Abstract
Objective
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) is a potent natural inhibitor of hematopoietic stem cell proliferation which is degraded mainly by angiotensin-converting enzyme (ACE). In vitro, Ac-SDKP inhibits collagen production by cardiac fibroblasts; while in vivo it blocks collagen deposition in the left ventricle (LV) of rats with hypertension or myocardial infarction (MI). In addition, it reportedly prevents and reverses macrophage infiltration in the LV of rats with MI. We tested the hypothesis that when Ac-SDKP is infused at doses that cause plasma concentrations similar to those observed after ACE inhibition, it mimics the anti-inflammatory and antifibrotic effects of ACE inhibitors (ACEi) in the heart, and, further, that these effects are independent of changes in blood pressure.
Design and methods
Rats were divided into five groups: (1) controls, (2) Ang II (750 μg/kg per day, s.c.), (3) Ang II + captopril (100 mg/kg per day in drinking water), (4) Ang II + Ac-SDKP (400 μg/kg per day, s.c.), and (5) Ang II + Ac-SDKP (800 μg/kg per day, s.c.). We measured LV cell proliferation, inflammatory cell infiltration, cytokine expression, hypertrophy and fibrosis.
Results
Plasma Ac-SDKP was five-fold higher in rats given ACEi and four- and ten-fold higher in rats given 400 and 800 μg/kg per day Ac-SDKP, respectively. ACEi significantly decreased Ang II-induced cell proliferation (Ki-67), LV macrophage/mast cell infiltration, transforming growth factor-β, connective tissue growth factor and collagen deposition without affecting hypertension, LV hypertrophy or myocyte cross-sectional area, and these effects were mimicked by exogenous Ac-SDKP (400 μg/kg per day) which raised plasma Ac-SDKP to levels similar to ACEi. BP was not decreased by either ACEi or Ac-SDKP.
Conclusions
We concluded that Ac-SDKP may be an important mediator of the anti-inflammatory and antifibrotic effects of ACEi in hypertension independent of its hemodynamic effects.
Keywords: N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP), angiotensin, angiotensin-converting enzyme inhibitor, collagen, heart, hypertension, inflammation
Introduction
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) is a natural inhibitor of pluripotent hematopoietic stem cell proliferation [1,2] and is normally present in human plasma and circulating mononuclear cells [3]. It is ubiquitously distributed in mouse tissues, including the lung, kidney and heart [4], and is cleaved to an inactive form by the NH2-terminal catalytic domain of angiotensin-converting enzyme (ACE) [5]. Captopril, an ACE inhibitor (ACEi), prevented degradation of endogenous Ac-SDKP and raised its circulating concentrations about five-fold in volunteers [5,6]. Ac-SDKP has a 4.5 min half-life in the circulation and is probably released continuously [6]. We found that Ac-SDKP not only inhibited rat cardiac fibroblast proliferation and collagen synthesis in vitro [7,8] but also prevented left ventricular (LV) fibrosis in hypertensive rats in vivo [9,10]. On the other hand, ACEi significantly attenuated cardiac fibrosis in rats with heart failure induced by myocardial infarction (MI) [11], spontaneously hypertensive rats (SHR) [12] and rats with mineralocorticoid hypertension [13]. Angiotensin II (Ang II)-induced hypertension has been associated with not only fibroblast proliferation and interstitial/perivascular fibrosis, but also myocardial invasion by inflammatory cells such as macrophages and lymphocytes that persists for least 6 weeks after the start of Ang II infusion [14]. Mast cells are another type of inflammatory cell highly correlated with the severity of fibrosis in diseases such as scleroderma, idiopathic pulmonary fibrosis, neurofibromas and some forms of eosinophilic myocarditis (for review, see [15]). ACEi-treated SHR exhibited significantly lower LV mast cell density and fibrosis, suggesting that mast cells may play a role in the development of ventricular myocardial fibrosis in hypertension [15]. Treatment of renovascular hypertensive rats with an inhibitor of mast cell degranulation markedly attenuated LV fibrosis [16]. However, it is not known whether Ac-SDKP interferes with the pro-inflammatory and profibrotic effects of Ang II in vivo. Ang II is also known to stimulate expression of transforming growth factor-β1 (TGF-β1) in cardiac fibroblasts and myofibroblasts [17]. Most of the effects of TGF-β1 are believed to be mediated by another cytokine named connective tissue growth factor (CTGF) [18], and both of these cytokines play a central role in the development of fibrosis [19]. We hypothesized that when Ac-SDKP is infused at doses that cause plasma concentrations similar to those observed after ACE inhibition, it mimics the anti-inflammatory and antifibrotic effects of ACE inhibitors (ACEi) in the heart, and, further, that these effects are independent of changes in blood pressure. We examined whether: (1) ACEi increase plasma Ac-SDKP, which in turn blunts cell proliferation, LV inflammatory cell infiltration and collagen deposition; (2) exogenous Ac-SDKP mimics the anti-inflammatory and antifibrotic effects of ACEi; and (3) the mechanism by which ACEi and Ac-SDKP inhibit cardiac collagen is associated with inhibition of cell proliferation, TGF-β and CTGF expression and infiltration of cardiac tissue by inflammatory cells. Since reports have suggested that the antifibrotic effect of ACEi is not associated with hemodynamic changes in Ang II-induced hypertension [20], we selected this model to test our hypothesis.
Methods
This study was approved by the Henry Ford Hospital Institutional Animal Care and Use Committee.
Animals and experimental design
Male Sprague–Dawley rats weighing 200–255 g (Charles River, Wilmington, Delaware) were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). A small incision was made between the shoulder blades and a pocket created subcutaneously, just large enough to hold an osmotic minipump (Alzet 2 ML4). The pump was implanted to deliver Ang II and/or Ac-SDKP (synthesized at Dr Domenico Regoli’s laboratory, University of Sherbrooke, Canada) or saline plus 0.01 N acetic acid. Captopril was given in drinking water. Treatment with Ac-SDKP or captopril was begun simultaneously with Ang II and continued for 4 weeks. Rats were divided into five groups: (1) sham, (2) Ang II + vehicle (saline + 0.01 acetic acid), (3) Ang II + captopril at 100 mg/kg per day, (4) Ang II + Ac-SDKP at 400 μg/kg per day and (5) Ang II + Ac-SDKP at 800 μg/kg per day.
Systolic blood pressure (SBP) was measured by tail cuff twice a week for 4 weeks. At the end of the experiment, animals were anesthetized with 50 mg/kg pentobarbital sodium, and blood from the aorta was collected in a heparinized tube. The heart was stopped at diastole with an intraventricular injection of 15% KCl and then rapidly excised along with the right kidney for histological analysis. The LV (including the septum) was weighed and sectioned transversely from apex to base.
Hydroxyproline assay
Collagen content of myocardial and renal tissue was determined by hydroxyproline assay as described previously [10,21]. Briefly, tissue was freeze-dried, homogenized, and hydrolyzed with 6 N HCl for 16 h at 110 °C. A standard curve of 0 to 5 μg hydroxyproline was used, and data were expressed as μg collagen/mg dry wt, assuming that collagen contains an average of 13.5% hydroxyproline [22].
