Abstract
Pressure-overload left ventricular hypertrophy (LVH) is characterized by an increase in myocyte size and fibrosis. However, it is not clear how each of these components affects hypertensive heart disease (HHD). We have shown in 2 different rat models of hypertension that cardiac fibrosis can be reduced with N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP), an antifibrotic peptide normally present in mammals. To assess how inhibition of fibrosis affects HHD, spontaneously hypertensive rats (SHR) and normotensive controls (WKY) were treated with Ac-SDKP or vehicle. Cardiac systolic and diastolic function were assessed using in vivo pressure-volume (PV) analysis. Left ventricle passive compliance was also determined ex vivo. We found that in SHR, Ac-SDKP normalized left ventricle total collagen content and interstitial collagen fraction without changing myocyte diameter or left ventricle mass. In WKY, collagen did not change significantly after treatment. Ac-SDKP did not affect left ventricle diastolic function, determined in vivo and ex vivo in SHR and WKY, whereas systolic function was significantly decreased in SHR treated with Ac-SDKP and unchanged in treated WKY. We concluded that in adult SHR, reducing left ventricle collagen deposition with Ac-SDKP does not improve diastolic function, whereas it decreases systolic performance. These findings suggest that total left ventricle collagen reduction per se does not necessarily benefit cardiac function. In HHD, other factors besides collagen quantity, such as myocyte hypertrophy and/or collagen type or cross-link, might be targeted to improve cardiac function.
Keywords: hypertension, rats, spontaneously hypertensive
Left ventricular hypertrophy (LVH) associated with hypertension is an independent cardiovascular risk factor that, if left untreated, will lead to heart failure (HF).1–3 Transition from LVH to HF is characterized by increases in myocyte size and interstitial fibrillar collagen. In addition, changes in the expression of contractile proteins from mature to fetal isoforms may interfere with normal cardiovascular performance.4,5 Many LVH-targeted treatments modify myocyte contractile protein synthesis and fibrosis while lowering arterial blood pressure (BP), making it difficult to elucidate the specific role played by each of these components in the development of HF.6–8
In the heart, fibrosis, composed mainly of fibrillar types I and III collagen, forms a delicate sheath that interconnects bundles of contractile units. Although the excessive cardiac collagen accumulation that develops in hypertension has been implicated in the pathogenesis of hypertensive heart disease (HHD), published data disagree regarding the impact of total collagen reduction on heart function. Kim et al showed that prolonged overexpression of cardiac matrix metalloproteinase-1 in mice led to loss of interstitial collagen with deterioration of contractile function.9 Other investigators also reported that in cats with pressure-overload LVH, acute regression of fibrosis decreased systolic performance without altering isolated myocyte contractility, suggesting that collagen deposition could play an important role in optimizing myocardial systolic performance.10 However, several studies have shown that regression of fibrosis ameliorates systolic and/or diastolic dysfunction.11,12 Some of these studies have involved drugs commonly used in the clinical arena, such as angiotensin-converting enzyme inhibitors, which modify the expression of contractile and noncontractile elements as well, reducing ventricular mass and/or BP in addition to collagen reduction.8,13 In the present study, we tested the hypothesis that reduction of total cardiac collagen accumulation without changing either myocyte hypertrophy or BP will negatively affect cardiac function in spontaneously hypertensive rats (SHR), a model that in certain ways resembles hypertension in humans.14 To this end, we studied in vivo left ventricle (LV) function with a micromanometer-conductance catheter system at different preloads. To prevent collagen accumulation in the heart, we administered N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP),15 which is endogenously released from its protein precursor thymosin β-4 and inhibits entry of pluripotent stem cells into the S phase, maintaining them in the G0/G1 phase. This tetrapeptide is normally present in mammalian plasma and in tissue where angiotensin-converting enzyme is present and is hydrolyzed almost exclusively by angiotensin-converting enzyme.16 Long-term or short-term administration of the angiotensin-converting enzyme inhibitor captopril prevented degradation of endogenous Ac-SDKP and raised its circulating concentration 5-fold. We have previously demonstrated that Ac-SDKP reduced LV fibrosis in 2 different rat models of hypertension,17,18 suggesting that this peptide could partially mediate the anti-fibrotic effects of angiotensin-converting enzyme inhibitors. Interestingly, these effects were achieved without altering either BP or ventricular mass, making it an ideal compound to test our hypothesis.
Methods
Animals and Procedures
The 9-month-old SHR and normotensive controls (WKY) were acquired from Taconic Farms (Germantown, NY) and maintained at a constant room temperature (22°C) and 12-hour light/dark cycle. We decided to test our hypothesis in rats of this age because they exhibit a marked difference in LV collagen content compared with WKY, and we have previously found that at this age, SHR have diastolic dysfunction with supranormal systolic performance,19 probably reflecting a pre-HF stage.
