95:267–274. doi: 10.1097/TP.0b013e3182708ea8. [DOI] [PubMed] [Google Scholar]
- 16.Merion RM, Goodrich NP, Johnson RJ, et al. Kidney transplant graft outcomes in 379 257 recipients on 3 continents. Am J Transplant. 2018;18:1914–1923. doi: 10.1111/ajt.14694. [DOI] [PubMed] [Google Scholar]
- 17.Tanzi MG, Undre N, Keirns J, Fitzsimmons WE, Brown M, First MR. Pharmacokinetics of prolonged-release tacrolimus and implications for use in solid organ transplant recipients. Clin Transplant. 2016;30:901–911. doi: 10.1111/ctr.12763. [DOI] [PubMed] [Google Scholar]
- 18.Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520–2531. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 19.National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Estimating glomerular filtration rate (GFR). NIDDK. 2017. https://www.niddk.nih.gov/health-information/communication-programs/nkdep/laboratory-evaluation/glomerular-filtration-rate/estimating. Accessed 14 Mar 2019.
- 20.Alloway R, Steinberg S, Khalil K, et al. Conversion of stable kidney transplant recipients from a twice daily Prograf-based regimen to a once daily modified release tacrolimus-based regimen. Transplant Proc. 2005;37:867–870. doi: 10.1016/j.transproceed.2004.12.222. [DOI] [PubMed] [Google Scholar]
- 21.Van Hooff J, Van der Walt I, Kallmeyer J, et al. Pharmacokinetics in stable kidney transplant recipients after conversion from twice-daily to once-daily tacrolimus formulations. Ther Drug Monit. 2012;34:46–52. doi: 10.1097/FTD.0b013e318244a7fd. [DOI] [PubMed] [Google Scholar]
- 22.Guirado L, Cantarell C, Franco A, et al. Efficacy and safety of conversion from twice-daily to once-daily tacrolimus in a large cohort of stable kidney transplant recipients. Am J Transplant. 2011;11:1965–1971. doi: 10.1111/j.1600-6143.2011.03571.x. [DOI] [PubMed] [Google Scholar]
- 23.van Hooff JP, Alloway RR, Trunečka P, Mourad M. Four-year experience with tacrolimus once-daily prolonged release in patients from phase II conversion and de novo kidney, liver, and heart studies. Clin Transplant. 2011;25:1–12. doi: 10.1111/j.1399-0012.2010.01377.x. [DOI] [PubMed] [Google Scholar]
- 24.Caillard S, Moulin B, Buron F, et al. Advagraf®, a once-daily prolonged release tacrolimus formulation, in kidney transplantation: literature review and guidelines from a panel of experts. Transpl Int. 2016;29:860–869. doi: 10.1111/tri.12674. [DOI] [PubMed] [Google Scholar]
- 25.Hanaway MJ, Woodle ES, Mulgaonkar S, et al. Alemtuzumab induction in renal transplantation. N Engl J Med. 2011;364:1909–1919. doi: 10.1056/NEJMoa1009546. [DOI] [PubMed] [Google Scholar]
- 26.Silva HT, Jr, Yang HC, Abouljoud M, et al. Erratum: one-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant. 2007;7:595–608. doi: 10.1111/j.1600-6143.2007.01661.x. [DOI] [PubMed] [Google Scholar]
- 27.Wlodarczyk Z, Squifflet JP, Ostrowski M, et al. Pharmacokinetics for once- versus twice-daily tacrolimus formulations in de novo kidney transplantation: a randomized, open-label trial. Am J Transplant. 2009;9:2505–2513. doi: 10.1111/j.1600-6143.2009.02794.x. [DOI] [PubMed] [Google Scholar]
- 28.Guirado L, Burgos D, Cantarell C, et al. Medium-term renal function in a large cohort of stable kidney transplant recipients converted from twice-daily to once-daily tacrolimus. Transplant Direct. 2015;1:e24. doi: 10.1097/TXD.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muduma G, Odeyemi I, Pollock RF. Evaluating the economic implications of non-adherence and antibody-mediated rejection in renal transplant recipients: the role of once-daily tacrolimus in the UK. J Med Econ. 2015;18:1050–1059. doi: 10.3111/13696998.2015.1074584. [DOI] [PubMed] [Google Scholar]
- 30.Muduma G, Odeyemi I, Smith-Palmer J, Pollock RF. Budget impact of switching from an immediate-release to a prolonged-release formulation of tacrolimus in renal transplant recipients in the UK based on differences in adherence. Patient Prefer Adherence. 2014;8:391–399. doi: 10.2147/PPA.S60213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abecassis MM, Seifeldin R, Riordan ME. Patient outcomes and economics of once-daily tacrolimus in renal transplant patients: results of a modeling analysis. Transplant Proc. 2008;40:1443–1445. doi: 10.1016/j.transproceed.2008.03.090. [DOI] [PubMed] [Google Scholar]
- 32.Gaynor JJ, Ciancio G, Guerra G, et al. Graft failure due to noncompliance among 628 kidney transplant recipients with long-term follow-up. Transplantation. 2014;97:925–933. doi: 10.1097/01.TP.0000438199.76531.4a. [DOI] [PubMed] [Google Scholar]
- 33.Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009;9:2597–2606. doi: 10.1111/j.1600-6143.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- 34.Ichimaru N, Kakuta Y, Abe T, et al. Treatment adherence in renal transplant recipients: a questionnaire survey on immunosuppressants. Transplant Proc. 2008;40:1362–1365. doi: 10.1016/j.transproceed.2008.02.083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access to anonymized individual patient-level data will not be provided for this study as it meets one or more of the exceptions described under the Sponsor Specific Information for Astellas on http://www.clinicalstudydatarequest.com.