Abstract
N-Acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) is a natural inhibitor of pluripotent hematopoietic stem cell entry into the S phase of the cell cycle and is normally present in human plasma. Ac-SDKP is exclusively hydrolyzed by ACE, and its plasma concentration is increased 5-fold after ACE inhibition in humans. We examined the effect of 0.05 to 100 nmol/L Ac-SDKP on 24-hour 3H-thymidine incorporation (DNA synthesis) by cardiac fibroblasts both in the absence and presence of 5% FCS. Captopril (1 μmol/L) was added in all cases to prevent the degradation of Ac-SDKP. Treatment of cardiac fibroblasts with 5% FCS increased thymidine incorporation from a control value of 12 469±594 to 24 598±1051 cpm (P<0.001). Cotreatment with 1 nmol/L Ac-SDKP reduced stimulation to control levels (10 373±200 cpm, P<0.001). We measured hydroxyproline content and incorporation of 3H-proline into collagenous fibroblast proteins and found that Ac-SDKP blocked endothelin-1 (10−8 mol/L)–induced collagen synthesis in a biphasic and dose-dependent manner, causing inhibition at low doses, whereas high doses had little or no effect. It also blunted the activity of p44/p42 mitogen-activated protein kinase in a biphasic and dose-dependent manner in serum-stimulated fibroblasts, suggesting that the inhibitory effect of DNA and collagen synthesis may depend in part on blocking mitogen-activated protein kinase activity. Participation of p44/p42 in collagen synthesis was confirmed, because a specific inhibitor for p44/p42 activation (PD 98059, 25 μmol/L) was able to block endothelin-1–induced collagen synthesis, similar to the effect of Ac-SDKP. The fact that Ac-SDKP inhibits DNA and collagen synthesis in cardiac fibroblasts suggests that it may be an important endogenous regulator of fibroblast proliferation and collagen synthesis in the heart. Ac-SDKP may participate in the cardioprotective effect of ACE inhibitors by limiting fibroblast proliferation (and hence collagen production), and therefore it would reduce fibrosis in patients with hypertension.
Keywords: amino acid, angiotensin-converting enzyme inhibitors, fibroblasts, collagen, endothelin, protein kinases
N-Acetyl-Ser-Asp-Lys-Pro (Ac-SDKP) is endogenously released from its protein precursor thymosin-β4 in bone marrow cells.1,2 It naturally inhibits entry of pluripotent hematopoietic stem cells into the S phase, maintaining them in the G0/G1 phase and thus blocking DNA synthesis.3,4 This tetrapeptide is normally present in human plasma and circulating mononuclear cells.5 In addition, Ac-SDKP and its precursor are ubiquitously distributed in mouse tissues, including the lung (0.86±0.25 nmol/g), kidney (0.43±0.02 nmol/g), and heart (0.88±0.36 nmol/g).6 ACE has 2 homologous NH2- and COO-terminal catalytic domains. Ac-SDKP is found in tissues where ACE is present and hydrolysed almost exclusively by ACE,7 showing a 50-fold higher affinity for the N-domain than for the C-domain of recombinant mutant ACE.8 This peptide has a 4.5-minute half-life in the circulation and thus is probably released continuously.9 However, chronic or acute administration of captopril, an ACE inhibitor (ACEI), reportedly prevented the degradation of endogenous Ac-SDKP and raised its circulating concentrations by ≈5-fold in volunteers, while it inhibited the hydrolysis of 3H-Ac-SDKP in plasma by 90% to 99%.7,10
The antiproliferative effects of Ac-SDKP are not limited to the hematopoietic system. When administered after two-thirds hepatectomy in rats, it reduces hepatocyte proliferation by up to 50% as assessed by 3H-thymidine incorporation,11 suggesting that it may also inhibit the growth of other types of cells. In addition, Ac-SDKP suppressed the proliferation of renal fibroblasts, suggesting that it may be an endogenous modulator of renal cell proliferation.12 Chronic ACE inhibition also regressed abnormally increased interstitial collagen deposition within the myocardium in spontaneously hypertensive rats (SHR)13 and prevented myocardial fibrosis in Lewis rats with heart failure induced by myocardial infarction. 14 We wanted to determine whether Ac-SDKP inhibits cardiac fibroblast proliferation and collagen turnover in vitro so we would know whether this tetrapeptide plays a role in the cardioprotective effects of ACEIs.
Mitogen-activated protein kinases (MAPKs) may be important post–receptor signaling pathways through which growth factors stimulate cardiac fibroblast proliferation.15,16 However, it is not known whether Ac-SDKP interferes with the activation of MAPK pathways in cardiac fibroblasts or other cells. Endothelin-1 (ET-1), a 21-amino-acid peptide, is produced by endothelial cells, smooth muscle cells, fibroblasts, and cardiomyocytes. ET-1 gene upregulation may occur in response to stretching the cell wall,17 ischemia,18 or angiotensin II (Ang II) acting via Ang II type 1 (AT1) receptors.19 ET-1 is a potent stimulator of collagen synthesis in cultured adult cardiac fibroblasts, causing a dose-dependent increase in collagen production with a threshold dose approaching 1 nmol/L.20 Using cultured adult rat cardiac fibroblasts (passage 2), we tested whether Ac-SDKP (1) inhibits cardiac fibroblast proliferation (3H-thymidine incorporation) when stimulated with FCS, (2) blocks collagen synthesis by cardiac fibroblasts stimulated with ET-1 in vitro (hydroxyproline assay and 3H-proline incorporation into collagenase-sensitive proteins), or (3) prevents activation of p44/p42 MAPK. We also tested whether (1) PD98059, a p44/p42 inhibitor; (2) SB203580, a p38 inhibitor; or (3) curcumin, a c-Jun kinase (JNK) inhibitor, blocks ET-1–induced collagen synthesis.
