Abstract
While early studies focused on the potential roles in health and disease of provitamin A carotenoids, such as β-carotene, research over the past decade has provided a framework for our understanding of the functions of non-provitamin A carotenoids such as lycopene, especially in regards to its association with a reduced risk of a number of chronic diseases, including cancer. Recent data suggests that lycopene metabolites may possess specific biological activities on several important cellular signaling pathways and molecular targets. Carotenoid metabolites may have more important biological roles than their parent compounds in human health and disease. This notion has been reinforced by the observation of both beneficial and detrimental effects of carotenoid metabolites in cancer prevention.
Keywords: biological activity, cancer prevention, lycopene metabolites
INTRODUCTION
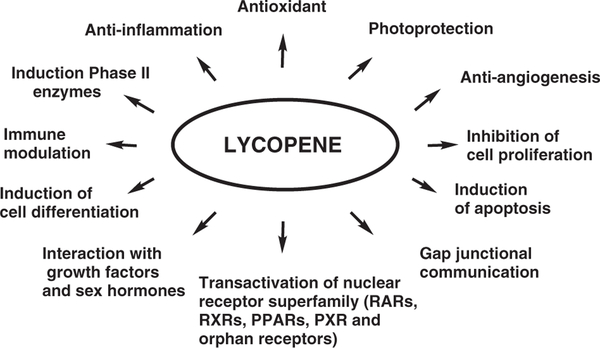
Considerable interest and research efforts have been expended in an effort to uncover the potential roles of carotenoids in human health and disease. While early studies focused on pro-vitamin A carotenoids, more recent research efforts have focused on the potential roles in health and disease of the non-provitamin A carotenoids, such as lycopene. Lycopene has been implicated as having a potential beneficial impact in a number of chronic diseases including cancer. Although evidence from epidemiological and animal studies supports a potential chemopreventive role of lycopene,1–5 the biochemical mechanisms behind such beneficial effects have, as of yet, not been well defined. Several reports have demonstrated potential beneficial effects of lycopene, especially in respect to the following: antioxidant function; enhanced cellular gap junction communication; induction of phase II enzymes through activation of the antioxidant response element (ARE) transcription system; suppression of insulin-like growth factor-1 stimulated cell proliferation by induced insulin-like growth factor binding protein; anti-angiogenesis and inhibition of cell proliferation; and induction of apoptosis (Figure 1). With the cloning and characterization of two distinct carotenoid cleaving enzymes, recent research has focused on the metabolic fate of lycopene and the subsequent metabolites created. Several reports, including our own, suggest that the biological activities of lycopene may be mediated, in part, by lycopene metabolites. Lycopene metabolites and carotenoid metabolites in general, can possess either more or less activity than the parent compound or can have an entirely independent function. This review highlights the chemical and biological metabolism of lycopene and the potential actions of lycopene and its metabolites on chemoprevention.
Figure 1. Possible biological functions of lycopene.
LYCOPENE METABOLISM
Carotenoids are a class of lipophilic compounds with a polyisoprenoid structure. Most carotenoids contain a series of conjugated double bonds, which are sensitive to oxidative modification and cis-trans isomerization. There are six major carotenoids (β-carotene, α-carotene, lycopene, β-cryptoxanthin, lutein, and zeaxanthin) that can be found routinely in human plasma and tissues. Among them, β-carotene has been the most extensively studied. More recently, lycopene has attracted considerable attention due to its association with a decreased risk of a number of chronic diseases, including cancers. Considerable efforts have been expended in order to identify its biological and physiochemical properties. Relative to β-carotene, lycopene has the same molecular mass and chemical formula, yet lycopene is an open-polyene chain lacking the β-ionone ring structure. While the metabolism of β-carotene has been studied extensively, the metabolism of lycopene remains poorly understood.
Chemical oxidation of lycopene
There have been a number of reports studying the formation of lycopene metabolites and oxidation products in vitro. Many of these studies have utilized various oxidizing systems and have identified several unique metabolites. Kim et al.6 identified oxidative products after incubation of lycopene at 37°C for 72 h under atmospheric oxygen. Using three separate solubilization schemes (toluene, aqueous Tween 40, and liposomal suspension) eight oxidative products were identified: 3,7,11-trimethyl-2, 4, 6, 10-dodecatetraen-1-al, 6,10,14-trimethyl-3,5,7,9,13-pentadecapentaen-2-one,acycloretinal, apo-14′-lycopenal, apo-12′-lycopenal, apo10′-lycopenal, apo-8′-lycopenal, and apo-6′-lycopenal. It was subsequently demonstrated that acyclo-retinal could be oxidized to the corresponding acyclo-retinoic acid when incubated with pig liver homogenate,6 indicating potential in vivo formation. Incubation of deuterated lycopene with rat intestinal post-mitochondrial fractions and soy lipoxygenase led to the identification of several additional lycopene metabolites and oxidative products.7 The cleavage products identified were 3-keto-apo-13lycopenone and 3,4-dehydro-5, 6-dihydro-15,15′-apolycopenal, while the four oxidative metabolites identified were: 2-apo-5,8-lycopenal-furanoxide,lycopene-5,6,5′,6′diepoxide, lycopene-5,8-furanoxide, and 3-ketolycopene-5′,8′-furanoxide. Zhang et al.8 isolated and identified a cleavage product of lycopene from an autooxidation mixture of lycopene products. Lycopene was oxidized by exposure to atmospheric oxygen and perfusion of ozone. A lycopene oxidative product was tentatively identified as (E, E, E)-4-methyl-8-oxo-2,4,6nonatrienal.8 Using a seperate oxidizing system, Aust et al.9 identified an additional lycopene oxidative metabolite. Lycopene was completely oxidized using a combination of hydrogen peroxide and osmium tetroxide. The oxidized lycopene mixture was separated and a new metabolite was tentatively identified as 2,7,11-trimethyltetradecahexaene-1,14-dial.9 Utilizing yet another oxidizing system, Caris-Veyrat et al.10 identified an extensive number of lycopene oxidative products. Oxidation of lycopene by potassium permanganate produced eight apo-lycopenals and three apo-lycopenones,as detected by HPLC-DAD-MS, including acyclo-retinal and apo-10′-lycopenal, which are the predicted central and excentric enzymatic cleavage products. Six apo-carotendials were also detected. Although a number of putative lycopene oxidative metabolites have been identified and characterized in vitro, few products identified in these reports have been identified in vivo. However, taken together, the results from these studies suggest that the susceptibility of carbonyl compounds to cleavage by autooxidation, radical-mediated oxidation, and singlet oxygen occurs in carotenoids with a long chain of conjugated double bonds. These products may be produced in vivo if the tissues are exposed to oxidative stress such as smoking and drinking. While oxidative metabolites are inevitably produced in vivo, the importance of such metabolites remains poorly understood.