All animals were given standard rat chow and water ad libitum. After remaining in the animal facility for 1 week, BP was measured by tail-cuff once per week for 3 weeks and every 2 weeks thereafter until the end of the protocol. At age 10 months, SHR were randomized to either Ac-SDKP (800 μg/kg per day; obtained from Drs Domenico Regoli and Witold Neugebauer, University of Sherbrooke, Canada) diluted in 0.01 N acetic acid (Ac-SDKP; n=10) or 0.01 N acetic acid alone (vehicle; n=11). This dose was selected based on previous experience from our laboratory.18
Drugs were administered via osmotic minipumps (Alzet) implanted subcutaneously under anesthesia (methohexital 50 mg/kg IP) and replaced every 4 weeks. After 4 months, when they were 14 months old, rats were anesthetized with the long-lasting agent thiobutabarbital (Inactin, 100 mg/kg IP). Additional boluses of 10 mg/kg were administered if necessary to stabilize anesthesia, pinching the tail with a forceps until no movement was observed. The abdomen, chest, and neck were shaved and in vivo cardiac pressure-volume (PV) loops and ex vivo PV relationships were obtained as described. After the experiments, hearts were dissected, weighed, and preserved for determination of hydroxyproline, interstitial collagen volume fraction (CVF), and myocyte cross-sectional area (MCSA). 10-month-old WKY (n=6) and SHR (n=7) were also euthanized, and their hearts were removed, weighed, and preserved for baseline measurements.
In Vivo PV Loops
Rats were placed on a warm pad (37°C) and ventilated. A 2-French miniaturized combined conductance catheter-micromanometer (model SPR-819; Millar Instruments, Houston, Tex) connected to a pressure-conductance unit (MPCU-200; Millar) was advanced into the LV as described previously.19 PV loops were recorded at baseline and during unloading by gently occluding the inferior vena cava with a cotton swab. Time–varying volume was obtained as described previously20,21 according to the formula:
where V indicates volume; Gtot, total conductance; Gp, parallel conductance of surrounding structures; L, distance between catheter electrodes (9 mm); and ρ, conductivity of blood.
Volume was calibrated as described previously, using simultaneous Doppler echocardiography (Sequoia C 256 with a 15-MHz transducer; Acuson). Parallel conductance from surrounding structures was calculated by the hypertonic saline method as described previously.19,22
Data were analyzed using Millar conductance data acquisition and analysis software. The following parameters were calculated: end-diastolic PV relationship (EDPVR) and the time constant of LV pressure decay, tau (τ), as determinants of end-diastolic stiffness and relaxation, respectively; maximum dP/dt/instant developed pressure ( + dP/dt/iP) as an index of isovolumic contraction; and preload recruitable stroke work as a determinant of LV systolic performance.
Ex Vivo PVR
Immediately after terminating each in vivo experiment, hearts were stopped with 15% KCl and removed. A PE-50 tube connected to a pressure transducer (Gould Instruments, Cleveland, Ohio) was passed through the aorta into the LV. The atrioventricular groove was sealed with 4–0 silk. A 23-gauge needle was inserted into the LV apex and saline solution (0.9% NaCl) infused through it at a constant rate (675 μL/min) after aspirating the LV to generate a negative pressure of −5 mm Hg as described by Raya et al.23 The RV was incised to avoid fluid accumulation. At least 2 PV curves (from −5 to 35 mm Hg) were obtained from each heart within 10 minutes after arrest. Volumes were corrected for chamber volume at 0 mm Hg pressure.
Hydroxyproline Assay, CVF, and MCSA Analysis
Each LV was weighed and sectioned into 3 slices. The slices were rapidly frozen in isopentane precooled in liquid nitrogen and stored at −70°C. One LV sample was stored at −20°C until the hydroxyproline assay.
Collagen content of myocardial tissue was determined by hydroxyproline assay as described elsewhere.18 Briefly, samples were freeze-dried, weighed, and pulverized. Each sample was homogenized in 0.1 mol/L NaCl and 5 mmol/L NaHCO3, washed with the same solution 5 times, and hydrolyzed in 1 mL 6N HCl for 16 hours at 110°C. Samples were filtered, vacuum-dried, and dissolved in distilled water. Hydroxyproline was measured using a colorimetric assay.17 Data were expressed as micrograms of collagen per milligram of dry weight, assuming that collagen contains an average of 13.5% hydroxyproline.
Sections 6-μm thick were cut from each frozen slice and stained separately with: (1) fluorescein-labeled peanut agglutinin (Vector Laboratories) after pretreatment with 3.3 U/mL neuroaminidase type V (Sigma) to delineate myocyte cross-sectional area (as an indicator of myocyte volume) and the interstitial space (consisting of collagen and capillaries); and (2) rhodamine-labeled Griffonia simplicifolia lectin I to show only the capillaries, because it selectively binds to capillaries. Three radially oriented microscopic fields were selected at random from each section and photographed using a fluorescence microscope (Nikon) equipped with a spot digital camera (X100; Diagnostic Instruments). Each field contained ≥100 myocytes. Total surface area, MCSA, interstitial space (collagen plus capillaries), and area occupied by capillaries alone were measured by computer-assisted videodensitometry (JAVA; SPSS). Interstitial CVF was calculated as percent surface area occupied by the interstitial space minus percent surface area occupied by capillaries. CVF and MCSA were averaged using data from all 3 slices.
Statistical Analysis
Results are expressed as mean±SE. Differences between groups were compared by ANOVA followed by Schaffe test, considering P<0.05 as statistically significant.