Methods
Cell Culture
Primary cultures of cardiac fibroblasts were derived from adult Sprague-Dawley (SD) rats weighing 200 to 250 g by a modification of a method previously described by Eghbali et al.21 Briefly, hearts were rapidly excised from 2 or 3 ether-anesthetized rats, and the atrial tissue was removed. Ventricles were minced and placed in tubes containing 5 mL calcium bicarbonate–free Hanks’ solution with HEPES (CBFHH) and 0.067% collagenase B (Boehringer Mannheim) and then gently stirred. After each 15-minute period of digestion, 5 mL CBFHH buffer was added to each tube, and the minced tissue was pipetted up and down 30 times with a wide-bore pipette. Cell suspensions from 3 consecutive digestions were pooled together in a 50-mL tube containing 10% FCS and kept on ice until pelleting at 1000 rpm for 5 minutes. The pellet was washed twice with DMEM (Gibco) containing 10% FCS, 100 IU/L penicillin, and 0.1 g/L streptomycin. Cell suspensions were passed through a 60-mesh screen (Sigma) into a 100-mm culture dish and incubated for 45 minutes at 37°C. Unattached cells were discarded, whereas attached cells were washed twice with 10% FCS/DMEM and allowed to grow to confluence before passage, in trypsin-based solution in 1:3 dilutions. All cells used in these experiments were taken from passage 2. This protocol was approved by the Henry Ford Hospital Committee for the Care and Use of Experimental Animals.
3H-Thymidine Incorporation
Fibroblasts were seeded onto 6-well plates containing DMEM supplemented with 10% FCS at a density of 0.5×105 cells per well and allowed to grow until subconfluent, occupying 60% to 70% of the total surface of the plate. Cells were cultured in serum-free DMEM for 24 hours and then treated with 5% FCS either alone or combined with Ac-SDKP (Bachem) (0.1 to 1000 nmol/L) for 24 hours in DMEM containing 3H-thymidine (1 mCi/mL) and 10−6 mol/L captopril. Control cells were also treated with captopril. Each well was washed once with 1 mL ice-cold PBS, and then 1 mL ice-cold 10% trichloroacetic acid (TCA) was added. The plates were scraped, and cell lysates were transferred to Eppendorf vials, then vacuum-filtered through Whatman filters and washed 3 times with 5 mL ethanol/TCA (70:5%). Filters were counted for 1 minute in vials containing 4 mL scintillation cocktail.
Collagen Synthesis
Collagen synthesis was estimated with 2 different methods: 3Hproline incorporation by collagenous proteins and hydroxyproline assay.
3H-Proline Incorporation
Collagen synthesis by confluent cardiac fibroblasts was measured according to the method previously described by Brilla et al.22 Briefly, fibroblasts from passage 2 (1×105 cells/well) were seeded onto 6-well plates containing 10% FCS/DMEM and allowed to grow until confluent, making close contact with each other. Cells were cultured in serum-free DMEM for at least 48 hours before the medium was replaced with 0.4% FCS/DMEM containing 0.15 mmol/L L-ascorbic acid and 10−6 mol/L captopril. We used endothelin-1 (ET-1) to stimulate collagen synthesis, because (1) ET-1 is a potent stimulus for collagen synthesis in fibroblasts20; (2) endogenous ET-1 has been shown to play an important role in Ang II–induced myocyte hypertrophy in vitro,23 hypertension in vivo,24 and renal25 and cardiac fibrosis26 in vivo, suggesting that ET-1 could be a second messenger for Ang II; and (3) we found that either Ang II or its aminopeptidase-resistant analog, Sar1-Ang II, failed to increase collagen synthesis as measured by either proline incorporation or hydroxyproline assay. We are not the first to obtain negative results with Ang II; Agocha et al27 and Pathak et al28 both found that Ang II was unable to stimulate collagen synthesis in adult rat cardiac fibroblasts under normal conditions. Cells were treated with Ac-SDKP (1 nmol/L) for 30 minutes, and then ET-1 (10−8 mol/L) was added for 48 hours, followed by an 18-hour exposure to 3H-proline (14 μCi/well) in fresh serum-free DMEM containing 0.1% BSA, 0.04% proline, 0.15 mmol/L L-ascorbic acid, and 10−8 mol/L ET-1 either alone or combined with Ac-SDKP. After incubation, cells were sonicated on ice, and TCA (final concentration 10% wt/vol) was used to precipitate proteins in the presence of 0.04% proline and 0.1% BSA. The samples were allowed to stand overnight at 4°C before centrifugation. Protein pellets were washed 3 times with 1 mL of 5% TCA/1 mmol/L proline, and the final pellet was dissolved in 1 mL of 0.2 mol/L NaOH. Fibroblast proteins were incubated with 1 mmol/L CaCl2 and 2.5 mmol/L N-ethylmaleimide in the presence of either collagenase type III (50 U/mL; Calbiochem) or 2 mmol/L Tris (pH 7.6) and 0.2 mmol/L CaCl2 for 90 minutes at 37°C. The vials were placed on ice, and 0.5 mL of 20% TCA/0.5% tannic acid was added to precipitate protein for 1 hour. Supernatants were transferred to scintillation vials together with 0.5 mL of 5% TCA after centrifugation at 10 000 rpm for 5 minutes. Scintillation fluid (10 mL) was added to each sample, and radioactivity was determined with a liquid scintillation counter. The supernatant from the noncollagenase group served as background, and the supernatant from the group treated with collagenase was counted for collagen; the pellet was also counted to determine nonsensitive collagenase proteins. Percent collagen synthesis was calculated as [C/P ratio/ 5.4×(1−C/P ratio)+C/P ratio]×100, where C is collagenase-releasable counts, P is total proteins (supernatant plus pellet), and 5.4 is a correction factor for noncollagen protein used to adjust for the relative abundance of proline and hydroxyproline in proteins that contain collagen.