ENZYMATIC CLEAVAGE OF LYCOPENE
Carotene-15, 15′-oxygenase
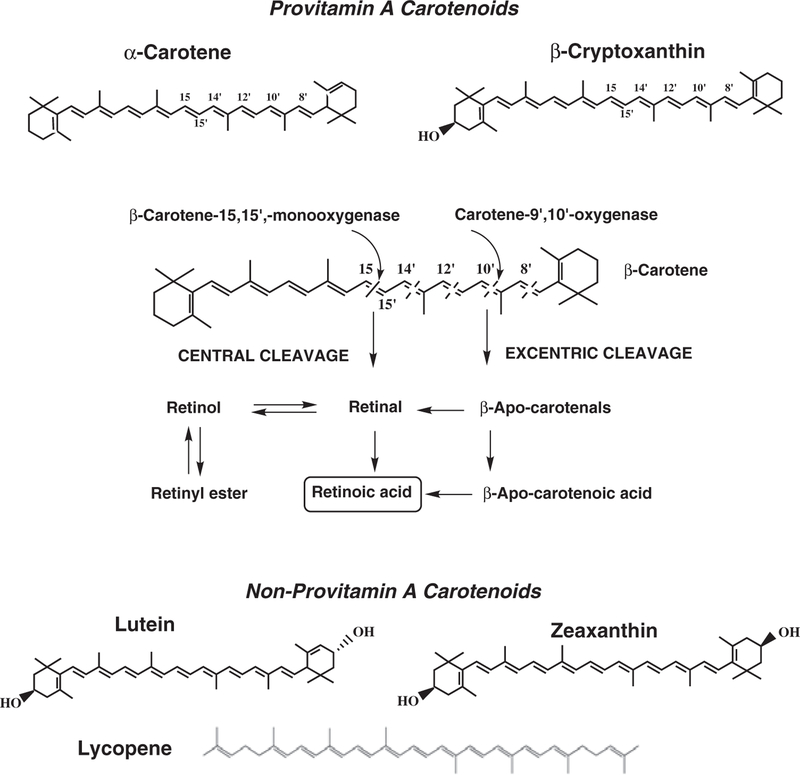
For provitamin A carotenoids, such as β-carotene, α-carotene, and β-cryptoxanthin, central cleavage is a major pathway leading to vitamin A formation (Figure 2).11–13 The carotene 15,15′-oxygenase (CMO1) gene, which is responsible for central cleavage at the 15, 15′ double bond,14,15 has been cloned and characterized in a number of species including the human and mouse.16–20 With the molecular characterization of CMO1, it has been definitively shown that CMO1 catalyzes the central cleavage of β-carotene to yield two molecules of retinal, thus contributing to vitamin A stores.21 While many reports have focused exclusively on the ability of CMO1 to cleave β-carotene, few have explored other carotenoids such as lycopene as potential substrates, and those that have, have had mixed results. Using the murine CMO1, Redmond et al.18 demonstrated cleavage activity towards lycopene. Using lycopene-accumulating Escherichia coli-expressing mouse CMO1, they observed a distinct bleaching of color when induced with L-arabinose, suggesting cleavage of lycopene. In addition, purified recombinant mouse CMO1 displayed cleavage activity towards lycopene. However, acyclo-retinal was only detected when the lycopene concentrations used were 2.5–3 times higher than the observed Km for β-carotene (Km = 6 μmol). In contrast, Yan et al.20 observed no detectable activity of human retinal pigment epithelium CMO1 towards lycopene or lutein. Their report supported the data of previous reports. For example, when lycopene was incubated with the Drosohpila homologue of CMO114 and crude preparations of rat liver and intestine,22 no lycopene cleavage products were detected. Additionally, Lindqvist and Andersson16, using a purified recombinant CMO1 isolated from a human liver cDNA library, demonstrated cleavage activity towards both β-carotene and β-crytoxanthin but no activity towards lycopene or zeaxanthin. Although CMO1 was shown to cleave β-cryptoxanthin, analysis of the apparent Km revealed an approximate fourfold lower affinity towards β-cryptoxanthin (Km = 30.0 ± 3.8 μM) than towards β-carotene (Km = 7.1 ± 1.8 μM).16 These authors concluded that the presence of at least one unsubstituted b-ionone ring appears to be sufficient for catalytic cleavage of the central carbon 15,15′ double bond. Taken together, these studies suggest that lycopene is a poor substrate for CMO1. Yet, this fact has not hindered research into the potential biological activity of acyclo-retinoids.
Figure 2. Metabolic pathway of β-carotene, and chemical structures of provitamin A carotenoids (β-carotene, α-carotene, and β-cryptoxanthin) and non-provitamin A carotenoids (lutein, zeaxanthin, and lycopene).