Results
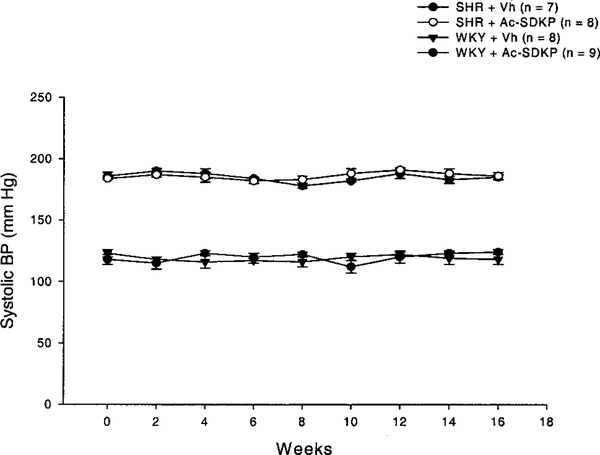
General characteristics of WKY and SHR are shown in Table. At this age, SHR are smaller and exhibit LVH and delayed LV relaxation compared with WKY. Ac-SDKP did not affect heart rate, body weight, left ventricular weight/body weight, left ventricle end-diastolic volume, or MCSA in either strain. Ac-SDKP did not affect systolic BP levels during the 4-month treatment period as shown in Figure 1.
HR, BW, LVW/BW, LVEDV, MCSA, and τ of WKY and SHR at Baseline and After 4 Months of Treatment With Ac-SDKP or Vehicle
| Baseline |
After 4 Months |
|||||
|---|---|---|---|---|---|---|
| Parameters | WKY-10 (n=6) | SHR-10 (n=7) | WKY-14 (Vh) (n=8) | WKY-14 (Ac) (n=9) | SHR-14 (Vh) (n = 7) | SHR-14 (Ac) (n=8) |
| HR | 257 ±12 | 326 ±24* | 315±30 | 340±13 | 392 ±47* | 408± 23* |
| BW (g) | 674±41 | 384 ±14* | 719±31 | 691±17 | 395 ±45* | 427 ±14* |
| LVW/BW (mg/g) | 1.7±0.1 | 2.5±0.1* | 1.54±0.1 | 1.56±0.2 | 2.9±0.1* | 2.8±0.1* |
| LVEDV (μL) | 690 ±56 | 610±31 | 719±45 | 760 ±25 | 650 ±33* | 684 ±19* |
| MCSA (μm2) | 380 ±22 | 480 ±12* | 485±13 | 479±17 | 551 ±27* | 525 ±49* |
| τ (msec) | 12±0.2 | 15±0.3* | 12±0.4 | 11.6±0.7 | 16.3±1.3* | 17.2±0.7* |
HR indicates heart rate; BW, body weight; LVW, left ventricular weight; LVEDV, left ventricle end-diastolic volume; MCSA, myocyte cross-sectional area; t, a determinant of LV end-diastolic relaxation; WKY, Wistar-Kyoto Rats; SHR, spontaneously hypertensive rats; Vh, vehicle; Ac, Ac-SDKP.
P<0.05 vs age-matched WKY. At this age, WKY are larger than SHR. Treatment did not significantly affect hypertrophy, LVDV, or relaxation in either strain.
Figure 1.
Systolic BP measured by tail-cuff during the treatment period. Vh indicates vehicle. Ac-SDKP did not alter BP in either strain.
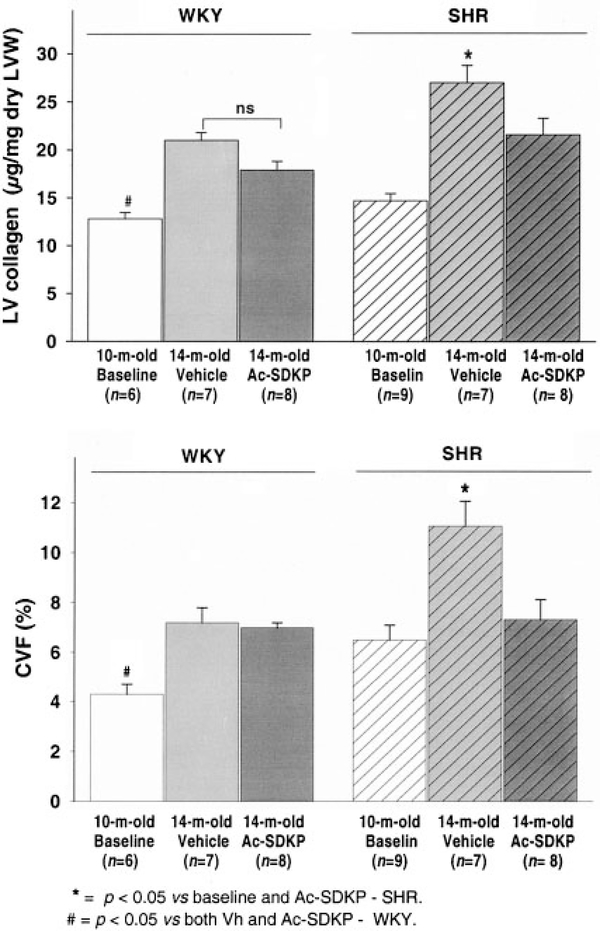
Figure 2 shows LV collagen for WKY and SHR at baseline and after 4 months of either Ac-SDKP or vehicle. In WKY, baseline LV collagen was 12.8±0.66 μg/mg dry weight and it increased to 22±0.8 after 4 months. This increase in LV fibrosis is probably secondary to aging and has already been described in this strain.24 Ac-SDKP tended to decrease collagen in these rats (17.9±1.9 μg/mg dry weight), although this reduction did not reach statistical significance.
Figure 2.
LV total collagen (upper panel) and collagen volume fraction (CVF, lower panel) in WKY receiving vehicle (Vh, light gray bars) or Ac-SDKP (dark gray), as well as SHR treated with Vh (hatched light gray) or Ac-SDKP (hatched dark gray). After 4 months of treatment, Ac-SDKP significantly reduced LV collagen deposition in the hypertensive rats. Baseline 10-month-old WKY and SHR are represented by white and hatched white bars, respectively.
