Abstract
Introduction
A water-soluble Cremophor EL-free formulation of paclitaxel, in which retinoic acid derivates solubilize paclitaxel by forming micelles (paclitaxel micellar), was studied for the first time in man to establish the maximum tolerated dose (MTD) and to characterize the pharmacokinetics (PK).
Methods
This was an open-label, one-arm, dose-escalating study in patients with advanced solid malignant tumours, for which no standard therapy was available or had failed. Paclitaxel micellar was given as 1-h intravenous infusion every 21 days for 3 cycles, mainly without premedication. Plasma samples were collected during 24 h at the first cycle and paclitaxel concentrations were assayed by high-performance liquid chromatography. PK was evaluated using a two-compartment model.
Results
Thirty-four patients received paclitaxel micellar at doses ranging between 90 and 275 mg/m2. MTD was established as 250 mg/m2. Fatigue and neuropathy were the most frequent dose-limiting toxicities. No hypersensitivity reactions were observed. PK of paclitaxel was evaluated in 25 data sets. Paclitaxel micellar had a rapid initial distribution phase, mean half-life 0.55 h, estimated to be completed 3 h after dosing and a mean terminal half-life of 8.8 h. Mean clearance was 13.4 L/h/m2 with fivefold interindividual variability. The residual areas after 10 h and 24 h were 15.7 ± 8.6% and 5.7 ± 3.9% of the area under the plasma concentration–time curve to infinite time (AUCinf), respectively.
Conclusion
No new side effects unknown for paclitaxel were observed. Maximum plasma concentration (Cmax) and AUCinf showed a tendency to increase linearly with dose within the 150–275 mg/m2 dose range. The possibility to administer paclitaxel micellar without steroid premedication makes it an attractive candidate for further studies in combination with immunotherapy.
Trial Registration
EudraCT no: 2004-001821-54.
Funding
Oasmia Pharmaceutical AB.
Keywords: Cancer, Dose-finding, First-in-man, Nano-sized micelles, Paclitaxel micellar, Paclitaxel, XR17, Pharmacokinetics
Introduction
Paclitaxel plays a major role in treatment of ovarian cancer, lung cancer, and breast cancer, not only in the palliative but also in the adjuvant setting. Paclitaxel has a very low solubility in water, with an estimated value less than 0.1 µg/mL [1]. In the first marketed paclitaxel formulation, approved in the USA in 1992, a mixture of Cremophor EL and ethanol is used as solubilizer to enable parenteral administration of paclitaxel [2]. However, the use of Cremophor EL as a solubilizer can cause anaphylaxis and severe hypersensitivity reactions, such as dyspnoea, hypotension, angioedema and generalized urticaria, which occurred in 2–4% of patients receiving Cremophor EL formulated paclitaxel (Cremophor EL paclitaxel) in clinical trials [3]. Consequently, all patients receiving Cremophor EL paclitaxel should be pre-treated with corticosteroids and antihistamines (H1 and H2 antagonists) and patients who experience severe hypersensitivity reactions to Cremophor EL paclitaxel should not be re-challenged with the drug [3]. Cremophor EL is also responsible for the non-linear pharmacokinetic (PK) behaviour of paclitaxel for Cremophor EL paclitaxel at clinical doses [4]. In addition, premedication with corticosteroids may potentially interfere with the immune response elicited by check-point inhibitors [5] and potentially affect efficacy in combination treatment including chemotherapy and immune therapy, suggesting that chemotherapy not requiring corticosteroid premedication may potentially be advantageous in that specific setting. Therefore, the main goals in the development of novel formulations of paclitaxel is to reduce toxicities associated with Cremophor EL and to decrease the need for premedication with corticosteroids [6, 7].
Extensive research has been performed to find competitive formulations to Cremophor EL paclitaxel and some of them have been more successful than others. For example, two of them have received market authorizations: nanoparticle albumin-bound paclitaxel in the USA (2005) and the European Union (2008) and paclitaxel micellar in the Russian Federation (2015) and European Union (2018) [8, 9]. In addition, several other formulations are in late stage clinical development such as nanoparticle polymer-based paclitaxel, liposomal paclitaxel, paclitaxel injection concentrate for nanodispersion (PICN) as well as nanosomal paclitaxel lipid suspension (NPLS) [6, 8].
Paclitaxel micellar is a Cremophor EL-free formulation of paclitaxel, in which two isoforms of N-retinoyl-l-cysteic acid methyl ester sodium salt (XR17) are used as micelle-forming excipients to make paclitaxel water soluble. When the lyophilized powder, containing paclitaxel and XR17, is dissolved in an aqueous medium, paclitaxel-containing micelles with sizes of 20–30 nm are formed. Paclitaxel micellar has been investigated in several clinical studies during the last decade. However, the results of the clinical trials have not been reported in any publication before.
The primary aim of this first-in-man study was to define the maximum tolerated dose (MTD) of paclitaxel micellar in patients with recurrent solid tumours. Secondary objectives were to describe the PK of increasing doses of paclitaxel when administered as this new micellar formulation.
Methods
Study Design
This was an open-label dose-escalation study. Patients were enrolled at the Department of Oncology at Lund University Hospital and at Norrlands University Hospital, Umeå, Sweden, between November 2004 and May 2007. The protocol was approved by the Swedish Medical Products Agency (EudraCT number: 2004-001821-54) and the Regional Ethical Review Board in Lund, Sweden (Dnr 542/2004). The study was conducted in accordance with the protocol, regulatory requirements, Good Clinical Practice and the ethical principles of the Declaration of Helsinki.
Male or female patients of at least 18 years old with a histologically proven solid malignant tumour for which no standard therapy was available or had failed were eligible. Other inclusion criteria were Eastern Cooperative Oncology Group (ECOG) performance status of up to 2, and life expectancy of at least 12 weeks. Patients with significant abnormalities in blood chemistry, haematology or renal function, neuropathy (sensory or motor) of at least grade 2, or autoimmune disease were not included.