Adapted from Merntiz et al. (2007).11
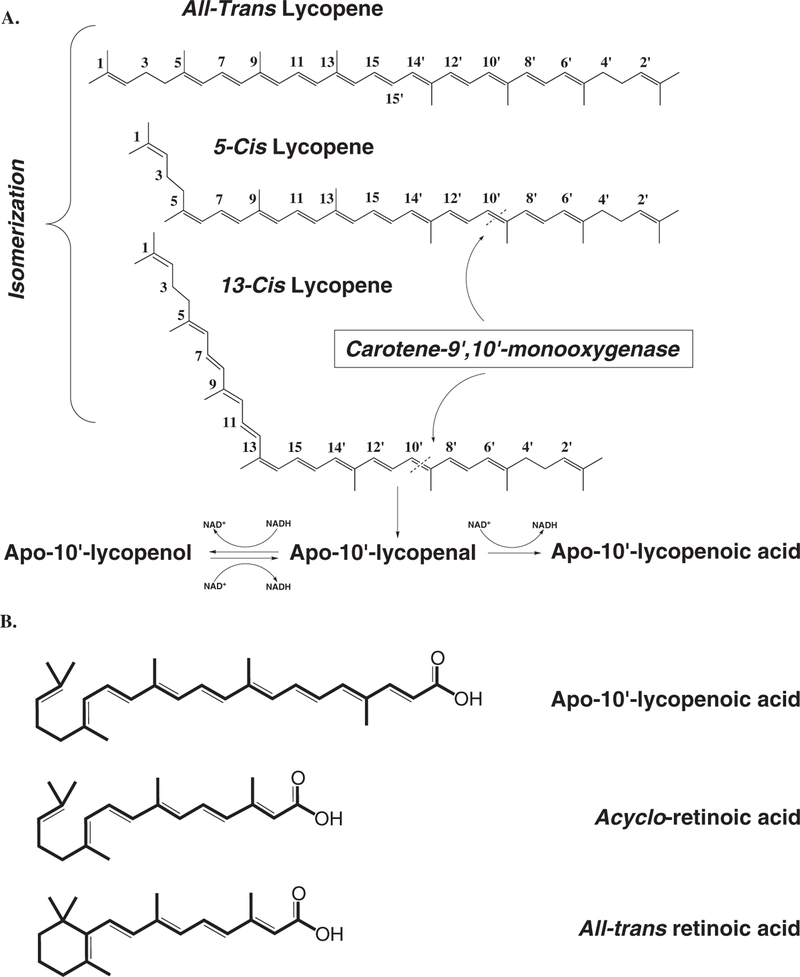
Although previous observations provide little evidence for lycopene as a substrate for CMO1, the previous observation that cis-lycopene isomers were superior substrates for CMO2 compared to all-trans lycopene23 brings about an important question. Does CMO1 cleave cis-lycopene isomers to acyclo-retinoids? Previous in vivo reports using all-trans lycopene as a supplement observed dramatic increases in the cis-isomers of lycopene, including 5-cis, 13-cis, and 9-cis isomers.24–27 From the previous in vitro kinetic analyses,16,18,20 it is unclear if all-trans lycopene was used as the substrate in determination of CMO1 activity towards lycopene. The chemical structures of cis-lycopene isomers could mimic the unsubstituted β-ionone ring structures of other carotenoid molecules and fit into the enzyme-substrate pocket enabling central cleavage (Figure 3). More understanding of the significance of the metabolism of cis-lycopene will help further elucidate the biological functions of lycopene.
Figure 3. Schematic illustration of lycopene metabolic pathway by CMO2.
(A) 5-cis and 13-cis lycopene are preferentially cleaved by CMO2 at 9′10′-double bond. The cleavage product, apo-10′-lycopenal, can be further oxidized to apo-10′-lycopenol or reduced to apo-10′-lycopenoic acid, dependent on the presence of NADH. (B) Chemical structures of apo-10′-lycopenoic acid, acycloretinoic acid, and all-trans retinoic acid. Adapted from Hu et al. (2006).23
Carotene-9′, 10′-oxygenase
In addition to the central cleavage pathway, an alternative pathway for carotenoid metabolism in mammals, termed the excentric cleavage pathway, remained a controversial issue for several decades. The controversy centered on the existence of a dedicated enzyme responsible for excentric carotenoid metabolism. We had previously demonstrated the random cleavage of β-carotene and identified a series of homologous carbonyl cleavage products, including β-apo-14′-, 12′-, 10′-, and 8′-carotenals, β-apo-13carotenone, and retinoic acid in tissue homogenates of humans, ferrets, and rats.28–30 This controversy was put to rest with the cloning and characterization of the murine carotene-9′, 10′-oxygenase by Kiefer et al.,31 thus confirming the existence of the asymmetric cleavage pathway of carotenoids. Keifer et al.31 isolated the murine CMO2 and demonstrated enzymatic cleavage of both β-carotene and lycopene. Using β-carotene as the substrate, HPLC analysis revealed that no retinoid compounds were formed, yet a peak matching the retention time of the β-apo-10′-carotenal standard was detected. Cleavage of β-carotene was further demonstrated using beta-carotene synthesizing and accumulating Escherichia coli strains expressing the mouse CMO2. A similar E. coli model, which synthesizes and accumulates lycopene, was used to demonstrate cleavage of lycopene by CMO2. When CMO2 was induced, a distinct color shift from red to white occurred, indicating cleavage. HPLC analysis revealed the formation of several putative apo-lycopenals. Although the authors state that significant amounts of apo-lycopenals were detected and tentative identifications were made based upon UV-visible spectra, no data was showntoaccompanytheseobservations.31 Given the putative observations regarding lycopene and cleavage by CMO2, we sought to further characterize the relationship of lycopene as a possible substrate for CMO2.
Because ferrets (Mustela putorius furo) and humans are similar in terms of carotenoid absorption, tissue distribution and concentrations, and metabolism,32,33 we cloned and characterized the ferret CMO2 gene.23 Using the reported cDNA sequence for a carotene excentric cleavage enzyme from humans, we cloned a full-length carotene-9′, 10′-oxygenase in ferrets that encodes a protein of 540 amino acids and has 82% identity with human carotene, 9′, 10′-oxygenase. Further analysis revealed that the enzyme is expressed in the testis, liver, lung, prostate, intestine, stomach, and kidneys of ferrets, similar to the expression pattern of human CMO2.34 Using the recombinant ferret CMO2 expressed in Spodoptera frugiperda (Sf9) insect cells for kinetic analysis, we found that the cleavage of carotenoids by the ferret CMO2 occurs in a pH, incubation time, protein dose, and substrate dose-dependent manner.23 Notably, the optimum pH for CMO2 is 8.5, which differs from the optimum pH (7.7) for the activity of CMO1, the central cleavage enzyme for carotenoids.16 This difference in optimum pH between these two carotenoid cleavage enzymes may indicate different roles of the two pathways in carotenoid metabolism or different functions in various pathophysiological conditions, which needs further investigation. Nonetheless, similar to the CMO1, we found that the cleavage activity of ferret CMO2 for both β-carotene and lycopene was iron-dependent, indicating that iron is an essential cofactor for the enzymatic cleavage activity of carotenoids. This is supported by the existence of four conserved histidine residues in the ferret CMO2.23 These data are in agreement with previous observations demonstrating these conserved histidines act as putative iron-binding residues for iron coordination in apocarotenoid 15,15′-oxygenase35 and CMO1,36 supporting the notion that the entire superfamily of oxygenases shares a common structure.36
Interestingly, we demonstrated that the recombinant ferret CMO2 catalyzes the excentric cleavage of all-trans β-carotene and cis-lycopene isomers effectively but not all-trans lycopene at the 9′, 10′ double bond.23 While we estimated a Km of 3.5 μM for all-trans β-carotene based on the CMO2 expressed in SF9 cells, we could not calculate the kinetic constants of CMO2 for lycopene due to difficulty in controlling auto-isomerization, thus necessitating the use of mixed isomers of lycopene as the substrate for kinetic analysis. Since the lycopene substrate mixture contains only ~20% as cis isomers and considering the ferret CMO2 would not cleave all-trans lycopene, we speculate that the Km for cis-lycopene is actually much lower than that of the lycopene isomer mixture. This indicates that cis-lycopene may act as a better substrate than all-trans β-carotene for the ferret CMO2. The mechanism whereby ferret CMO2 preferentially cleaves the 5-cis and 13-cis-isomers of lycopene into apo-10′-lycopenal but not all-trans lycopene is currently unknown. One possible explanation is that the chemical structure of cis isomers of lycopene could mimic the ring structure of the β-carotene molecule and fit into the substrate-enzyme binding pocket (Figure 3). Although this hypothesis warrants further investigation, the observation that supplementation of all-trans lycopene results in a significant increase in cis-lycopene tissue concentration in ferrets underlies the significance of this observation.24–26
