Abstract
Introduction
Homozygous familial hypercholesterolaemia (HoFH) is a rare, autosomal disease affecting the clearance of low-density lipoprotein cholesterol (LDL-C) from circulation, and leading to early-onset atherosclerotic cardiovascular disease (ASCVD). Treatment consists mainly of statins, lipoprotein apheresis (LA) and, more recently, the microsomal triglyceride transfer protein inhibitor lomitapide. Lomitapide is not licensed for use in children, but has been made available through an expanded access programme or on a named patient basis.
Methods
This case series includes 11 HoFH patients in 10 different centres in eight countries, less than 18 years of age (mean 11.6 ± 1.1 years, 64% male), with signs of ASCVD, and who have received treatment with lomitapide (mean dose 24.5 ± 4.3 mg/day; mean exposure 20.0 ± 2.9 months). Background lipid-lowering therapy was given according to local protocols. Lomitapide was commenced with a stepwise dose escalation from 2.5 mg or 5 mg/day; dietary advice and vitamin supplements were provided as per the product label for adults. Laboratory analysis was conducted as part of regular clinical care.
Results
In the 11 cases, mean baseline LDL-C was 422 ± 245.4 mg/dL and was markedly reduced by lomitapide to a nadir of 192.2 ± 163.2 mg/dL (56.7 ± 21.7% decrease). Six patients achieved recommended target levels for children below 135 mg/dL, three of whom had LA frequency reduced and a further three stopped LA. In one case, LDL-C levels were close to target when lomitapide was started but remained stable despite 75% reduction in LA frequency (from twice weekly to biweekly). Adverse events were mainly gastrointestinal in nature, occurred early in the treatment course and were well managed. Three patients with excursions in liver function tests were managed chiefly without intervention; two patients had decreases in lomitapide dose.
Conclusions
Lomitapide demonstrated promising effectiveness in paediatric HoFH patients. Adverse events were manageable, and the clinical profile of the drug is apparently similar to that in adult patients.
Funding
Amryt Pharma.
Keywords: Adverse events, Atherosclerosis, Cardiology, Homozygous familial hypercholesterolaemia, Lipidology, Lomitapide, Low-density lipoprotein cholesterol, Paediatric, Real-world data, Patient cases
Introduction
Homozygous familial hypercholesterolaemia (HoFH) is a rare, genetic autosomal co-dominant disease, with mutated alleles in the genes involved in the low-density lipoprotein receptor (LDL-R) pathway, including LDL-R, apolipoprotein B (ApoB) and pro-protein convertase subtilisin/kexin type 9 (PCSK9), and the recessive LDL-R adapter protein 1 (LDLRAP1).
Patients with the HoFH phenotype present with very high LDL cholesterol (LDL-C) levels from birth [1]. This leads to early onset atherosclerotic cardiovascular disease (ASCVD), as well as aortic or supra aortic valve disease, which in turn causes a range of associated life-threatening cardiac conditions [1]. If HoFH goes undetected or untreated, the average age at which patients develop ASCVD is 12.5 years and mean survival is 18 years [2].
Clearly, there is a need to not only diagnose HoFH promptly in childhood but also to provide effective treatments. For paediatric patients with HoFH, this is particularly problematic. Statins can be used, and recent data have shown that there are no concerns regarding growth rate and plasma levels of creatinine kinase, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in children receiving these drugs [3]. However, as a result of the genetic profile of HoFH whereby some patients can have extremely low levels of LDL-R functionality [1], statins may not be as effective as they would be in an individual with residual LDL-R activity. Furthermore, non-statin lipid-lowering therapies like ezetimibe and PCSK9 inhibitors seldom bring LDL-C to recommended levels despite additional cholesterol lowering [4].
Lipoprotein apheresis (LA) is a mainstay of lipid-lowering therapy (LLT) for patients with HoFH. The procedure involves the extracorporeal removal of cholesterol and in children this can present technical, clinical and social challenges such as mild hypotension, pain, venous access, iatrophobia, trypanophobia and time away from normal activities [5]. Nevertheless, LA has been successfully applied in children as young as 3 years [5].
LA is an effective treatment, and can reduce LDL-C levels by more than 50% and delay the onset of ASCVD [6–8]. However, LDL-C levels rebound to baseline within 2 weeks of an LA procedure [9]. A systematic analysis of LA in children found that only selective LA techniques were capable of enabling patients to reach at least 70% acute reduction in LDL-C per session [10]. Therefore, even with the application of LA, patients will experience long-term elevated levels of LDL-C, which continually exposes them to risks of developing premature ASCVD [11]. For this reason, clinical recommendations suggest weekly LA regimens. However, for some HoFH patients, LA is a tremendous burden on time, school and working patterns. There can be additional problems with the ability to attend treatment centres, and in some countries, LA is not available at all [12].
Although not in full-time work, as children become older, they need to be able to devote increasing amounts of time to schooling, social activity and sports. Given the problems of applying LA in the very young, increasing time commitments as age advances, and the reduced effectiveness of statins, other therapies are needed in paediatric patients with HoFH.
Lomitapide is a microsomal triglyceride transfer protein (MTP) inhibitor that reduces plasma LDL-C levels independently of LDL-R activity. Lomitapide is approved as an adjunct to lipid-lowering treatment, with or without apheresis, in adult patients with HoFH [13, 14]. In clinical trials, patients receiving lomitapide, added to standard of care, achieved mean reductions in LDL-C from 40% to 50% and a mean reduction of up to 76.5% in patients in ‘real-world’ use [15–17]. Up to 51–68% patients achieved the European Atherosclerosis Society (EAS) recommended LDL-C target for adults of less than 100 mg/dL; 40–42% patients achieved less than 70 mg/dL [1, 15, 18, 19]. Notably, in the extension phase of the lomitapide phase 3 clinical trial, 14 (74%) and 11 (58%) of the 19 patients who remained in the extension study after week 126 reached LDL-C targets of 100 mg/dL and 70 mg/dL, respectively, at least once during the study period [20]. Lomitapide has been made available to paediatric patients in response to requests from physicians as part of an expanded access programme or on a named patient basis. Recently the first case of lomitapide use for over 4 years in a HoFH child was published [21].
In this paper, we have gathered data from some of the paediatric patients treated with lomitapide and evaluated the overall effectiveness. The cases described here have been deemed ‘urgent to treat’ due to the early presence of ASCVD, lack of sufficient response to standard-of-care treatment, and inability to wait for a paediatric trial that is due to commence in 2019 and enrol patients from at least 5 to at most 18 years of age.
Methods
Physicians involved in the treatment of HoFH requested access to treat their paediatric patients with lomitapide either as part of an expanded access programme or on a named patient basis. Some of the physicians had prior experience of lomitapide use in adult patients. Patients were treated in 10 different centres in eight countries (Qatar, Spain, Greece, Denmark, Norway, Argentina, Israel and Brazil).
In all cases, lomitapide was commenced at a low dose, and gradually escalated. The starting dose was as low as 2.5 mg/day in some children (e.g. those below 10 years of age) and was selected on the basis of the protocol of the planned paediatric study using the calculated doses of both physiologically based pharmacokinetic modelling and allometric scaling, based on adult references. Lomitapide administration does not involve weight-based dosing. Efficacy, hepatic safety and tolerability guide dose escalation. Therefore, any differences in exposure that may be affected by body weight are accommodated in the dosing schedule. Background LLT was provided according to local protocols and treatment availability. Dietary counselling and vitamin E, omega 3 and omega 6 supplements were provided as per the adult product label for lomitapide, but at lower doses of vitamin E in some cases, as required. Patients and carers were advised that lomitapide should be accompanied with a low-fat diet whereby less than 20% of total daily energy is derived from fat. Patients also underwent regular monitoring of liver function, and had laboratory measurements, including lipid panels recorded during the normal course of their treatment.
Data were recorded according to local practice; therefore, there is some variation in the type, presentation and time period of data between the patients. For this reason, data are presented chiefly as individual patient profiles rather than cohort-based statistics. Baseline LDL-C readings were recorded from samples taken at the clinic visit immediately prior to the commencement of lomitapide. LDL-C nadir reading values were defined as the lowest level achieved after commencement of lomitapide.
Compliance with Ethics Guidelines
The cases described in this paper were treated in the normal course of care, and not as part of a clinical trial requiring IRB or ethical committee approval. All of the included patients have provided verbal consent for the data to be published, and a consent form has been lodged in the patient case notes for parental/guardian consent documentation.
Results
Case 1
This is 13-year-old girl with a homozygous LDLR c.313+5G>A mutation and evidence of an aortic root plaque. Her parents are first-degree cousins, both with heterozygous FH (HeFH).
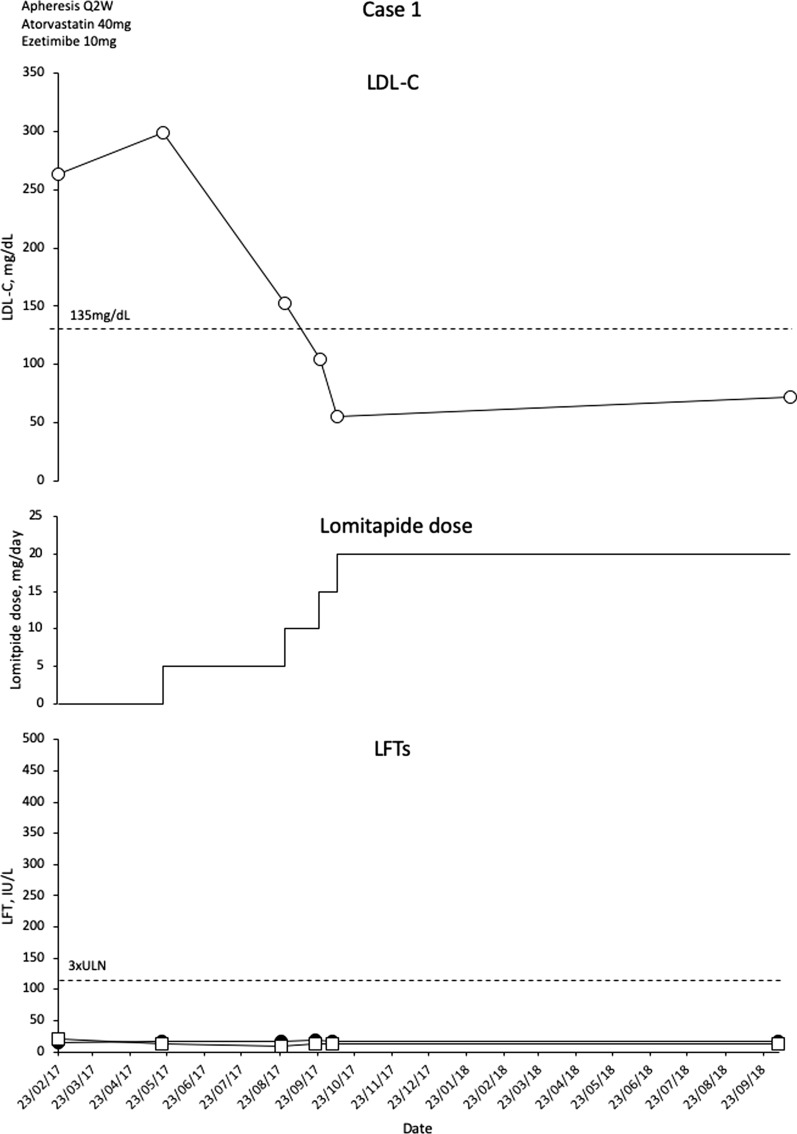
This patient was initially treated with statins, ezetimibe and biweekly LA but the patient remained with increased LDL-C and evidence of atherosclerotic disease in the form of an aortic root plaque. Evolocumab was tried, but only for 3 months because of lack of response. With a background therapeutic regimen of atorvastatin 40 mg/day, ezetimibe 10 mg/day and biweekly (Q2W) LA, mean interval LDL-C levels were 263 mg/dL. The patient was commenced on lomitapide 5 mg/day. The patient initially suffered from nausea, occasional vomiting, reduced appetite and increased stool frequency; however, these symptoms became tolerable and no other adverse events were observed. Liver enzymes remained within normal limits. The patient was gradually escalated to lomitapide 20 mg/day and atorvastatin 40 mg/day with cessation of LA. Mean interval LDL-C levels are now maintained at 72 mg/dL (76% decrease) without the need for LA (Fig. 1). The patient has been treated for over 18 months with no alteration in liver enzymes (Fig. 1).