Drug Preparation
Lyophilized powder of paclitaxel micellar (Apealea®/Paclical, Oasmia Pharmaceutical AB, Sweden) was dissolved in a sterile aqueous saline solution (150 mmol/L) with calcium chloride (2.3 mmol/L) for the first 9 patients, and in Ringer-acetate for injection for the remaining 25 patients to a concentration of 1 mg/mL. The paclitaxel micellar solution was thereafter administered as a 1-h intravenous infusion for 3 cycles every 21 days.
Dosing Procedure and Definition of MTD
A stepwise dose escalation scheme was used starting at a dose of 90 mg/m2 (see Fig. 1). Briefly, the first two patients received increasing doses; thereafter, cohorts of three patients were included at each dose level for three 3-week cycles. If dose limiting toxicity (DLT) was recorded in one patient at one dose level, additionally three patients were treated at that same dose level. If at least two of the initial three patients or two of the six patients in the extended cohort experienced DLT, the MTD was defined as the dose level below that dose. Thereafter, six additional patients were administered the MTD for three treatment cycles. DLT was defined as one or more of the following adverse events (AEs): any life-threatening event possibly related to the study drug, any common grade 3 or 4 toxicity considered possibly related to the study drug, grade 4 neutropenia lasting for at least 7 days, grade 4 neutropenia with a fever over 38 °C, grade 3 thrombocytopenia lasting for at least 7 days, any grade 4 thrombocytopenia, grade 3 or worse non-haematological toxicity, except for nausea, vomiting and alopecia. Toxicity criteria were assessed according to National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
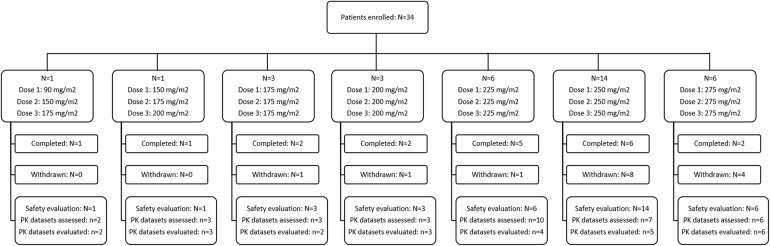
Fig. 1.
Patient disposition. N number of patients, n number of data sets. Of the 34 PK data sets assessed, nine were excluded from the PK evaluation because of the following reasons: missing actual sampling times (n = 1, 175 mg/m2), insufficient data for the PK model (i.e. only 2 post-infusion samples; n = 1, 225 mg/m2), poor fit to the model (n = 2, 225 mg/m2, and n = 2, 250 mg/m2) or when PK data sets were assessed repeatedly for the same patient at the same dose level (n = 3, 225 mg/m2)
Premedication with corticosteroids, H1 and H2 blockers was not given routinely but corticosteroids could be given at the discretion of the investigator. Patients were followed until 3 weeks after the last drug administration (end of trial visit at week 9), if not prematurely withdrawn.
PK of Paclitaxel
Blood Sampling
Blood samples to determine plasma levels of paclitaxel were collected before infusion and at 5 min, 15 min, 30 min, 60 min, 3 h, 6 h, 8 h, 10 h, 12 h and 24 h after start of infusion. Blood samples for PK analysis were taken during all 3 cycles in the first two patients, and in the first two patients who had been administered 225 mg/m2. For all other patients, samples were taken during the first treatment cycle only. The blood samples were collected in 5-mL sodium-heparin vacutainers, centrifuged and thereafter the plasma was transferred to cryovials and stored at −70 °C until analysis.
Paclitaxel Concentration Analysis
Paclitaxel concentrations in plasma were determined by Quintiles AB (Uppsala, Sweden) using a modification of a high-performance liquid chromatography (HPLC) method previously described by Sparreboom et al. [10]. In short, the assay consists of a rapid and highly selective liquid–liquid extraction step followed by HPLC using the mobile phases A) methanol/acetate buffer pH 5/tetrahydrofuran (40:60:2, v/v) and B) methanol/acetate buffer pH 5/tetrahydrofuran (80:20:2, v/v). One-millilitre human plasma samples were spiked with internal standard (docetaxel) followed by extraction with 3 mL of acetonitrile/1-chlorobutane (2:8, v/v) during 1 min on a Vortex mixer. The organic phase was separated and evaporated and the residue re-dissolved in mobile phase A before being injected onto the HPLC column. Isocratic elution with 36% mobile phase A and 64% mobile phase B for 20 min (flow rate 0.5 mL/min) was used, at a column temperature of 60 °C. Detection was done with ultraviolet light at 230 nm, and quantification was based on peak height ratios of paclitaxel to the internal standard. The calibration was obtained by a linear curve fit of the peak height ratios versus the concentration with a weighting factor of 1/concentration. The method was validated in the range 10–500 ng/mL with an inter-assay repeatability of 1.7–4.5% and an accuracy better than 4%. The lower limit of quantification of paclitaxel in human plasma was 10 ng/mL. It was ascertained that the XR17 component of the formulation did not interfere with the measurements.