REGULATION OF CAROTENE OXYGENASES
Unlike vitamin A, high-dose β-carotene supplementation does not result in hypervitaminosis A, indicating that cleavage of β-carotene to vitamin A is tightly regulated. A number of animal studies have demonstrated that CMO1 activity is affected by vitamin A status.37,38 Other studies have indicated that expression of CMO1 may be regulated at the transcriptional level through feedback regulatory mechanisms via interactions between retinoic acid and its nuclear receptors.39,40 Recent molecular studies of the mouse and human CMO1 promoter demonstrated the presence of a peroxisome proliferator response element (PPRE).41,42 PPARγ and RXRα agonists were shown to transactivate the CMO1 promoter-reporter when cotransfected with the corresponding nuclear receptor.41 Analysis of the human CMO1 promoter identified an additional enhancer element. A myocyte enhancer factor-2 (MEF2) binding site was identified and when mutated reduced luciferase activity by ~30%.42 The in vivo importance of the MEF2 binding site is not fully understood. Nonetheless, the regulation by PPAR and RXR indicates a regulatory link between carotenoid and lipid metabolism. Two recent reports have shed some light onto this relationship. In F344 rats supplemented with lycopene, CMO1 expression was significantly decreased in the adrenal gland and kidney.43 Interestingly, fatty acid binding protein-3 (FABP-3), a PPARγ target gene, was downregulated in parallel with CMO1. While vitamin A production from β-carotene was abolished in CMO1-knockout (KO) mice, lipid metabolism was significantly altered.21 There was a significant increase in several fatty acid metabolism genes, including CD36 and fatty acid binding protein-4 (FABP4) expression, both PPARγ target genes, in visceral adipose tissue. Additionally, there were significant increases in serum free fatty acids and total lipids resulting in hepatic steatosis. It has been suggested that cleavage products of β-carotene may fine-tune the crosstalk between the nuclear receptors that regulate lipid metabolism.44–46 The relationship between carotenoid and lipid metabolism deserves further inquiry.
Regulation of CMO2 is less well understood. A recent analysis failed to identify a PPRE within the mouse CMO2 promoter.43 Work in our laboratory also failed to identify any potential nuclear receptor response elements or any other enhancer sequences within the ferret CMO2 promoter (unpublished data). Recent in vivo studies have provided evidence of an effect of lycopene on CMO2 expression. After 9 weeks of lycopene supplementation, we observed an ~4-fold increase in CMO2 expression in the lungs of ferrets.23 In F344 rats, however, there was no significant effect of lycopene on CMO2 expression in several tissues, including lungs.43 β-carotene supplementation also had no significant effect on CMO2 expression. In ferrets supplemented with low- and high-dose β-carotene and exposed to cigarette smoke for 6 weeks, we observed no change in lung and liver CMO2 expression.47 Similar findings were observed using CMO1knockout mice. CMO1-KO mice supplemented with β-carotene accumulated significant amounts of β-carotene in various tissues.21 However, there were no changes in CMO2 protein expression in any tissues analyzed. Clearly, more research is needed in order to gain a better understanding of the transcriptional regulatory mechanisms of CMO2.
LYCOPENE METABOLITES IN VIVO
While in vitro oxidation studies have yielded a large number of oxidative metabolites, in vivo studies have yielded a much smaller catalogue of lycopene metabolites.48,49 Khachiket al.50–52 first identified 5,6-dihydroxy-5, 6-dihydrolycopene in human serum. It was proposed that formation of this metabolite results from oxidation of lycopene forming 5,6 epoxide, which is then reduced to the 5,6-dihydroxy-5, 6-dihydrolycopene metabolite. The same group also identified epimeric 2,6-cyclolycopene-1, 5-diols in human milk and serum.53 Based upon studies of lycopene oxidation with m-chloroperbenzoic acid,54,55 it was proposed that lycopene is first oxidized at 1, 2 – and 5, 6 –positions to form lycopene 1,2-epoxide and lycopene 5,6-epoxide. Due to the instability of the epoxide rearrangement products, cyclization occurs and results in the corresponding diols. Interestingly, epimeric 2,6cyclolycopene −1,5-diols are also found in low concentrations in tomato-based products.55 Whether the presence of these metabolites results from consumption of tomato-based products or from in vivo oxidation or both is not fully understood.
The use of animal models has proven to be useful in the identification of in vivo lycopene metabolites. A study in preruminant cattle identified as 5, 6-dihydrolycopene and 5, 6-dihydro-5-cis lycopene in serum after 2 weeks of lycopene supplementation.56 Using 14C-labeled lycopene, Gajic et al.57 detected both apo-8′-lycopenal and apo-12′-lycopenal in rat liver 24 h post dosing. In addition, a large quantity of very polar, unidentified short-chain compounds was detected. We have recently identified apo10′-lycopenol in ferret lungs, which is the predicted cleavage product of CMO2, and certain unidentified compounds in the HPLC profiles of ferret lungs after lycopene supplementation for 9 weeks.23 Since we did not detect apo-10′-lycopenal or apo-10′-lycopenoic acid in the lung tissues of lycopene-supplemented ferrets, it is likely that apo-10′-lycopenal is a short-lived intermediate compound and can be reduced to its alcohol form in vivo, and apo-10′-lycopenoic acid may be present at too low a concentration to be detected in our HPLC system. This was supported by our subsequent demonstrations that incubation of apo-10′-lycopenal with the post-nuclear fraction of hepatic tissue of ferrets resulted in both apo10′-carotenol and apo-10′-lycopenoic acid, depending upon the presence of either NAD+ or NADH. In the presence of NADH, apo-10′-lycopenal was converted to both the alcohol and acid forms (Figure 3). The latter could be due to the consumption of NADH for the reduction reaction, which makes NAD+ available for the oxidation of apo-10′-lycopenal. Nonetheless, the presence of specific metabolites has not been consistent across different animal models. Whether these differences are due to different methodological approaches, species used, or tissues analyzed should be ascertained.