Cross-sectional area of LV myocytes
Sections, 10 pm thick, from each frozen slice were stained with fluorescein-labeled peanut agglutinin (Vector Laboratories, Burlingame, California, USA) after pretreatment with 3.3 U/ml neuroaminidase V type (Sigma, St Louis, Missouri, USA) to delineate myocyte cross-sectional area (an indicator of myocyte volume) [11]. Stained sections were photographed on 35-mm film. Each field contained ⩾ 100 myocytes. Images were projected with a photo-magnifier, and myocyte cross-sectional area (radial fields) and length (longitudinal fields) were determined using computer-based planimetry (SigmaScan). An average cross-sectional area was calculated using data from all three slices.
LV interstitial collagen fraction
Total surface area (microscopic field), interstitial space (collagen plus capillaries) and area occupied by capillaries alone were measured with computer-assisted videodensitometry (JAVA; SPSS; Chicago, Illinois, USA). Interstitial collagen fraction was calculated by the per cent total surface area occupied by the interstitial space minus the per cent total surface area occupied by the capillaries. The average interstitial collagen fraction was calculated using data obtained from all three slices [23].
Determination of cell proliferation (Ki-67)
Sections measuring 6 μm were heated for 30 min in a 37°C oven, deparaffinized, rehydrated and boiled in a microwave oven for 17 min. They were incubated overnight at 4°C with a polyclonal anti Ki-67 antibody (1 : 33; Santa Cruz Biotechnology, California, USA) followed by a biotinylated secondary antibody and stained with anti-rabbit IgG labeled by FITC for 1 h at 37°C [24,25]. Ki-67-positive cells in half of the LV were counted and expressed as cells/mm2.
LV macrophage/monocyte and mast cell infiltration
LV sections were fixed in 2.5% paraformaldehyde and mounted in a paraffin block. Sections 4 μm thick were deparaffinized, rehydrated, boiled in 0.2% citric acid (pH 6.0) for 10 min for antigen retrieval, and washed three times in phosphate-buffered saline (PBS) for 5 min each time. Sections were preincubated with blocking serum (1% normal serum) for 30 min and incubated with a mouse monoclonal antibody against rat macrophages/monocytes (ED1, 1 : 1000 dilution; Chemicon, Temecula, California, USA) at room temperature for 30 min. Each section was washed three times in PBS, and ED1 was assayed (Vectastain ABC kit; Vector Laboratories). Sections were developed with diaminobenzidine substrate (Vector) and counterstained with hematoxylin. To determine mast cell infiltration, we stained 4-μm sections (prepared as described above) with toluidine blue O (Sigma) [26,27]. A Nikon microscope was attached to a charge-coupled device video camera (Optronics) and the images sent to a computer fitted with a Bioquant NOVA image analysis system (R&M Biometrics, Nashville, Tennessee, USA). Stained cells were counted at ×40; and because every selected image occupied the entire window, we measured window size with the same objective. Cell density was calculated as the number of cells per window area (1 mm2), which was fixed at 0.022194 mm2. For each sample, 12 randomly selected fields were examined.
Immunohistochemical staining for TGF-β and CTGF in the left ventricle
Sections 5 μm thick were deparaffinized, rehydrated and quenched with endogenous peroxide (3% hydrogen peroxide) and boiled in 0.2% citric acid (pH 6.0) for 10 min for antigen retrieval. They were washed twice in PBS for 5 min each time, preincubated with blocking serum (1% normal serum) for 30 min, then incubated with a mouse monoclonal antibody against TGF-β (1 : 1000 dilution; R&D Systems, Minneapolis, Minnesota) or an affinity-purified rabbit polyclonal antibody against residues 81–94 of human CTGF (1 ng/ml) at 4°C overnight [28]. Each section was washed three times in 1% Tween PBS and assayed with a Vectastain ABC kit. Sections were developed with diaminobenzidine substrate and counterstained with hematoxylin. For each sample, 16 randomly selected fields in the LV were examined. TGF-β-positive staining was found in the interstitium. TGF-β was localized within the cells and in the extracellular space. Thus, we felt the best way to quantitate the results was to measure per cent total myocardial area as reported by others [29]. CTGF-positive staining was intracellular, making it possible to count the cells; therefore, CTGF-positive cells in half of the LV were counted and expressed as cells/mm2.
Ac-SDKP plasma levels
Arterial blood mixed with lisinopril (final concentration, 10 μmol/l) was centrifuged at 2000 g for 15 min at 4°C. Plasma was stored at −70°C until the assay. Plasma Ac-SDKP was quantified using a competitive enzyme immunoassay kit (SPI-BIO, Massey Cedex, France) and expressed as nm/l [3].
Statistical analysis
Analysis of variance (ANOVA) was used to test for differences among the five different groups. Hochberg’s method was used to adjust the a level of significance. Values are expressed as mean ± SEM. P < 0.05 was considered significant.
Results
SBP, heart rate, left ventricular hypertrophy and myocyte cross-sectional area
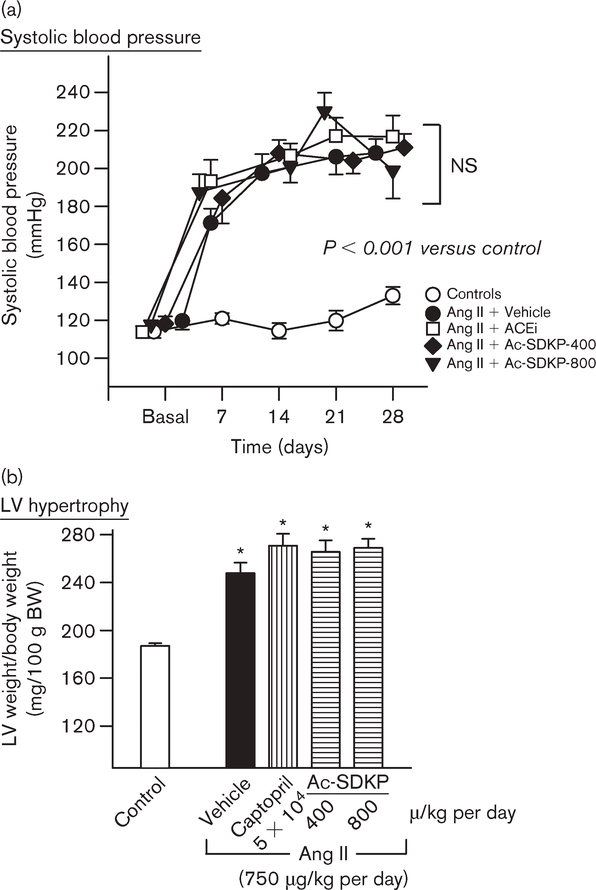
One week after Ang II infusion, SBP in the Ang II + vehicle group was significantly increased compared with the control group (P < 0.005) and remained at this plateau for 3 weeks. Neither captopril (100 mg/kg per day) nor Ac-SDKP at 400 or 800 μg/kg per day for 4 weeks had any effect on the development of hypertension (Fig. 1). Heart rate was unchanged and was comparable in all groups. The ratio of LV weight to body weight was significantly increased in the Ang II + vehicle group (P < 0.001), and neither captopril nor Ac-SDKP suppressed this increase. Myocyte cross-sectional area was also significantly increased in the Ang II + vehicle group (455 ± 14 versus 346 ± 12 μm2 for control; P < 0.0005). It was not affected by either captopril (434 ± 3 μm2) or Ac-SDKP (461 ± 12) and was consistently higher than control (P < 0.0005).
Fig. 1.
