Abstract
Sharma U, Rhaleb NE, Pokharel S, Harding P, Rasoul S, Peng H, Carretero OA. Novel anti-inflammatory mechanisms of N-Acetyl-Ser-Asp-Lys-Pro in hypertension-induced target organ damage. Am J Physiol Heart Circ Physiol 294: H1226–H1232, 2008. First published January 4, 2008; doi:10.1152/ajpheart.00305.2007.— High blood pressure (HBP) is an important risk factor for cardiac, renal, and vascular dysfunction. Excess inflammation is the major pathogenic mechanism for HBP-induced target organ damage (TOD). N-acetyl-Ser-Asp-Lys-Pro (Ac-SDKP), a tetrapeptide specifically degraded by angiotensin converting enzyme (ACE), reduces inflammation, fibrosis, and TOD induced by HBP. Our hypothesis is that Ac-SDKP exerts its anti-inflammatory effects by inhibiting: 1) differentiation of bone marrow stem cells (BMSC) to macrophages, 2) activation and migration of macrophages, and 3) release of the proinflammatory cytokine TNF-α by activated macrophages. BMSC were freshly isolated and cultured in macrophage growth medium. Differentiation of murine BMSC to macrophages was analyzed by flow cytometry using F4/80 as a marker of macrophage maturation. Macrophage migration was measured in a modified Boyden chamber. TNF-α release by activated macrophages in culture was measured by ELISA. Myocardial macrophage activation in mice with ANG II-induced hypertension was studied by Western blotting of Mac-2 (galectin-3) protein. Interstitial collagen deposition was measured by picrosirius red staining. We found that Ac-SDKP (10 nM) reduced differentiation of cultured BMSC to mature macrophages by 24.5% [F4/80 positivity: 14.09 ± 1.06 mean fluorescent intensity for vehicle and 10.63 ± 0.35 for Ac-SDKP; P ˂ 0.05]. Ac-SDKP also decreased galectin-3 and macrophage colony-stimulating factor-dependent macrophage migration. In addition, Ac-SDKP decreased secretion of TNF-α by macrophages stimulated with bacterial LPS. In mice with ANG II-induced hypertension, Ac-SDKP reduced expression of galectin-3, a protein produced by infiltrating macrophages in the myocardium, and interstitial collagen deposition. In conclusion, this study demonstrates that part of the anti-inflammatory effect of Ac-SDKP is due to its direct effect on BMSC and macrophage, inhibiting their differentiation, activation, and cytokine release. These effects explain some of the anti-inflammatory and antifibrotic properties of Ac-SDKP in hypertension.
Keywords: macrophages, inflammation, activation, angiotensin II
HYPERTENSION LEADS TO CARDIAC, renal, and vascular damage. The mechanisms of target organ damage have not been fully elucidated. There is evidence that inflammation contributes to end organ damage (20). We have shown that in ANG II-induced hypertension (32), as well as in renovascular hypertension (25, 35), mineralocorticoid-salt hypertension (26, 28), spontaneously hypertensive rats (9), and heart failure postmyo-cardial infarction (24), N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) not only prevents but also reverses inflammation, cell proliferation, and fibrosis in the heart and kidney.
Ac-SDKP is an endogenous peptide released from its precursor (thymosin-β4) by proline oligopeptidase (9, 12). Ac-SDKP is a natural inhibitor of pluripotent hematopoietic stem cell proliferation (5) and is normally found in human plasma and circulating monocyte cells (2). Early on, Ac-SDKP was shown to originate from bone marrow (19), but it has recently been shown that in mice both Ac-SDKP and its precursor are ubiquitously distributed in tissues including the lung, kidney, and heart (31). Ac-SDKP is mainly hydrolyzed by angiotensin converting enzyme (ACE), and ACE inhibitors (ACEi) prevent its hydrolysis and raise its concentration in plasma four- to fivefold (2).
Ac-SDKP prevents and reverses macrophage infiltration into the left ventricle (LV) of rats with myocardial infarction (42) and 2-kidney-1 clip hypertension (35). Myocardial fibrosis and the number of infiltrated macrophages are positively correlated (3). Macrophages release several proinflammatory cytokines and growth factors, including galectin-3, IL-6, transforming growth factor-β (TGF- β), and TNF-α, that lead to cell proliferation, fibrosis, organ damage, and dysfunction (37). We hypothesize that the inhibitory effect of Ac-SDKP on inflammation, especially in macrophage infiltration, is multiphasic, inhibiting differentiation of bone marrow stem cells (BMSC) to macrophages, macrophage migration, and activation. Accordingly, we examined in vitro (cell culture) the effect of Ac-SDKP on BMSC differentiation to macrophages (F4/80 expression) and macrophage migration and activation (production of TNF-α). We also examined in mice with ANG II-induced hypertension the effects of Ac-SDKP on cardiac macrophage infiltration and activation (galectin-3 expression) and fibrosis.
METHODS
BMSC Isolation and Culture
This protocol was approved by the Henry Ford Hospital Institutional Animal Care and Use Committee. BMSC were isolated from mouse femurs and tibias as reported previously (16). Hind legs from two mice were cleaned thoroughly, and marrow was flushed from the femora and tibias using ice-cold PBS. The collected bone marrow cells were pooled, washed in ice-cold PBS, and resuspended in macrophage growth medium (MGM; BioVeris) with the following composition: human colony stimulating factors [granulocyte macrophage (GM)-CSF, granulocyte (G)-CSF, and macrophage colony-stimulating factor (M-CSF)] and cytokines in Iscove’s modified Dulbecco’s medium (IMDM; HEPES buffered) with 5% (vol/vol) FBS (heat inactivated) and kanamycin sulfate. MGM was supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin (Fisher).