In SHR receiving vehicle, collagen increased from 14.7±0.74 to 27±1.85 μg/mg dry tissue after 4 months. In SHR receiving Ac-SDKP, collagen content was significantly less (21.6±0.23 μg/mg dry tissue, the same as age-matched WKY). CVF was also lower in Ac-SDKP-treated SHR than in the vehicle group (11.08%±1.09% versus 7.34%±0.8%; P<0.05), whereas no significant change was observed after treatment in WKY (7.1%±0.4% versus 6.5%±0.6%; P=NS). There was no difference in capillary density between vehicle-treated and Ac-SDKP-treated groups (1506±174 versus 1390±197 cap/mm2; P=NS).
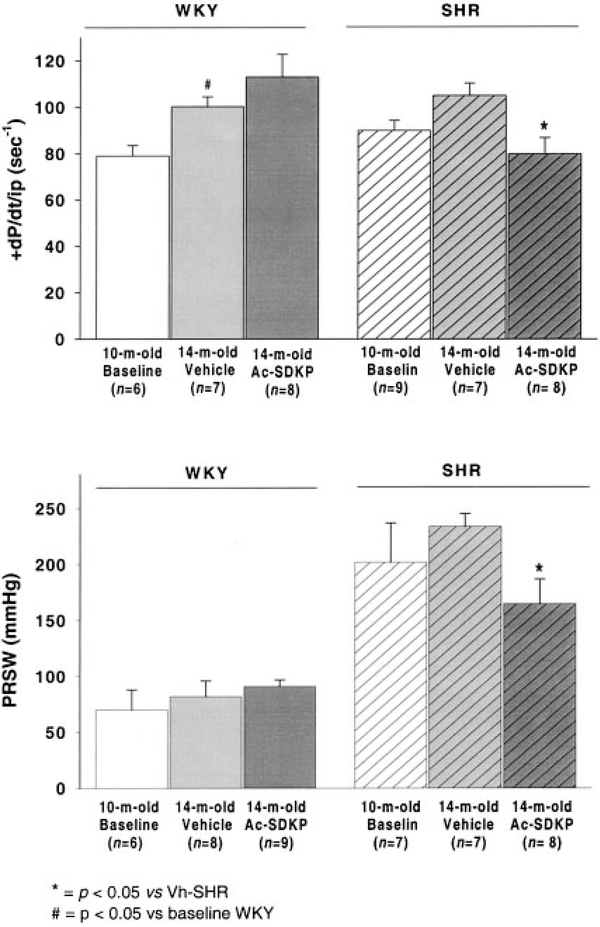
Figure 3 shows systolic function in WKY and SHR at baseline and after 4 months of vehicle or Ac-SDKP. In WKY, +dP/dt/iP, an index of isovolumic contractility, was not affected by Ac-SDKP (100.3 ±4.1 versus 113 ± 9.7 seconds−1; P=ns); however, it was significantly reduced in SHR treated with Ac-SDKP (105.1±5.2 versus 79.9±6.9 seconds−1, vehicle, and Ac-SDKP; P<0.05). Preload recruitable stroke work, a load-independent index of chamber performance, was also significantly decreased in the Ac-SDKP group (234±11.5 versus 165±22 mm Hg; P<0.05) and showed no change after treatment in WKY (82±14 versus 91±6 mmHg, Ac-SDKP, and vehicle; P=ns).
Figure 3.
LV maximum dP/dt/instant developed pressure (+dP/dt/iP, upper panel) and preload recruitable stroke work (PRSW, lower panel) in WKY and SHR at baseline and after 4 months of vehicle or Ac-SDKP. Both systolic function indices were decreased in SHR receiving Ac-SDKP; however, the peptide did not affect these parameters in WKY.