(a) Systolic blood pressure (SBP) and (b) ratio of left ventricular (LV) weight to body weight in rats with angiotensin II (Ang II)-induced hypertension (750 μg/kg per day) treated with either captopril at 100 mg/kg per day or N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) at 400 or 800 μg/kg per day. Ang II infusion significantly increased SBP and induced LV hypertrophy. Neither captopril nor Ac-SDKP affected SBP or LV hypertrophy. *P < 0.005 for all groups versus control. ACEi, angiotensin-I converting enzyme inhibitors; NS, not significant.
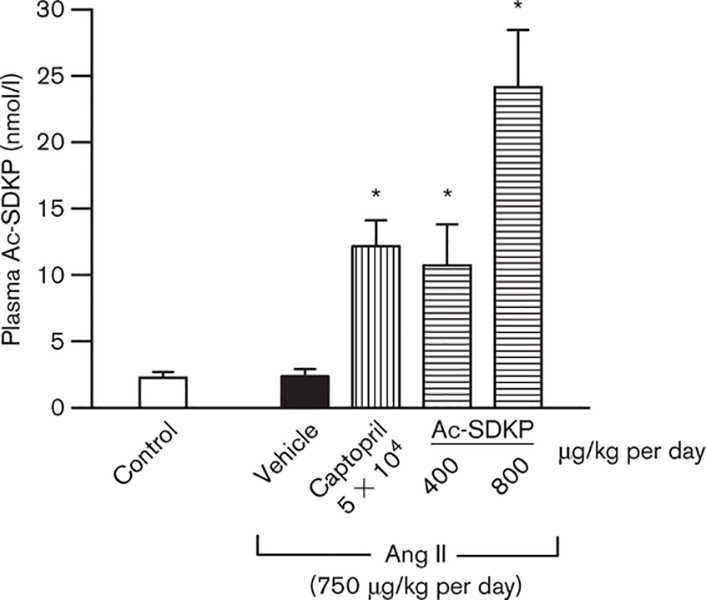
Ac-SDKP plasma concentration
Ac-SDKP plasma concentration was the same for Ang II + vehicle and control (Fig. 2). However, as expected, plasma Ac-SDKP was five-fold higher in rats given captopril (P < 0.008). Exogenous infusion of Ac-SDKP (400 μg/kg per day) also generated higher plasma Ac-SDKP compared with control and Ang II + vehicle (P < 0.008), but similar to Ang II + ACEi. Ac-SDKP at 800 μg/kg per day increased plasma Ac-SDKP 10-fold.
Fig. 2.
Plasma N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) in controls and angiotensin II (Ang II)-infused rats after 4 weeks of treatment with captopril or Ac-SDKP. Ac-SDKP was not affected by chronic Ang II treatment; however, it was five-fold higher in rats given captopril (100 mg/kg per day) and four- and ten-fold higher in rats given Ac- SDKP at 400 and 800 μg/kg per day, respectively. *P < 0.008 for all groups versus control.
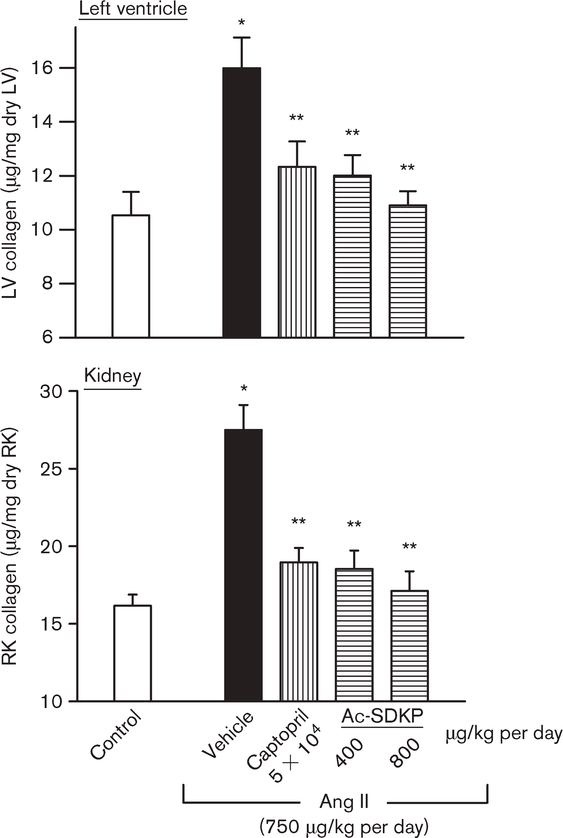
LV and kidney collagen content
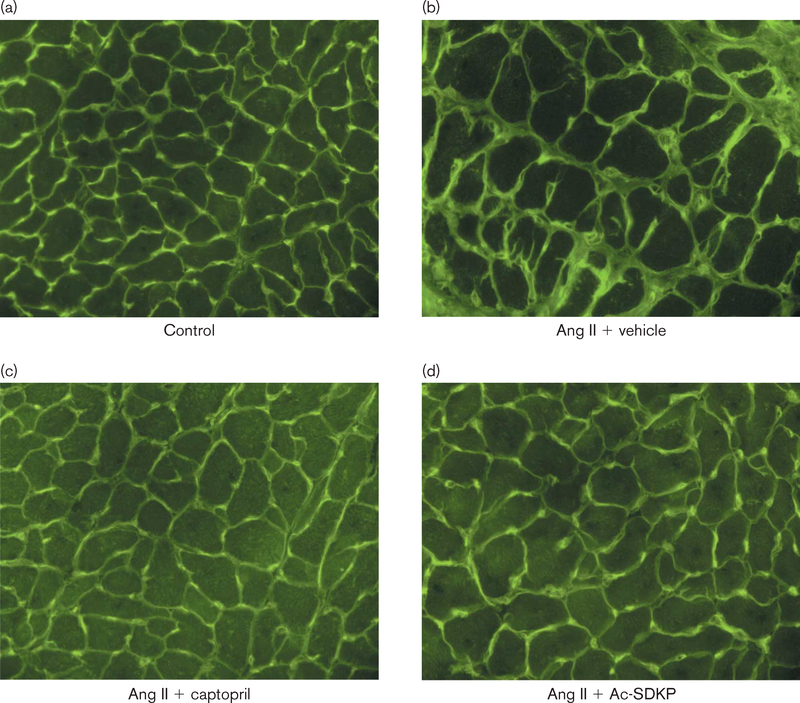
LV collagen was significantly increased in the Ang II + vehicle group (15.9 ± 1.8 μg/mg dry LV weight) compared with control (8.0 ± 0.3; P < 0.001), and this increase was significantly prevented by captopril (10.5 ± 0.4; P < 0.05) and by Ac-SDKP at 400 (11.4 ± 0.9; P < 0.001) and 800 μg/kg per day (9.97 ± 0.4; P < 0.001) (Fig. 3). Figure 4 shows representative histological sections of myocyte cross-sectional area and interstitial collagen deposition from controls and Ang II-hypertensive rats treated with either vehicle, ACEi or Ac-SDKP. We also observed a significant increase in renal collagen in the Ang II + vehicle group (28.11 ± 2.58 μg/mg dry kidney weight) compared with control (14.93 ± 1.72; P < 0.001), which was significantly attenuated by captopril (18.0 ± 0.72; P < 0.001) and Ac-SDKP at 400 (17.24 ± 0.42; P < 0.001) and 800 μg/kg per day (16.38 ± 0.73; P < 0.001) (Fig. 3).
Fig. 3.