PK Data Analysis
PK analysis was performed with individual plasma concentration–time data from each patient using WinNonlin PK software (Pharsight Corporation, Mountain View, CA, USA). In all PK calculations the actual given dose, infusion times and blood sampling times were used. If PK samples were collected for one patient at multiple cycles at the same dose level, the first evaluable data set was used in the PK evaluation. The data were found to be adequately described by a two-compartment model with a constant input rate during the infusion phase. All individual model-generated plasma concentration–time profiles were fitted to the input data by examining different weighting schemes and it was judged that a weight of 1/(Yhat)2 was most appropriate. If PK parameters could not be evaluated because of poor fits to the model (identified by visual inspection) these data sets were excluded from the evaluation. The following primary parameters were used to describe the two-compartment model: Vc, K10, K12 and K21, where Vc is the apparent volume of the central compartment, K10 is the rate constant for elimination from the central compartment, K12 is the rate constant for drug transport from the central to the peripheral compartment and K21 is the rate constant for the opposite transport, i.e. from the peripheral back to the central compartment.
Secondary PK parameters obtained from the two-compartment model were the following: total area under the plasma concentration–time curve to infinite time (AUCinf), maximum plasma concentration (Cmax), clearance (CL), the distribution rate constant α, the distribution half-life (t1/2α), the terminal elimination rate constant β, the terminal elimination half-life (t1/2β), the distribution volume during the terminal elimination phase (Vz) and the distribution volume during steady state (Vss). AUCinf was calculated from the following equation:
| 1 |
Note that these parameter values are based on the fitted model and therefore may differ numerically from traditional non-compartmental values, which are calculated directly from the raw data.
The rate at which the micelle formulation disintegrates in vivo was estimated with the assumption that the diameter of the micelles (20–30 nm) is too large to allow them to leave the blood intact by diffusion through pores in the capillary walls. The amount of drug in plasma during the infusion, when little drug has been eliminated from the body, will therefore to a large extent reflect the stability of the micelles. To characterize the micelle stability, the amount of drug in plasma at half the infusion time, tmid, was divided by the given dose, Dosemid, at the same time point according to the following equation:
| 2 |
where the amount of paclitaxel present in plasma is calculated from the plasma concentration at tmid (Cp, mid), and the plasma volume (Vp) is approximated to be 1.7 L/m2, assuming a total plasma volume of 3 L and a body surface area of 1.73 m2. Dosemid was estimated by the following equation:
| 3 |
where Tinf is the total infusion time. When Tinf deviated from 1 h and/or sampling time deviated from 0.5 h, the sample taken closest to Tinf/2 was selected.
Since paclitaxel is available in plasma in both micellar and non-micellar form, this “remaining dose fraction in plasma” is the upper limit of the amount of paclitaxel in plasma that theoretically can be in its micellar form.
In order to characterize the contributed exposure over time, the areas under the concentration–time curve (AUC) during the first 10 h (AUC10h) or 24 h (AUC24h) were calculated in each subject according to Eq. 4:
| 4 |
where AUCt is the AUC at time t and Ct is the plasma concentration at time t (Ct/β corresponds to the residual area). The contribution of exposure over time was thereafter calculated as a percentage of AUCinf.
All data in the present study were summarized descriptively using STATA v 11 (STATA corporation).
Results
Patient Characteristics
Thirty-four Caucasian patients (32–79 years) were included and received at least one dose of paclitaxel micellar. Nineteen patients (56%) received all three treatment cycles. Demographic data for all included patients as well as for the patients included in the PK analysis are presented in Table 1. The most common primary tumours were ovarian cancer (N = 6), malignant melanoma (N = 5), colon cancer (N = 4) and uterine cancer (N = 4). Patients were administered doses ranging between 90 and 275 mg/m2 as described in Fig. 1. Premedication was given before drug administration in four patients at 7 of 80 treatment cycles (9%) in total.
Table 1.
Characteristics of all patients and patients included in the PK evaluation
| All patients (N = 34) | PK set (N = 22) | |
|---|---|---|
| Sex (female/male) | 15 (44%)/19 (56%) | 8 (36%)/14 (64%) |
| Age (years) | 60 ± 11 | 62 ± 10 |
| Height (cm) | 172 ± 8 | 173 ± 8 |
| Weight (kg) | 73 ± 14 | 72 ± 11 |
| Body surface area (m2) | 1.9 ± 0.2 | 1.9 ± 0.2 |
| ECOG performance status | ||
| 0 | 9 (26%) | 7 (32%) |
| 1 | 19 (56%) | 12 (55%) |
| 2 | 6 (18%) | 3 (14%) |
Data are presented as mean ± standard deviation (continuous data) or counts and percentages (categorical data)
ECOG Eastern Cooperative Oncology Group
MTD and Safety
Safety was evaluated in all 34 enrolled patients. DLTs occurred in three of six patients at a dose of 275 mg/m2 and MTD was established as 250 mg/m2. The MTD was validated in six patients. A total of 11 different preferred terms (PTs) were reported as 20 DLTs in eight patients. Fatigue and neuropathy (neuropathy, neuropathy peripheral or peripheral sensory neuropathy) were the most frequently occurring DLTs (N = 4 [12%], respectively), followed by leukopenic events (leukopenia or febrile neutropenia) and stomatitis (N = 2 [6%], respectively). The remaining DLTs occurred in single patients (small intestinal obstruction, arthralgia, myalgia, skin reaction). All DLTs occurred at paclitaxel doses of at least 225 mg/m2; one patient (17%) at 225 mg/m2, four patients (29%) at 250 mg/m2 and three patients (50%) at 275 mg/m2.
All 34 included patients experienced one or more AE and 12 patients (35%) experienced a serious adverse event (SAE). The number of patients with SAE listed by preferred terms are presented in Table 2. There were no deaths during the study. Fifteen patients (44%) discontinued the study after first or second treatment cycle, mainly because of AE with DLT and/or disease progression. Overall, the most common AE was fatigue (N = 23 [68%]). Other frequently occurring AEs were alopecia (N = 18 [53%]), leukopenia (N = 16 [47%]), neutropenia (N = 15 [44%]), peripheral sensory neuropathy (15 [44%]), nausea (14 [41%]) and pyrexia (13 [38%]). AEs of neuropathy were reported in a total of 24 patients. Fourteen patients had a previous history of neuropathy and 11 of them experienced exacerbation or aggravation of such symptoms during treatment. No hypersensitivity reactions were observed relating to administration of paclitaxel micellar.