Macrophage Differentiation
BMSC were treated with either Ac-SDKP or PBS and cultured for 7 days in MGM. Ac-SDKP was added daily to overcome degradation by ACE and maintain Ac-SDKP concentration, according to previous reports by others (4, 6, 11, 36). In addition, ACEi was not used in this study since captopril, an ACEi, has been shown to directly inhibit proliferation of hematopoietic stem and progenitor cells in murine bone marrow cultures (8). On day 8, cells were harvested with PBS supplemented with 10 mM EDTA and 4 mg/ml lidocaine. Typically, marrow from the femurs and tibias of one mouse yielded 2×107 to 6×107 macrophages after 7 days in culture. Macrophages were stained with FITC-conjugated F4/80 as the primary antibody and anti-mouse IgG2α-FITC as the isotype control. Approximately 3,000 cells were analyzed, and only viable cells were gated. Surface expression of F4/80 by mature macrophages was quantified by flow cytometry using the FL1 channel, and data were expressed as mean fluorescence intensity (MFI).
Macrophage Migration Assays
Macrophage migration studies were performed in a modified Boyden chamber. We used a Chemicon QCM 96-well migration assay with an 8-μm pore size, which is appropriate for monocytes and macrophages. The upper chamber was filled with a macrophage suspension (10×104 cells per well) derived from mouse spleens, and 150 μl of serum-free medium were added to the lower chamber in the presence or absence of a chemoattractant (3 μM galectin-3) or M-CSF (100 ng/ml). For the inhibition studies with Ac-SDKP, gradually increasing doses of Ac-SDKP (0.01, 0.1, 10, and 100 nM) were added and the samples were preincubated for 30 min before the chemoattractant was added. Cells were incubated for 12 h, and those attached to the bottom of the porous membrane were detached using a prewarmed buffer. The detached cells were incubated in lysis buffer-dye solution, and the fluorescence emitted by CyQuant GR dye was quantified by a fluorescence plate reader using a 480/520 nm filter set. We found that Ac-SDKP was most effective at 10 nM, and thus we used this dose in all subsequent experiments.
Macrophage Activation In Vivo and In Vitro
TNF-α expression by activated macrophages in vitro.
Macrophages obtained from differentiation of BMSC were used for this study according to a method described previously (1). The mature macrophages were stimulated by a purified endotoxin (10 μg/ml LPS) with and without Ac-SDKP (1 and 10 nM) for 48 h. TNF-α in the cell supernatant was quantified by ELISA according to the manufacturer’s instructions (R&D Systems). The viable cells present in each culture well were stained with crystal violet, and absorbance was measured according to manufacturer’s instructions. The TNF-α level in the supernatant was normalized to the absorbance recorded from the corresponding culture wells.
Infiltration by activated macrophages in vivo.
Male C57Bl6/J mice (12- to 14-wk-old) were divided into three groups: 1) vehicle (n = 5), 2) ANG II (n = 7), and 3) ANG II + Ac-SDKP (n = 7). Mice were anesthetized with sodium pentobarbital (50 mg/kg ip). A small incision was made between the shoulder blades, and a pocket was created subcutaneously, just large enough to hold an osmotic minipump (Alzet 2004). The pump was implanted to administer ANG II (750 μg·kg·−1·day−1) and/or Ac-SDKP (800 μg·kg·−1·day−1; synthesized by W. Neugebauer, University of Sherbrooke, Canada) or saline plus 0.01 N acetic acid. Mice were treated for 8 wk. Systolic blood pressure was measured by tail cuff twice a week. At the end of the experiment, animals were anesthetized with 50 mg/kg sodium pentobarbital. The heart was stopped at diastole with an intraventricular injection of 15% KCl and rapidly excised. The LV (including the septum) was weighed and sectioned transversely from apex to base.
Picrosirius red staining for detection of collagen in the heart.
Sections measuring 6 μm were deparaffinized, rehydrated, and stained with picrosirius red using a modification of the method of Sweat et al. (39). Briefly, sections were postfixed in Bouin’s fluid, followed by iron hematoxylin stain to show the nuclei. They were then stained with 0.1% picrosirius red for 1 h and washed twice with 0.5% acetic acid. Images were obtained with a computerized digital camera (SPOT, Diagnostic Instruments), and collagen was examined under a microscope (Nikon E600) using normal light and analyzed with SigmaScan Pro (Jandel Scientific).
Western blot for detection of galectin-3 in the LV and kidney.
In all three groups, galectin-3 protein expression in myocardial tissue was detected by Western blot. A total of 60 μg protein from the LV extract was subjected to 12% SDS-PAGE under reducing conditions. Proteins were transferred to a nitrocellulose membrane. The primary antibody was a mouse monoclonal antibody against full-length recombinant human galectin-3 (1 μg/ml, BioReagents). The membrane was incubated with the primary antibody at 4°C overnight, followed by anti-mouse IgG, HRP-linked antibody at room temperature for 1 h. ECL Western kit (GE Healthcare) was used to visualize the bands. Bands were quantified with a bioscanner, and the results were normalized to actin and expressed as fold increase compared with sham.