Collagen deposition in the left ventricle (LV) and right kidney (RK) in controls and angiotensin II (Ang II)-infused rats after 4 weeks of treatment with either captopril or N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP). Collagen deposition was significantly higher in the Ang II + vehicle groups. Captopril (100 mg/kg per day) and Ac-SDKP at 400 and 800 μg/kg per day significantly lowered collagen deposition. *P < 0.002 for Ang II + vehicle versus control. **P < 0.001 for Ang II + angiotensin-I converting enzyme inhibitors (ACEi) or Ac-SDKP versus Ang II + vehicle.
Fig. 4.
Representative histological sections showing myocyte cross-sectional area, interstitial collagen deposition (green) and capillaries (yellow; since images were exposed under fluorescent light, the red color given by rhodamine is not apparent) from (1) controls and rats infused with angiotensin (Ang II) plus either (2) vehicle, (3) captopril (100 mg/kg per day) or (4) N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP, 800 μg/kg per day).
Effect of captopril and Ac-SDKP on cell proliferation in the LV
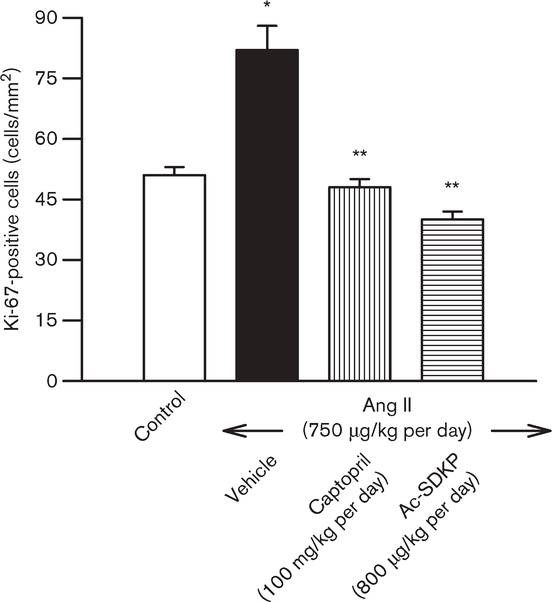
Few Ki-67-positive cells were seen in the controls. In the Ang II + vehicle group, Ki-67-positive cells were largely restricted to the interstitial and perivascular spaces but were significantly increased compared with control (P < 0.01). Treatment with ACEi or Ac-SDKP significantly lowered the number of Ki-67-positive cells in the LV (P < 0.01) (Fig. 5).
Fig. 5.
Immunohistochemical staining for Ki-67-positive cells in the left ventricle (LV) of controls and angiotensin II (Ang II)-infused rats after 4 weeks of treatment with either captopril or N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP). The number of Ki-67-positive cells was significantly higher in the Ang II + vehicle group. Captopril (100 mg/kg per day) and Ac-SDKP at 800 μg/kg per day significantly lowered cell proliferation. *P < 0.01 for Ang II + vehicle versus control. **P < 0.01 for Ang II + ACEi or Ac-SDKP versus Ang II + vehicle.
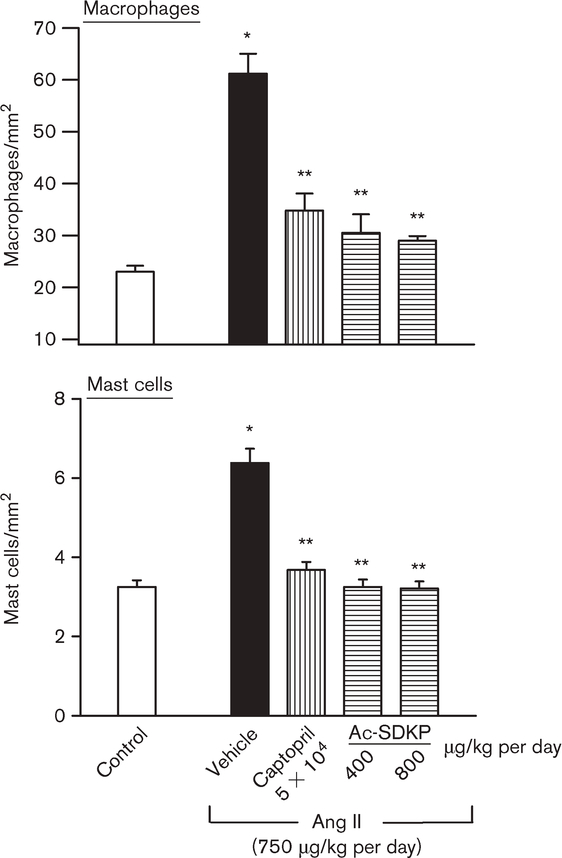
Effect of captopril and Ac-SDKP infusion on monocyte/macrophage (ED1) and mast cell infiltration in the LV interstitium
ED1-positive cells were significantly increased in the Ang II + vehicle group compared with control (P < 0.001). Treatment with captopril and Ac-SDKP (at both doses) significantly reduced the number of ED1-positive cells in the LV (P < 0.001) (Figs 6 and 7). There were also significantly more mast cells in the LV in the Ang II + vehicle group than control (P < 0.001); captopril and Ac-SDKP kept mast cell infiltration at normal levels (Figs 6 and 7).
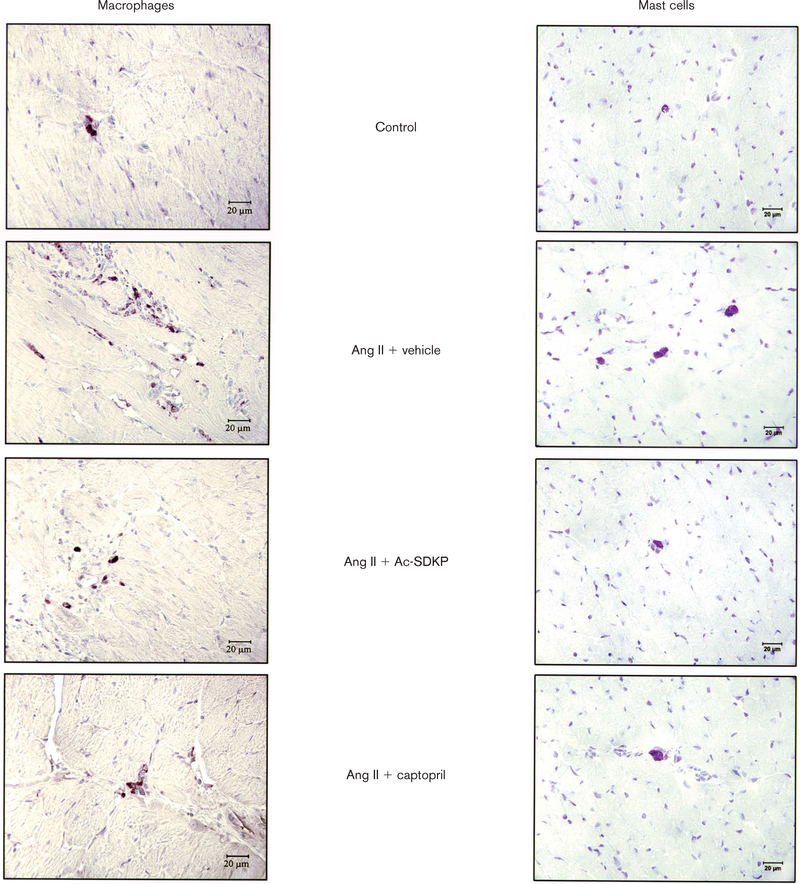
Fig. 6.