Table 2.
Number of patients (%) with serious adverse events listed by preferred term and dose of paclitaxel micellar
| Preferred term | Dose of paclitaxel micellar | All patients (N = 34) | ||||
|---|---|---|---|---|---|---|
| 175 mg/m2 (N = 5)a | 200 mg/m2 (N = 3) | 225 mg/m2 (N = 6) | 250 mg/m2 (N = 14) | 275 mg/m2 (N = 6) | ||
| Ileus | 1 (20%) | 1 (33%) | 2 (6%) | |||
| Subileus | 1 (20%) | 1 (3%) | ||||
| Stomatitis | 1 (7%) | 1 (3%) | ||||
| Small intestinal obstruction | 1 (17%) | 1 (3%) | ||||
| Pyrexia | 1 (20%) | 1 (17%) | 1 (17%) | 3 (9%) | ||
| Abdominal infection | 1 (20%) | 1 (3%) | ||||
| Sepsis | 1 (7%) | 1 (3%) | ||||
| Haemoglobin decreased | 1 (7%) | 1 (17%) | 2 (6%) | |||
| Arthralgia | 1 (7%) | 1 (3%) | ||||
| Myalgia | 1 (7%) | 1 (3%) | ||||
| Syncope | 1 (7%) | 1 (3%) | ||||
| Urinary retention | 1 (7%) | 1 (3%) | ||||
| Dyspnoea | 1 (7%) | 1 (3%) | ||||
| Thrombosis | 1 (17%) | 1 (3%) | ||||
aData from the 2 patients receiving dose escalations are included at dose level 175 mg/m2
PK of Paclitaxel
PK Evaluation
A total of 34 sets of plasma concentration time data were obtained from 27 patients, at a total of seven dose levels (90–275 mg/m2). Nine data sets were excluded from the PK evaluation because of missing actual sampling time recordings (n = 1, 175 mg/m2), insufficient data for the PK model (i.e. only 2 post-infusion samples; n = 1, 225 mg/m2), poor fit to the model (n = 2, 225 mg/m2, and n = 2, 250 mg/m2) or when PK data sets were repeatedly assessed for the same patient at the same dose level (n = 3, 225 mg/m2). The remaining 25 data sets were obtained from 22 subjects (see Figs. 1, 2). The 90 mg/m2 dose, which was outside the clinical dose range, was only administered to one single patient and mainly used to assess safety. Therefore, this dose level is only included in Fig. 2b and in the dose-independent summary of PK parameters for all data sets (last column in Table 3). All infusions were administered during 0.95–1.15 h, except on three occasions (1.30, 2.03, and 2.07 h).
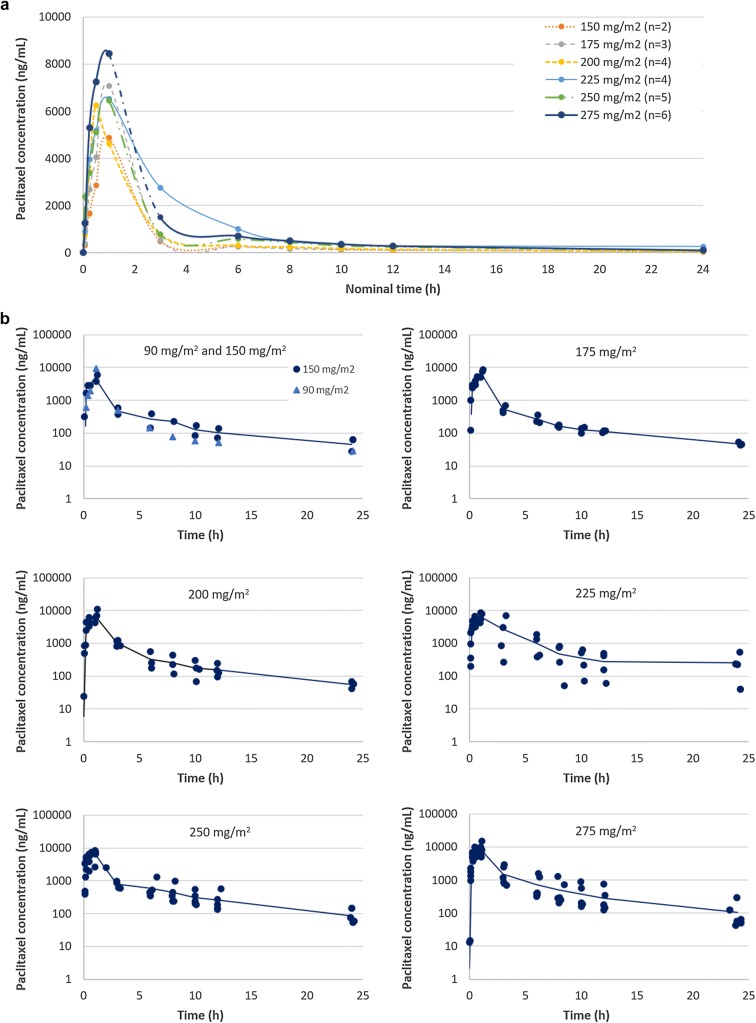
Fig. 2.
Plasma concentration–time curves for total paclitaxel at 1-h infusion of paclitaxel micellar at doses ranging between 90 and 275 mg/m2. a Mean concentrations for each dose level on a linear–linear scale, excluding the 90 mg dose (n = 1) since it is outside the clinical dose range and mainly used to assess safety. b Individual (dots) and mean concentrations (continuous line) of data sets included in the PK evaluation, presented per dose level on log-linear scales. n number of data sets
Table 3.