Fig. 1.
Evolution of LDL-C values in case 1 with lomitapide therapy. Upper panel shows mean interval LDL-C levels for patient 1. Middle panel shows lomitapide dose changes over time. Lower panel shows corresponding ALT (closed circles) and AST (open squares) levels over the same period. Dotted line on upper panel shows EAS targets for LDL-C levels in children with HoFH. Dotted line on lower panel indicates 3× upper limit of normal for LFTs; ALT alanine aminotransferase, AST aspartate aminotransferase, EAS European Atherosclerosis Society, HoFH homozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, LFTs liver function tests, Q2W every 2 weeks, ULN upper limit of normal
Case 2
This is a 12-year-old boy with a homozygous LDLR c.682G>T mutation. Both parents have HeFH. The patient has left ventricle dilatation, mild aortic regurgitation and atherosclerotic plaques (1.5–2.1 mm thick) in both carotid bulbs, and in the common and internal carotid arteries. Imaging revealed diffuse atherosclerotic involvement of all coronary arteries, but this was not clinically significant.
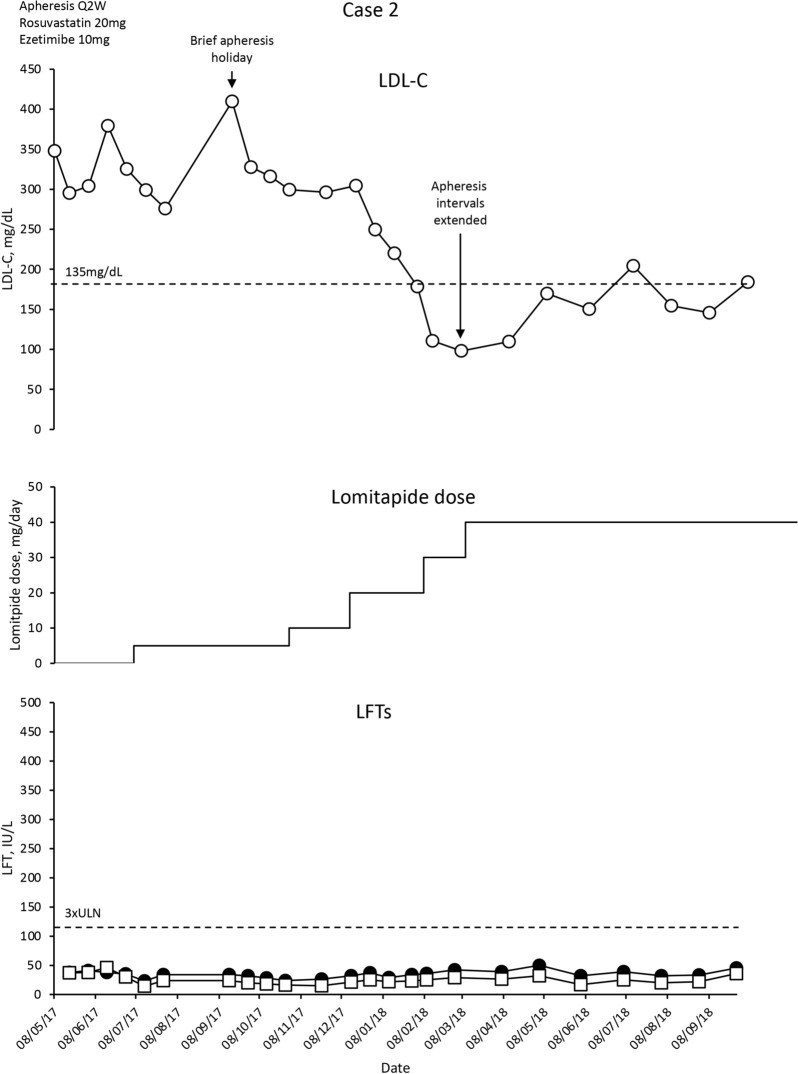
The patient was treated with rosuvastatin 20 mg/day, ezetimibe 10 mg/day, LA Q2W, but mean interval LDL-C levels remained increased at approximately 300 mg/dL. The patient was commenced on lomitapide 5 mg/day following a normal FibroScan test. Rosuvastatin 20 mg/day, ezetimibe 10 mg/day and LA Q2W were maintained.
Mean interval LDL-C levels decreased modestly over 2 months (Fig. 2). The patient then took a vacation with no LA, and experienced an increase in LDL-C levels that was gradually brought under control with escalating doses of lomitapide. Overall, LDL-C levels decreased by 50–70%. There has been no evidence of adverse events to lomitapide, and liver function tests (LFTs) remain normal (Fig. 2). Gastrointestinal (GI) complaints were only present when dietary advice was not followed. The patient achieved an LDL-C nadir of 98 mg/dL (70% reduction). LA intervals have been gradually extended to Q4W with a modest increase in LDL-C levels to 184 mg/dL (44% reduction from baseline).
Fig. 2.
Evolution of LDL-C values in case 2 with lomitapide therapy. Upper panel shows mean interval LDL-C levels for patient 2. Middle panel shows lomitapide dose changes over time. Lower panel shows corresponding ALT (closed circles) and AST (open squares) levels over the same period. Dotted line on upper panel shows EAS targets for LDL-C levels in children with HoFH. Dotted line on lower panel indicates 3× upper limit of normal for LFTs; ALT alanine aminotransferase, AST aspartate aminotransferase, EAS European Atherosclerosis Society, HoFH homozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, LFTs liver function tests, Q2W every 2 weeks, ULN upper limit of normal
Case 3
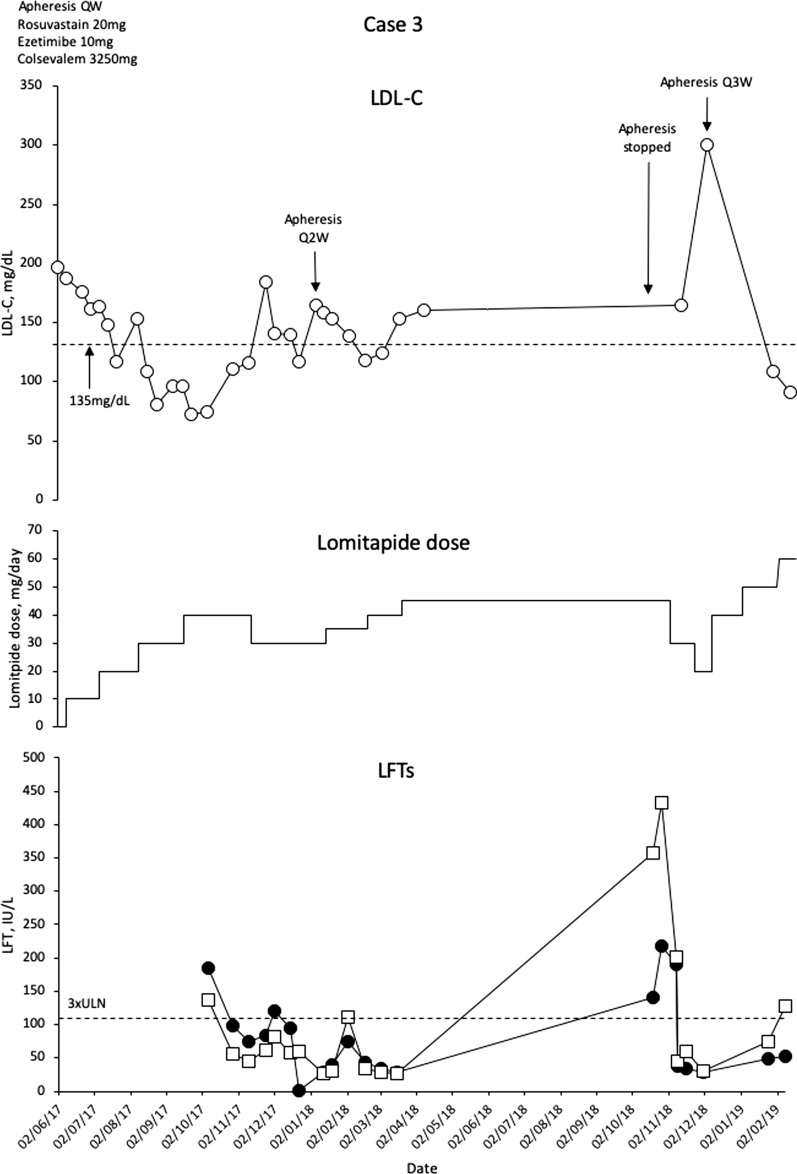
This is a 16-year-old boy diagnosed with HoFH at the age of 10 months on the basis of development of xanthomas, LDL-C levels 981 mg/dL, and LDLR genotyping with the finding of homozygosity for the LDLR mutation c.119_1207del. Both parents have HeFH, and the patient has a right coronary lesion evident on computed tomography, which has been treated with percutaneous revascularisation (at the age of 14 years). The patient was commenced on statins at the age of 2 years, which was gradually escalated to rosuvastatin 20 mg/day, ezetimibe 10 mg/day and colesevelam 3250 mg/day. At the age of 5 years, weekly LA was commenced, which resulted in regression of xanthomas by the age of 10 years, but without complete control of LDL-C levels (mean interval LDL-C 168 mg/dL).