Immunohistochemical staining for monocytes/macrophages (ED1-positive cells; brown) and toluidine blue staining for mast cells (dark pink cells with characteristic cytoplasmic granules) in the left ventricle (LV) 4 weeks after treatment in controls, rats given angiotensin II (Ang II) + vehicle and rats given Ang II + captopril or N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP, 400 or 800 μg/kg per day). Magnification × 400.
Fig. 7.
Effect of captopril and N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) on macrophage and mast cell infiltration in the left ventricle (LV) in controls and angiotensin II (Ang II)-infused rats after 4 weeks of treatment. There were significantly more macrophages and mast cells in rats given Ang II + vehicle than in the controls. Captopril and Ac-SDKP significantly inhibited inflammatory cell infiltration in the LV. *P < 0.001 for Ang II + vehicle versus controls. **P < 0.001 for Ang II + angiotensin-I converting enzyme inhibitors (ACEi) or Ac-SDKP versus Ang II + vehicle.
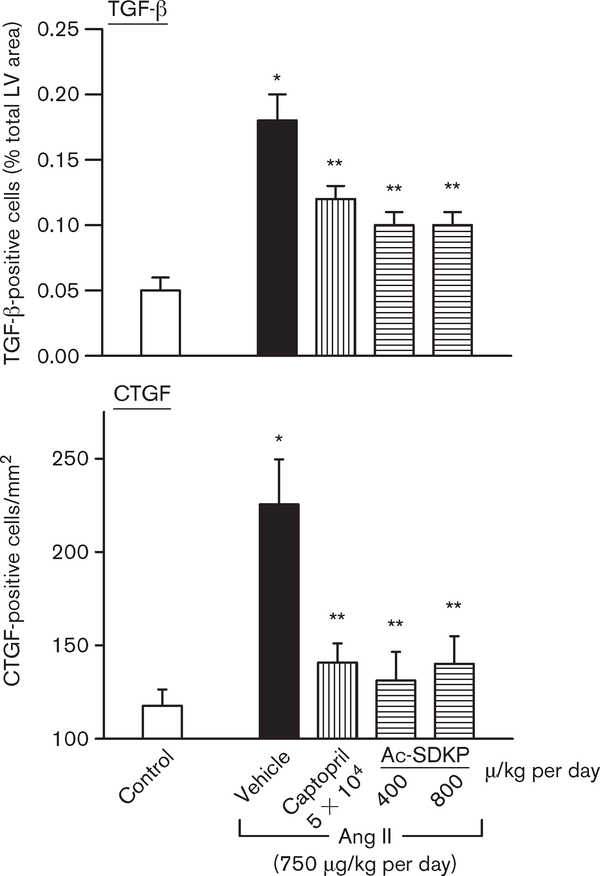
Effect of captopril and Ac-SDKP infusion on TGF-β and CTGF expression in the LV
TGF-β expression was significantly higher in the Ang II + vehicle group (0.18 ± 0.02% total myocardium) compared with control (0.05 ± 0.01%; P < 0.001). Treatment with captopril and Ac-SDKP at 400 and 800 μg/kg per day inhibited TGF-β expression (0.12 ± 0.01, P < 0.01; 0.10 ± 0.01, P < 0.01; and 0.10 ± 0.01, P < 0.007, respectively) (Figs 8 and 9). CTGF expression was also higher with Ang II + vehicle, and treatment with captopril and Ac-SDKP reduced it as well (Figs 8 and 9).
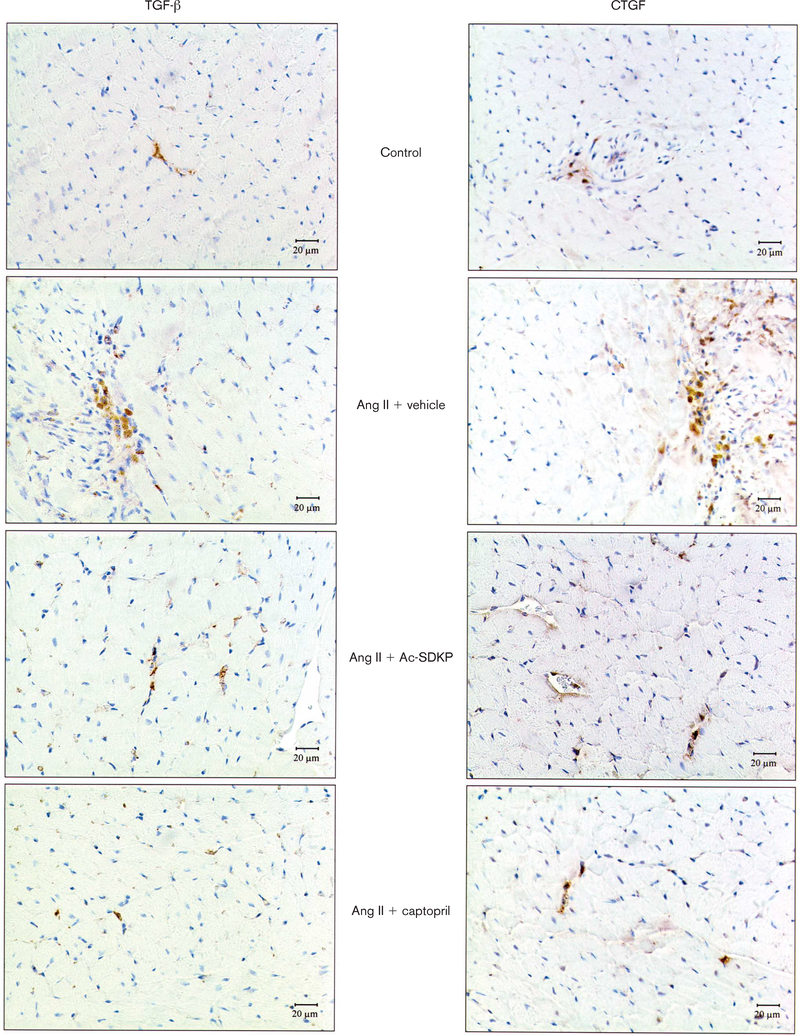
Fig. 8.
Immunohistochemical staining for transforming growth factor-β (TGF-β)-positive staining and connective tissue growth factor (CTGF)-positive cells in the left ventricle (LV) 4 weeks after treatment in controls, rats given angiotensin II (Ang II) + vehicle and rats given Ang II + captopril or N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) (400 or 800 μg/kg per day). Magnification ×400.
Fig. 9.
Effect of captopril and N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) on transforming growth factor-β (TGF-β)- and connective tissue growth factor (CTGF)-positive cells in the left ventricle (LV) in controls and angiotensin II (Ang II)-infused rats after 4 weeks of treatment. There were significantly more TGF-β- and CTGF-positive cells in rats given Ang II + vehicle than in the controls. Captopril and Ac-SDKP significantly inhibited cytokine expression in the LV. *P < 0.001 for Ang II + vehicle versus controls. **P < 0.05 for Ang II + ACEi or Ac-SDKP versus Ang II + vehicle.