Primary and secondary parameters of total paclitaxel PK obtained from the two-compartment model by dose level
| Dose levela | All data sets (n = 25) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 150 mg/m2 (n = 2) | 175 mg/m2 (n = 3) | 200 mg/m2 (n = 4) | 225 mg/m2 (n = 4) | 250 mg/m2 (n = 5) | 275 mg/m2 (n = 6) | Median (min–max) | Mean ± SD | ||
| Primary PK parametersb | |||||||||
| Vc (L/m2) | 16.9 (11.9–21.8) | 13.9 (12.5–16.5) | 15.4 (8.7–21.9) | 17.7 (9.8–23.7) | 13.7 (8.2–20.3) | 12.8 (11.0–19.3) | 13.7 (6.9–23.7) | 14.8 ± 4.9 | |
| K10 (h–1) | 0.93 (0.92–0.93) | 1.05 (0.86–1.13) | 0.85 (0.62–1.06) | 0.56 (0.31–2.31) | 1.21 (0.77–1.40) | 0.95 (0.46–1.24) | 0.93 (0.31–2.31) | 0.96 ± 0.40 | |
| K12 (h–1) | 0.43 (0.36–0.50) | 0.38 (0.29–0.61) | 0.32 (0.16–0.60) | 0.36 (0.11–1.12) | 0.67 (0.44–2.08) | 0.40 (0.28–0.62) | 0.42 (0.11–2.08) | 0.53 ± 0.42 | |
| K21 (h–1) | 0.13 (0.12–0.14) | 0.13 (0.11–0.13) | 0.11 (0.04–0.33) | 0.13 (0.04–0.26) | 0.15 (0.14–0.28) | 0.16 (0.13–0.21) | 0.14 (0.04–0.33) | 0.15 ± 0.07 | |
| Secondary PK parametersc | |||||||||
| Cmax (ng/mL) | 5354 (3893–6815) | 7264 (5220–7609) | 7713 (5198–13,716) | 7746 (5363–12,863) | 8692 (3183–9589) | 10,398 (8227–15,466) | – | – | |
| AUCinf (ng h/mL) | 10,551 (7416–13,686) | 12,367 (10,063–14,666) | 15,396 (14,145–23,571) | 22,979 (9951–51,154) | 15,985 (14,632–24,945) | 17,783 (15,932–48,203) | – | – | |
| t1/2α (h) | 0.50 (0.47–0.52) | 0.45 (0.40–0.59) | 0.55 (0.46–0.71) | 0.93 (0.20–1.63) | 0.33 (0.20–0.51) | 0.52 (0.36–0.84) | 0.47 (0.20–1.63) | 0.55 ± 0.32 | |
| t1/2β (h) | 8.04 (7.88–8.20) | 8.93 (7.39–8.96) | 8.83 (4.75–18.47) | 9.75 (4.82–23.07) | 7.16 (6.93–10.80) | 6.76 (5.54–9.40) | 7.72 (4.75–23.10) | 8.83 ± 4.10 | |
| CL (L/h/m2) | 15.6 (11.0–20.2) | 14.2 (11.9–17.3) | 12.7 (8.5–14.1) | 13.0 (4.4–22.6) | 15.6 (10.0–17.1) | 15.5 (5.7–17.3) | 14.1 (4.4–22.6) | 13.4 ± 4.8 | |
| Vz (L/m2) | 182 (125–239) | 182 (127–224) | 165 (93–226) | 140 (125–206) | 165 (104–243) | 145 (62–185) | 155 (62–243) | 155 ± 49 | |
| Vss (L/m2) | 70.4 (54.1–86.7) | 57.4 (44.8–96.6) | 57.2 (41.1–61.7) | 66.9 (50.9–76.0) | 70.0 (50.2–165.0) | 54.5 (31.4–67.9) | 59.8 (23.8–165.0) | 63.3 ± 26.9 | |
Data are presented as median (min–max). n number of data sets
aData at dose level 90 mg/m2 (n = 1) are not shown individually but are included in the last column presenting median (min–max) and mean (SD) values for all data sets
bVc apparent volume of the central compartment, K10 rate constant for elimination from the central compartment, K12 rate constant for drug transport from the central to the peripheral compartment, K21 rate constant for the drug transport from the peripheral to the central compartment
cAUCinf total area under the plasma concentration–time curve to infinite time, Cmax maximum plasma concentration, CL clearance, t1/2α distribution half-life, t1/2β terminal elimination half-life, Vz distribution volume during the terminal elimination phase, Vss distribution volume during steady state
The plasma log concentration–time curves observed after a 1-h infusion of the paclitaxel micellar exhibited a biphasic shape (Fig. 2), consistent with the two-compartment model. The primary and secondary PK parameters obtained from the two-compartment model are presented in Table 3. The two-compartment analysis of paclitaxel in plasma demonstrated that the drug is rapidly leaving the central compartment, and distribution should be virtually complete 3 h after dosing, as the mean alpha half-life of paclitaxel was 0.55 h.
The tissue distribution was extensive according to the large Vss, with a mean value of 63.3 ± 26.9 L/m2 or about 120 L for the average patient with a mean body surface area of 1.9 m2. The elimination half-life (beta half-life) varied from 4.75 h to 23.10 h, with a mean value of 8.83 ± 4.10 h. CL varied from 4.4 to 22.6 L/h/m2 with a mean value of 13.4 ± 4.8 L/h/m2.
Micelle Disintegration
The remaining dose fraction of total paclitaxel present in plasma in the middle of the infusion interval was estimated in patients having received paclitaxel by a 1-h infusion (range 0.95–1.15 h) at doses ranging from 90 to 275 mg/m2. The remaining dose fraction at mid-infusion was 7.8 ± 2.1% (range 4.6–13.2%). This shows that persistent plasma levels of paclitaxel in its micellar form during the infusion are insignificant, indicating a rapid release from the formulation. There was no indication that this fraction was dose-dependent.