BIOLOGICAL ACTIVITY OF LYCOPENE METABOLITES
The identification of lycopene metabolites in vitro and in vivo raises the question of whether lycopene metabolites, similar to other carotenoid metabolites that can possess either more or less activity than the parent compound or have entirely different functions,58 may contribute, at least in part, to the biological functions ascribed to lycopene.
Antioxidant properties
Much of the biological activity ascribed to lycopene has been attributed to its antioxidant capabilities. Indeed, antioxidant properties of many carotenoids have been long believed to play critical roles in their anticarcinogenic actions.59 Lycopene is a powerful in vitro antioxidant. Among naturally occurring carotenoids, lycopene has shown the strongest ability to scavenge free radicals60 and chemically quench singlet oxygen,61,62 being 2-fold and 10-fold more effective at quenching singlet oxygen than β-carotene and α-tocopherol,respectively.62 Accordingly, several epidemiological studies have evaluated the role of lycopene as a potential in vivo antioxidant. Using tomatoes or tomato products, numerous studies have demonstrated decreased DNA damage,63,64 decreased susceptibility to oxidative stress in lymphocytes,65,66 and decreased LDL oxidation67 or lipid peroxidation.67,68 However, most in vivo studies have used tomato products, which also contain various micronutrients and phytochemicals, including other carotenoids, polyphenols, vitamin C, and vitamin E. Caution must be taken when attributing the beneficial effects of tomatoes and tomato products solely to lycopene. Data regarding the antioxidative effects of lycopene alone in biological systems are limited.
While evidence suggests that intact lycopene functions as an antioxidant, especially in vitro, there is little evidence to support an antioxidant role of lycopene metabolites. However, recent evidence suggests that lycopene metabolites may indeed possess putative antioxidant functions. We have recently provided evidence of a possible indirect antioxidant effect of the excentric cleavage product apo-10′lycopenoic acid in BEAS-2B cells. After 24 h of treatment with apo-10′-lycopenoic acid (3–10 μM), we observed a dose-dependent decrease in endogenous ROS production.69 This decrease in ROS was comparable to control cells treated with tert-butylhydroquinone (tBHQ). We next determined if apo-10′-lycopenoic acid had any effect on H2O2-induced oxidative damage, as measured by lactate dehydrogenase release (LDH). Pre-treating BEAS-2B cells with apo-10′lycopenoic acid (3–10 μM) for 24 h resulted in a dose-dependent inhibition of LDH release. These results were comparable to control cells pre-treated with tHBQ.69 Taken together, our data suggests that lycopene metabolites in general, and apo-10′-lycopenoic acid in particular, may possess indirect antioxidant functions. More research is needed.
Gap-junction communication
Gap junctions are cell-to-cell channels that enable connected cells to exchange nutrients, waste products, and information. Each gap junction is derived from six connexin proteins from each adjacent cell for a total of 12 connexin proteins. The connexin family has >20 connexins that are expressed in mammals with both cell and developmental specificity of expression.70 Although there are >20 connexins, connexin 43 (Cx43) is the most widely expressed connexin. More interestingly, Cx43 is the connexin most often induced by retinoids and carotenoids. Gap junction communication (GJC) has been implicated in the control of cell growth via adaptive responses, i.e., differentiation, proliferation, and apoptosis.71 A large body of evidence now indicates loss of GJC is a hallmark of carcinogenesis.72
Targeting connexins as a possible strategy for chemoprevention has been suggested.72 Retinoids and carotenoids have been shown to inhibit carcinogenesis. Moreover, retinoids and carotenoids increase GJC between normal and transformed cells.73,74 Interestingly, it was demonstrated that both pro-vitamin A and non-provitamin A carotenoids inhibited carcinogen-induced neoplastic transformation75 and upregulated Cx43 mRNA expression.73,74 Furthermore, whereas treatment with retinoic acid increased Cx43 expression within 6 h, carotenoid treatment required approximately three times longer and produced the same response.76,77 This lag in activity is often attributed to the formation of active metabolites.
Several lines of in vitro evidence indicate that carotenoid oxidative products/metabolites may be responsible for increased GJC, especially in the case of lycopene. After complete oxidation of lycopene with hydrogen peroxide and osmium tetroxide, Aust et al.9 isolated an oxidative metabolite that effectively increased GJC. The compound, identified as 2,7,11-trimethyl-tetradecahexaene-1, 14-dial, induced GJC to a level comparable to retinoic acid. The oxidative metabolite lycopene-5,6-epoxide, which is found in tomatoes, was showntoincreaseCx43expression in human keratinocytes.52 Stahl et al.78 demonstrated that the central cleavage product of lycopene, acyclo-retinoic acid (ACR), could increase GJC. However, an effect of ACR on GJC was only achieved at high concentrations, indicating that the contribution of ACR to the activity of lycopene on GJC appears to be minimal. More recently, we demonstrated cleavage of lycopene to apo-10′-lycopenal by ferret CMO2.23 While the Cx43 promoter does not contain an RARE, it has been reported that RAR antagonists inhibited upregulation by retinoids and had no effect on the effect of carotenoids.79 This is interesting due to the effect of both oxidative metabolites and enzymatic cleavage metabolites of lycopene on modulating GJC, which could provide two separate pathways of increasing GJC. Considering the bio-conversion of lycopeneintoapo-10′-lycopenoids, whether apo-10-lycopenoids contribute to lycopene activity on GJC warrants further study.