Discussion
We tested whether chronic infusion of exogenous Ac-SDKP or administration of an ACEi would result in similar rises in plasma Ac-SDKP, leading to similar antiproliferative, anti-inflammatory and antifibrotic effects in rats made hypertensive by Ang II infusion. We found that Ac-SDKP plasma concentration was the same for Ang II + vehicle and control, suggesting that Ac-SDKP synthesis and degradation may not be affected by Ang II hypertension. However, plasma Ac-SDKP was five-fold higher in rats given ACEi compared with controls, confirming others’ findings [5,6]. Exogenous infusion of a low dose of Ac-SDKP (400 μg/kg per day) generated plasma levels of Ac-SDKP similar to Ang II + ACEi. Interestingly, both ACEi and low-dose Ac-SDKP produced a very similar decrease in the number of infiltrating inflammatory cells (monocytes/macrophages and mast cells), expression of TGF-β and CTGF, and collagen deposition in the LV of Ang II hypertensive rats without affecting blood pressure, cardiac hypertrophy or myocyte cross-sectional area. Doubling the dose of exogenous Ac-SDKP produced twice the level of plasma Ac-SDKP compared with Ang II + ACEi or Ang II + Ac-SDKP (400 μg/kg per day), and yet anti-inflammatory and antifibrotic effects were similar to ACEi or low-dose Ac-SDKP. At week 4 of Ang II infusion, the number of cells positive for Ki-67 (a marker for cell proliferation) was significantly higher in the Ang II + vehicle group compared with control. This finding is different from Campbell et al., who reported that most PCNA-positive staining (another marker for cell proliferation) in rats with Ang II-induced hypertension was seen as early as 2 days and lasted up to 14 days, but then faded [14]. It could be that PCNA is also detectable in quiescent cells, probably because of its involvement in nucleotide excision repair mechanisms, while Ki-67 antigen, a nuclear protein of unknown function, was shown to be strictly expressed when cells passed from G0 to G1 with continued expression throughout the cell cycle. Indeed, Ki-67 has been used as a preferential marker for cell proliferation of solid tumors and some hematological malignancies [30–32]. The advantage of this method over BrdU or 3H-thymidine incorporation is that markers are not needed prior to isolating the cells (e.g. cardiac fibroblasts or vascular smooth muscle cells) or tissue (e.g. heart). Ac-SDKP (high dose) and ACEi prevented cell proliferation (Ki-67) in a very similar fashion. The antifibrotic effect of Ac-SDKP has also been shown in rats on aldosterone-salt [10] or two-kidney, one-clip hypertension (2K-1C) [9] and in rat hearts post-MI [33], and Ac-SDKP is known to prevent macrophage/monocyte infiltration in the LV of 2K-1C hypertensive rats and rat hearts post-MI [9,33].
Inflammatory cells (macrophages and mast cells) are reportedly associated with cardiovascular disease. Myocardial fibrosis and the density of infiltrating macrophages are positively correlated in SHR [34]. Nicoletti et al. [35] reported that myocardial macrophages (ED1-positive cells) were significantly increased in rats with 2K-1C hypertension and co-localized with collagen-synthesizing fibroblasts. Inflammatory cells could promote fibrosis by releasing growth factors or cytokines such as TGF-β which act on fibroblasts and/or myofibroblasts. Mast cells are increased in the right and left ventricles of hypertensive rats with myocardial fibrosis [15] and infarction [36] and in the lungs of patients with fibrosis [37]. Mast cells may also play a role in cardiovascular disease, since they are present in human heart tissue [38,39] and in the adventitia of diseased coronary arteries [40–42]. Mast cell density and histamine concentration are both increased in the coronary arteries of cardiac patients [40,41,43], whose arteries become hyper-responsive to histamine [40]. Furthermore, in vivo histamine and other mast cell-derived mediators (peptide LTC4) cause significant cardiovascular effects [44–46]. Mast cell-derived mediators are mitogens and co-mitogens for human fibroblasts [47–50] and stimulate synthesis and accumulation of collagen, a hallmark of ischemic and dilated cardiomyopathy [51]. In addition, mast cells are an important source of monocyte chemoattractant protein-1 (MCP-1), which when released can recruit more macrophages to the injured myocardium. Thus inhibition of macrophages/monocytes and mast cells by ACEi (probably mediated by Ac-SDKP) and exogenous Ac-SDKP may indicate that their antifibrotic action is at least partially mediated by their anti-inflammatory effect.
TGF-β expression could be enhanced in the hypertensive heart, either because of increased infiltrating inflammatory cells (macrophages) or the action of Ang II on cardiac fibroblasts and myofibroblasts [17]. Lee et al. [52] reported that Ang II stimulates autocrine production of TGF-β in adult rat cardiac fibroblasts and suggested that its effect on the adult myocardium may be mediated in part by autocrine/paracrine mechanisms, including production and release of TGF-β by cardiac fibroblasts. In turn, TGF-β induces expression of another downstream factor, CTGF, which promotes proliferation and extracellular matrix production in connective tissue and was found to be overexpressed in fibrotic disorders [19,53]. CTGF is a 38-kD protein belonging to the insulin-like growth factor family and is a mitogenic and chemotactic factor for cultured fibroblasts [54,55]. It has been shown to promote proliferation and production of extracellular matrix in the heart [19]. As expected, we found that CTGF was markedly increased in the LV of Ang II hypertensive rats, and that Ac-SDKP significantly inhibited overexpression of CTGF in the heart. Therefore, inhibition of cardiac fibrosis was associated with suppression of increased LV TGF-β and CTGF. Ac-SDKP could inhibit the increase in CTGF by blocking TGF-β production, because CTGF is a downstream component of the TGF-β signaling pathway [19]; or it could do so by inhibiting cardiac fibroblast proliferation [7] and thus CTGF production, since fibroblasts can also produce CTGF [54,55]. CTGF is probably induced following TGF-β binding to its receptor(s), triggering specific signals such as Smads and leading to activation of transcriptional factors. Indeed, Ac-SDKP has been shown to inhibit TGF-β signaling, resulting in inhibition of Smad activation in rat cardiac fibroblasts [8] as well as human mesangial cells [56].
In summary, inhibition of TGF-β and CTGF expression by Ac-SDKP in the LV of Ang II-infused hypertensive rats may be an important factor in mediating its antifibrotic effect. We found that an ACEi increased plasma Ac-SDKP in a manner similar to exogenous Ac-SDKP. The ACEi also resembled Ac-SDKP in several other ways: (1) inhibition of cell proliferation, (2) inhibition of LV inflammatory cell infiltration (macrophages/monocytes and mast cells), (3) reduction of TGF-β and CTGF expression in the LV, and (4) prevention of cardiac and renal fibrosis resulting from Ang II infusion. These findings suggest that Ac-SDKP prevents cardiac fibrosis by blocking cell proliferation and collagen production and also inhibits inflammation in Ang II-hypertensive rats. ACEi increase plasma [6] and tissue Ac-SDKP [57] and decrease cardiac and renal fibrosis [11–13,58,59]. In the future, development of an Ac-SDKP antagonist or an inhibitor of Ac-SDKP synthesis would be valuable in determining what role Ac-SDKP might play in the anti-inflammatory/antifibrotic effect of ACEi in cardiovascular disease.
Acknowledgments
Sponsorship: This study was supported by AHA grant 0130128N and NIH grants HL 71806-01 (N.E.R.) and HL 28982 (O.A.C.).