Hydroxyproline Assay
Collagen synthesis was measured with an established hydroxyproline assay29 adapted for use under tissue culture conditions by Villarreal et al.30 Cardiac fibroblasts were placed in a 100-mm culture dish and grown until confluent in DMEM supplemented with 10% FCS, then serum-starved for 48 hours. Cells were cultured in fresh 0.4% FCS/DMEM containing 0.15 mmol/L L-ascorbic acid and 10−6 mol/L captopril and treated with Ac-SDKP (0 to 10 nmol/L) for 30 minutes before the addition of ET-1 (10−8 mol/L) for 48 hours. At the end of the experiment, medium was precipitated in 2 volumes of absolute ethanol at −20°C for 24 hours. After precipitation, samples were centrifuged at 16 000 rpm for 30 minutes, and the pellet was air-dried. Each precipitant was homogenized in a tube containing 1 mL of 0.1 mmol/L NaCl and 5 mmol/L NaHCO3 and then washed 5 times with the same solution. Each pellet was hydrolyzed in 500 μL of 6N HCl for 16 hours at 110°C. The samples were filtered, dried under nitrogen gas, and dissolved in 400 μL of water. Hydroxyproline content was determined with a color-based reaction as described by Stegemann and Stalder,31 using a standard curve for 0 to 5 mg hydroxyproline.
Plates containing 500 μL lysis buffer were scraped, and the cell lysate was homogenized for protein determination using a Bio-Rad protein assay kit. Data were expressed as micrograms of collagen produced in the medium per milligram of fibroblast proteins, assuming that collagen contains 13.5% hydroxyproline.32
Effect of Ac-SDKP on p44/p42 Mitogen-Activated Protein Kinase Activity
MAPK activity was measured after selective precipitation of active MAPK and detection of MAPK-induced phosphorylation of the transcription factor Elk-1 at Ser383 according to the method described by Gille et al.33 Adult cardiac fibroblasts were grown to confluence with 10% FCS/DMEM in a 100×20-mm culture dish and then serum-deprived for 48 hours. Cells were treated with Ac-SDKP (0 to 10 nmol/L) for 30 minutes before the addition of 10% FCS for 5 minutes. The medium was removed, and cells were washed once with ice-cold PBS, then sonicated for 15 seconds on ice. Samples were stored at −80°C until assayed. MAPK activity was measured with a p44/p42 MAPK assay kit and a phototope-horseradish peroxidase (HRP) Western blot detection kit (New England BioLabs). p44/p42 MAPK was measured by Western blotting with a p44/p42 MAPK antibody (rabbit polyclonal IgG; New England BioLabs). p44/p42 MAPK activity was normalized to p44/p42 MAPK protein and expressed as fold increase compared with control. To further assess the role of MAPK in ET-1–induced collagen synthesis, cells were pretreated for 1 hour with (1) the MAPK kinase (MEK) inhibitor PD98059 (25 mmol/L), which is an upstream activator of p42/p44, (2) SB203580 (25 μmol/L), which inhibits p38 kinase, or (3) curcumin (25 μmol/L), which inhibits JNK. After this pretreatment, ET-1 was added for 48 hours. Samples were harvested and processed for hydroxyproline content.
Statistical Analysis
Data from multiple experiments were expressed as mean±SEM, and differences in mean values were analyzed by 1-way ANOVA with pairwise multiple comparisons made by the Student-Newman-Keuls method. P<0.05 was considered significant (compared with control unless otherwise specified).
Results
Effect of Ac-SDKP on3H-Thymidine Incorporation by Cardiac Fibroblasts In Vitro
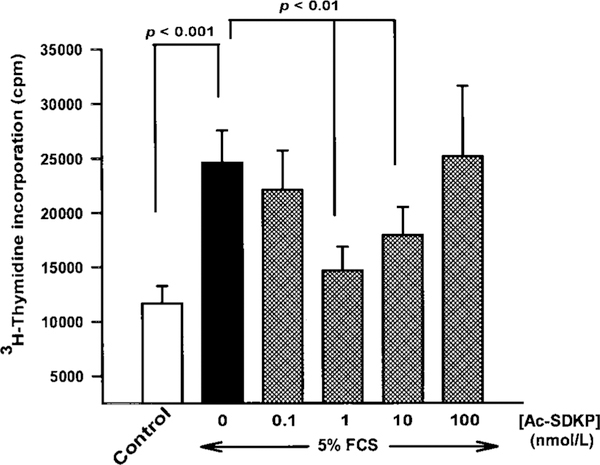
FCS induced a 100% increase in 3H-thymidine incorporation by rat cardiac fibroblasts (Figure 1). Ac-SDKP inhibited DNA synthesis in a biphasic and dose-dependent fashion. Thymidine incorporation was blunted in fibroblasts pretreated with Ac-SDKP, with maximum inhibition occurring at 1 nmol/L; however, at doses higher than 1 nmol/L, inhibition was diminished. A dose of 1 μmol/L had no apparent inhibitory effect.