In 2016, at age 14 years, the patient was commenced on evolocumab 420 mg QW with no effect on LDL-C levels. The following year, lomitapide was commenced at 5 mg/day, and gradually escalated to 40 mg/day. Mean interval LDL-C levels were reduced to 75–83 mg/dL, which represents a greater than 60% reduction in LDL-C (Fig. 3).
Fig. 3.
Evolution of LDL-C values in case 3 with lomitapide therapy. Upper panel shows mean interval LDL-C levels for patient 3. Middle panel shows lomitapide dose changes over time. Lower panel shows corresponding ALT (closed circles) and AST (open squares) levels over the same period. Dotted line on upper panel shows EAS targets for LDL-C levels in children with HoFH. Dotted line on lower panel indicates 3× upper limit of normal for LFTs; ALT alanine aminotransferase, AST aspartate aminotransferase, EAS European Atherosclerosis Society, HoFH homozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, LFTs liver function tests, Q2W every 2 weeks, Q3W every 3 weeks, ULN upper limit of normal
The patient began to have some recurrent issues with elevated liver enzymes, possibly related to rosuvastatin, which had caused this in the past. Lomitapide dose was reduced to 30 mg/day, and rosuvastatin briefly stopped while the LFTs resolved. Eventually, LA intervals were extended to every 2–3 weeks and rosuvastatin was permanently stopped. The patient remained on lomitapide 40 mg/day. Increasing lomitapide dose to 45 mg/day was associated with an increase in LFTs that was brought under control with dose reduction and gradual re-escalation. The patient is now doing well on lomitapide 60 mg/day with an LDL-C level of just 90.8 mg/dL and LA Q2W. There has been no evidence of GI adverse events apart from mild flatulence.
Case 4
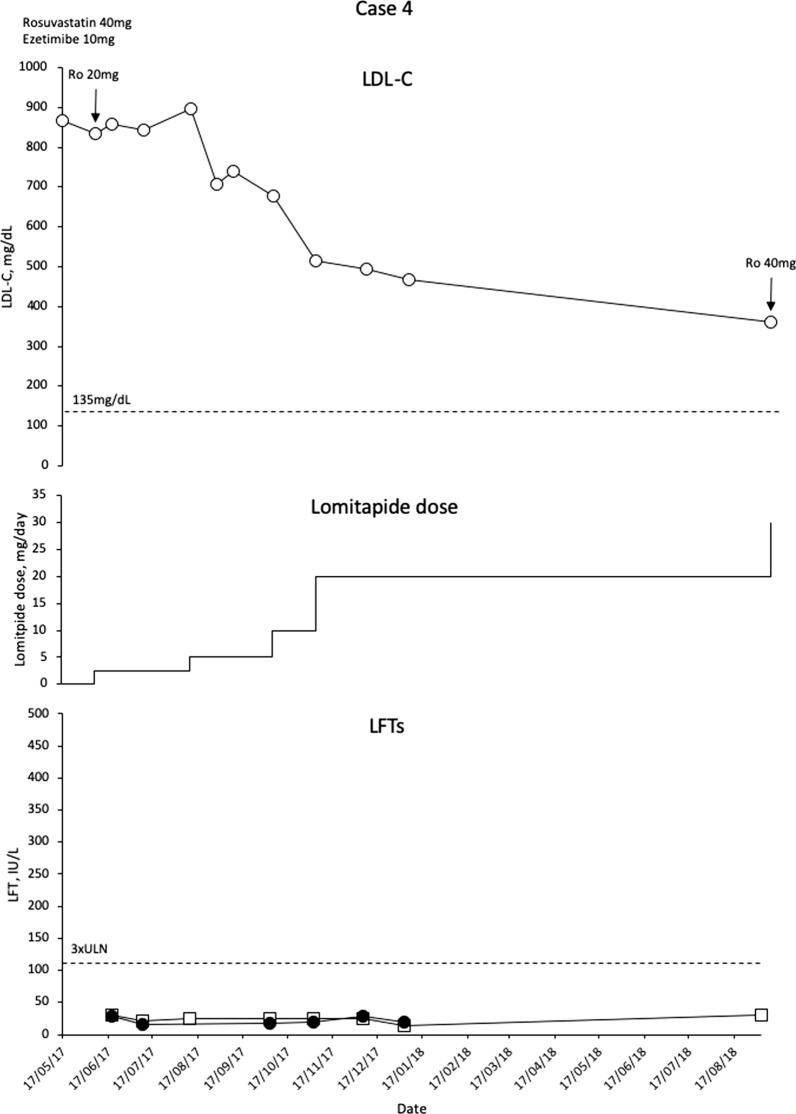
This patient is a 7-year-old boy with compound heterozygote mutations in LDLR c.666C>A and c.1646C>A. The patient has a sister with HoFH who is on LA and not part of this patient series. Both parents have HeFH. The patient has a large aortic plaque extending the length of the thorax with 25–50% narrowing of the lumen.
Before lomitapide, the patient was treated with rosuvastatin 40 mg/day, ezetimibe 10 mg/day and colesevelam 625 mg/day. No LA was used as a result of venous access issues. LDL-C levels were increased at 833 mg/dL. The patient underwent dose reduction of rosuvastatin (20 mg) and was commenced on lomitapide 2.5 mg/day, which was gradually escalated to 30 mg/day in 5-mg increments. LDL-C levels reduced by 57% to 360 mg/dL (Fig. 4). Notably, this patient has been treated for over 18 months and has not experienced any side effects of lomitapide.
Fig. 4.
Evolution of LDL-C values in case 4 with lomitapide therapy. Upper panel shows mean interval LDL-C levels for patient 4. Middle panel shows lomitapide dose changes over time. Lower panel shows corresponding ALT (closed circles) and AST (open squares) levels over the same period. Dotted line on upper panel shows EAS targets for LDL-C levels in children with HoFH. Dotted line on lower panel indicates 3× upper limit of normal for LFTs; ALT alanine aminotransferase, AST aspartate aminotransferase, EAS European Atherosclerosis Society, HoFH homozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, LFTs liver function tests, ULN upper limit of normal
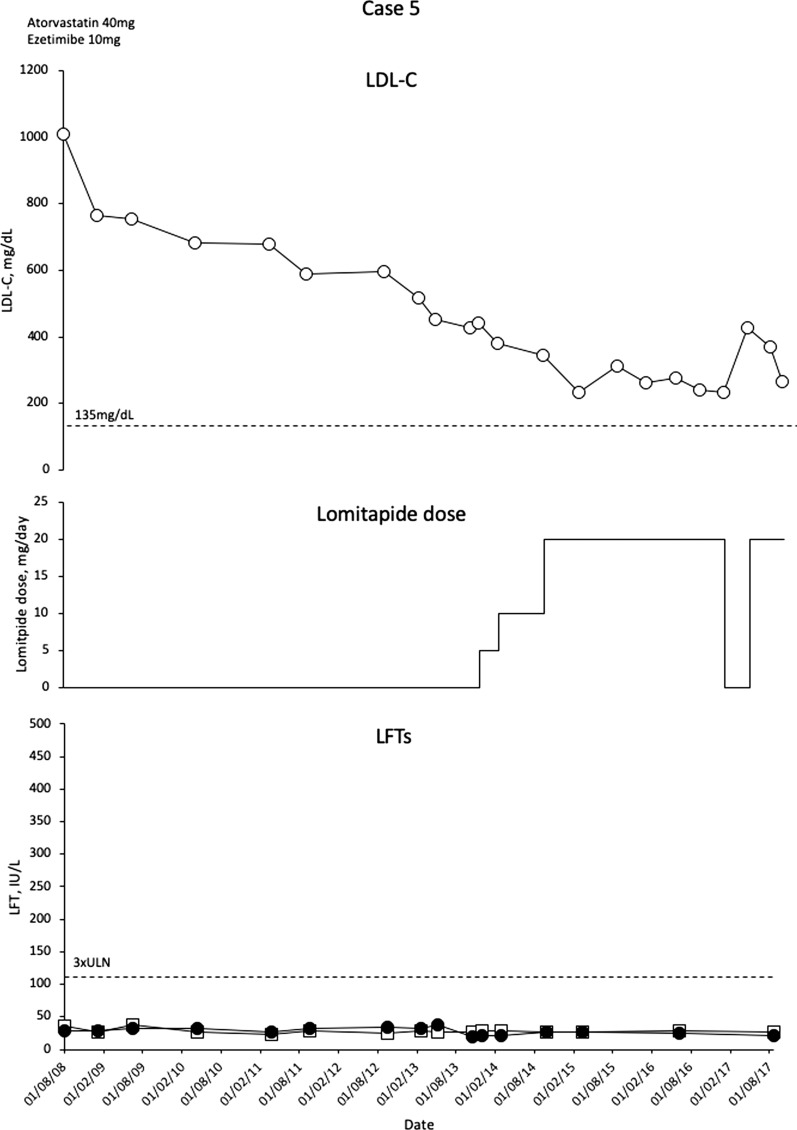
Case 5
This patient is an 11-year-old girl with a homozygous duplication spanning the promoter region to exon 6 of LDLR (c.-187-? 940+? Dup) [21]. Family history includes sudden death in an older brother aged 17. Another brother has HoFH with aortic stenosis and a non-critical obstruction of the right coronary artery. The patient presented with non-critical aortic stenosis/supra aortic stenosis, and non-obstructive plaques in the carotid arteries. Her computed tomography angiography showed no signs of coronary artery disease.
The patient commenced LLT at the age of 2 years when LDL-C levels were 1009 mg/dL. Up until the age of 7, she was treated with atorvastatin 40 mg/day and ezetimibe 10 mg/day and her LDL-C level was 453 mg/dL. LA is not available in the patient’s home country.
At age 7, atorvastatin dose was decreased to 10 mg/day, and lomitapide was commenced at a dose of 5 mg/day. Over the next year, lomitapide was escalated to 30 mg/dL but this dose was reduced back to 20 mg/day as a result of diarrhoea. LDL-C levels were effectively reduced with treatment (nadir 231 mg/dL; 48% reduction) with no elevations in LFTs (Fig. 5). A trip to a remote region of Brazil led to brief discontinuation of lomitapide with a consequent increase in LDL-C levels. Following resolution of the issues, reintroduction of lomitapide resulted in the expected decrease in LDL-C levels.
Fig. 5.