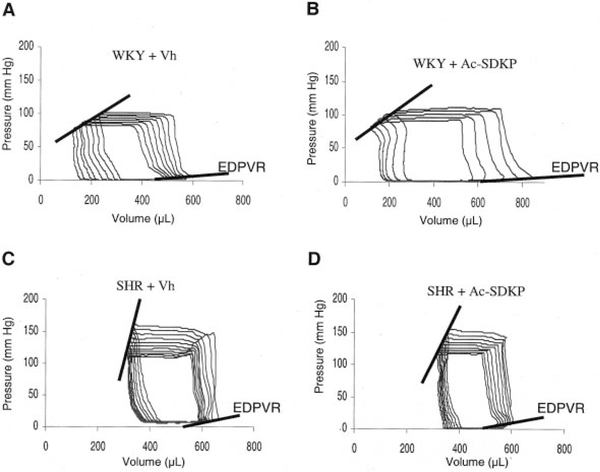
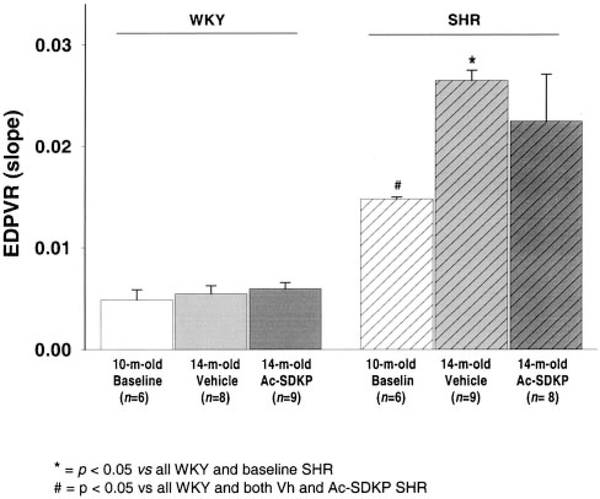
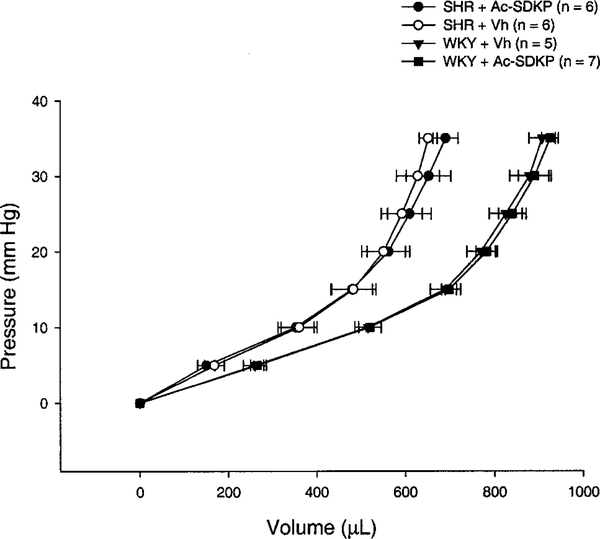
Diastolic function was evaluated in vivo and ex vivo. In the beating heart, Ac-SDKP did not affect relaxation (τ=12±0.4 versus 11.6±0.7 ms for untreated and treated WKY and 16.3±1.3 versus 17.2±0.7 ms for SHR; P=NS, treated versus untreated) (Table). Typical PV loops from vehicle-treated and Ac-SDKP-treated WKY and SHR are shown in Figure 4. Judged by EDPVR, there was no difference in end-diastolic stiffness between vehicle and Ac-SDKP groups. Values for EDPVR (slopes) are shown in Figure 5. The ex vivo LV passive PV curves (Figure 6) again showed no significant difference between untreated and treated rats, indicating that Ac-SDKP did not affect passive stiffness despite the reduction in fibrosis.
Figure 4.
Representative PV loops from 14-month-old WKY receiving vehicle (A), WKY receiving Ac-SDKP (B), SHR treated with vehicle (C), and SHR treated with Ac-SDKP (D). Although the EDPVR is steeper in SHR than in WKY, reflecting increased chamber stiffness in the hypertensive rats, treatment with Ac-SDKP did not change the slope of the EDPVR.
Figure 5.
Overall results of EDPVR for untreated and treated WKY and SHR. Even though Ac-SDKP reduced LV collagen in SHR, end-diastolic stiffness was not affected by the treatment.
Figure 6.
Ex vivo passive LV PV curves in KCl-arrested hearts of vehicle (○) and Ac-SDKP SHR (●) as well as age-matched WKY receiving vehicle (▼) or Ac-SDKP (■). Despite the LV total collagen reduction achieved in SHR receiving Ac-SDKP, curves were similar to nontreated SHR, suggesting that at our experimental pressures, total collagen is not a major determinant of passive stiffness. 0 μL=initial volume reached at 0 mm Hg pressure.
Discussion
We found that long-term treatment with Ac-SDKP normalized total cardiac collagen accumulation in adult SHR without affecting the diastolic dysfunction that characterizes this model.19 Furthermore, systolic function was slightly impaired by this antifibrotic treatment, suggesting that factors other than total LV collagen must be targeted to improve cardiac function. To our knowledge, this is the first report to evaluate the impact of total collagen reduction on cardiac function using pressure-conductance methodology in these animals. This technique allowed us to study systolic and diastolic functions simultaneously at varying preloads in the beating heart.
The pathological LVH that accompanies HHD typically involves increases in myocyte hypertrophy and collagen content. In our study, SHR treated with Ac-SDKP evidenced decreased systolic function, suggesting that in this model reducing total collagen without reducing BP or LV mass may interfere with optimal performance. Although cardiomyocytes are known to be central to the contractile function of the myocardium, the extracellular matrix provides a scaffolding for myocytes and vasculature, maintaining the appropriate geometric structure of the heart25 and proper alignment of myocytes and enabling optimal transduction of the coordinated force generated by cardiac contraction.9 Systolic performance was assessed by PV analysis, a load-independent method. Because there was no change in heart rate between Ac-SDKP and vehicle groups, our results really represent changes in the contractile state of the chamber. These findings are in agreement with Baicu et al, who found that in hypertrophied papillary muscles from cats, acute regression of fibrosis with plasmin through activation of matrix metalloproteinases negatively affected systolic function.10 In Baicu’s study, when contractility was assessed in isolated myocytes exposed to the same treatment, it was no different from nontreated myocytes, suggesting that collagen may play an important role in interaction between myocytes and thereby optimize ventricular systolic function. Alternatively, others have suggested that disruption of the extracellular matrix could interfere with compensatory cellular signaling processes that may occur naturally in LVH, ultimately affecting cardiac function.9 Although we cannot completely exclude the possibility that the effects observed in our study were secondary to mechanisms other than collagen reduction, the fact that Ac-SDKP did not modify systolic function in WKY (in which collagen was not significantly reduced) suggests that in treated SHR, the reduction in systolic performance is caused by reduced LV total collagen and not to a direct effect on cardiac contractility. However, it is important to emphasize that the effects of fibrosis reduction on systolic function may differ during other stages of hypertrophy. The model we studied represents a compensated stage of hypertrophy in which fibrosis may play a crucial role in coordinating the contractile forces of these hypertrophied myocytes. Interestingly, there was no significant difference in volume of the hearts between treated and untreated SHR, suggesting that the reduction in contractility was not accompanied by chamber dilatation.