Figure 1.
Effect of Ac-SDKP on 3H-thymidine incorporation by adult cardiac fibroblasts stimulated with 5% FCS. Ac-SDKP at 1 nmol/L had the greatest inhibitory effect. As the concentration of Ac-SDKP increased, the inhibitory effect diminished. Each column represents mean±SEM of 5 to 18 observations.
Effect of Ac-SDKP on Collagen Synthesis by Cardiac Fibroblasts In Vitro
Hydroxyproline Assay
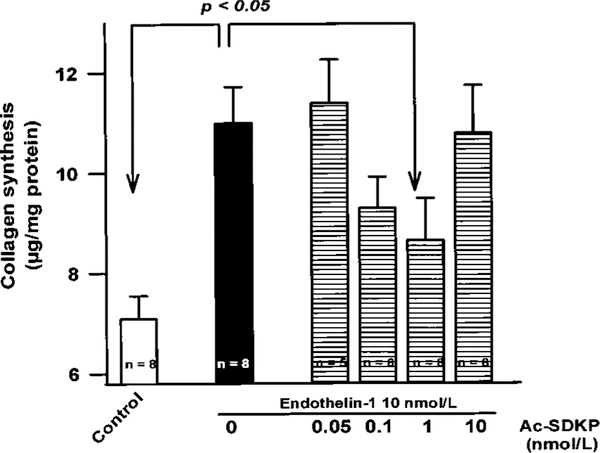
Collagen synthesis was measured in confluent adult rat cardiac fibroblasts (passage 2) incubated with ET-1 (10−8 mol/L) either alone or combined with different concentrations of Ac-SDKP and expressed as micrograms of collagen per total fibroblast proteins (mg) (Figure 2). ET-1 significantly enhanced collagen production from 7.1±0.5 (control) to 10.9±0.7 μg/mg. Ac-SDKP blocked ET-1–stimulated collagen production in a biphasic and dose-dependent manner, with maximum inhibition at 1 nmol/L; 10 nmol/L failed to prevent collagen production.
Figure 2.
Effect of Ac-SDKP on collagen synthesis by quiescent cardiac fibroblasts incubated with 10−8 mol/L ET-1. The maximum inhibitory effect of Ac-SDKP was obtained at 1 nmol/L. Each column represents mean±SEM of 5 to 8 observations.
3H-Proline Incorporation
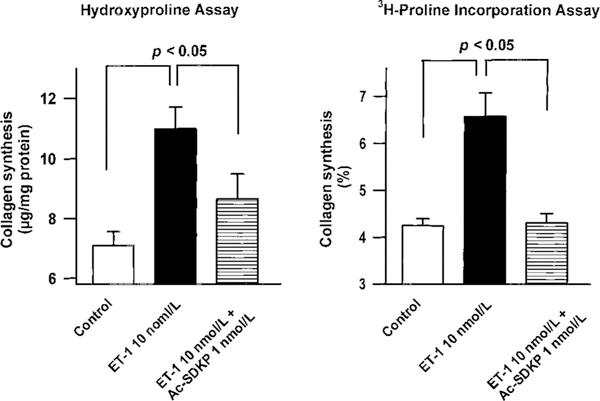
We examined the effect of Ac-SDKP on collagen synthesis stimulated with ET-1 (10−8 mol/L), measuring the incorporation of 3H-proline by collagen protein. Increased collagen synthesis induced by ET-1 (10−8 mol/L) was abolished in the presence of Ac-SDKP (1 nmol/L) (Figure 3, right). When some of the data from Figure 2 were used to compare the 2 methods of estimating collagen synthesis, we found that both 3H-proline incorporation and hydroxyproline assay gave very similar results (Figure 3, left).
Figure 3.
Effect of Ac-SDKP on collagen synthesis by quiescent cardiac fibroblasts incubated with 10−8 mol/L ET-1. Collagen synthesis was estimated by hydroxyproline content (left) and 3H-proline incorporation into collagenase-sensitive proteins (right). Both methods showed that ET-1 significantly enhances collagen synthesis, which was blunted by concomitant addition of Ac-SDKP (1 nmol/L). Each column represents mean±SEM of 6 to 8 in each group.