Dose Linearity
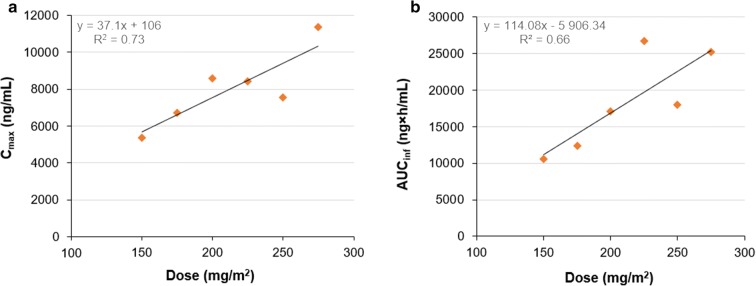
Mean values of Cmax and AUCinf for the different dose groups (excluding the single subject that was studied at 90 mg/m2) were analysed by plotting them as a function of given dose and subjecting them to linear regression analysis (Fig. 3). Cmax apparently increased in direct proportion to dose, although there was a considerable spread of the data points at the higher dose levels (R2 = 0.73) (Fig. 3a). In a similar way, AUCinf was seemingly linearly correlated to dose (R2 = 0.66) (Fig. 3b).
Fig. 3.
Relationship between given dose and a mean Cmax or b mean AUCinf after a 1-h infusion for doses between 150 mg/m2 and 275 mg/m2 of paclitaxel micellar
Estimations of Residual Area
To characterize the generation of AUC with time, the residual area (the extrapolated AUC) was calculated in each subject using the 10-h or 24-h sample as the last sampling point on the curve. These estimates showed that the majority of the total AUCinf was generated during the first 10 h, as the mean residual area (AUC10h-inf) accounted for only 15.7 ± 8.6% of AUCinf (n = 25). The mean residual area at 24 h accounted for 5.7 ± 3.9% (n = 23). Hence the area from 10 h to 24 h is only 10% of the total AUCinf.
Discussion
This study was the first time the novel micellar formulation of paclitaxel was administered to man and the MTD was established as 250 mg/m2. This is higher than the dose indicated for Cremophor EL paclitaxel [3], even if doses up to 250 mg/m2 have been administered [11]. A higher tolerated dose of paclitaxel formulated without Cremophor EL is in line with what previously has been established for nanoparticle albumin-bound paclitaxel (nab-paclitaxel), 260 mg/m2 [12], another Cremophor-free formulation of paclitaxel.
As a result of the absence of Cremophor, it was anticipated that hypersensitivity events would be less frequent with paclitaxel micellar. Therefore, no dexamethasone or antihistamine premedication was given in 90% of the treatment cycles. Exceptions were four patients who received premedication on the initiative of the investigator early in the study, since this was a first-in-man study. The most frequent AEs were in agreement with those reported in other studies for paclitaxel [3, 11–13], and no new side effects unknown for paclitaxel were observed. Fatigue and neuropathy were the most frequent DLTs. All neuropathies were experienced on dose level 225 mg/m2 or higher. Five patients had a grade 3 neuropathy (all at doses 250 mg/m2 or higher), of which all but one (275 mg/m2) had neurological symptoms already at inclusion. Haematological AEs were reported in 21 patients and were dose limiting in two patients (both on 250 mg/m2 dose level). Several patients experienced mild to moderate gastrointestinal tract symptoms (mucositis, nausea and diarrhoea) and gastrointestinal symptoms were classified as dose limiting in three patients.
The micellar formulation of paclitaxel was designed to make the lipophilic paclitaxel water soluble. The composition of the formulation does not indicate that it will act as a slow-release formulation of paclitaxel. However, there are no studies conducted on the stability of these paclitaxel-containing micelles in plasma. In the present study, the maximum amount of drug located in micelles in plasma during infusion was estimated to be only 7.8% (range 4.6–13.2%), suggesting that the micelles rapidly disintegrate in vivo.
The Cmax and AUCinf tended to increase linearly with dose, although the data obtained in the present study are limited. In this context it is interesting to compare Cmax of paclitaxel given as paclitaxel micellar with another rapidly dissolving formulation of paclitaxel, nab-paclitaxel. Data on mean Cmax at different dose levels received during 0.5-h infusions of nab-paclitaxel were extracted from a comprehensive review by Stage et al. [14] citing six studies and from a study by Nyman et al. [15] and plotted as a function of dose (Fig. 4). In order to make data from the present study comparable to those from the literature, individual Cmax after 0.5-h infusion of paclitaxel micellar was estimated by a simulation based on the two-compartment model. The estimated mean values of Cmax after 0.5-h infusion of increasing doses of paclitaxel micellar are in line with data for nab-paclitaxel in the 150–275 mg/m2 dose range (Fig. 4). In fact, Fig. 4 indicates a linear relation between dose and Cmax for these two rapidly dissolving formulations of paclitaxel up to a dose of about 300 mg/m2.
Fig. 4.