Retinoid activity
Retinoids, the most important oxidative products of provitamin A carotenoids, play an essential role in several critical processes, including vision, reproduction, metabolism, differentiation, hematopoiesis, bone development, and pattern formation during embryogenesis.80 Considerable evidence supports the role of retinoids in the management of several human chronic diseases, including cancer.81 The mechanism by which retinoids elicit these responses is through their ability to regulate gene expression at specific target sites within the body. Beta-carotene and its excentric cleavage metabolites can serve as direct precursors for all-trans and 9-cis-retinoic acid,30,82,83 which are ligands for both retinoic acid receptors (RAR) and retinoid X receptors (RXR). Retinoid receptors function as ligand-dependent transcription factors and regulate gene expression by binding as dimeric complexes to the retinoic acid response element (RARE) and the retinoid X response element (RXRE), which are located in the 5′ promoter region of responsive genes. RXR can not only form dimeric complexes with RAR, it can also dimerize with other members of the nuclear hormone receptor superfamily, such as thyroid hormone receptors, the vitamin D receptor (VDR), peroxisome proliferators-activated receptors (PPAR), and possibly other receptors with unknown ligands designated orphan receptors.
Upregulation of retinoid receptor expression and function by provitamin A carotenoids may play a role in mediating the growth inhibitory effects of retinoids in cancer cells.84,85 However, it is unclear if non-provitamin A carotenoids and their metabolites may function in a similar fashion. Several reports have evaluated the ability of the CMO1 cleavage product, acyclo-retinoic acid (ACR), to transactivate the RARE. Using a RARE reporter gene, Ben-Dor et al.86 demonstrated that ACR transactivates the RARE-reporter gene through an interaction with RARα. However, the potency of activation was approximately 100-fold lower than retinoic acid. Binding affinity studies indicated that ACR bound RXRα with no appreciable affinity, whereas ACR bound RARα with an equilibrium dissociation constant in the range of 50–150 nM – two orders of magnitude lower than all-trans retinoic acid. Intact lycopene did not show any significant binding to either receptor. However, intact lycopene displayed a weak transactivation of the RARE reporter gene.86 Stahl et al.78 demonstrated similar findings with the RAR-β2 promoter. Only when ACR was provided at concentrations 500-fold higher than retinoic acid was an effect on luciferase and β-galactosidase reporter activity observed.78 There was no effect of intact lycopene on reporter transactivation at the concentrations used in this study. ACR was also found to have no significant effect on transactivation of RAR and RXR reporter systems in yet another study.87 While an effect of ACR on retinoid signaling has not been substantiated in vivo, a synthetic acyclic retinoid, lacking one or two double bonds, has been shown to transactivate retinoid reporter systems with potential benefits in the treatment of hepatocellular carcinoma.88–90
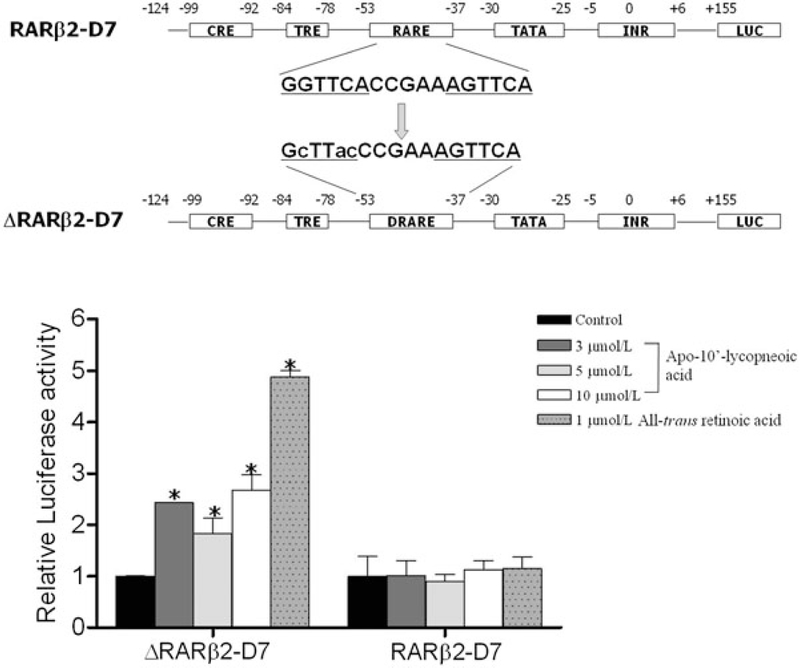
Because of the similarity in chemical structures among apo-10′lycopenoic acid, acyclo-retinoic acid, and all-trans retinoic acid (Figure 3), we questioned whether apo-10′-lycopenoic acid is an activator of RARs. We hypothesized that the antiproliferative effects of the excentric cleavage product apo-10′-lycopenoic acid91 may be mediated through induced expression of the RARβ receptor, which has been suggested to be a tumor suppressor gene playing a critical role in mediating the growth inhibitory effects of retinoids in various cancer cell lines.92 Three cell lines – NHBE, a normal human bronchial epithelial cell line, BEAS-2B, an immortalized human bronchial epithelial cell line, and A549 cells, a non-small-cell lung cancer cell – representing different stages of lung carcinogenesis were incubated with increasing concentrations of apo-10′-lycopenoic acid (3–5 μmol/L).91 After 48 h, we observed a dose-dependent increase in RARβ mRNA expression in both NHBE and BEAS-2B cell lines. The effect of apo-10′lycopenoic acid was similar to that of all-trans-retinoic acid. However, there was only a slight increase in expression of RARβ in A549 cells. Compared to NHBE and BEAS-2B cells, A549 cells were less sensitive to apo-10′-lycopenoic acid and retinoic acid in terms of RARβ expression. We next sought to determine if increased RARβ mRNA expression was due to increased transactivation of the RARβ promoter region. A series of reporter constructs containing serially deleted fragments of the RARβ promoter were constructed. Treatment with apo-10′-lycopeneoic acid increased luciferase activity in all constructs. However, the magnitude of the increase in luciferase was less than that induced by retinoic acid. The deletion of the promoter region between −1500 and −124 bp did not affect the transactivation activity of apo10′-lycopenoic acid. To investigate the involvement of the RARE in the promoter, located between −53 and −37 bp, site-directed mutagenesis was utilized to abolish the RAR binding site (Figure 4). Both apo-10′-lycopenoic acid and retinoic acid significantly increased luciferase activity in the presence of the wild-type RARE. However, mutation of the RAR binding site completely abolished promoter activity induction by both retinoic acid and apo-10′lycopenoic acid (Figure 4). These results suggest that the growth inhibitory actions of apo-10′-lycopenoic acid may be mediated through retinoid signaling.