Effect of Ac-SDKP on p42/44 Kinase Activity in FCS-Stimulated Adult Rat Cardiac Fibroblasts
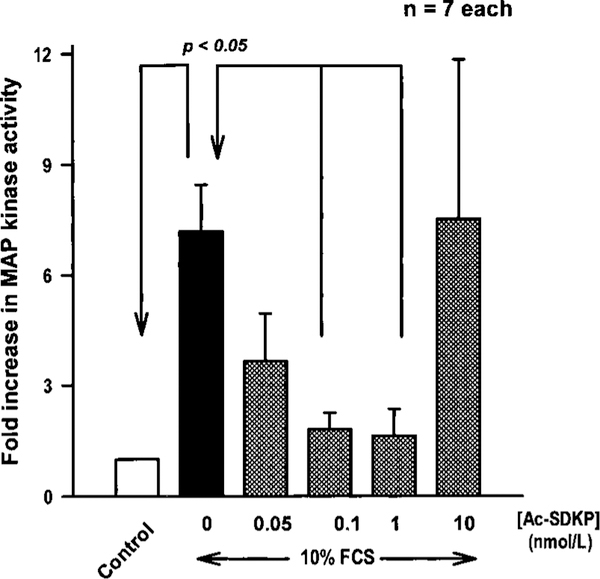
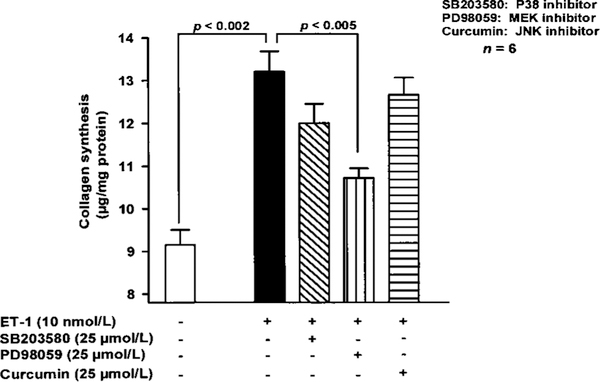
FCS at 10% increased p42/p44 kinase activity by 7-fold compared with untreated cells (P<0.01). Ac-SDKP did not affect total MAPK protein but consistently inhibited MAPK activity in a biphasic and dose-dependent fashion; 10 nmol/L had no inhibitory effect (Figure 4). PD98059, an inhibitor of p42/p44 MAPK activation, significantly decreased ET-1–stimulated collagen production, whereas SB203580 and curcumin, which inhibit p38 and JNK, respectively, were inactive (Figure 5).
Figure 4.
Effect of Ac-SDKP on MAPK activation in cardiac fibroblasts incubated with 10% FCS. Fibroblasts were incubated with different doses of Ac-SDKP for 30 minutes before stimulation with FCS for 5 minutes. MAPK activity was associated with phosphorylation of Elk-1 at Ser383, which was assayed by 10% SDS-PAGE followed by immunoblotting with phospho-Elk-1 antibody. Phosphorylation of Elk-1 was quantified and plotted as the fold increase compared with control. Each column represents mean±SEM.
Figure 5.
Effect of (1) PD98059, an inhibitor of p42/p44 MAPK activation; (2) SB203580, a p38 inhibitor; and (3) curcumin, a JNK inhibitor, on ET-1–induced collagen synthesis by cardiac fibroblasts. Only PD98059 significantly inhibited collagen synthesis. Each column represents mean±SEM.
Discussion
Fibroblasts constitute the vast majority (>90%) of nonmyocyte cells in the heart.34 Cardiac fibroblasts increase the production of fibronectin and collagen when the heart is exposed to a variety of injuries, such as myocardial infarction, pressure overload, and myocarditis. It is believed that an increase in both the number of cardiac fibroblasts and the content of extracellular matrix proteins during cardiac remodeling is one of the major causes of cardiac dysfunction.14,35,36 The cardioprotective effects of ACEIs in myocardial infarction, pressure overload, and myocarditis associated with left ventricular hypertrophy and fibrosis are due in part to inhibition of Ang II formation.14,37,38 However, other mechanisms such as increased plasma and/or tissue kinins and Ac-SDKP10,14,36,38,39 may also be involved. Our data show that Ac-SDKP inhibits (1) serum-stimulated 3H-thymidine incorporation, (2) ET-1–induced collagen synthesis, and (3) p44/p42 MAPK activation by serum in cultured rat adult cardiac fibroblasts. The biphasic and dose-dependent inhibitory effect of Ac-SDKP we observed was very similar to the pharmacological profile previously reported for human bone marrow mononuclear cells (CD34+)4 and human and chicken lymphocytes,40 occurring at low concentrations and disappearing at higher concentrations. Although we could not explain this biphasic effect, such a bell-shaped curve might suggest that Ac-SDKP activates multiple receptors or enzymes, with each of them leading to either inhibition or stimulation.
Ang II is one of several agents that stimulate synthesis and secretion of ET via the activation of AT1 receptors. Ang II has been shown to increase expression of preproET-1 mRNA in cultured ventricular myocytes,18 suggesting that ET-1 might be an important mediator of Ang II in cardiac hypertrophy and fibrosis in animals or patients with Ang II–dependent hypertension. ET-1 is a potent stimulator of collagen synthesis in cultured fibroblasts, with a maximum effect at 10−7 mol/L.20 Ac-SDKP maximally inhibited ET-1–induced collagen synthesis at the same dose that blocked DNA synthesis in fibroblasts, but at present the mechanisms of the inhibitory effects of Ac-SDKP are unknown. Two possible causes are (a) inhibition of enzymes responsible for collagen synthesis and maturation and (2) activation of metalloproteinases such as MMP-1. It is unlikely that collagen synthesis would depend on cell proliferation, because we used confluent adult rat cardiac fibroblasts, which form a typical monolayer cell culture, are contact-inhibited, and do not grow in multiple layers like vascular smooth muscle cells or other cell types.22 In addition, Ac-SDKP does not seem to have a cytotoxic effect on fibroblasts, because cell number was not reduced and the medium did not become turbulent when Ac-SDKP alone was incubated with quiescent fibroblasts (Rhaleb et al, unpublished observations). For this reason, we expressed collagen synthesis as micrograms of collagen per milligram of fibroblast proteins.