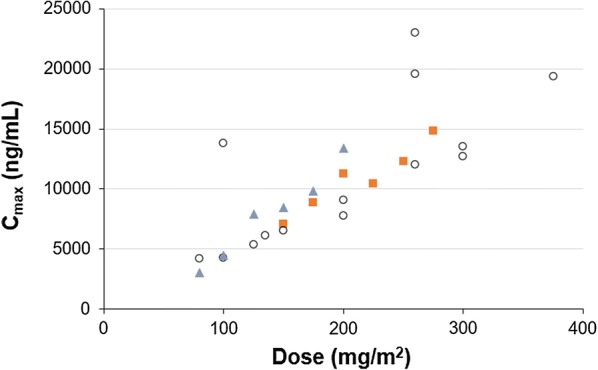
Mean Cmax of paclitaxel after 0.5-h infusions of paclitaxel micellar (orange squares) and nab-paclitaxel (empty circles, blue triangles) at various dose levels. Nab-paclitaxel data were extracted from six studies compiled by Stage et al. (empty circles; [14]) and one study by Nyman et al. (blue triangles; [15]). Number of patients per dose level of paclitaxel micellar was as follows: N = 2 (150 mg/m2), N = 3 (175 mg/m2), N = 4 (200 mg/m2), N = 4 (225 mg/m2), N = 5 (250 mg/m2), N = 6 (275 mg/m2)
The majority of the AUCinf, about 84%, was generated during the first 10 h. This number may be critical in designing future PK studies of paclitaxel micellar, as the European Medicines Agency (EMA) guideline on the investigation of bioequivalence requires that at least 80% of AUC is covered by the sampling scheme [16].
The mean CL values of paclitaxel when given as paclitaxel micellar in the present study should not be compared with the CL of paclitaxel given in Cremophor EL formulation. This is because the total paclitaxel, and not unbound paclitaxel, concentrations were measured in the present study. It has been reported that Cremophor EL paclitaxel has a non-linear PK which is explained by the fact that stable Cremophor EL-containing micelles are retained in plasma, in contrast to paclitaxel which is easily being distributed in the body. However, some paclitaxel will remain bound to the Cremophor EL micelles. Higher doses will generate higher plasma concentrations of Cremophor EL that will result in a higher proportion paclitaxel being bound to the Cremophor EL micelles [4]. This is equivalent to a lower apparent volume of distribution as well as a lower CL when total drug in plasma is measured, while PK parameters based on unbound drug levels will remain unaffected, according to a theory outlined for low clearance drugs like paclitaxel [17]. Total paclitaxel will appear to have non-linear PK, which is a mere consequence of the formulation rather than a true characteristic of paclitaxel itself [4]. Therefore, the true PK of paclitaxel in Cremophor EL formulations should be based on measurement of unbound drug concentrations in plasma [18]. However, total paclitaxel PK of Cremophor EL-free formulations should be comparable. The mean CL of paclitaxel including all dose levels in the present study was 13.4 L/h/m2. This can be compared with mean CL of nab-paclitaxel ranging from 11.9 L/h/m2 in four subjects receiving 375 mg/m2 [19] to 24.8 L/h/m2 in six subjects receiving 125 mg/m2 [20], and a majority in the range 20–25 L/h/m2 [14]. Mean CL of nab-paclitaxel tended to decrease at higher doses [14], which was not seen for paclitaxel micellar, probably as a result of the more limited dose range studied. There was a fivefold range, from 4.4 to 22.6 L/h/m2, in CL of paclitaxel micellar. Relatively few studies of nab-paclitaxel report on interindividual variability in CL, but ranges between 8.8 and 16.7 L/h/m2 in 14 patients after doses of 250 mg/m2 [21] and between 8.7 and 43.4 L/h/m2 in 14 patients receiving doses of 260 mg/m2 [22] have been reported. The latter variability is in line with that observed for paclitaxel micellar.
The present study was conducted in patients with metastatic malignant solid tumours for which they previously had been extensively treated. This contributes to a large interindividual variability, which further implicates some limitations for the study. The large interindividual variability prevents the establishment of dose linearity in the present study as the sample size within each dose level is probably too small to establish the true relationship between dose and Cmax or AUCinf. Thus, the present study alone cannot be used to ascertain the dose linearity of paclitaxel micellar. However, the large variability in CL may be expected for paclitaxel as a combined consequence of interindividual differences in physiology, metabolic capacity and disease state of the patients. For example, it has been shown by simulations that polymorphic enzymes altering the metabolic capacity cannot be the sole explanation of this variability [23]. The physical status of the patients together with the small study population and short study duration also generate a lack of valid efficacy assessments.
Since the present first-in-man study was conducted, paclitaxel micellar has undergone later stages of clinical development. This includes a pivotal phase III study in platinum-sensitive ovarian cancer patients where paclitaxel micellar was compared with Cremophor EL paclitaxel (both in combination with carboplatin), and a PK study comparing paclitaxel micellar with nab-paclitaxel. Paclitaxel micellar is now approved as an option to treat ovarian cancer patients in several markets.
Conclusions
This first-in-man study with the novel micellar formulation of paclitaxel established an MTD of 250 mg/m2. Cmax and AUCinf of total paclitaxel tended to increase linearly with dose after 1-h infusions in the dose range (150–275 mg/m2). Simulation-derived estimates of Cmax after 0.5-h infusions showed approximately the same maximum levels for paclitaxel micellar as for nab-paclitaxel. In plasma, the micellar formulation appeared to disintegrate rapidly, the distribution phase that followed was short and the majority of AUCinf was generated during the first 10 h. Thus, paclitaxel has a rapid and extensive tissue distribution. In the studied dose range, there was no indication that CL decreased at higher doses. In agreement with previous data with nab-paclitaxel, interindividual variability in CL of paclitaxel micellar was also large. The fact that paclitaxel micellar safely can be given without premedication with corticosteroids makes it an attractive component in future studies combining chemotherapy with immune check-point inhibitors.
Acknowledgements
The authors would like to thank all participating patients and their families.
Funding
This study and the journal's article processing charges and Open Access fee were funded by Oasmia Pharmaceutical AB. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Olof Borgå is a salaried consultant at Oasmia Pharmaceutical AB. Roger Henriksson was an investigator in the clinical trial. Helena Bjermo is a full-time employee at Oasmia Pharmaceutical AB. Elsa Lilienberg is a full-time employee at Oasmia Pharmaceutical AB. Nina Heldring is a full-time employee at Oasmia Pharmaceutical AB. Niklas Loman was an investigator in the clinical trial.