Evolution of LDL-C values in case 5 with lomitapide therapy. Upper panel shows mean interval LDL-C levels for patient 5. Middle panel shows lomitapide dose changes over time. Lower panel shows corresponding ALT (closed circles) and AST (open squares) levels over the same period. Dotted line on upper panel shows EAS targets for LDL-C levels in children with HoFH. Dotted line on lower panel indicates 3× upper limit of normal for LFTs; ALT alanine aminotransferase, AST aspartate aminotransferase, EAS European Atherosclerosis Society, HoFH homozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, LFTs liver function tests, ULN upper limit of normal
The patient is now maintained on atorvastatin 60 mg/day, ezetimibe 10 mg/day and lomitapide 20 mg/day and has been treated with lomitapide for 4 years. She continues to develop normally and had her menarche at the age of 11 years. LDL-C levels are 266 mg/dL (38% reduction from pre-lomitapide values). A routine check-up revealed no calcification in the coronary arteries; however, there were signs of aortic stenosis and supra aortic stenosis, and some non-obstructive plaques in the carotid arteries, but these are not considered to be life-threatening. There were no elevations of liver enzymes during treatment. A detailed history of this patient has been published by Chacra et al. [21].
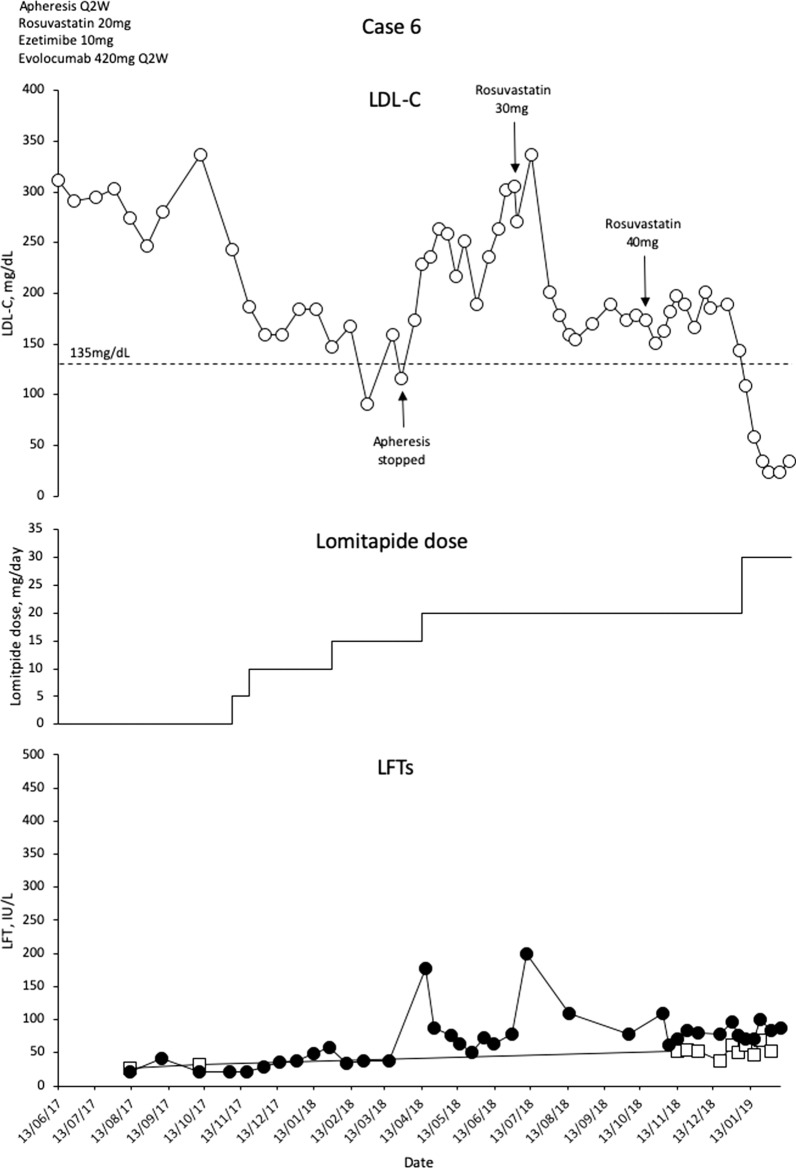
Case 6
This is a 16-year-old boy with compound heterozygote mutations LDLR c.131G>A and c.2043C>A and a history of HeFH in both parents (LDL-C levels at 271 mg/dL and 387 mg/dL). The patient has carotid plaques occluding 25–30% of the carotid lumina, but with no impairment of perfusion. The patient was diagnosed with HoFH at the age of 9 years with Achilles’ xanthomata and LDL-C levels at 900 mg/dL. LLT with rosuvastatin 20 mg/day, ezetimibe 10 mg/day and weekly apheresis was started, with dietary modifications to reduce fat intake to 15% of total energy. On this regimen, mean interval LDL-C levels reduced to 206 mg/dL.
As the patient got older, issues with schooling meant that there was a desire to extend the LA interval to biweekly. This change was made, and evolocumab 420 mg Q2W was added to the background LLT. However, as a result of the mutation profile of the patient, evolocumab did not work (mean interval LDL-C 329 mg/dL), and the decision was taken to prescribe lomitapide. The patient was commenced on lomitapide at 5 mg/day with escalation to 15 mg/day over 3 months (Fig. 6). Apheresis was stopped, lomitapide was increased to 20 mg, rosuvastatin was increased to 40 mg and ezetimibe doses were maintained. LDL-C levels remained at approximately 190 mg/dL for more than 6 months and therefore lomitapide was increased to 30 mg/day and rosuvastatin reduced to 35 mg/day. Figure 6 shows that LDL-C levels continued to rise after commencement of lomitapide, followed by a subsequent marked decrease. There is no direct explanation for this other than the cessation of apheresis.
Fig. 6.
Evolution of LDL-C values in case 6 with lomitapide therapy. Upper panel shows mean interval LDL-C levels for patient 6. Middle panel shows lomitapide dose changes over time. Lower panel shows corresponding ALT (closed circles) and AST (open squares) levels over the same period. Dotted line on upper panel shows EAS targets for LDL-C levels in children with HoFH. Dotted line on lower panel indicates 3× upper limit of normal for LFTs; ALT alanine aminotransferase, AST aspartate aminotransferase, EAS European Atherosclerosis Society, HoFH homozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, LFTs liver function tests, Q2W every 2 weeks, ULN upper limit of normal
Mean interval LDL-C levels are now at 34.8 mg/dL (a remarkable 87.3% decrease), with no LA currently ongoing. Early GI issues resolved. There were no other adverse events, other than slightly depressed alkaline phosphatase levels. Alanine aminotransferase briefly increased by more than three times the upper limit of normal (ULN) on two occasions, and reduced to less than three times the ULN without intervention. All other laboratory parameters remain normal and there is no evidence of hepatic steatosis.
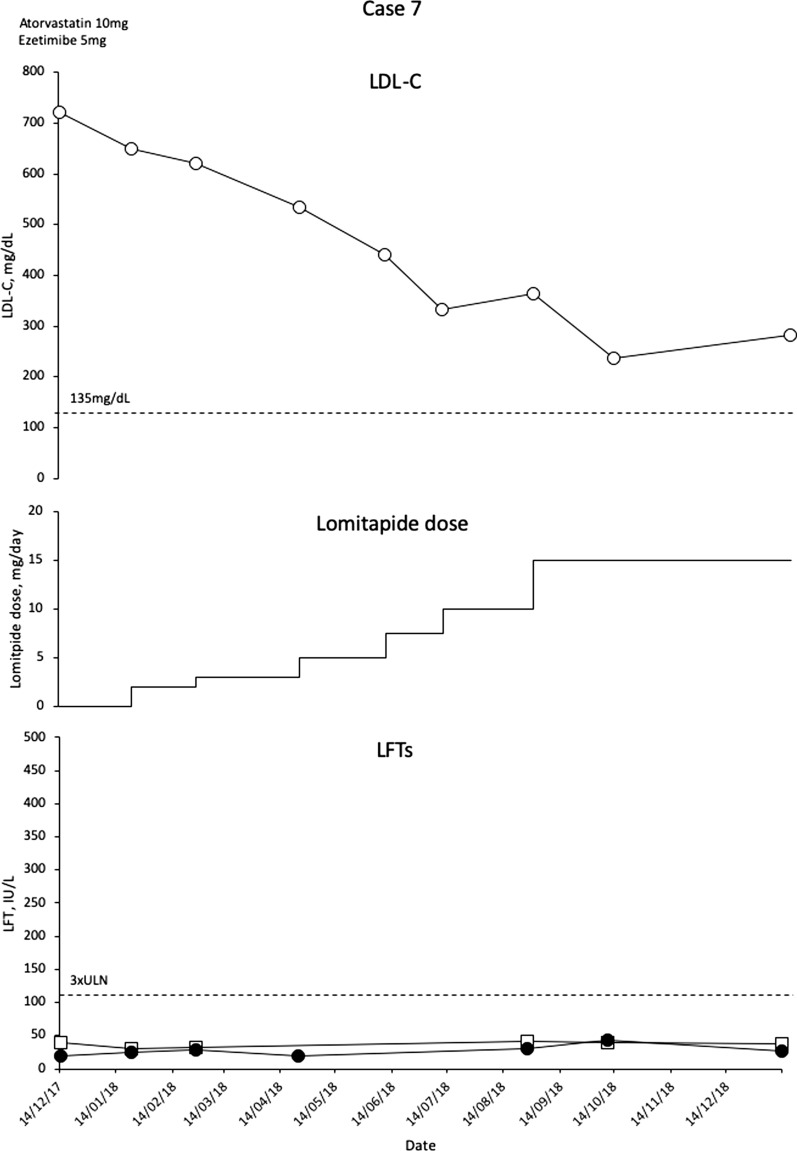
Case 7
This is a 4-year-old girl with a homozygous c.2043C>A mutation who presented at the age of 3 with an LDL-C level of 739 mg/dL. Her father has a severe form of HeFH. At diagnosis, the patient had normal echocardiography; but, by 2016, she had mild aortic thickening and some mild aortic valve regurgitation. There was evidence of a pedunculated atheroma at the aortic arch that had potentially embolised as it was no longer apparent on a later scan. For this reason, the treating physician decided that the patient was a candidate for aggressive LLT, but low body weight meant that LA was not suitable. LDL-C levels were reduced very slightly to 685 mg/dL with atorvastatin 10 mg/day and ezetimibe 5 mg/day, and the decision was taken to intensify LLT with lomitapide. Doses were escalated gradually from 2.5 mg/day in 2.5-mg increments given the young age of the patient and low body weight, and LDL-C levels became reduced. By the time the dose was escalated to 15 mg/day in August 2018 (patient now 5 years old), LDL-C had reached a nadir of 236 mg/dL (Fig. 7). There have been no side effects in this patient apart from one episode of a loose stools when pancakes were eaten. No liver pathology is evident on ultrasound. The patient has recently had a significant reduction in triglycerides to 21 mg/dL, and so the levels of fat-soluble vitamins are being checked prior to any further dose increase given the young age of the child.
Fig. 7.