The p42/p44, JNK, and p38 MAPK pathways are distinct serine-threonine kinase cascades, each consisting of 3 enzymes: MAPK kinase kinase (MAPKKK), MAPK kinases (MEK, MKK), and MAPK. Upstream activators of the MAPK pathways include small GTPases of the Ras family, and downstream effectors include transcription factors and other kinases.41,42,43 p42/p44 MAPK activity has been extensively studied in rat cardiac fibroblasts,44,45 and stimulation of fibroblasts with Ang II or platelet-derived growth factor is known to stimulate MAPK activity, fibroblast proliferation, and extracellular matrix formation.46,47 ET-1 has been shown to activate p42/p44 MAPK in cardiac myocytes (reviewed in Clerk and Sugden)48 and rat cardiac fibroblasts (Rhaleb et al, unpublished observations). Treatment of cardiac fibroblasts with serum activated p42/44 MAPK, and this effect was ameliorated by pretreatment with Ac-SDKP, which suggests that the MAPK pathway may mediate proliferation and collagen accumulation in cultured fibroblasts. However, inhibition of MAPK activity occurred at a 10-fold lower dose of Ac-SDKP than that needed for inhibition of cell proliferation and collagen synthesis, suggesting that p42/p44 MAPK is not the only pathway involved in these processes. Inhibition of p38 MAPK and JNK had no effect on ET-stimulated collagen synthesis. Other signaling pathways known to be activated by ET include protein kinase C, phosphatidylinositol-3 kinase, protein kinase B, and the nonreceptor tyrosine kinase Src.48 Thus, it is possible that serum-stimulated proliferation and ET-stimulated collagen synthesis utilize one or more of these other kinases, making them potential targets for the inhibitory effect of Ac-SDKP.
In summary, Ac-SDKP inhibits cardiac fibroblast proliferation, collagen synthesis, and activation of p42/p44 MAPK activity. Considering that inhibition of ACE substantially increases plasma Ac-SDKP levels,7,10 our study suggests that Ac-SDKP may be considered a new endogenous peptide by which ACEIs prevent or regress cardiac fibrosis in hypertension.
Acknowledgments
This study was supported by American Heart Association Grant-in-Aid 29GS978 and NIH HL-2898219.
References
- 1.Grillon C, Reiger K, Bakala J, Schott D, Morgat JL, Hannappel E, Voelter W, Lenfant M. Involvement of thymosin β4 and endoproteinase asp-N in the biosynthesis of the tetrapeptide Ac-Ser-Asp-Lys-Pro, a regulator of the haematopoietic system. FEBS Lett. 1990;274:30–34. [DOI] [PubMed] [Google Scholar]
- 2.Wdzieczak-Bakala J, Fache MP, Lenfant M, Frindel E, Sainteny F.Ac-SDKP, an inhibitor of CFU-S proliferation, is synthesized in mice under steady-state conditions and secreted by bone marrow in long term culture. Leukemia. 1990;4:235–237. [PubMed] [Google Scholar]
- 3.Lenfant M, Wdzieczak-Bakala J, Guittet E, Prome JC, Sotty D, Frindel E. Inhibitor of hematopoietic pluripotent stem cell proliferation: purification and determination of its structure. Proc Natl Acad Sci U S A. 1989;86: 779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet D, Lemoine FM, Pontvert-Delucq S, Baillou C, Najman A,Guigon M. Direct and reversible inhibitory effect of the tetrapeptide acetyl-N-Ser-Asp-Lys-Pro (seraspenide) on the growth of human CD34+ subpopulation in response to growth factors. Blood. 1993;82:3307–3314. [PubMed] [Google Scholar]
- 5.Pradelles P, Frobert Y, Créminon C, Liozon E, Massé A, Frindel E. Negative regulator of pluripotent hematopoietic stem cell proliferation in human white blood cells and plasma as analyzed by enzyme immunoassay. Biochem Biophys Res Commun. 1990;170:986–993. [DOI] [PubMed] [Google Scholar]
- 6.Pradelles P, Frobert Y, Créminon C, Ivonine H, Frindel E. Distribution of a negative regulator of haematopoietic stem cell proliferation (AcSDKP) and thymosin β4 in mouse tissues. FEBS Lett. 1991;289:171–175. [DOI] [PubMed] [Google Scholar]
- 7.Azizi M, Rousseau A, Ezan E, Guyene T-T, Michelet S, Grognet J-M, Lenfant M, Corvol P, Ménard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-ser-aspartyl-lysyl-proline. J Clin Invest. 1996;97:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rousseau A, Michaud A, Chauvet M-T, Lenfant M, Corvol P. Thehemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensinconverting enzyme. J Biol Chem. 1995;270:3656–3661. [DOI] [PubMed] [Google Scholar]