Diastolic dysfunction is a complex alteration characterized by an abnormal LV filling pattern. Although this condition has been studied for many years, its pathophysiology remains unclear.26 Alterations in diastole can be caused by abnormalities in relaxation, stiffness, or both, although recent evidence shows that the mechanisms involved are far more complex and may include changes in arterial compliance as well.27 Relaxation, an active process, is determined primarily by calcium uptake by the sarcoplasmic reticulum and usually takes place early in diastole. Classically, it is assessed by the time constant of LV isovolumic pressure decay (τ).28 Accurate evaluation of end-diastolic stiffness is more complicated and is better assessed by EDPVR,22 as in the present study. This index represents the slope of the line (or curve) connecting different end-diastolic PV points at varying preloads. Ac-SDKP did not affect end-diastolic stiffness in WKY or SHR compared with nontreated rats. Neither the in vivo EDPVR nor the ex vivo passive PV relationship was different in Ac-SDKP-treated animals compared with vehicle. It is generally accepted that stiffness depends primarily on passive structural components of the myocardium;12,26,29,30 however, some of the studies that led to this conclusion evaluated the impact of fibrosis regression on cardiac function using drugs that targeted not only fibrosis but also myocyte hypertrophy, or assessed diastolic compliance using methods that may not necessarily reflect true in vivo intracavitary mechanics.11,12,31 Our ex vivo PV relationship curves showed no significant difference between SHR receiving vehicle and Ac-SDKP; nevertheless, the curve for the vehicle group was slightly steeper (representing greater stiffness) at pressures >30 mm Hg, suggesting that the chamber could be more compliant in the collagen-targeted treatment group at supranormally higher diastolic pressures. The lower portion of these curves more likely reflects the in vivo EDPVR, in which no changes were evident. Our results are in agreement with other investigators who found no difference in the passive force-sarcomere length relationship between intact rat trabeculae and skinned myofibrils (lacking the extracellular matrix) throughout the normal myocyte operating length.32 A steeper curve was seen in the intact trabecula only when it was overstretched.
The mechanism of Ac-SDKP action remains unknown. In addition to inhibiting stem cells in the bone marrow, it inhibits fibroblast proliferation and therefore collagen production. The dose we used produces a similar amount of Ac-SDKP in plasma to that produced when angiotensin-converting enzyme inhibitors block its breakdown, which is important because this dose should not have any significant effect on hematopoietic precursor cells. Recently, it has been reported that Ac-SDKP inhibits transforming growth factor (TGF)-β1-stimulated phosphorylation of Smad2 in cardiac fibroblasts,33 this being one of the pathways by which this peptide may exert its antifibrotic effects. Kanasaki et al also showed that Ac-SDKP inhibits TGF-β-mediated plasminogen activator inhibitor-1 expression via inhibition of the Smad pathway in human mesangial cells.34
Ac-SDKP may also have other effects, such as inhibiting inflammatory responses. We have previously found that in a rat model of heart failure after myocardial infarction, Ac-SDKP not only reversed cardiac fibrosis but also significantly reduced macrophage infiltration and TGF-β expression.35 Although we did not study the anti-inflammatory effect of Ac-SDKP on the hearts of SHR, this model is not characterized by marked inflammation in the absence of triggers such as myocardial infarction or stroke, making it less likely that this may have affected our results. Another aspect that we explored is the possible effect of Ac-SDKP on neovascularization, which could be important in terms of oxygen supply to the cardiac muscle. In our study, capillary density was unchanged between treated and untreated SHR, suggesting that this was not a confounding factor in our contractility data.
One important limitation of our study is the fact that we did not determine type I/III or cross-linked collagen. It has been suggested that differences in the quality and spatial orientation of collagen more likely to affect diastolic stiffness than is total collagen.36,37 It is possible that other investigators’ opposite results regarding the improved diastolic compliance that accompanies collagen reduction could be because of the specific cross-linked or type I collagen regression they achieved.8,38–40 However, because type I is the predominant collagen phenotype in the LV, it is unlikely that our treatment exclusively targeted other types of collagen. Another limitation of the present work is the fact that we did not evaluate isolated myocyte shortening, which would have been helpful in clarifying whether Ac-SDKP can negatively affect the cellular contractile machinery. However, the fact that the peptide did not modify systolic function indices in normotensive rats strongly suggests that a negative inotropic effect was not responsible for our results.
In summary, treatment of adult SHR with Ac-SDKP normalized LV collagen content without decreasing BP or myocyte hypertrophy. This intervention led to diminished systolic performance without improving diastolic dysfunction.
Perspectives
LVH associated with hypertension is characterized by myocyte hypertrophy and increased interstitial fibrosis. This remodeling of the cardiac chamber is an independent mortality risk factor. Treatment of hypertension must be directed toward adequate control of BP to prevent or reverse target organ damage. Different drugs commonly used in clinical practice have diverse effects on cardiac collagen accumulation and myocyte hypertrophy. Our data suggest that total collagen-targeted treatment per se does not necessarily benefit cardiac function when coexisting BP and/or myocyte hypertrophy are not reduced.