References
- 1.Lenfant M, Wdzieczak-Bakala J, Guittet E, Prome JC, Sotty D, Frindel E. Inhibitor of hematopoietic pluripotent stem cell proliferation: purification and determination of its structure. Proc Natl Acad Sci USA 1989; 86:779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnet D, Lemoine FM, Pontvert-Delucq S, Baillou C, Najman A, Guigon M. Direct and reversible inhibitory effect of the tetrapeptide acetyl-N-Ser-Asp-Lys-Pro (Seraspenide) on the growth of human CD34+ subpopula-bpopulations in response to growth factors. Blood 1993; 82:3307–3314. [PubMed] [Google Scholar]
- 3.Pradelles P, Frobert Y, Créminon C, Liozon E, Massé A, Frindel E. Negative regulator of pluripotent hematopoietic stem cell proliferation in human white blood cells and plasma as analysed by enzyme immunoassay. Biochem Biophys Res Commun 1990; 170:986–993. [DOI] [PubMed] [Google Scholar]
- 4.Pradelles P, Frobert Y, Créminon C, Ivonine H, Frindel E. Distribution of a negative regulator of haematopoietic stem cell proliferation (AcSDKP) and thymosin b4 in mouse tissues. FEBS Lett 1991; 289:171–175. [DOI] [PubMed] [Google Scholar]
- 5.Azizi M, Rousseau A, Ezan E, Guyene T-T, Michelet S, Grognet JM, et al. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest 1996; 97:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azizi M, Ezan E, Nicolet L, Grognet JM, Menard J. High plasma level of N-acetyl-seryl-aspartyl-lysyl-proline: a new marker of chronic angiotensin-converting enzyme inhibition. Hypertension 1997; 30:1015–1019. [DOI] [PubMed] [Google Scholar]
- 7.Rhaleb N-E. Peng H, Harding P, Tayeh M, LaPointe MC, Carretero OA. Effect of N-acetyl-seryl-aspartyl-lysyl-proline on DNA and collagen synthesis in rat cardiac fibroblasts. Hypertension 2001; 37:827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pokharel S, Rasoul S, Roks AJM, Van Leeuwen REW, Van Luyn MJA, Deelman LE, et al. N-acetyl-Ser-Asp-Lys-Pro inhibits phosphorylation of Smad2 in cardiac fibroblasts. Hypertension 2002; 40:155–161. [DOI] [PubMed] [Google Scholar]
- 9.Rhaleb N-E, Peng H, Yang X-P, Liu Y-H, Mehta D, Ezan E, et al. Long-term effect of N-acetyl-seryl-aspartyl-lysyl-proline on left ventricular collagen deposition in rats with 2-kidney, 1-clip hypertension. Circulation 2001; 103:3136–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng H, Carretero OA, Raij L, Yang F, Kapke A, Rhaleb N-E. Antifibrotic effects of N-acetyl-seryl-aspartyl-lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension 2001; 37:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y-H, Yang X-P, Sharov VG, Nass O, Sabbah HN, Peterson E, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure: role of kinins and angiotensin II type 2 receptors. J Clin Invest 1997; 99:1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks WW, Bing OH, Robinson KG, Slawsky MT, Chaletsky DM, Conrad CH. Effect of angiotensin-converting enzyme inhibition on myocardial fibrosis and function in hypertrophied and failing myocardium from the spontaneously hypertensive rat. Circulation 1997; 96: 4002–4010. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Ratajska A, Zhou G, Weber KT. Angiotensin-converting enzyme and myocardial fibrosis in the rat receiving angiotensin II or aldosterone. J Lab Clin Med 1993; 122:395–403. [PubMed] [Google Scholar]
- 14.Campbell SE, Janicki JS, Weber KT. Temporal differences in fibroblast proliferation and phenotype expression in response to chronic administration of angiotensin II or aldosterone. J Mol Cell Cardiol 1995; 27: 1545–1560. [DOI] [PubMed] [Google Scholar]
- 15.Panizo A, Pardo Mindán FJ, Galindo MF, Cenarruzabeitia E, Hernández M. Díez J. Are mast cells involved in hypertensive heart disease? J Hypertens 1995; 13:1201–1208. [DOI] [PubMed] [Google Scholar]
- 16.Hocher B, Godes M, Olivier J, Weil J, Eschenhagen T, Slowinski T, et al. Inhibition of left ventricular fibrosis by tranilast in rats with renovascular hypertension. J Hypertens 2002; 20:745–751. [DOI] [PubMed] [Google Scholar]
- 17.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-β1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol 1997; 29:1947–1958. [DOI] [PubMed] [Google Scholar]
- 18.Kothapalli D, Hayashi N, Grotendorst GR. Inhibition of TGF-β-stimulated CTGF gene expression and anchorage-independent growth by cAMP identifies a CTGF-dependent restriction point in the cell cycle. FASEB J 1998; 12:1151–1161. [DOI] [PubMed] [Google Scholar]
- 19.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF-β in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol 2000; 32:1805–1819. [DOI] [PubMed] [Google Scholar]
- 20.Crawford DC, Chobanian AV, Brecher P. Angiotensin II induces fibronectin expression associated with cardiac fibrosis in the rat. Circ Res 1994; 74:727–739. [DOI] [PubMed] [Google Scholar]
- 21.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol 1995; 147: 325–338. [PMC free article] [PubMed] [Google Scholar]
- 22.Chiariello M, Ambrosio G, Cappelli-Bigazzi M, Perrone-Filardi P, Brigante F, Sifola C. A biochemical method for the quantitation of myocardial scarring after experimental coronary artery occlusion. J Mol Cell Cardiol 1986; 18:283–290. [DOI] [PubMed] [Google Scholar]
- 23.Sabbah HN, Sharov VG, Lesch M, Goldstein S. Progression of heart failure: a role for interstitial fibrosis. Mol Cell Biochem 1995; 147:29–34. [DOI] [PubMed] [Google Scholar]
- 24.Schlüter C, Duchrow M, Wohlenberg C, Becker MHG, Key G, Flad H-D, et al. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol 1993; 123:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gore SD, Weng L-J, Burke PJ. Validation of flow-cytometric determination of Ki67 expression as a measure of growth factor response in acute myelogenous leukemia. Exp Hematol 1993; 21:1702–1708. [PubMed] [Google Scholar]
- 26.The staining of mast cells. In: Sagher F, Even-Paz Z (editors): Mastocytosis and the mast cell. Chicago, Illinois: Year Book Medical Publishers; 1967, pp. 269–277. [Google Scholar]
- 27.Roberts ISD, Brenchley PEC. Mast cells: the forgotten cells of renal fibrosis. J Clin Pathol 2000; 53:858–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brigstock DR, Steffen CL, Kim GY, Vegunta RK, Diehl JR, Harding PA. Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids. Identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem 1997; 272:20275–20282. [DOI] [PubMed] [Google Scholar]
- 29.Lim D-S, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 2001; 103:789–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis PA, Smith IE, Detre S, Burton SA, Salter J, A’hern R, et al. Reduced apoptosis and proliferation and increased Bcl-2 in residual breast cancer following preoperative chemotherapy. Breast Cancer Res Treat 1998; 48:107–116. [DOI] [PubMed] [Google Scholar]