Statistical analyses
Data are means ± SE. A paired t-test followed by Hochberg’s adjustment was used to judge statistical significance between treatment groups, with P<0.05 considered as significant.
RESULTS
Effect of Ac-SDKP on Differentiation of BMSC to Macrophages
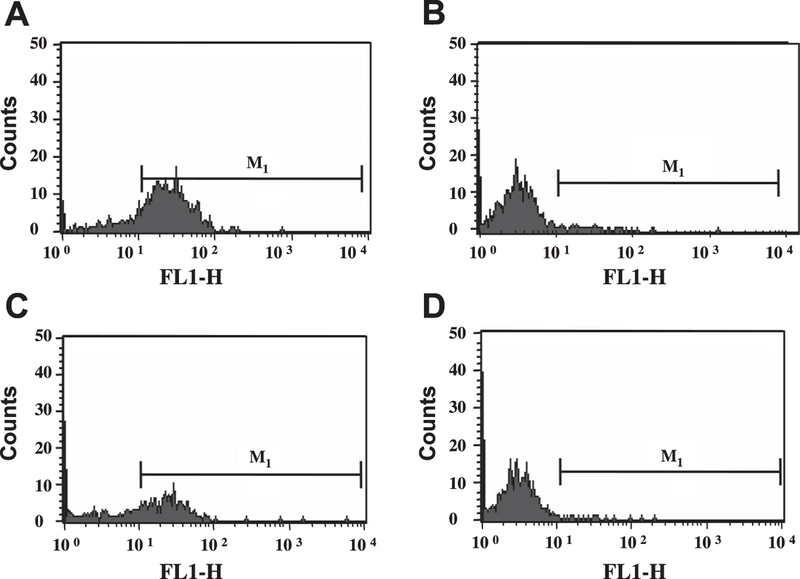
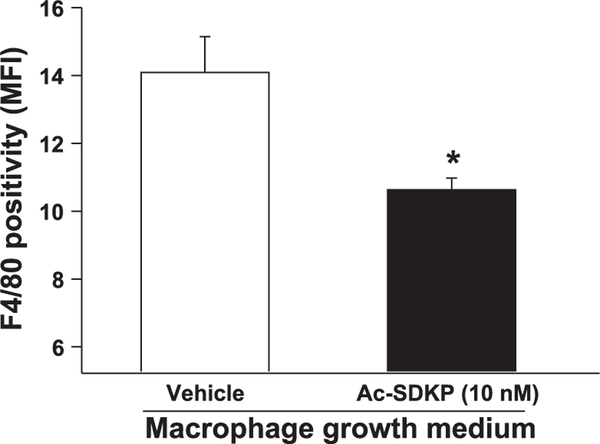
BMSC isolated from mouse bones were treated with Ac-SDKP. Cells treated with MGM developed podocytic processes and strongly expressed the mature macrophage-specific marker F4/80 after 7 days (Fig. 1 A). Adding 10-nM Ac-SDKP to the medium every 24 h for 7 days significantly reduced surface expression of F4/80 (Fig. 1 C; Fig. 2), while maintaining the number of viable cells, suggesting that Ac-SDKP inhibits differentiation of BMSC to mature macrophages (geometric mean of F4/80 positivity: vehicle, 14.09 ± 1.06 MFI; Ac-SDKP, 10.63 ± 0.35 MFI; P < 0.05).
Fig. 1.
FACS analysis to compare expression of F4/80 by macrophages. A: surface expression of F4/80 by macrophages obtained by treating bone marrow stem cells (BMSC) with macrophage growth medium (MGM). B: isotype control for A. C: surface F4/80 expression in cells treated with MGM + N-acetyl-serylaspartyl-lysyl-proline (Ac-SDKP; 10 nM). D: isotype control for C. FL1-H, fluorescence intensity on the FL1, (FITC) channel; M1, marker used to define positive events.
Fig. 2.
Quantitative analysis of the effect of Ac-SDKP on decreased F4/80 surface expression. BMSC incubated in MGM for 7 days demonstrated surface expression of F4/80. F4/80 expression was significantly reduced by concomitant Ac-SDKP treatment for 7 days; n = 3. *P < 0.05, vehicle versus Ac-SDKP.
Effect of Ac-SDKP on Macrophage Migration
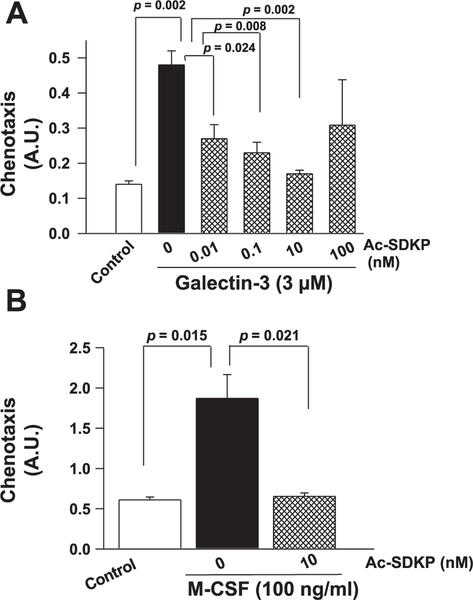
Exogenous recombinant galectin-3 (3 μM) increased macrophage migration (Fig. 3A). Ac-SDKP at increasing concentrations caused significant inhibition of galectin-3-induced macrophage migration, except the 100-nM dose, at which the effects of Ac-SDKP were lessened (Fig. 3A). Similarly, compared with vehicle-treated cells, mouse recombinant M-CSF at a concentration of 100 ng/ml showed an increase in macrophage migration, which was significantly reduced by Ac-SDKP (Fig. 3B).