Evolution of LDL-C values in case 7 with lomitapide therapy. Upper panel shows mean interval LDL-C levels for patient 7. Middle panel shows lomitapide dose changes over time. Lower panel shows corresponding ALT (closed circles) and AST (open squares) levels over the same period. Dotted line on upper panel shows EAS targets for LDL-C levels in children with HoFH. Dotted line on lower panel indicates 3× upper limit of normal for LFTs; ALT alanine aminotransferase, AST aspartate aminotransferase, EAS European Atherosclerosis Society, HoFH homozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, LFTs liver function tests, ULN upper limit of normal
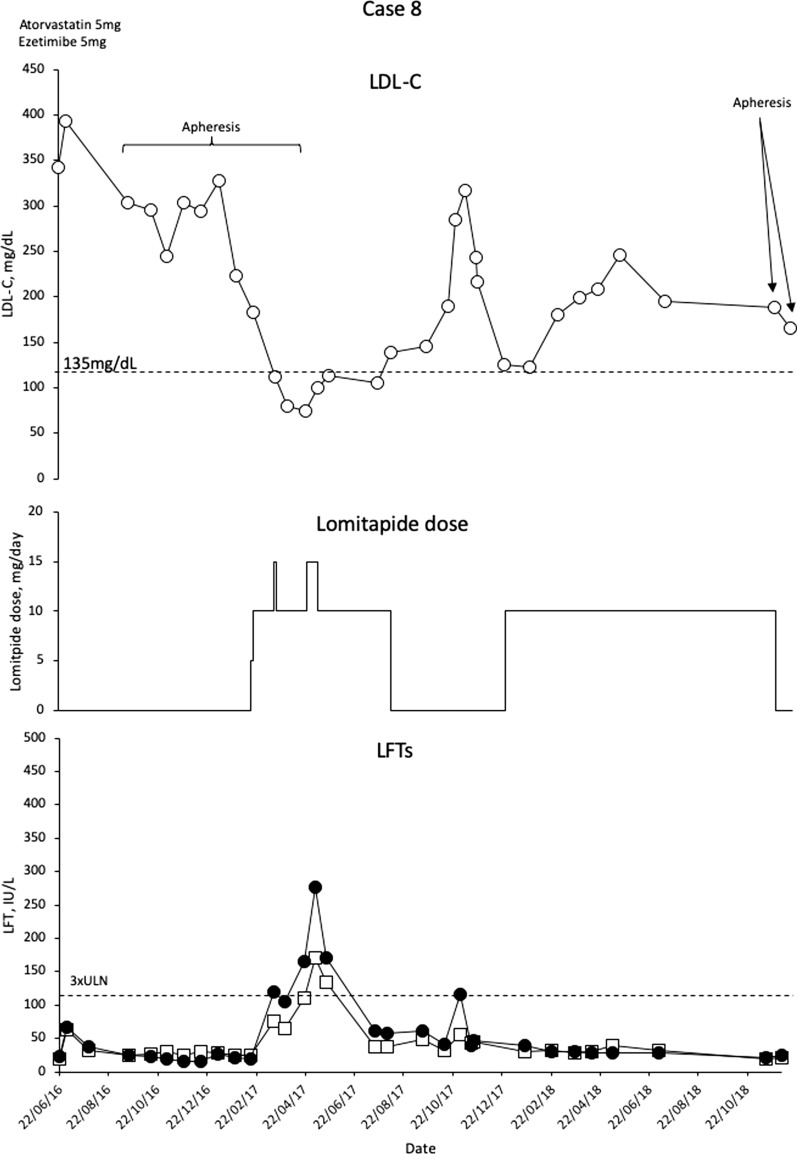
Case 8
This is a 14-year-old boy with an extensive family history of HeFH and a brother with HoFH. The boy has a compound heterozygous for the LDLR mutations c.1846-? 2311+?del and c.1895A>T (variant of unknown significance). The boy presented with xanthomas and hypercholesterolemia at 4.8 years of age. High dose (for age) statins and ezetimibe were started (atorvastatin 20–30 mg/day; ezetimibe 10 mg/day). At the age of 8 years, mild aortic regurgitation was evident. Treatment with plasma exchange (PE) was commenced every 15–20 days. Three years later, the patient required composite graft replacement of the aortic valve, aortic root and ascending aorta, with re-implantation of the coronary arteries (Bentall procedure).
At this point, lomitapide was initiated as add-on to the previous treatment regimen, escalating from 5 to 10 mg/day. LDL-C levels decreased to almost 100 mg/dL (Fig. 8). Lomitapide dose was increased to 15 mg/day and then briefly escalated to 20 mg, but the patient experienced elevated transaminases, so the dose was reverted with consequent normalization of LFTs. The LA frequency was altered a number of times in this patient (Q2W–Q6W), and was eventually stopped once the patient was on the 15 mg dose of lomitapide. The response to lomitapide was a 66.4% reduction, and apheresis has been discontinued, apart from two emergency sessions when an insurance issue interrupted the lomitapide dosing (Fig. 8).
Fig. 8.
Evolution of LDL-C values in case 8 with lomitapide therapy. Upper panel shows mean interval LDL-C levels for patient 8. Middle panel shows lomitapide dose changes over time. Lower panel shows corresponding ALT (closed circles) and AST (open squares) levels over the same period. Dotted line on upper panel shows EAS targets for LDL-C levels in children with HoFH. Dotted line on lower panel indicates 3× upper limit of normal for LFTs; ALT alanine aminotransferase, AST aspartate aminotransferase, EAS European Atherosclerosis Society, HoFH homozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, LFTs liver function tests, ULN upper limit of normal
Case 9
This is a 15-year-old boy with compound heterozygous LDLR c.313+1G>A and a deletion spanning exon 1–6. Both parents have HeFH with no evidence of cardiovascular disease (CVD). The patient was diagnosed at the age of 2 years old as a result of the presence of xanthomas. LDL-C levels were found to be elevated to 982 mg/dL, and diagnosis was confirmed via genetic testing. Asymptomatic aortic insufficiency was evident. Medication was commenced with atorvastatin 40 mg/day plus ezetimibe 10 mg/day. The patient received once-weekly lipoprotein apheresis, but LDL-C levels remained elevated (mean interval LDL-C 197 mg/dL). At the age of 8.5 years, chest pain led to a diagnosis of angina and a coronary bypass operation. LA was intensified to two times per week.
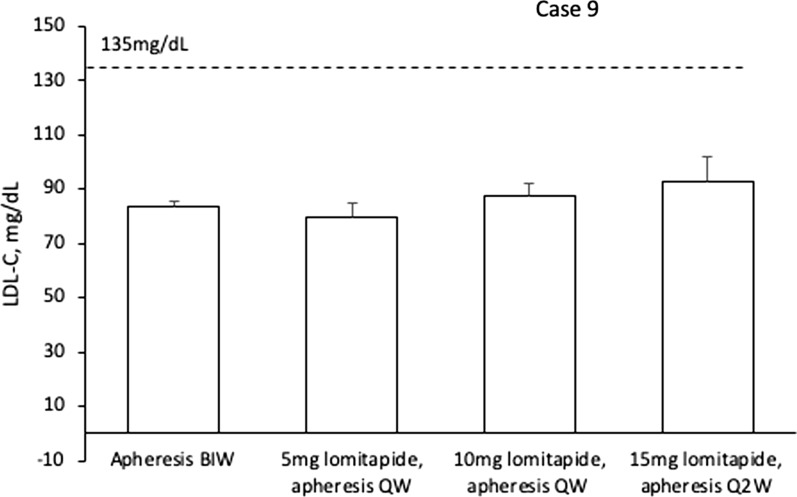
At 13.5 years of age, prior to the initiation of lomitapide, mean interval LDL-C levels were at 85 mg/dL with LA twice weekly, and therefore well below current treatment target. Unlike the other patients in this case series, the treatment plan for this patient was to attempt to maintain the LDL-C levels at target but to reduce the apheresis burden. Lomitapide was commenced at a dose of 5 mg/day and apheresis was decreased to once weekly. After 6 months, the lomitapide dose was intensified to 10 mg/day followed by 15 mg/day and after an additional 5 months, apheresis was reduced to Q2W. Through these modifications, LDL-C levels remained under control (nadir 62 mg/dL) (Fig. 9).
Fig. 9.
LDL-C levels per therapy period for case 9. Values are mean interval LDL-C ± SD for each apheresis treatment period. Dotted line shows EAS targets for LDL-C levels in children with HoFH. LDL-C low-density lipoprotein cholesterol, BIW twice weekly, QW once weekly, Q2W once every 2 weeks
No adverse events have been reported for lomitapide, liver enzymes and imaging are normal, and the patient has reported improved quality of life due to less disruption from apheresis sessions resulting in less time away from school, sports and other leisure activities. As a result, the LDL-C target levels have been maintained despite reducing apheresis burden by 75%.
Cases 10 and 11
These two patients (both homozygous for the LDLR c.1731G>T mutation) are siblings born to consanguineous parents with HeFH. They have one other sibling with HeFH, and without the disease. The girl presented at age 2 with high LDL-C and xanthomas, but with normal cardiac status. Her brother with HoFH was referred at age 9 on the basis of the family history, and has mild, stable aortic insufficiency and focal thickening of the right common carotid artery. Development was normal in both patients. The patients were managed with atorvastatin 40 mg/day, ezetimibe 10 mg/day, a low-fat diet, cholestyramine 4 g/day and aspirin 75 mg QD.