- 9.Erdös EG. News about ACE, or, the separate lives of “Siamese twin”domains. J Clin Invest. 1996;97:588 Editorial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azizi M, Ezan E, Nicolet L, Grognet JM, Ménard J. High plasma level of N-acetyl-seryl-aspartyl-proline: a new marker of chronic angiotensin-converting enzyme inhibition. Hypertension. 1997;30:1015–1019. [DOI] [PubMed] [Google Scholar]
- 11.Lombard M-N, Sotty D, Wdzieczak-Bakala J, Lenfant M. In vivo effect of the tetrapeptide, N-acetyl-Ser-Asp-Lys-Pro, on the G1-S transition of rat hepatocytes. Cell Tissue Kinet. 1990;23:99–103. [DOI] [PubMed] [Google Scholar]
- 12.Yoshioka T, Iwamoto N, Nitta K, Irie K, Xiao HJ, Ito K, Muraki T. AcSDKP is a novel anti-proliferative peptide for renal fibroblasts. Naunyn-Schmiedebergs Arch Pharmacol. 1998;358:R742. Abstract. [Google Scholar]
- 13.Brilla CG, Matsubara L, Weber KT. Advanced hypertensive heart disease in spontaneously hypertensive rats: lisinopril-mediated regression of myocardial fibrosis. Hypertension. 1996;28:269–275. [DOI] [PubMed] [Google Scholar]
- 14.Liu YH, Yang XP, Sharov VG, Nass O, Sabbah HN, Peterson E, Carretero OA. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. J Clin Invest. 1997;26:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagès G, Lenormand P, L’Allemain G, Chambard JC, Meloche S, Pouysségur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci U S A. 1993;90: 8319–8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schorb W, Conrad KM, Singer HA, Dostal DE, Baker KM. Angiotensin II is a potent stimulator of MAP-kinase activity in neonatal cardiac fibroblasts. J Mol Cell Cardiol. 1995;27:1151–1160. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Hiroi Y, Mizuno T, Maemura K, Kurihara H, Aikawa R, Takano H, Yazaki Y. Endothelin-1 is involved in mechanical stress-induced cardiomyocyte hypertrophy. J Biol Chem. 1996;271:3221–3228. [DOI] [PubMed] [Google Scholar]
- 18.Ito H, Adachi S, Tamamori M, Fujisaki H, Tanaka M, Lin M, Akimoto H, Marumo F, Hiroe M. Mild hypoxia induces hypertrophy of cultured neonatal rat cardiomyocytes: a possible endogenous endothelin-1mediated mechanism. J Mol Cell Cardiol. 1996;28:1271–1277. [DOI] [PubMed] [Google Scholar]
- 19.Ito H, Hirata Y, Adachi S, Tanaka M, Tsujino M, Koike A, Nogami A, Marumo F, Hiroe M. Endothelin-1 is an autocrine/paracrine factor in the mechanism of angiotensin II-induced hypertrophy in cultured rat cardiomyocytes. J Clin Invest. 1993;92:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guarda E, Katwa LC, Myers PR, Tyagi SC, Weber KT. Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc Res. 1993;27:2130–2134. [DOI] [PubMed] [Google Scholar]
- 21.Eghbali M, Tomek R, Sukhatme VP, Woods C, Bhambi B. Differential effects of transforming growth factor-β1 and phorbol myristate acetate on cardiac fibroblasts: regulation of fibrillar collagen mRNAs and expression of early transcription factors. Circ Res. 1991;69:483–490. [DOI] [PubMed] [Google Scholar]
- 22.Brilla CG, Zhou G, Matsubara L, Weber KT. Collagen metabolism in cultured rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol. 1994;26:809–820. [DOI] [PubMed] [Google Scholar]
- 23.Pönicke K, Heinroth-Hoffmann I, Becker K, Brodde OE. Trophic effect of angiotensin II in neonatal rat cardiomyocytes: role of endothelin-1 and non-myocyte cells. Br J Pharmacol. 1997;121:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajagopalan S, Laursen JB, Borthayre A, Kurz S, Keiser J, Haleen S, Giaid A, Harrison DG. Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension. 1997;30:29–34. [DOI] [PubMed] [Google Scholar]
- 25.Herizi A, Jover B, Bouriquet N, Mimran A. Prevention of the cardiovascular and renal effects of angiotensin II by endothelin blockade. Hypertension. 1998;31:10–14. [DOI] [PubMed] [Google Scholar]
- 26.Hocher B, George I, Rebstock J, Bauch A, Schwartz A, Neumeyer H-H, Bauer C. Endothelin system–dependent cardiac remodeling in renovascular hypertension. Hypertension. 1999;33:816–822. [DOI] [PubMed] [Google Scholar]
- 27.Agocha A, Lee HW, Eghbali-Webb M. Hypoxia regulates basal and induced DNA synthesis and collagen type I production in human cardiac fibroblasts: effects of transforming growth factor-beta1, thyroid hormone, angiotensin II and basic fibroblast growth factor. J Mol Cell Cardiol. 1997;9:2233–2244. [DOI] [PubMed] [Google Scholar]
- 28.Pathak M, Sil P, Young D, Sen S, Angiotensin II-stimulated collagen production influenced by fibroblast crosstalk. Circulation. 1998;98(suppl I):I-624 Abstract. [Google Scholar]
- 29.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys. 1961;93:440–447. [DOI] [PubMed] [Google Scholar]