Acknowledgments
This work was supported by National Institutes of Health grants HL-28982 (O.A.C.) and HL-71806 (N.-E.R).
References
- 1.Wachtell K, Palmieri V, Olsen MH, Gerdts E, Papademetriou V, Nieminen MS, Smith G, Dahlöf B, Aurigemma GP, Devereux RB. Change in systolic left ventricular performance after 3 years of antihypertensive treatment. The Losartan Intervention for Endpoint (LIFE) Study. Circulation. 2002;106:227–232. [DOI] [PubMed] [Google Scholar]
- 2.Lip GYH. Regression of left ventricular hypertrophy and improved prognosis. Some HOPE now … or hype? [editorial]. Circulation. 2001; 104:1582–1584. [PubMed] [Google Scholar]
- 3.Liebson PR, Grandits GA, Dianzumba S, Prineas RJ, Grimm RH Jr., Neaton JD, Stamler J, for the Treatment of Hypertension Study Research Group. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995;91:698–706. [DOI] [PubMed] [Google Scholar]
- 4.Boluyt MO, Bing OHL. Matrix gene expression and decompensated heart failure: the aged SHR model. Cardiovasc Res. 2000;46:239–249. [DOI] [PubMed] [Google Scholar]
- 5.Boluyt MO, O’Neill L, Meredith AL, Bing OHL, Brooks WW, Conrad CH, Crow MT, Lakatta EG. Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure. Marked upregulation of genes encoding extracellular matrix components. Circ Res. 1994;75:23–32. [DOI] [PubMed] [Google Scholar]
- 6.Lindholm LH, Ibsen H, Dahlöf B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman J, Snapinn S, for the LIFE study group. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–1010. [DOI] [PubMed] [Google Scholar]
- 7.Mayet J, Ariff B, Wasan B, Chapman N, Shahi M, Poulter NR, Sever PS, Foale RA, Thom SAM. Improvement in midwall myocardial shortening with regression of left ventricular hypertrophy. Hypertension. 2000;34: 755–759. [DOI] [PubMed] [Google Scholar]
- 8.Brilla CG, Janicki JS, Weber KT. Cardioreparative effects of lisinopril in rats with genetic hypertension and left ventricular hypertrophy. Circulation. 1991;83:1771–1779. [DOI] [PubMed] [Google Scholar]
- 9.Kim HE, Dalal SS, Young E, Legato MJ, Weisfeldt ML, D’Armiento J. Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J Clin Invest. 2000;106:857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baicu CF, Stroud JD, Livesay VA, Hapke E, Holder J, Spinale FG, Zile MR. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol. 2003;284:H122–H132. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto K, Masuyama T, Sakata Y, Nishikawa N, Mano T, Yoshida J, Miwa T, Sugawara M, Yamaguchi Y, Ookawara T, Suzuki K, Hori M. Myocardial stiffness is determined by ventricular fibrosis, but not by compensatory or excessive hypertrophy in hypertensive heart. Cardiovasc Res. 2002;55:76–82. [DOI] [PubMed] [Google Scholar]
- 12.Brilla CG, Janicki JS, Weber KT. Impaired diastolic function and coronary reserve in genetic hypertension. Role of interstitial fibrosis and medial thickening of intramyocardial coronary arteries. Circ Res. 1991; 69:107–115. [DOI] [PubMed] [Google Scholar]
- 13.Brooks WW, Bing OHL, Boluyt MO, Malhotra A, Morgan JP, Satoh N, Colucci WS, Conrad CH. Altered inotropic responsiveness and gene expression of hypertrophied myocardium with captopril. Hypertension. 2000;35:1203–1209. [DOI] [PubMed] [Google Scholar]
- 14.Bing OHL, Brooks WW, Robinson KG, Slawsky MT, Hayes JA, Litwin SE, Sen S, Conrad CH. The spontaneously hypertensive rat as a model of the transition from compensated left ventricular hypertrophy to failure. J Mol Cell Cardiol. 1995;27:383–396. [DOI] [PubMed] [Google Scholar]
- 15.Vazeux G, Cotton J, Cuniasse P, Dive V. Potency and selectivity of RXP407 on human, rat, and mouse angiotensin-converting enzyme. Biochem Pharmacol. 2001;61:835–841. [DOI] [PubMed] [Google Scholar]
- 16.Junot C, Gonzales M-F, Ezan E, Cotton J, Vazeux G, Michaud A, Azizi M, Vassiliou S, Yiotakis A, Corvol P, Dive V. RPX 407, a selective inhibitor of the N-domain of angiotensin I-converting enzyme, blocks in vivo the degradation of hemoregulatory peptide acetyl-Ser-Asp-Lys-Pro with no effect on angiotensin I hydrolysis. J Pharmacol Exp Ther. 2001;297:606–611. [PubMed] [Google Scholar]
- 17.Rhaleb N-E, Peng H, Yang X-P, Liu Y-H, Mehta D, Ezan E, Carretero OA. Long-term effect of N-acetyl-seryl-aspartyl-lysyl-proline on left ventricular collagen deposition in rats with 2-kidney, 1-clip hypertension. Circulation. 2001;103:3136–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng H, Carretero OA, Raij L, Yang F, Kapke A, Rhaleb N-E. Anti-fibrotic effects of N-acetyl-seryl-aspartyl-lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension. 2001;37: 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cingolani OH, Yang X-P, Cavasin MA, Carretero OA. Increased systolic performance with diastolic dysfunction in adult spontaneously hypertensive rats. Hypertension. 2003;41:249–254. [DOI] [PubMed] [Google Scholar]