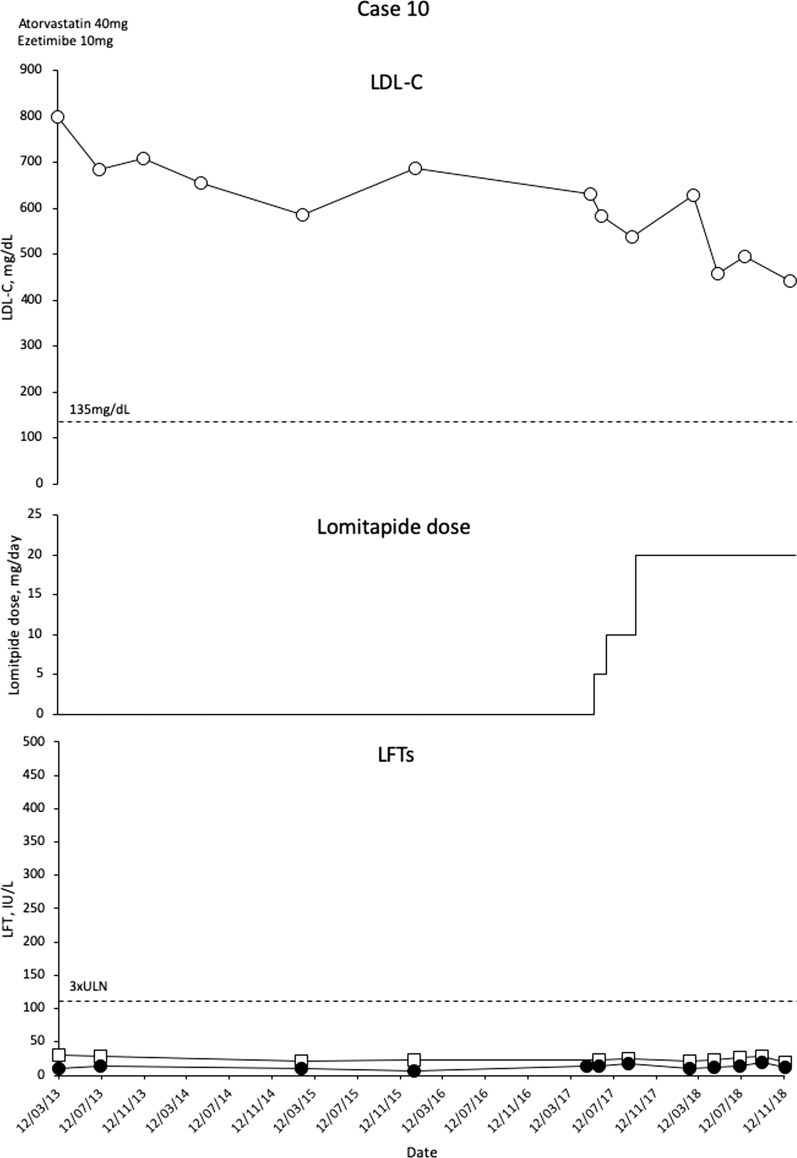
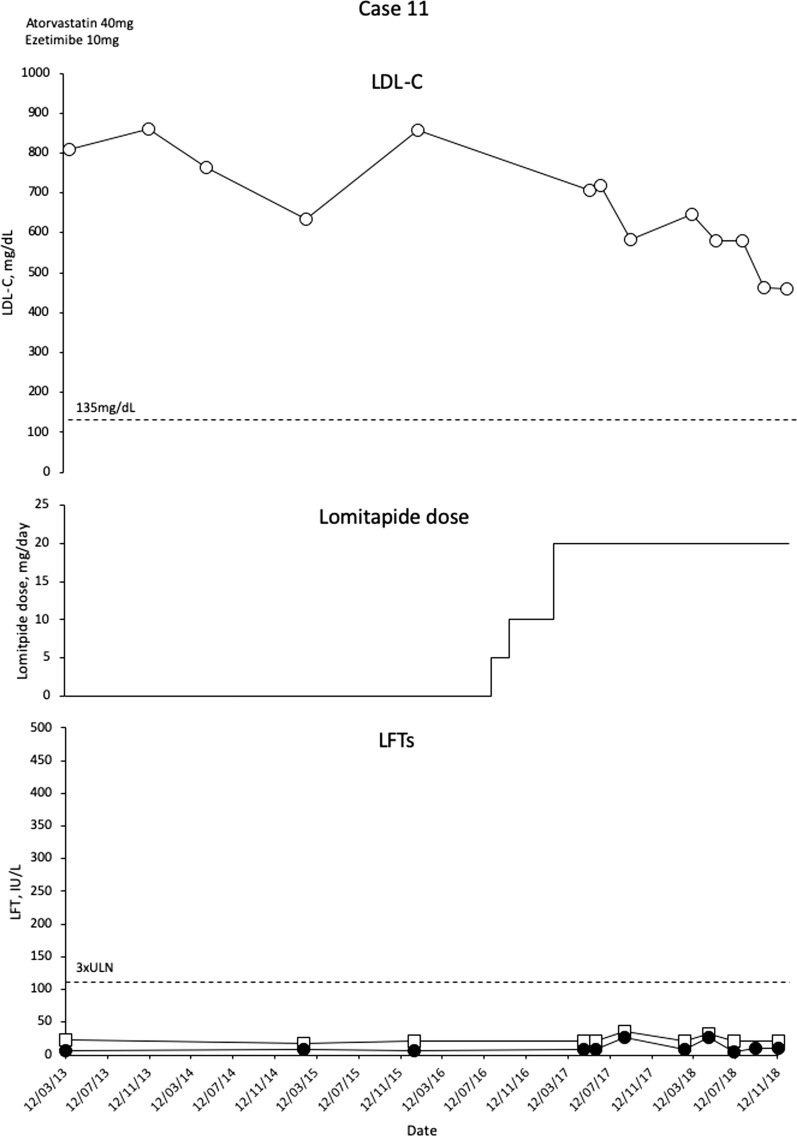
In September 2017 (girl aged 11 with LDL-C 684 mg/dL, boy aged 9 with LDL-C 705 mg/dL) the children commenced lomitapide with rapid escalation from 5 to 20 mg/day. Initial LDL-C decreases were modest (Figs. 10 and 11), and investigations revealed that the parents were not administering the medication regularly to the children. After counselling the parents on the burden of HoFH and the need to adhere to therapy, LDL-C levels have decreased but remained above 440 mg/dL in November 2018. Lomitapide has not been associated with side effects in either patient. In the female patient, an echocardiogram conducted in March 2018 revealed a supra aortic stenosis with a peak of gradient of 50 mmHg, mild tricuspid regurgitation with normal right ventricular systolic pressure and normal biventricular function. Ultrasound Doppler of carotid arteries was normal at the same date. For the male patient, there was aortic insufficiency evident in 2011 that progressed slightly to 2013 but remained stable and mild. An ultra-sounded Doppler of carotid arteries in 2013 showed focal intimal thickening of the right common carotid artery. An echocardiogram conducted in March 2018 showed thickened tricuspid aortic valve leaflets. Development has been normal in the girl. The boy is in second grade school with below average performance.
Fig. 10.
Evolution of LDL-C values in case 10 with lomitapide therapy. Upper panel shows mean interval LDL-C levels for patient 10. Middle panel shows lomitapide dose changes over time. Lower panel shows corresponding ALT (closed circles) and AST (open squares) levels over the same period. Dotted line on upper panel shows EAS targets for LDL-C levels in children with HoFH. Dotted line on lower panel indicates 3× upper limit of normal for LFTs; ALT alanine aminotransferase, AST aspartate aminotransferase, EAS European Atherosclerosis Society, HoFH homozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, LFTs liver function tests, ULN upper limit of normal
Fig. 11.
Evolution of LDL-C values in case 11 with lomitapide therapy. Upper panel shows mean interval LDL-C levels for patient 11. Middle panel shows lomitapide dose changes over time. Lower panel shows corresponding ALT (closed circles) and AST (open squares) levels over the same period. Dotted line on upper panel shows EAS targets for LDL-C levels in children with HoFH. Dotted line on lower panel indicates 3× upper limit of normal for LFTs; ALT alanine aminotransferase, AST aspartate aminotransferase, EAS European Atherosclerosis Society, HoFH homozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, LFTs liver function tests, ULN upper limit of normal
Summary of the Case Series
A summary of baseline, nadir and current LDL-C values is presented in Table 1. Patients 1–8 show substantial reductions in LDL-C levels. Patient 9 was at target LDL-C levels, and the aim of treatment with lomitapide was to reduce LA frequency from twice per week, so the LDL-C decrease is more modest than for other patients. In patients 10 and 11 compliance with lomitapide therapy was suboptimal, and they have yet to reach stable LDL-C levels.
Table 1.
Individual data for the 11 patients
| Parameter | Patient | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Sex | Female | Male | Male | Male | Female | Male | Female | Male | Male | Female | Male |
| Age, years | 13 | 12 | 16 | 7 | 11 | 16 | 4 | 14 | 15 | 11 | 9 |
| Genetic variant |
LDLR c.313 + 5 G > A |
LDLR c.682G > T | LDLR c.119_1207del | LDLR c.666C > A, c.1646C > A | c.-187-? 940 + ? Dup | LDLR c.131G > A, c.2043C > A | LDLR c.2043C > A | LDLR c.1846-? 2311 + ?del, c.1895A > T |
LDLR c.313 + 1 G > A, del exon 1–6 |
LDLR c.1731G > T | LDLR c.1731G > T |
| LDL-C at diagnosis, mg/dL | 799 | 672 | 981 | 1008 | 1009 | 901 | 739 | 474 | 982 | 1002 | 824 |
| LLT prior to lomitapide | Statins, ezetimibe, LA | Statins, ezetimibe, LA | Statins, ezetimibe, LA, EV | Statins, ezetimibe, bile acid sequestrant | Statins, ezetimibe | Statins, ezetimibe, LA, Ev | Statins, ezetimibe | Statins, ezetimibe | Statins, ezetimibe, LA, Ev | Statins, ezetimibe, bile acid sequestrant | Statins, ezetimibe, bile acid sequestrant |
| Duration of therapy prior to lomitapide, years | 11 | 2 | 14 | 3 | 8 | 6 | < 1 | 6 | 11 | 8 | 8 |
| LDL-C prior to lomitapide, mg/dL | 299 | 326 | 187 | 833 | 443 | 274 | 649 | 223 | 81 | 630 | 705 |
| LDL-C at nadir, mg/dL | 56 | 98 | 73 | 360 | 231 | 23 | 236 | 75 | 62 | 441 | 460 |
| Concomitant LLT |
Atv 40mg Ez 10mG LA Q2W |
Ro 20mg Ez 10mg LA Q15D |
Ro 20mg Ez 10mg Ev 420mg QW Co 3250mg LA Q1W |
Ro 20mg Ez 10mg Co 625mg |
Atv 10mg Ez 10mg |
Ro 20mg Ez 10mg LA Q2W |
Atv 10mg Ez 5mg |
Ro 30mg Ez 10mg |
Atv 40mg Ez 10mg LA 2xW |
Atv 40mg Ez 10mg Cholestyramine 4g |
Atv 40mg Ez 10mg Cholestyramine 4g |
| Maximal reduction with lomitapide, % | 81 | 70 | 61 | 57 | 48 | 92 | 64 | 66 | 24 | 27 | 34 |
| Maximum dose of lomitapide, mg/day | 20 | 40 | 60 | 30 | 20** | 30 | 15 | 15*** | 15 | 20 | 20 |
| Length of lomitapide exposure, months | 17 | 15 | 20 | 15 | 48 | 15 | 12 | 22 | 18 | 19 | 19 |
| Change in concomitant LLT |
Ev stopped§ LA stopped |
Ev stopped§ LA reduced to Q4W |
Ev stopped§ Ro stopped LA reduced to Q2W |
None |
Atv 40mg† Atv 60mg† |
Ev stopped§ Ro 30mg Ro 40mg**** LA stopped |
None |
Ez stopped§ LA stopped |
LA reduced 75% Ev stopped§ |
None | None |
| Liver Status | Liver enzymes normal | Liver enzymes normal | Elevated liver enzymes resolved after Ro stopped | Liver enzymes normal | Liver enzymes normal | Minimal ALT increase resolved without intervention | Liver enzymes normal | ALT increases managed with lomitapide dose reduction | Liver enzymes and liver imaging normal | Liver enzymes normal | Liver enzymes normal |
| Adverse events¶ | Nausea, vomiting, Diarrhoea, frequent bowel movements | Diarrhoea, vomiting | Flatulence, hypertransaminasaemia | None | Diarrhoea | Gastrointestinal pain, Hhpertransaminasaemia | Diarrhoea | Hypertransaminasaemia | None | None | None |
AE, adverse events; ALT, alanine aminotransferase; At, atorvastatin; Co, colesevalem; Ev, evolocumab (all Ev stopped prior to lomitapide); Ez, ezetimibe; GI, gastrointestinal; LA lipoprotein apheresis; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapies.