Figure 4. The involvement of RARE on apo-10′-lycopenoic acid-transactivated RARβ expression.
Upper panel shows a diagram of the construction of the RARβ reporter vector with wild-type or mutated RARE. Lower panel, RARβ reporter vector was treated with apo-10′-lycopenoic acid or all-trans retinoic acid for 24 h. Luciferase activities were measured by dual-luciferase reporter system. Values are means ± SEM of three replicate assays.
*Statistically different, as compared with control in the same group, P < 0.05.
Adapted from Lian et al. (2007).91
Induction of phase II enzymes
The drug-metabolizing phase I and II enzymes respond to various compounds, including drugs, environmental compounds, pollutants, carcinogens, and dietary and endogenous compounds. Phase I enzymes, such as cytochrome P450, catalyze the addition of oxygen to carcinogens, thereby increasing the reactivity of carcinogens and the formation of DNA adducts, known as bioactivation.93 In general, the action of phase II enzymes increases the hydrophilicity of carcinogens and enhances their detoxification and excretion.94 Evidence in recent years has begun to accumulate indicating the beneficial effect of lycopene may be due, in part, to induction of phase II detoxification enzymes.95 Induction of phase II enzymes is mediated through cis-regulatory DNA sequences located in the promoter or enhancer region, which are known as antioxidant response elements (ARE).96 The major ARE transcription factor Nrf2 (nuclear factor E2-related factor 2) is a primary factor involved in induction of antioxidant and detoxifying enzymes97 and is essential for the induction of several phase II enzymes, including glutathione S-transferases, NAD(P)H:quinone oxidoreductase.98
Many carotenoids, including lycopene, have been shown to induce several phase I and II enzymes both in vivo and in vitro.43,99–101 Gradelet et al.99 observed an induction in the phase II enzymes p-nitrophenol-UDP-glucuronosyltransferase and quinone oxidoreductase in rats fed various carotenoids. While canthaxanthin and astaxanthin induced phase II activity, lycopene and lutein had no effect after 15 days of feeding. In another study, Breinholt et al. demonstrated a dose-dependent induction of several phase I and II enzymes in female Wistar rats supplemented with lycopene at doses ranging from 0.001 to 0.1 g/kg body weight for 2 weeks.100 Hepatic ethoxyresorufiin O-dealkylase (EROD) and benzyloxyresorufin O-dealkylase (BROD) increased approximately 2-fold and 50%, respectively, suggesting activation of cytochrome P450 enzyme (CYP) 1A (CYP1A) enzymes. In addition, several liver and red blood cell phase II enzyme activities, such as glutathione reductase, glutathione S-transferase, and quinone reductase, were significantly increased by lycopene feeding. The induction of phase II enzymes by lycopene has been reported in other animal studies.43,102 However, it is unclear if induction of xenobiotic metabolizing enzymes is due to intact lycopene or lycopene metabolites. Ben-Dor et al. showed that lycopene induced phase II enzymes by activating Nrf2 transcription factor. More interestingly, an ethanolic extract of lycopene containing unidentified hydrophilic derivatives activated ARE-driven reporter gene at a similar potency to lycopene alone.101 Although the identity of the lycopene oxidative derivatives is unknown, this study suggested that lycopene oxidative metabolites might be responsible for the induction of phase II enzymes through ARE-induced expression.
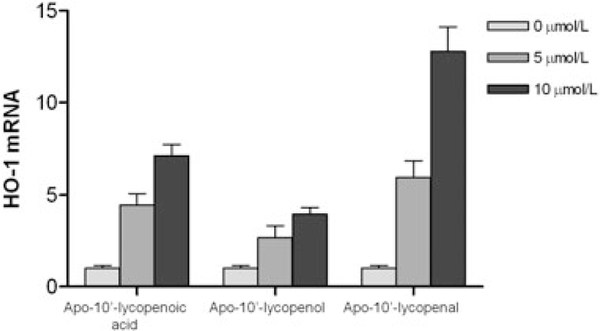
We have recently provided evidence that the lycopene CMO2 cleavage product, apo-10′-lycopenoic acid, induces phase II enzyme expression in vitro. Using BEAS-2B human bronchial epithelial cells, we demonstrated a dose- and time-dependent increase in nuclear Nrf2 protein accumulation with apo-10′-lycopenoic acid treatment.69 In addition, apo-10′-lycopenoic acid significantly induced mRNA expression of several phase II enzymes, including heme-oxygenase-1 (HO-1), quinone oxidoreductase, glutathione S-transferase, glutathione reductase, glutamate-cysteine ligase (catalytic unit, GCLC; and modifier unit, GCLM), microsomal epoxide hydrolase 1, and UDP glucuronosyltransferase 1 family, polypeptide A6 compared to THF alone.69 Additionally, all three apo-10′-lycopenoids were effective in activating the Nrf2-mediated induction of HO-1 (Figure 5). Although the exact mechanisms behind activation of the Nrf2-dependent HO-1 induction by these three lycopenoids remain unknown, the chemical properties of these compounds may contribute to the activation of Nrf2, which is controlled through multiple regulatory mechanisms, including Keap1-mediated ubiquitination and degradation, subcellular distribution, and phosphorylation. Notably, apo-10′-lycopenal showed the strongest induction of HO-1 as compared with apo-10′-lycopenoic acid and apo-10′-lycopenol.This may be due to the highly reactive aldehyde group in the compound, which is capable of Schiff base formation with the amino groups of protein and reactive with cellular macromolecules (e.g., directly modifying the reactive cysteine residues in Keap1 and interrupting Keap1-mediated Nrf2 ubiquitination and degradation).It is also possible that these lycopenoids affect upstream signaling pathways, such as mitogen-activated protein kinases, phosphoinositol 3-kinase, epidermal growth factor receptor, and protein kinase C, which all have been shown to play a role in the regulation of Nrf2-ARE in lung epithelial cells. Clearly, these hypotheses warrant further investigation.
Figure 5. Effect of apo-10’-lycopenoic acid, apo10’-lycopenol, and apo-10’-lycopenal on hemooxygenase-1 gene expression.