- 20.Georgakopoulos D, Mitzner WA, Chen C-H, Byrne BJ, Millar HD, Hare JM, Kass DA. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol. 1998;274: H1416–H1422. [DOI] [PubMed] [Google Scholar]
- 21.Feldman MD, Erikson JM, Mao Y, Korcarz CE, Lang RM, Freeman GL. Validation of a mouse conductance system to determine LV volume: comparison to echocardiography and crystals. Am J Physiol Heart Circ Physiol. 2000;279:H1698–H1707. [DOI] [PubMed] [Google Scholar]
- 22.Kass DA. Clinical evaluation of left heart function by conductance catheter technique. Eur Heart J. 1992;13(Suppl E):57–64. [DOI] [PubMed] [Google Scholar]
- 23.Raya TE, Gay RG, Lancaster L, Aguirre M, Moffett C, Goldman S. Serial changes in left ventricular relaxation and chamber stiffness after large myocardial infarction in rats. Circulation. 1988;77:1424–1431. [DOI] [PubMed] [Google Scholar]
- 24.Susic D, Varagic J, Frohlich ED. Isolated systolic hypertension in elderly WKY is reversed with L-arginine and ACE inhibition. Hypertension. 2001;38:1422–1426. [DOI] [PubMed] [Google Scholar]
- 25.Weber KT. Cardioreparation in hypertensive heart disease. Hypertension. 2001;38:588–591. [DOI] [PubMed] [Google Scholar]
- 26.Burkhoff D, Maurer MS, Packer M. Heart failure with a normal ejection fraction. Is it really a disorder of diastolic function? [editorial]. Circulation. 2003;107:656–658. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction. Implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. [DOI] [PubMed] [Google Scholar]
- 28.Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest. 1976;58:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimura H, Kubo S, Nishioka A, Imamura K, Kawamura K, Hasegawa M. Left ventricular diastolic function of spontaneously hypertensive rats and its relationship to structural components of the left ventricle. Clin Sci. 1985;69:571–580. [DOI] [PubMed] [Google Scholar]
- 30.Camilión de Hurtado MC, Portiansky EL, Pérez NG, Rebolledo OR, Cingolani HE. Regression of cardiomyocyte hypertrophy in SHR following chronic inhibition of the Na+/H+ exchanger. Cardiovasc Res. 2002;53:862–868. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Bell SP, Trombitas K, Witt CC, Labeit S, LeWinter MM, Granzier H. Changes in titin isoform expression in pacing-induced cardiac failure give rise to increased passive muscle stiffness. Circulation. 2002;106: 1384–1389. [DOI] [PubMed] [Google Scholar]
- 32.Kass DA. Myocardial mechanics In: Poole-Wilson PA, Colucci WS, Massie BM, Chatterjee K, Coats AJS, eds. Heart Failure. Scientific Principles and Clinical Practice. New York: Churchill Livingstone; 1997:87–108. [Google Scholar]
- 33.Pokharel S, Rasoul S, Roks AJM, van Leeuwen REW, van Luyn MJA, Deelman LE, Smits JF, Carretero O, van Gilst WH, Pinto YM. N-acetyl-Ser-Asp-Lys-Pro inhibits phosphorylation of Smad2 in cardiac fibroblasts. Hypertension. 2002;40:155–161. [DOI] [PubMed] [Google Scholar]
- 34.Kanasaki K, Koya D, Sugimoto T, Isono M, Kashiwagi A, Haneda M. N-acetyl-seryl-aspartyl-lysyl-proline inhibits TGF-β-mediated plasminogen activator inhibitor-1 expression via inhibition of Smad pathway in human mesangial cells. J Am Soc Nephrol. 2003;14:863–872. [DOI] [PubMed] [Google Scholar]
- 35.Yang F, Yang X-P, Liu Y-H, Xu J, Cingolani O, Rhaleb N-E, Carretero OA. Ac-SDKP reverses inflammation and fibrosis in rats with heart failure after myocardial infarction. Hypertension. 2004;43:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukherjee D, Sen S. Collagen phenotypes during development and regression of myocardial hypertrophy in spontaneously hypertensive rats. Circ Res. 1990;67:1474–1480. [DOI] [PubMed] [Google Scholar]
- 37.Woodiwiss AJ, Tsotetsi OJ, Sprott S, Lancaster EJ, Mela T, Chung ES, Meyer TE, Norton GR. Reduction in myocardial collagen cross-linking parallels left ventricular dilatation in rat models of systolic chamber dysfunction. Circulation. 2001;103:155–160. [DOI] [PubMed] [Google Scholar]
- 38.Norton GR, Tsotetsi J, Trifunovic B, Hartford C, Candy GP, Woodiwiss AJ. Myocardial stiffness is attributed to alterations in cross-linked collagen rather than total collagen or phenotypes in spontaneously hypertensive rats. Circulation. 1997;96:1991–1998. [DOI] [PubMed] [Google Scholar]
- 39.Cingolani HE, Rebolledo OR, Portiansky EL, Pérez NG, Camilión de Hurtado MC. Regression of hypertensive myocardial fibrosis by Na+/H+ exchange inhibition. Hypertension. 2003;41:373–377. [DOI] [PubMed] [Google Scholar]
- 40.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–1393. [DOI] [PubMed] [Google Scholar]