Fig. 3.
Measurement of macrophage migration in the Boyden chamber. Cells were plated on the upper surface of the Boyden chamber and a chemoattractant [galectin-3 or macrophage colony-stimulating factor (M-CSF)] with and without Ac-SDKP was added to the lower chamber. A: effects of Ac-SDKP on macrophage migration stimulated with galectin-3. B: effects of Ac-SDKP on macrophage migration stimulated with M-CSF. Ac-SDKP inhibited both galectin-3- and M-CSF-dependent macrophage migration in vitro. A.U., arbitrary units; n = 4.
Effect of Ac-SDKP on TNF-α Release by Activated Macrophages
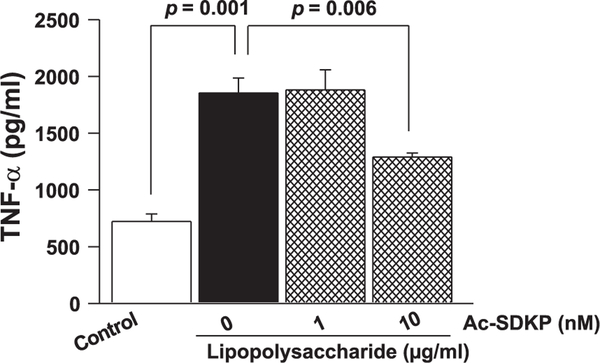
To test for changes in TNF-α release by LPS-activated macrophages in culture, we treated macrophages with bacterial LPS for 48 h and measured TNF-α in the supernatant by quantitative ELISA. As shown in Fig. 4, LPS increased TNF-α release (TNF-α: vehicle, 721.8 ± 66.1 pg/ml; LPS, 1,853.9 ± 132.2 pg/ml; P = 0.001), and 10 nM Ac-SDKP significantly decreased LPS-induced TNF-α release (1,289.8 ± 34.6 pg/ml; P = 0.006 ).
Fig. 4.
Effect of Ac-SDKP on TNF-α release by cultured macrophages stimulated with LPS. Macrophages were grown in the 96-well culture plate; TNF-α levels were measured in the culture supernatant and were normalized to the viable cells present on the corresponding culture wells. Bacterial LPS increased the release of TNF-α by mature macrophages, which was significantly reduced by Ac-SDKP; n = 6.
Effect of Ac-SDKP on Blood Pressure, Cardiac Hypertrophy, and Infiltration of Activated Macrophages Into the Myocardium of ANG II- Hypertensive Mice
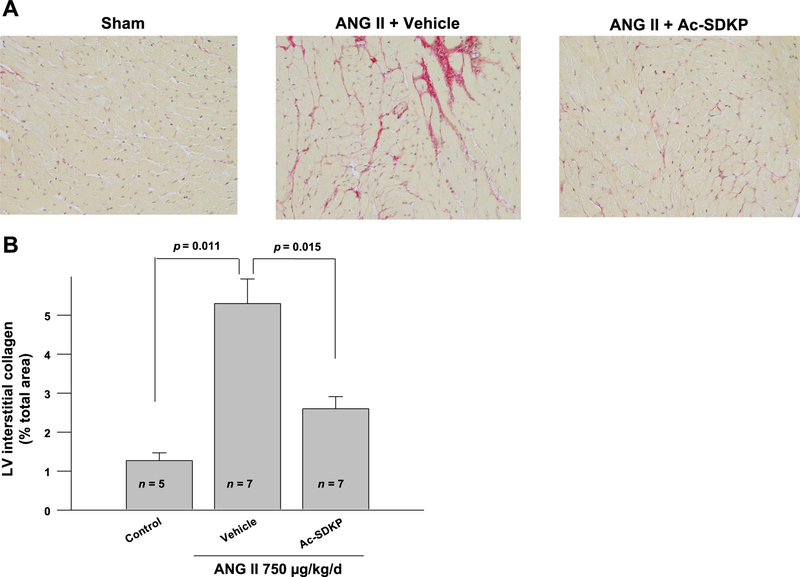
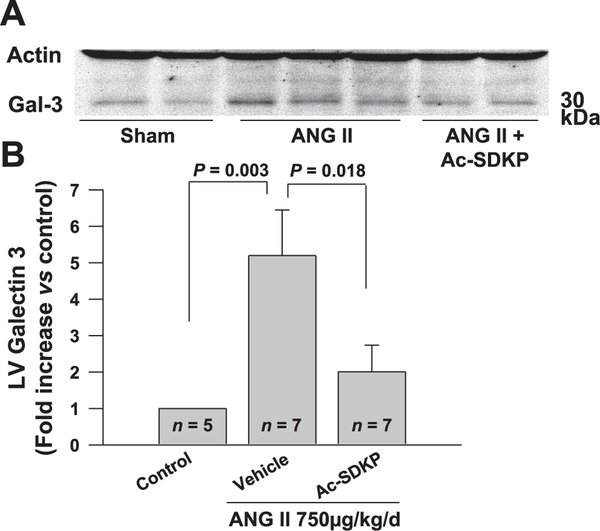
We treated mice with vehicle, ANG II, or ANG II + Ac-SDKP. As expected, ANG II significantly increased systolic blood pressure, heart rate, and LV weight, which were not altered by Ac-SDKP (Table 1). We also found that in mice treated with ANG II + Ac-SDKP the collagen deposition in the LV was significantly decreased compared with mice given ANG II + vehicle (Fig. 5). Subsequently, we measured Mac-2 (galectin-3) protein levels in the myocardium of ANG II-treated hypertensive mice and controls by Western blotting. Galectin-3 expression was increased in the myocardium of ANG II-treated mice. Ac-SDKP infusion significantly decreased ANG II-induced galectin-3 production in the myocardium (Fig. 6). Decreased galectin-3 expression in the myocardium after Ac-SDKP treatment suggested decreased infiltration of activated macrophages.