Compliance with Ethics Guidelines
The protocol was approved by the Swedish Medical Products Agency (EudraCT number: 2004-001821-54) and the Regional Ethical Review Board in Lund, Sweden (Dnr 542/2004). All procedures performed in the study were in accordance with the protocol, regulatory requirements and Good Clinical Practice. They also adhered to the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available due to confidentiality of Oasmia Pharmaceutical AB but are available from the corresponding author upon reasonable request.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7707836.
References
- 1.Konno T, Watanabe J, Ishihara K. Enhanced solubility of paclitaxel using water-soluble and biocompatible 2-methacryloyloxyethyl phosphorylcholine polymers. J Biomed Mater Res Part A. 2003;65(2):209–214. doi: 10.1002/jbm.a.10481. [DOI] [PubMed] [Google Scholar]
- 2.Safavy A. Recent developments in taxane drug delivery. Curr Drug Deliv. 2008;5(1):42–54. doi: 10.2174/156720108783331005. [DOI] [PubMed] [Google Scholar]
- 3.FDA, US Label. Taxol ® (paclitaxel) injection (Patient Information Included). Bristol-Myers Squibb Company. 2011. Reference ID:2939751. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf. Accessed 8 Oct 2018.
- 4.Van Tellingen O, Huizing MT, Nannan Panday VR, Schellens JHM, Nooijen WJ, Beijnen JH. Cremophor EL causes (pseudo-) non-linear pharmacokinetics of paclitaxel in patients. Br J Cancer. 1999;81(2):330–335. doi: 10.1038/sj.bjc.6690696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 6.Rajappa S, Joshi A, Doval DC, Batra U, Rajendranath R. Novel formulations of docetaxel, paclitaxel and doxorubicin in the management of metastatic breast cancer. Int J Nanomed. 2018;10:3757–3769. doi: 10.3892/ol.2018.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar P, Raza K, Kaushik L, Malik R, Arora S, Prakash Katare O. Role of colloidal drug delivery carriers in taxane-mediated chemotherapy: a review. Curr Pharm Des. 2016;22:5127–5143. doi: 10.2174/1381612822666160524144926. [DOI] [PubMed] [Google Scholar]
- 8.Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20–30. doi: 10.1016/j.addr.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Assessment report for Apealea. https://www.ema.europa.eu/documents/assessment-report/apealea-epar-public-assessment-report_en.pdf. Accessed 2 Feb 2019.
- 10.Sparreboom A, de Bruijn P, Nooter K, Loos WJ, Stoter G, Verweij J. Determination of paclitaxel in human plasma using single solvent extraction prior to isocratic reversed-phase high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl. 1998;705(1):159–164. doi: 10.1016/S0378-4347(97)00502-1. [DOI] [PubMed] [Google Scholar]
- 11.Omura GA, Brady MF, Look KY, et al. Phase III trial of paclitaxel at two dose levels, the higher dose accompanied by filgrastim at two dose levels in platinum-pretreated epithelial ovarian cancer: an intergroup study. J Clin Oncol. 2003;21(15):2843–2848. doi: 10.1200/JCO.2003.10.082. [DOI] [PubMed] [Google Scholar]
- 12.FDA, US Label. Highlights of prescribing information. Abraxane® for injectable suspension (paclitaxel protein-bound particles for injectable suspension). Celgene. 2018. Reference ID: 4307748. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021660s045lbl.pdf. Accessed 8 Oct 2018.
- 13.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 14.Stage TB, Bergmann TK, Kroetz DL. Clinical pharmacokinetics of paclitaxel monotherapy: an updated literature review. Clin Pharmacokinet. 2018;57(1):7–19. doi: 10.1007/s40262-017-0563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyman DW, Campbell KJ, Hersh E, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23(31):7785–7793. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 16.European Medicines Agency. Guideline on the investigation of bioequivalence. CPMP/EWP/QWP/1401/98 Rev 1/Corr**. 2010;1–27.
- 17.Rowland M, Tozer TN. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott William & Wilkins; 1995. pp. 164–165.
- 18.Brouwer E, Verweij J, De Bruijn P, et al. Measurement of fraction unbound paclitaxel in human plasma. Drug Metab Dispos. 2000;28(10):1141–1145. [PubMed] [Google Scholar]
- 19.Ibrahim NK, Desai N, Legha S, et al. Phase I and pharmacokinetic study of ABI-007, a cremophor-free, protein-stabilized, nanoparticl formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–1044. [PubMed] [Google Scholar]
- 20.Ando M, Yonemori K, Katsumata N, et al. Phase I and pharmacokinetic study of nab-paclitaxel, nanoparticle albumin-bound paclitaxel, administered weekly to Japanese patients with solid tumors and metastatic breast cancer. Cancer Chemother Pharmacol. 2012;69(2):457–465. doi: 10.1007/s00280-011-1726-5. [DOI] [PubMed] [Google Scholar]
- 21.Gardner ER, Dahut WL, Scripture CD, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14(13):4200–4205. doi: 10.1158/1078-0432.CCR-07-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sparreboom A, Scripture CD, Trieu V, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in cremophor (Taxol) Clin Cancer Res. 2005;11(11):4136–4143. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 23.De Graan AJM, Elens L, Smid M, et al. A pharmacogenetic predictive model for paclitaxel clearance based on the DMET platform. Clin Cancer Res. 2013;19(18):5210–5217. doi: 10.1158/1078-0432.CCR-13-0487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available due to confidentiality of Oasmia Pharmaceutical AB but are available from the corresponding author upon reasonable request.