All oral drug doses are daily
**patient briefly received 30mg/day before back-titration to 20mg/day
***patient briefly received 20mg/day before back-titration to 15mg/day
****subsequent, post-hoc reduction to Ro 35mg
†atorvastatin dose changes – Atv dose increased to 60mg near end of observation period
§patient had also received evolocumab (no response), which had been stopped before commencement on lomitapide
¶MedDRA preferred term
Table 2 provides summary descriptive statistics for all 11 patients. Baseline LDL-C was 422.7 ± 245.4 mg/dL. The mean at nadir was 192.2 ± 163.2 mg/dL, representing a 56.7 ± 21.7% reduction in LDL-C. Note that patients 9–11 had modest decreases in LDL-C levels (patient 9 was treated to reduce LA frequency, and patients 10 and 11 had compliance issues). These LDL-C reductions were achieved with a mean dose of lomitapide 25.0 ± 13.8 mg/day over a mean period of 20.0 ± 2.9 months. Most adverse events (Table 2) resolved without intervention.
Table 2.
Summary data for the 11 patients
| Parameter | Age | Baseline LDL-C, mg/dL | Nadir LDL-C, mg/dL | Percentage reduction in LDL-C from baseline to nadir, % | Lomitapide dose, mg/day | Lomitapide exposure, months |
|---|---|---|---|---|---|---|
| Mean | 11.6 | 422.7 | 192.2 | 56.7 | 25.0 | 20.0 |
| Median | 12.0 | 325.5 | 98.0 | 61.2 | 20.0 | 18.2 |
| SD | 3.8 | 245.4 | 163.2 | 21.7 | 13.8 | 9.5 |
LDL-C, low-density lipoprotein cholesterol; SD, standard deviation
Discussion
This report is the first available of a case series in paediatric patients with HoFH receiving lomitapide, and it shows that, as in adult patients, marked reductions in LDL-C are possible in these patients. In all cases, the LDL-C values at diagnosis were very high—some as high as 1000 mg/dL. Even with the use of statins, ezetimibe and in some cases LA, LDL-C levels remained highly elevated in most cases. Notably, some patients received the PCSK9 inhibitor evolocumab, with little effect. The failure of evolocumab to lower LDL-C levels in some patients with HoFH is likely to be due to its mechanism of action. PCSK9 inhibitors prevent the binding of PCSK9 to the LDL-C/LDL receptor complex and thereby prevent the degradation of the LDL receptors, ultimately increasing their recycling to the cell surface [22]. This means that PCSK9 inhibitors require a functional LDL-R to exert their effect. Since LDL-R activity is impaired or absent in HoFH, PCSK9 inhibitors have reduced effectiveness in comparison to that seen in heterozygous familial hypercholesterolaemia or other dyslipidaemia patients in whom LDL receptor functionality is maintained. In the TAUSSIG study of evolocumab in FH patients, LDL-C was reduced on average by 25%, but with no effect in patients with null mutations (i.e. LDL-R functionality < 2%) [23]. This is borne out by data from patients 1, 2 and 6 where LDL-C levels remained very high (263–430 mg/dL), despite use of a PCSK9 inhibitor; patients 2 and 6 had null mutations.
Exposure to high levels of LDL-C for an extended period presents a high risk of ASCVD. In patients with HoFH, the LDL-C levels are so high that the threshold exposure level whereby cardiovascular disease can occur is reached by the age of 12 years, in contrast to 55 years for individuals without FH [2]. All of the patients in the present report were 16 years of age or younger at the start of treatment (range 4–16 years), and all but case 10 had evidence of cardiovascular disease. The youngest patient was case 7, and even she had evidence of thickened aortic cusps and a possible mobile atheroma at the age of 3 years old. Case 9 was diagnosed at the age of 2 years, when there was already subclinical aortic insufficiency.
The early-onset cardiovascular and valvular heart diseases in HoFH demand early lipid-lowering intervention such as statins, ezetimibe, PCSK9 inhibitors and LA that are nevertheless often not sufficient to achieve proposed target LDL-C levels in HoFH patients. Early access to lomitapide, either through an expanded access programme or on a named patient basis, has provided unique opportunities for early-life access to this MTP inhibitor. Introduction and dose escalation of lomitapide was associated with marked decreases in LDL-C levels. For cases 1–8, percentage reductions (nadir) were in the range 44–91%. Six of the patients (cases 1–3, 6, 8 and 9) were able to achieve EAS targets for LDL-C levels in children with HoFH (nadir < 135 mg/dL) [1]. For case 9, baseline LDL-C values were already at target but the patient needed to undergo LA twice weekly which was very burdensome on the patient and became problematic with increasing demands of schooling (Table 1). This patient was able to reduce his LA frequency from an unmanageable twice weekly to a more satisfactory biweekly (75% reduction in apheresis) and still maintain target levels.
Case 9 was not the only patient able to maintain control of LDL-C levels with extended LA intervals. Case 1 has stopped LA, and continues to do well. Case 2 has extended intervals from Q2W to Q4W with LDL-C levels at about 150 mg/dL. Case 3 extended intervals from QW to Q2W. No LA was used in cases 4 and 5; and case 6 has stopped LA for a year, and has LDL-C levels of 34.8 mg/dL (87% reduction from baseline). Case 8 had multiple changes to LA frequency prior to lomitapide, but was able to stop the therapy between April 2017 and July 2018 with the availability of lomitapide. Alterations to LA frequency have been observed in the phase 3 clinical trial of lomitapide and in real-world, adult case series of lomitapide use in HoFH [15, 24]. In the phase 3 study, six of the 13 patients on LA from weeks 26 to 78 underwent permanent changes to their LA regimens [25], some of whom were able to stop LA entirely [26]. Similarly, in a real-word cohort of HoFH adult patients undergoing lomitapide therapy, eight of the 10 patients (80%) on LA were able to stop the treatment, and a further patient was able to reduce LA frequency by 50% [15]. LA presents a huge burden of time, discomfort and expense on patients with HoFH [12], and is also a psychological burden for the patients and their families. This would particularly affect children who need to increase their involvement in education and other activities as they get older. Development of additional effective pharmacotherapies has the potential to reduce or eliminate the constraints of regular LA on patients [27]. The data from this case series suggest that lomitapide may be useful to reduce LA frequency in this particularly difficult-to-treat patient population.
Blunted LDL-C responses in cases 10 and 11 underscore the need for patients to remain compliant with all aspects of their therapy, including low-fat diet, lifestyle modifications and lomitapide dosing.
In the phase 3 clinical trial of lomitapide, there was a dose escalation protocol that increased lomitapide doses until the maximum tolerated dose up to 60 mg/day was reached. This resulted in a median dose of 40 mg/day (mean 44.0 ± 3.8 mg/day) and a mean reduction in LDL-C of 50% versus baseline (baseline 336.4 ± 112.1 mg/dL; 26 weeks 116.3 ± 96.7 mg/dL) [25]. In the present case series, there was no such driver to increase lomitapide dose. Five of the patients described here are maintained on 20 mg/day, two on 15 mg/day, one on 10 mg/day, one on 30 mg/day, one on 40 mg/day and one on 60 mg/day. The mean dose was 24.5 mg/day (median 20 mg/day) resulting in a mean 58.4% reduction in LDL-C at nadir. In a real-world study where the treatment strategy was similar—i.e. to titrate the dose of lomitapide to LDL-C levels as opposed to a maximum tolerated dose—the mean LDL-C levels in a cohort of 15 Italian patients was 19 mg/day with a mean LDL-C reduction at nadir versus baseline of 76.5% (baseline 426.0 ± 204.0 mg/dL; nadir 81.9 ± 56.0 mg/dL) [15]. In the present paediatric case series, the LDL-C lowering is similar to the Italian cohort at a similar mean dose.
Through its mechanism of action, lomitapide results in a reduced absorption of fats in the intestine, resulting in the possibility of GI adverse effects. A corresponding block on the release of VLDL from the liver can result in increases in hepatic fat [16]. In the present case series, adverse events were consistent with those seen in the phase 3 study in adults and in real-world use. Those that did occur were nearly all GI complaints, presented early in the treatment course, and resolved with minimal active management. There were some increases in LFTs with patients 3, 6 and 8 experiencing transient elevations in LFTs above three times the ULN. In patient 3, the LFT increases were managed with a brief dose reduction to 30 mg/day, followed by gradual restoration to 40 mg/day, and later to 60 mg/day. In patient 6, LFT excursions resolved without intervention. Patient 8 had one episode controlled by a lomitapide dose reduction, and the second episode was due to a peritoneal infection.
This paediatric case series—the first of its kind for lomitapide in HoFH—demonstrates that lomitapide has been effective in reducing LDL-C in paediatric patients with HoFH, and suggests that the drug has a similar adverse event and usage profile to that observed in adult patients. Consistent with real-world data from adult patients [15, 28], this paediatric case series shows a greater reduction in LDL-C at a lower mean dose of lomitapide than in the phase 3 study. This is a curious finding but is replicated across data sets and may be explained by the titration of the dose of lomitapide to desired LDL-C reduction as opposed to the ‘forced’ escalation protocol based on tolerability as used in the phase 3 study. Irrespective of rationale, these data show a reduced level of adverse effects related to the lower mean dose and an improved benefit-to-risk profile compared with data from formal clinical trials.
A further benefit of lomitapide in HoFH is the ability for patients to reduce or stop LA, which is an important and positive outcome for many of the children in this case series. The efficacy and safety of lomitapide will be explored in a formal phase 3 study (registration pending) in patients from at least 5 to at most 18 years old, and will provide further evidence to explore the efficacy, tolerability and safety of lomitapide in this cohort. Mean exposure in this case series was 19.9 months (range 12–48 months). The phase 3 study will employ an efficacy period of 24 weeks and a safety phase of an additional 80 weeks, total study duration of 2 years and will assess growth, bone development and reproductive maturation which are all important safety aspects to consider in treating children from the ages of 5 to 18 years. One patient in this series underwent treatment during puberty and had a normal menarche, but further safety data are warranted.