Table 1.
Blood pressure, heart rate, left ventricular hypertrophy, and plasma Ac-SDKP in rats infused with vehicle, ANG II, or ANG II + Ac-SDKP
| Control | ANG II | ANG II + Ac-SDKP | |
|---|---|---|---|
| SBP, mmHg | 110±6 | 156±6* | 148±7* |
| HR, beats/min | 574±7 | 583±23 | 584±18 |
| LVW/BW | 31.2±1 | 41.1±2.1* | 43.8±1.5* |
| Plasma Ac-SDKP, nM | 1.67±0.21 | 1.88±0.11 | 15.02±10.14† |
Values are means ± SE. Ac-SDKP, N-acetyl-Ser-Asp-Lys-Pro; SBP, systolic blood pressure; HR, heart rate, LVW/BW, ratio of left ventricular weight to body weight.
P ≤ 0.005 vs. control;
P = 0.014 vs. control.
Fig. 5.
Effect of Ac-SDKP on ANG II-induced interstitial collagen deposition in the mice heart. A: representative images of the picrosirious-stained sections of the heart. B: quantification of the interstitial collagen contents in the picrosirious-stained sections. ANG II significantly increased interstitial collagen volume fraction in mice heart, which was inhibited by Ac-SDKP; n = 5–7.
Fig. 6.
Measurement of left ventricular (LV) galectin-3 levels in mice treated with vehicle (n = 5), ANG II (n = 7), or ANG II + Ac-SDKP (n = 7) for 8 wk. A: representative galectin-3 immunoblot on the protein extracted from mice heart treated with vehicle, ANG II, or ANG II + Ac-SDKP. Actin immunoblot was performed on the same sample concurrently to normalize the galectin-3 levels. B: quantification of galectin-3 levels in the immunoblots. ANG II treatment increased LV galectin-3 expression levels, which was significantly reduced by Ac-SDKP; n = 5–7.
DISCUSSION
We have evidence of the anti-inflammatory and antifibrotic properties of Ac-SDKP when administered chronically in a rat model of hypertension; however, the mechanism(s) of the anti-inflammatory effect of Ac-SDKP has not yet been established. In the present study, we found that in vitro Ac-SDKP inhibits 1) differentiation of BMSC to macrophages (F4/80 expression), 2) macrophage migration, and 3) macrophage activation (TNF-α). We also found that in mice with ANG II-induced hypertension Ac-SDKP inhibits galectin-3 (Mac-2) expression and cardiac collagen deposition without affecting blood pressure or cardiac hypertrophy.
Inflammation, scarring, and target organ damage are common consequences of hypertension. Typically, an inflammatory process involves the following mechanisms: 1) a stimulus to pluripotent BMSCs to direct increased differentiation towards monocyte and macrophage lineage, 2) site-directed attraction of macrophages to the area of tissue damage, and 3) activation of macrophage and release of the proinflammatory cytokines (17). We believe that this is the first study showing that Ac-SDKP can inhibit each of the three steps that play a crucial role in hypertension-induced inflammation and fibrosis.
Previous studies done in long-term bone marrow culture have shown that the initial process of macrophage development is mediated by M-CSF, primarily produced by local bone marrow stromal tissue. M-CSF upon binding to its single high-affinity receptor activates tyrosine kinase activity and specifically stimulates the BMSCs to differentiate into macrophages (10). CSF-1-deficient mice with unilateral renal obstruction show decreased inflammatory response and improved organ function (18). We found that Ac-SDKP can inhibit differentiation BMSC to macrophages and thus maintains primitive hematopoietic cells in an undifferentiated state. This effect probably contributes to the reduction of inflammatory infiltrates. The inhibitory effect of Ac-SDKP on BMSC differentiation is not surprising, since one of the early pharmacological effects described for Ac-SDKP was inhibition of hematopoetic stem cell proliferation by preventing entry into S phase (12, 22, 23).
In our previous studies, Ac-SDKP significantly decreased ANG II-induced LV monocyte/macrophage infiltration, cell proliferation, and TGF-β expression, and these effects were counteracted by a monoclonal Ac-SDKP-blocking antibody (25, 28). In the present study, by assessing the effects of Ac-SDKP on macrophage migration in vitro, we have uncovered that Ac-SDKP acts directly on macrophages, inhibiting their migration and thus tissue infiltration; this may explain in part the inhibitory effect of Ac-SDKP on macrophage infiltration seen in the heart, kidneys, and arteries of hypertensive animals (25–27, 32). Although the exact mechanism of chemokine-directed cell migration is still under investigation, local activation of cellular tyrosine kinase has been implicated (38). We recently demonstrated that Ac-SDKP binds specifically to binding sites located in the membranes of cardiac fibroblasts, enabling us to further postulate that Ac-SDKP may exert its anti-inflammatory effects via a receptor possibly expressed in the macrophages that have not yet been identified (43).