- 30.Villarreal FJ, Kim NN, Ungab GD, Printz MP, Dillmann WH. Identification of functional angiotensin II receptor on cardiac fibroblasts. Circulation. 1993;88:2849–2861. [DOI] [PubMed] [Google Scholar]
- 31.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. [DOI] [PubMed] [Google Scholar]
- 32.Chiariello M, Ambrosio G, Cappelli-Bigazzi M, Perrone-Filardi P, Brigante F, Sifola C. A biochemical method for the quantitation of myocardial scarring after experimental coronary artery occlusion. J Mol Cell Cardiol. 1986;18:283–290. [DOI] [PubMed] [Google Scholar]
- 33.Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE. ERK phosphorylation potentiates Elk-1 mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eghbali M Cardiac fibroblasts: function of gene expression, and phenotypic modulation. Basic Res Cardiol. 1992;87(suppl 2):183–189. [DOI] [PubMed] [Google Scholar]
- 35.Brilla CG, Maisch B. Regulation of the structural remodeling of the myocardium: from hypertrophy to heart failure. Eur Heart J. 1994;15: 45–52. [DOI] [PubMed] [Google Scholar]
- 36.Liu YH, Yang XP, Sharov VG, Sigmon DH, Sabbah HN, Carretero OA. Paracrine systems in the cardioprotective effect of angiotensin-converting enzyme inhibitors on myocardial ischemia/reperfusion injury in rats. Hypertension. 1996;27:7–13. [DOI] [PubMed] [Google Scholar]
- 37.Brooks WW, Bing OHL, Conrad CH, O’Neill L, Crow MT, Lakatta EG, Dostal DE, Baker KM, Boluyt MO. Captopril modifies gene expression in hypertrophied and failing hearts of aged spontaneously hypertensive rats. Hypertension. 1997;30:1362–1368. [DOI] [PubMed] [Google Scholar]
- 38.Araki M, Kanda T, Imai S, Suzuki T, Murata K, Kobayashi I. Comparative effects of losartan, captopril, and enalapril on murine acute myocarditis due to encephalomyocarditis. J Cardiovasc Pharmacol. 1995;26:61–65. [DOI] [PubMed] [Google Scholar]
- 39.Duncan AM, James GM, Anastasopoulos F, Kladis A, Briscoe TA, Campbell DJ. Interaction between neutral endopeptidase and angiotensin converting enzyme inhibition in rats with myocardial infarction: effects on cardiac hypertrophy and angiotensin and bradykinin peptide levels. J Pharmacol Exp Ther. 1999;289:295–303. [PubMed] [Google Scholar]
- 40.Volkov L, Quere P, Coudert F, Comte L, Praloran V. The tetrapeptide AcSDKP, a physiological inhibitor of normal cell proliferation, reduces the S phase entry of continuous cell lines. Exp Cell Res. 1996;223: 112–116. [DOI] [PubMed] [Google Scholar]
- 41.Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioassays. 1996;18:567–577. [DOI] [PubMed] [Google Scholar]
- 42.Fanger GR, Gerwins P, Widmann C, Jarpe MB, Johnson GL. MEKKs, GCKs, MLKs, PAKs, TAKs, and Tpls: upstream regulators of the c-Jun amino-terminal kinase. Curr Opin Genet Dev. 1997;7:67–74. [DOI] [PubMed] [Google Scholar]
- 43.LaPointe MC, Isenovič E. Interleukin-1b regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p42/44 and p38 MAPK signaling pathways in cardiac myocytes. Hypertension. 1999;33: 276–282. [DOI] [PubMed] [Google Scholar]
- 44.Thorburn J, Frost JA, Thorburn A. Mitogen-activated protein kinases mediate changes in gene expression, but not cytoskeletal organization associated with cardiac muscle cell hypertrophy. J Cell Biol. 1994;126: 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bogoyevitch MA, Glennon PE, Andersson MB, Clerk A, Lazou A, Marshall CJ, Parker PJ, Sugden PH. Endothelin-1 and fibroblast growth factors stimulate the mitogen-activated protein kinase signaling cascade in cardiac myocytes: the potential role of the cascade in the integration of two signaling pathways leading to myocyte hypertrophy. J Biol Chem. 1994;269:1110–1119. [PubMed] [Google Scholar]
- 46.Zou Y, Komuro I, Yamazaki T, Kudoh S, Aikawa R, Zhu W, Shiojima I,Hiro Y, Tobe K, Kadowaki T, Yazaki Y. Cell type-specific angiotensin II-evoked signal transduction: critical roles of Gβγ subunit, Src family, and Ras in cardiac fibroblasts. Circ Res. 1998;82:337–345. [DOI] [PubMed] [Google Scholar]
- 47.Moriguchi Y, Matsubara H, Mori Y, Murasawa S, Masaki H, Maruyama K, Tsutsumi Y, Shibasaki Y, Tanaka Y, Nakajima T, Oda K, Iwasaka T. Angiotensin II-induced transactivation of epidermal growth factor receptor regulates fibronectin and transforming growth factor-β synthesis via transcriptional and posttranscriptional mechanisms. Circ Res. 1999; 84:1073–1084. [DOI] [PubMed] [Google Scholar]
- 48.Clerk A, Sugden PH. Activation of protein kinase cascades in the heart by hypertrophic G protein-coupled receptor agonists. Am J Cardiol. 1999; 83:64H–69H. [DOI] [PubMed] [Google Scholar]