In this paediatric case series, all the patients except cases 10 and 11 already had atherosclerosis on screening and patients 3 and 9 already had revascularisation procedures at the ages of 12 and 8.5 years respectively. Modelling data in adult patients have shown that early intervention with lomitapide has the potential to increase life expectancy and delay the time to first major adverse cardiovascular event (MACE) [29]. Treatment of HoFH patients from very early life may have the potential to realise further outcome benefits through the prevention of atherosclerosis development and progression, thereby underscoring that effective treatments that can significantly lower LDL-C are valuable tools in the management of this severe, progressive and life-threatening condition.
This study, however, has several limitations: (1) the wide variations in underlying standard of care among the different centres, (2) the variability in time of exposure to lomitapide treatment, (3) issues with access and compliance to lomitapide therapy and (4) a non-randomized open design. However, despite these factors, this case series highlights the effectiveness of lomitapide in children with HoFH, and suggests that robust LDL-C reduction can be achieved with lower doses than used in the phase 3 clinical trial in adults and with generally good tolerability. With the exception of one case report [21], there are currently no data on the use of lomitapide in children with HoFH. Given that these patients have an urgent need for effective medical intervention, the information provided here is valuable to guide physicians considering lomitapide for paediatric patients. Further data are necessary to more fully determine the long-term efficacy tolerability and safety of lomitapide in this rare, life-threatening condition. A phase 3 study is planned for lomitapide in paediatric HoFH and this may result in a change to the licensed indication for the drug.
Conclusions
This case series of the real-world use of lomitapide in paediatric HoFH patients suggests that lomitapide can be efficacious as a treatment for HoFH, and that adverse events can be successfully managed by adjusting the patient’s diet and modifying the dose of lomitapide according to tolerability and safety. Overall, the data show that the clinical profile of lomitapide in paediatric patients is similar to that in adults patients with HoFH.
Acknowledgements
The authors would also like to thank the patients who contributed data for the paper and provided kind permission for publication.
Medical Writing and/or Editorial Assistance
The authors would like to thank Nigel Eastmond of Eastmond Medicomm Ltd for editorial support and data analysis that was funded by Amryt Pharma.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Luis Masana received lectures and advisory fees from Amgen, Sanofi, MSD, Myland and Daichii Sankyo. Genovefa Kolovou has given talks, attended conferences sponsored by Amgen, Angelini, MSD, Lilly, Vianex and Sanofi. Martin P. Bogsrud received honoraria relating consulting from Amgen, Sanofi, MSD, Boehringer Ingelheim and Kaneka. Osamah Hussein received honoraria relating to consulting for Megapharma. Daiana Ibarretxe received honoraria related speaker activities for Sanofi, MSD and Rubio. Raul D. Santos received honoraria related to consulting and speaker activities from AstraZeneca, Amgen, Akcea, Kowa, Esperion, MSD, Novo Nordisk and Sanofi. Raul D. Santos is a recipient of a scholarship from the Conselho Nacional de Pesquisa e Desenvolvimento Tecnologico (CNPq) process # 303734/2018-3. Tawfeg Ben-Omran, Gema Ariceta, F. Javier Nóvoa, Allan M. Lund, María Araujo and Rosa M. Sanchez-Hernández have nothing to disclose.
Funding
Amryt Pharma was not involved in design, data collection or decision to publish. Access to lomitapide was supported by Amryt Pharmaceuticals DAC. Article processing charges and open access fee were funded by Amryt Pharma. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Declarations
Conflict of Interest
This case series was not subject to ethic board approval as it includes cases treated in the normal course of care, but all patients provided permission for their data to be published.
Footnotes
This article has been revised due to correction in Figure 2 and Tables 1 and 2.
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8076275.
Tawfeg Ben-Omran, Luis Masana, Genovefa Kolovou, Gema Ariceta, F. Javier Nóvoa, Allan M. Lund, Martin P. Bogsrud, María Araujo, Osamah Hussein, Daiana Ibarretxe, Rosa M. Sanchez-Hernández and Raul D. Santos contributed equally to this work.
Change history
2/20/2024
A Correction to this paper has been published: 10.1007/s12325-023-02739-z
References
- 1.Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35(32):2146–2157. doi: 10.1093/eurheartj/ehu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34(45):3478–3490a. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphries SE, Cooper J, Dale P, Ramaswami U, FH Paediatric Register Steering Group The UK paediatric familial hypercholesterolaemia register: statin-related safety and 1-year growth data. J Clin Lipidol. 2018;12(1):25–32. doi: 10.1016/j.jacl.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos RD, Gidding SS, Hegele RA, et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016;4(10):850–861. doi: 10.1016/S2213-8587(16)30041-9. [DOI] [PubMed] [Google Scholar]
- 5.Stefanutti C, Di Giacomo S, Vivenzio A, et al. Low-density lipoprotein apheresis in a patient aged 3.5 years. Acta Paediatr. 2001;90(6):694–701. doi: 10.1080/080352501750258793. [DOI] [PubMed] [Google Scholar]
- 6.Gagné C, Gaudet D, Bruckert E, Ezetimibe Study Group Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation. 2002;105(21):2469–2475. doi: 10.1161/01.cir.0000018744.58460.62. [DOI] [PubMed] [Google Scholar]
- 7.Hudgins LC, Kleinman B, Scheuer A, White S, Gordon BR. Long-term safety and efficacy of low-density lipoprotein apheresis in childhood for homozygous familial hypercholesterolemia. Am J Cardiol. 2008;102(9):1199–1204. doi: 10.1016/j.amjcard.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 8.Thompson GR, Barbir M, Davies D, et al. Efficacy criteria and cholesterol targets for LDL apheresis. Atherosclerosis. 2010;208(2):317–321. doi: 10.1016/j.atherosclerosis.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Kroon AA, van’t Hof MA, Demacker PN, Stalenhoef AF. The rebound of lipoproteins after LDL-apheresis. Kinetics and estimation of mean lipoprotein levels. Atherosclerosis. 2000;152(2):519–526. doi: 10.1016/s0021-9150(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 10.Luirink IK, Determeijer J, Hutten BA, et al. Efficacy and safety of lipoprotein apheresis in children with homozygous familial hypercholesterolemia: a systematic review. J Clin Lipidol. 2018 doi: 10.1016/j.jacl.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Thompson GR, Catapano A, Saheb S, et al. Severe hypercholesterolaemia: therapeutic goals and eligibility criteria for LDL apheresis in Europe. Curr Opin Lipidol. 2010;21(6):492–498. doi: 10.1097/MOL.0b013e3283402f53. [DOI] [PubMed] [Google Scholar]
- 12.Bruckert E, Saheb S, Bonté JR, Coudray-Omnès C. Daily life, experience and needs of persons suffering from homozygous familial hypercholesterolaemia: insights from a patient survey. Atheroscler Suppl. 2014;15:46–51. doi: 10.1016/j.atherosclerosissup.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Aegerion Pharmaceuticals Inc. Juxtapid prescribing information 2013. http://www.juxtapid.com/sites/default/files/downloads/Prescribing_Information.pdf. Accessed 3 May 2019.
- 14.Amryt Pharmaceuticals DAC. Lojuxta summary of product characteristics 2015. https://www.medicines.org.uk/emc. Accessed 3 May 2019.
- 15.D’Erasmo L, Cefalu AB, Noto D, et al. Efficacy of lomitapide in the treatment of familial homozygous hypercholesterolemia: results of a real-world clinical experience in Italy. Adv Ther. 2017;34(5):1200–1210. doi: 10.1007/s12325-017-0531-x. [DOI] [PubMed] [Google Scholar]
- 16.Amryt Pharmaceuticals DAC. Lojuxta summary of product characteristics 2017. https://www.medicines.org.uk/emc. Accessed 3 May 2019.
- 17.Cuchel M, Blom DJ, Averna M, et al. Sustained LDL-C lowering and stable hepatic fat levels in patients with homozygous familial hypercholesterolemia treated with the microsomal transfer protein inhibitor lomitapide: results of an ongoing long-term extension study. American Heart Association Scientific Sessions, Dallas, Texas. 2013. Abstract 16516.
- 18.Underberg J, Cannon CP, Larrey D, Makris L, Jurecka A, Blom D. Long-term safety and efficacy of lomitapide in patients With homozygous familial hypercholesterolemia: three-year data from the Lomitapide Observational orldwide Evaluation Registry (LOWER). ACC18 67th Annual Scientific Session and Expo, 10–12 March, Orlando, FL. 2018. [DOI] [PubMed]
- 19.Blom DJ, Averna MR, Meagher EA, et al. Long-term efficacy and safety of the microsomal triglyceride transfer protein inhibitor lomitapide in patients with homozygous familial hypercholesterolemia. Circulation. 2017;136(3):332–335. doi: 10.1161/circulationaha.117.028208. [DOI] [PubMed] [Google Scholar]
- 20.Blom DJ, Cuchel M, Ager M, Phillips H. Target achievement and cardiovascular event rates with lomitapide in homozygous familial hypercholesterolaemia. Orphanet J Rare Dis. 2018;13(1):96. doi: 10.1186/s13023-018-0841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chacra AP, Ferrari MC, Rocha VZ, Santos RD. Case report: the efficiency and safety of lomitapide in a homozygous familial hypercholesterolemic child. J Clin Lipidol. 2019 doi: 10.1016/j.jacl.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50(Suppl):S172–S177. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raal FJ, Hovingh GK, Blom D, et al. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol. 2017 doi: 10.1016/s2213-8587(17)30044-x. [DOI] [PubMed] [Google Scholar]
- 24.Roeters van Lennep J, Averna M, Alonso R. Treating homozygous familial hypercholesterolemia in a real-world setting: experiences with lomitapide. J Clin Lipidol. 2015;9(4):607–617. doi: 10.1016/j.jacl.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Cuchel M, Meagher EA, du Toit Theron H, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381(9860):40–46. doi: 10.1016/S0140-6736(12)61731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Averna M, Cefalu AB, Stefanutti C, Di Giacomo S, Sirtori CR, Vigna GB. Individual analysis of patients with HoFH participating in a phase 3 trial with lomitapide: the Italian cohort. Nutr Metab Cardiovasc Dis. 2015;26(1):36–44. doi: 10.1016/j.numecd.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Moriarty PM, Parhofer KG, Babirak SP, et al. Alirocumab in patients with heterozygous familial hypercholesterolaemia undergoing lipoprotein apheresis: the ODYSSEY ESCAPE trial. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Real J, Arbona C, Goterris R, Ascaso JF. Management of homozygous familial hypercholesterolaemia in two brothers. BMJ Case Rep 2018. 2018 doi: 10.1136/bcr-2017-222155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leipold R, Raal F, Ishak J, Hovingh K, Phillips H. The effect of lomitapide on cardiovascular outcome measures in homozygous familial hypercholesterolemia: a modelling analysis. Eur J Prev Cardiol. 2017;24(17):1843–1850. doi: 10.1177/2047487317730473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.