Inflammation in cardiovascular diseases is associated with activation of and infiltration by macrophages, which express and secrete proinflammatory cytokines and chemokines (21, 37). The term “activated macrophages” denotes those macrophages that have acquired a heightened ability to perform specific immune functions. Typically, activated macrophages show higher levels of major histocompatibility complex class II, CD11b (Mac-1), and galectin-3 (Mac-2) gene expression. Previously, we observed macrophage infiltration and excess of galectin-3 expression in the heart of a transgenic rat model with excessive ANG II-induced hypertension [TGR (mREN2); ref. 37]. Chronic infusion of galectin-3 into the pericardial space caused cardiac fibrosis and LV dysfunction (37). We also reported that inflammation and fibrosis were decreased in Ac-SDKP-treated rats with 2-kidney-1 clip hypertension (35). These studies, along with our current data generated in an ANG II infusion model of hypertension, have advanced our previous findings, since we now know that the anti-inflammatory and antifibrotic effects of Ac-SDKP can be attributed at least in part to its inhibitory effects of macrophage activation and galectin-3 expression. The current study also supports our previous findings that Ac-SDKP does not have any effects on blood pressure or cardiac hypertrophy. The antifibrotic effects of this novel tetrapeptide can be attributed in part to its anti-inflammatory properties.
It is known that Ac-SDKP acts as a physiological regulator of hematopoiesis and inhibits S-phase entry of murine and human hematopoietic stem cells (23). Recently, we have shown that decreased basal levels of Ac-SDKP in rats treated with the POP inhibitor are associated with increased cardiac and renal perivascular fibrosis and renal glomerulosclerosis. This suggests that Ac-SDKP plays an important physiological role at basal concentrations, preventing organ collagen accumulation (7).
Several in vitro inhibitory effects of Ac-SDKP have been described, including inhibition of cardiac and renal fibroblast proliferation, collagen synthesis, and mesangial growth (13–15, 30, 34, 34, 40). Thus the anti-inflammatory and antifibrotic effects of Ac-SDKP may be complex, since Ac-SDKP influences a broad range of physiological activities both in vivo and in vitro by its inhibitory effects on inflammation, cell proliferation, and collagen synthesis, in addition to its neo-angiogenesis effects (9, 25, 28, 32–35, 41, 42). We have also shown that part of the anti-inflammatory effect of ACEi is mediated by Ac-SDKP (26, 29).
Study Limitations
BSMC differentiation and macrophage migration studies were performed in vitro. It is well understood that pharmacology of cells in vitro does not completely mimic the complex in vivo cell environment in a whole animal. On the other hand, the studies in vitro have allowed us to determine that Ac-SDKP has a direct effect on stem cell maturation into macrophages, macrophage migration, and cytokine release. The present data support in part the studies in vivo; however, in the intact animals additional factors, such as inhibition of expression of cell adhesion molecules, fibroblast proliferation, and collagen synthesis, could also a have significant effect on the antifibrotic effects of Ac-SDKP.
In conclusions, Inflammation and fibrosis in the heart, kidney, and arteries are among the known consequences of longstanding hypertension. Excess inflammatory cell infiltration and increased circulating proinflammatory cytokine levels are consistently reported in hypertensive end-organ damage. This study shows that Ac-SDKP can exert its anti-inflammatory effects at multiple stages of macrophage morphogenesis, including BMSC differentiation, migration, activation, and cytokine secretion. Since it is well documented that ACEi increases plasma and tissue Ac-SDKP, it can be concluded that the multiple effects of Ac-SDKP demonstrated in this study may point to a new direction in our understanding the mechanism by which ACEi counteracts the adverse outcomes of longstanding hypertension.
Acknowledgments
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-28982 (to O. A. Carretero) and HL-071806 (to N. E. Rhaleb).