- 31.Bottini A, Berruti A, Bersiga A, Brunelli A, Brizzi MP, Di Marco B, et al. Effect of neoadjuvant chemotherapy on Ki67 labelling index, c-erbB-2 expression and steroid hormone receptor status in human breast tumours. Anticancer Res 1996; 16:3105–3110. [PubMed] [Google Scholar]
- 32.Antoine N, Greimers R, De Roanne C, Kusaka M, Heinen E, Simar LJ, et al. AGM-1470, a potent angiogenesis inhibitor, prevents the entry of normal but not transformed endothelial cells into the G1 phase of the cell cycle. Cancer Res 1994; 54:2073–2076. [PubMed] [Google Scholar]
- 33.Yang F, Yang X-P, Liu Y-H, Xu J, Rhaleb N-E, Peng H-M, et al. Effects of Ac-SDKP, a natural inhibitor of hematopoietic stem cell proliferation, on cardiac collagen deposition in rats with myocardial infarction [abstract]. Hypertension 2000; 36:703. [Google Scholar]
- 34.Hinglais N, Heudes D, Nicoletti A, Mandet C, Laurent M, Bariéty J, et al. Colocalization of myocardial fibrosis and inflammatory cells in rats. Lab Invest 1994; 70:286–294. [PubMed] [Google Scholar]
- 35.Nicoletti A, Heudes D, Mandet C, Hinglais N, Bariety J, Michel JB. Inflammatory cells and myocardial fibrosis: spatial and temporal distribution in renovascular hypertensive rats. Cardiovasc Res 1996; 32: 1096–1107. [DOI] [PubMed] [Google Scholar]
- 36.Funck RC, Wilke A, Rupp H, Brilla CG. Regulation and role of myocardial collagen matrix remodeling in hypertensive heart disease In: Zanchetti A, Devereux RB, Hansson L Gorini S (editors): Hypertension and the heart. New York, NY: Plenum Press; 1997, pp. 35–44. [DOI] [PubMed] [Google Scholar]
- 37.Kawanami O, Ferrans VJ, Fulmer JD, Crystal RG. Ultrastructure of pulmonary mast cells in patients with fibrotic lung disorders. Lab Invest 1979; 40:717–734. [PubMed] [Google Scholar]
- 38.Dvorak AM. Mast-cell degranulation in human hearts. N Engl J Med 1986; 315:969–970. [DOI] [PubMed] [Google Scholar]
- 39.Sperr WR, Bankl HC, Mundigler G, Klappacher G, Grossschmidt K, Agis H, et al. The human cardiac mast cell: localization, isolation, phenotype, and functional characterization. Blood 1994; 84:3876–3884. [PubMed] [Google Scholar]
- 40.Kalsner S, Richards R. Coronary arteries of cardiac patients are hyperreactive and contain stores of amines: a mechanism for coronary spasm. Science 1984; 223:1435–1437. [DOI] [PubMed] [Google Scholar]
- 41.Forman MB, Oates JA, Robertson D, Robertson DM, Roberts LJ II, Virmani R Increased adventitial mast cells in a patient with coronary spasm. N Engl J Med 1985; 313:1138–1141. [DOI] [PubMed] [Google Scholar]
- 42.Kaartinen M, Penttila A, Kovanen PT. Mast cells of two types differing in neutral protease composition in the human aortic intima. Demonstration of tryptase- and tryptase/chymase-containing mast cells in normal intimas, fatty streaks, and the shoulder region of atheromas. Arterioscler Thromb 1994; 14:966–972. [DOI] [PubMed] [Google Scholar]
- 43.Kovanen PT, Kaartinen M, Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 1995; 92:1084–1088. [DOI] [PubMed] [Google Scholar]
- 44.Vigorito C, Russo P, Picotti GB, Chiariello M, Poto S, Marone G. Cardiovascular effects of histamine infusion in man. J Cardiovasc Pharmacol 1983; 5:531–537. [DOI] [PubMed] [Google Scholar]
- 45.Vigorito C, Poto S, Picotti GB, Triggiani M, Marone G. Effect of activation of the H1 receptor on coronary hemodynamics in man. Circulation 1986; 73:1175–1182. [DOI] [PubMed] [Google Scholar]
- 46.Marone G, Giordano A, Cirillo R, Triggiani M, Vigorito C. Cardiovascular and metabolic effects of peptide leukotrienes in man. Ann NY Acad Sci 1988; 524:321–323. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann T, Ruoss SJ, Raymond WW, Seuwen K, Caughey GH. Human tryptase as a potent, cell-specific mitogen: role of signaling pathways in synergistic responses. Am J Physiol 1992; 262:L528–L534. [DOI] [PubMed] [Google Scholar]
- 48.Baud L, Perez J, Denis M, Ardaillou R. Modulation of fibroblast proliferation by sulfidopeptide leukotrienes: effect of indomethacin. J Immunol 1987; 138:1190–1195. [PubMed] [Google Scholar]
- 49.Russel JD, Russell SB, Trupin KM. The effect of histamine on the growth of cultured fibroblasts isolated from normal and keloid tissue. J Cell Physiol 1977; 93:389–393. [DOI] [PubMed] [Google Scholar]
- 50.Hatamochi A, Fujiwara K, Ueki H. Effects of histamine on collagen synthesis by cultured fibroblasts derived from guinea pig skin. Arch Dermatol Res 1985; 277:60–64. [DOI] [PubMed] [Google Scholar]
- 51.Mukherjee D, Sen S. Alteration of collagen phenotypes in ischemic cardiomyopathy. J Clin Invest 1991; 88:1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee AA, Dillmann WH, McCulloch AD, Villarreal FJ. Angiotensin II stimulates the autocrine production of transforming growth factor-b1 in adult rat cardiac fibroblasts. J Mol Cell Cardiol 1995; 27:2347–2357. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Blom IE, Sa S, Goldschmeding R, Abraham DJ, Leask A. CTGF expression in mesangial cells: involvement of SMADs, MAP kinase, and PKC. Kidney Int 2002; 62:1149–1159. [DOI] [PubMed] [Google Scholar]
- 54.Moussad EEA, Brigstock DR. Connective tissue growth factor: what’s in a name? Mol Genet Metab 2000; 71:276–292. [DOI] [PubMed] [Google Scholar]
- 55.Steffen CL, Ball-Mirth DK, Harding PA, Bhattacharyya N, Pillai S, Brig-stock DR. Characterization of cell-associated and soluble forms of connective tissue growth factor (CTGF) produced by fibroblast cells in vitro. Growth Factors 1998; 15:199–213. [DOI] [PubMed] [Google Scholar]
- 56.Kanasaki K, Koya D, Sugimoto T, Isono M, Kashiwagi A, Haneda M. N-acetyl-seryl-aspartyl-lysyl-proline inhibits TGF-β-mediated plasminogen activator inhibitor-1 expression via inhibition of Smad pathway in human mesangial cells. J Am Soc Nephrol 2003; 14:863–872. [DOI] [PubMed] [Google Scholar]
- 57.Junot C, Nicolet L, Ezan E, Gonzales M-F. Menard J, Azizi M. Effects of angiotensin-converting enzyme inhibition on plasma, urine, and tissue concentrations of hemoregulatory peptide Acetyl-Ser-Asp-Lys-Pro in rats. J Cardiovasc Pharmacol 1999; 291:982–987. [PubMed] [Google Scholar]
- 58.Kim S, Ohta K, Hamaguchi A, Omura T, Yukimura T, Miura K, et al. Role of angiotensin II in renal injury of deoxycorticosterone acetate-salt hypertensive rats. Hypertension 1994; 24:195–204. [DOI] [PubMed] [Google Scholar]
- 59.Kaneto H, Morrissey J, McCracken R, Reyes A, Klahr S. Enalapril reduces collagen type IV synthesis and expansion of the interstitium in the obstructed kidney of the rat. Kidney Int 1994; 45:1637–1647. [DOI] [PubMed] [Google Scholar]