REFERENCES
- 1.Adams DO, Koren HS. BCG-activated macrophages: comparison as effectors in direct tumor-cell cytotoxicity and antibody dependent cell-mediated cytotoxicity. Adv Exp Med Biol 121B: 195–201, 1979. [DOI] [PubMed] [Google Scholar]
- 2.Azizi M, Ezan E, Nicolet L, Grognet JM, Menard J. High plasma level of N-acetyl-seryl-aspartyl-lysyl-proline: a new marker of chronic angiotensin-converting enzyme inhibition. Hypertension 30: 1015–1019, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Boluyt MO, O’Neill L, Meredith AL, Bing OHL, Brooks WW, Conrad CH, Crow MT, Lakatta EG. Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure. Marked upregulation of genes encoding extracellular matrix components. Circ Res 75: 23–32, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet D, Lemoine FM, Najman A, Guigon M. Comparison of the inhibitory effect of AcSDKP, TNF-α, TGF-β, and MIP-1α on marrow-purified CD34 progenitors. Exp Hematol 23: 551–556, 1995. [PubMed] [Google Scholar]
- 5.Bonnet D, Lemoine FM, Pontvert-Delucq S, Baillou C, Najman A, Guigon M. Direct and reversible inhibitory effect of the tetrapeptide acetyl-N-Ser-Asp-Lys-Pro (Seraspenide) on the growth of human CD34+ subpopulations in response to growth factors. Blood 82: 3307–3314, 1993. [PubMed] [Google Scholar]
- 6.Cashman JD, Eaves AC, Eaves CJ. The tetrapeptide AcSDKP specifically blocks the cycling of primitive normal but not leukemic progenitors in long-term culture: evidence for an indirect mechanism. Blood 84: 1534–1542, 1994. [PubMed] [Google Scholar]
- 7.Cavasin MA, Liao TD, Yang XP, Yang JJ, Carretero OA. Decreased endogenous levels of Ac-SDKP promote organ fibrosis. Hypertension 50: 130–136, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Chisi JE, Wdzieczak-Bakala J, Thierry J, Briscoe CV, Riches AC. Captopril inhibits the proliferation of hematopoietic stem and progenitor cells in murine long-term bone marrow cultures. Stem Cells 17: 339–344, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Cingolani OH, Yang XP, Liu YH, Villanueva M, Rhaleb NE, Carretero OA. Reduction of cardiac fibrosis decreases systolic performance without affecting diastolic function in hypertensive rats. Hypertension 43: 1067–1073, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dey A, She H, Kim L, Boruch A, Guris DL, Carlberg K, Sebti SM, Woodley DT, Imamoto T, Li W. Colony-stimulating factor-1 receptor utilizes multiple signaling pathways to induce cyclin D2 expression. Mol Biol Cell 11: 3835–3848, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudron S, Grillon C, Thierry J, Riches A, Wierenga PK, Wdzieczak-Bakala J. In vitro effect of acetyl-N-Ser-Asp-Lys-Pro (AcSDKP) analogs resistant to angiotensin I-converting enzyme on hematopoietic stem cell and progenitor cell proliferation. Stem Cells 17: 100–106, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Guigon M, Bonnet D, Lemoine FM, Kobari L, Permentier C, Mary JY, Najman A. Inhibition of human bone marrow progenitors by the synthetic tetrapeptide AcSDKP. Exp Hematol 18: 1112–1115, 1990. [PubMed] [Google Scholar]
- 13.Iwamoto N, Xano HJ, Yoshioka T, Shiraga H, Nitta K, Muraki T, Ito K. Acetyl-seryl-aspartyl-lysyl-proline is a novel natural cell cycle regulator of renal cells. Life Sci 66: PL221–PL226, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Kanasaki K, Haneda M, Sugimoto T, Shibuya K, Isono M, Isshiki K, Araki S, Uzu T, Kashiwagi A, Koya D. N-acetyl-seryl-aspartyl-lysyl-proline inhibits DNA synthesis in human mesangial cells via up-regulation of cell cycle modulators. Biochem Biophys Res Commun 342: 758–765, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Kanasaki K, Koya D, Sugimoto T, Isono M, Kashiwagi A, Haneda M. N-acetyl-seryl-aspartyl-lysyl-proline inhibits TGF-β-mediated plasminogen activator inhibitor-1 expression via inhibition of Smad pathway in human mesangial cells. J Am Soc Nephrol 14: 863–872, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Kanters E, Gijbels MJ, Van Der Made I, Vergouwe MN, Heeringa P, Kraal G, Hofker MH, de Winther MP. Hematopoietic NF-kappaB1 deficiency results in small atherosclerotic lesions with an inflammatory phenotype. Blood 103: 934–940, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. J Allergy Clin Immunol 120: 3–10, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Lenda DM, Kikawada E, Stanley ER, Kelly VR. Reduced macrophage recruitment, proliferation, and activation in colony-stimulating factor-1deficient mice results in decreased tubular apoptosis during renal inflammation. J Immunol 170: 3254–3262, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Lenfant M, Wdzieczak-Bakala J, Guittet E, Prome JC, Sotty D, Frindel E. Inhibitor of hematopoietic pluripotent stem cell proliferation: purification and determination of its structure. Proc Natl Acad Sci USA 86: 779–782, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luft FC, Mervaala E, Müller DN, Gross V, Schmidt F, Park JK, Schmitz C, Lippoldt A, Breu V, Dechend R, Dragun D, Schneider W, Ganten D, Haller H. Hypertension-induced end-organ damage. A new transgenic approach to an old problem. Hypertension 1999: 212–218, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Mann DL. Inflammatory mediators and the failing heart. Past, present, and the foreseeable future. Circ Res 91: 988–998, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Massé A, Ramirez LH, Bindoula G, Grillon C, Wdzieczak-Bakala J, Raddassi E, Deschamps de Paillette E, Mencia-Huerta JM, Koscielny S, Potier P, Sainteny F, Carde P. The tetrapeptide acetyl-N-Ser-AspLys-Pro (Goralatide) protects from doxorubicin-induced toxicity: improvements in mice survival and protection of bone marrow stem cells and progenitors. Blood 91: 441–449, 1998. [PubMed] [Google Scholar]
- 23.Monpezat JP, Frindel E. Further studies on the biological activities of the CFU-S inhibitory tetrapeptide AcSDKP. I. The precise point of the cell cycle sensitive to AcSDKP: studies on the effect of AcSDKP on GM-CFC and on the possible involvement of T-lymphocytes in AcSDKP response. Exp Hematol 17: 1077–1080, 1989. [PubMed] [Google Scholar]
- 24.Morimoto H, Takahashi M, Izawa A, Ise H, Hongo M, Kolattukudy PE, Ikeda U. Cardiac overexpression of monocyte chemoattractant protein-1 in transgenic mice prevents cardiac dysfunction and remodeling after myocardial infarction. Circ Res 99: 891–899, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Peng H, Carretero OA, Brigstock DR, Oja-Tebbe N, Rhaleb NE. Ac-SDKP reverses cardiac fibrosis in rats with renovascular hypertension. Hypertension 42: 1164–1170, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng H, Carretero OA, Liao TD, Peterson EL, Rhaleb NE. Role of Ac-SDKP in the antifibrotic effect of angiotensin-converting enzyme inhibitors in hypertension-induced target organ damage. Hypertension 49: 1–9, 2007.17145983 [Google Scholar]
- 27.Peng H, Carretero OA, Liao TD, Peterson EL, Rhaleb NE. Role of N-acetyl-seryl-aspartyl-lysyl-proline in the antifibrotic and anti-inflammatory effects of the angiotensin-converting enzyme inhibitor captopril in hypertension. Hypertension 49: 695–703, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng H, Carretero OA, Raij L, Yang F, Kapke A, Rhaleb NE. Antifibrotic effects of N-acetyl-seryl-aspartyl-lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension 37: 794–800, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng H, Carretero OA, Vuljaj N, Liao TD, Motivala A, Peterson EL, Rhaleb NE. Angiotensin-converting enzyme inhibitors. A new mechanism of action. Circulation 112: 2436–2445, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pokharel S, Rasoul S, Roks AJM, van Leeuwen REW, van Luyn MJA, Deelman LE, Smits JF, Carretero O, van Gilst WH, Pinto YM. N-acetyl-Ser-Asp-Lys-Pro inhibits phosphorylation of Smad2 in cardiac fibroblasts. Hypertension 40: 155–161, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Pradelles P, Frobert Y, Créminon C, Ivonine H, Frindel E. Distribution of a negative regulator of haematopoietic stem cell proliferation (AcSDKP) and thymosin 4 in mouse tissues. FEBS Lett 289: 171–175, 1991. [DOI] [PubMed] [Google Scholar]
- 32.Rasoul S, Carretero OA, Peng H, Cavasin MA, Zhuo J, SanchezMendoza A, Brigstock DR, Rhaleb NE. Antifibrotic effect of Ac-SDKP and angiotensin-converting enzyme inhibition in hypertension. J Hypertens 22: 593–603, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasoul S, Rhaleb NE, Peng H, Pinto YM, Carretero OA. N-acetylseryl-aspartyl-lysyl-proline (Ac-SDKP) inhibits macrophage activation and infiltration (Abstract). Circulation 104: II-278, 2001. [Google Scholar]
- 34.Rhaleb NE, Peng H, Harding P, Tayeh M, LaPointe MC, Carretero OA. Effect of N-acetyl-seryl-aspartyl-lysyl-proline on DNA and collagen synthesis in rat cardiac fibroblasts. Hypertension 37: 827–832, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhaleb NE, Peng H, Yang XP, Liu YH, Mehta D, Ezan E, Carretero OA. Long-term effect of N-acetyl-seryl-aspartyl-lysyl-proline on left ventricular collagen deposition in rats with 2-kidney, 1-clip hypertension. Circulation 103: 3136–3141, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson S, Lenfant M, Wdzieczak-Bakala J, Riches A. The molecular specificity of action of the tetrapeptide acetyl-N- Ser-Asp-Lys-Pro (AcSDKP) in the control of hematopoietic stem cell proliferation. Stem Cells 11: 422–427, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JPM, Schroen B, Andre S, Crijns HJGM, Gabius HJ, Maessen J, Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110: 3121–3128, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Shimokado K, Yokota K, Umezawa K, Sasaquri T, Oqata J. Protein tyrosine kinase inhibitors inhibit chemotaxis of vascular smooth muscle cells. Arterioscler Thromb 14: 973–981, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Sweat F, Puchtler H, Rosenthal SI. Sirius Red F3BA as a stain for connective tissue. Arch Pathol 78: 69–72, 1964. [PubMed] [Google Scholar]
- 40.Volkov L, Quéré P, Coudert F, Comte L, Antipov Y, Praloran V. The terapeptide AcSDKP, a negative regulator of cell cycle entry, inhibits the proliferation of human and chicken lymphocytes. Cell Immunol 168: 302–306, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Wang D, Carretero OA, Yang XY, Rhaleb NE, Liu YH, Liao TD, Yang XP. N-acetyl-seryl-aspartyl-lysyl-proline stimulates angiogenesis in vitro and in vivo. Am J Physiol Heart Circ Physiol 287: H2099–H2105, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang F, Yang XP, Liu YH, Xu J, Cingolani O, Rhaleb NE, Carretero OA. Ac-SDKP reverses inflammation and fibrosis in rats with heart failure after myocardial infarction. Hypertension 43: 229–236, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuo JL, Carretero OA, Peng H, Li XC, Regoli D, Neugebauer W, Rhaleb NE. Characterization and localization of N-acetyl-seryl-aspartyllysyl-proline (Ac-SDKP) receptor binding sites using 125I-Hpp-AcaSDKP in rat cardiac fibroblasts. Am J Physiol Heart Circ Physiol 292: H984–H993, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]