Values are means ± SEM of three replicate assays. Adapted from Lian et al. (2008).69
Interference with growth factors
It has been suggested that the insulin-like growth factors (IGF) signaling system may play a role in the biological action of lycopene.103,104 IGFs (IGF-1 and IGF-2) are mitogens that play a central role in the regulation of cellular proliferation, differentiation, and apoptosis.105 By binding to membrane IGF-1 receptors, IGFs activate intracellular phospatidylinositol 3′-kinase/Akt/protein kinase B and Ras/Raf/mitogen-activated protein kinase pathways, which regulate various biological processes such as cell cycle progression, survival, and transformation.106 IGFs are sequestered in circulation by a family of binding proteins (IGFBP1–IGFBP6), which regulate the availability of IGFs to bind to IGF receptors.106 Disruption of normal IGF signaling, leading to hyperproliferation and survival signal expression, has been implicated in the development of several tumor types.107 Indeed, strong positive associations have been found between plasma IGF-I levels and prostate cancer risk,108 breast cancer risk,109 and colorectal cancer risk.110 Recent epidemiological studies provide supportive evidence that lycopene may have a chemopreventive effect against a broad range of epithelial cancers, particularly prostate, breast, colorectal, and lung cancer.3,111–114 Sharoni et al. provided a potential mechanism whereby lycopene interfered with IGF-1-stimulated cell growth.103,104,115 They showed that IGF-1-stimulated cell growth, as well as DNA binding activity of the AP-1 transcription factor, were reduced by physiological concentrations of lycopene in endometrial, mammary (MCF-7), and lung (NCI-H226) cancer cell lines. Lycopene has been shown to inhibit IGF-1stimulated insulin receptor substrate 1 phosphorylation and cyclin D1 expression, block IGF-1-stimulated cell cycle progression,103,116 and increase membrane-associated IGFBPs.103,115 Consistent with previous in vitro findings, recent epidemiological studies demonstrated higher dietary intake of lycopene has been associated with lower circulating levels of IGF-1117 and higher levels of IGFBPs.118,119
We have examined the effect of lycopene on prevention of IGF signaling in cigarette smoke-related lung carcinogenesis in the ferret model.25 We found that plasma IGF-1 levels were not affected by cigarette smoke exposure or lycopene supplementation. However, IGFBP-3 levels were increased by lycopene supplementation and decreased by smoke exposure. Interestingly, lycopene increased plasma IGFBP-3 regardless of smoke exposure status. Increased plasma IGFBP-3 was associated with inhibition of cigarette smoke-induced lung squamous metaplasia, decreased proliferating cell nuclear antigen (PCNA), phosphorylated BAD levels, and cleaved caspase 3,suggesting inhibition of cell proliferation and induction of apoptosis.25 These results, along with others, suggest that interference of IGF-1 signaling may be an important mechanism by which lycopene exerts its anticancer activity. However, whether intact lycopene or its metabolites are responsible for the observed effects on IGF-1 signaling remains unknown. We recently provided evidence that lycopene metabolites may be partly responsible. Treatment with apo-10′-lycopenoic acid (5–20 μM) resulted in a dose-dependent increase in IGFBP-3 mRNA levels in THLE-2 human liver cells. Similar concentrations of retinoic acid, lycopene, and ACR showed no significant effect on induction of IGFBP-3 mRNA levels (unpublished results). Research into this area is ongoing in our laboratory.
Cell proliferation and apoptosis
The growth inhibitory effect of lycopene was first demonstrated by Levy et al., who showed that lycopene is a stronger cell growth inhibitor than β-carotene.104 This growth inhibitory effect was further observed in several cell lines, including breast cancer,104,120 prostate cancer,104 lung cancer,104 human colon cancer cells,121 and oral cavity cancer,122 as well as normal prostate epithelial cells.123 Cell proliferation is controlled by a series of cell cycle regulators, including cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors, which regulate cell cycle progression. Specifically, cyclin D regulates the transition from G0 to early G1 phase, while cyclin E regulates the transition of the cell from late G1 phase to S phase; p21 and p27 CDK inhibitors bind and inhibit the activity of cyclin E/CDK2 complex, blocking cell cycle progression in G1 phase. The growth inhibition of lycopene on MCF-7 breast cancer cells was associated with decreased G1-S cell cycle progression, decreased cyclin D1 expression, and stabilization of p27 in the cyclin E-CDK complex.116,120 Lycopene was recently associated with an inhibition of tumor invasion, cell proliferation, and angiogenesis in the lungs of nude mice injected with SK-Hep-1 human hepatoma cells.124 In addition to cell proliferation inhibition, the growth inhibitory effect of lycopene may also be attributed to induction of apoptosis. Hwang et al. observed that 1 μM water-soluble lycopene inhibited the growth of LNCaP prostate cancer cells while 5 μM lycopene blocked cells in G2/M phase and induced apoptosis, suggesting high concentrations of lycopene can induce DNA damage.125 In another study, physiological concentrations of lycopene (0.3–3 μM) did not affect proliferation of LNCaP cells but rather affected mitochondrial function and induced apoptosis.126 Palozza et al.127 reported that lycopene (0.5–2 μM) inhibited the growth of cigarette smoke condensate-exposed immortalized RAT-1 fibroblast cells by arresting cell cycle progression and inducing apoptosis.
Among identified lycopene metabolites, the central cleavage product, ACR, an analog of retinoic acid, is the best studied. It has been shown to inhibit cell proliferation86,128,129 and induce apoptosis130 in a variety of cell lines. Using lycopene, ACR, and retinoic acid, Ben-Dor et al. observed a decrease in cell growth and a decreased rate of cell cycle progression, especially G1 to S transition, in MCF-7 mammary cancer cells.86 Both ACR and retinoic acid inhibited cell growth with a similar potency (IC50 ~ 1–2 μM) to lycopene. Moreover, both ACR and retinoic acid decreased serum-stimulated cyclin D1 protein expression, a similar finding also observed with intact lycopene.120 In addition to ACR, the activity of other lycopene metabolites has been investigated. Zhang et al.8 identified an oxidative product of lycopene, (E, E, E)-4-methyl-8-oxo-2,4,6-nonatrienal, that induced apoptosis in HL-60 cells. A dose-dependent reduction of cell viability with a concomitant increase in chromatin condensation and nuclear fragmentation, characteristic of apoptosis, were observed. Further analysis revealed an increased ratio of sub-G1 cells and an increase in caspase-8 and caspase-9 activity. These apoptotic changes were accompanied by a decrease in Bcl2 and Bcl-XL protein expression but no changes in Bax expression. In spite of the evidence above, the physiologic role of these lycopene products remains unknown since none of these metabolites have been detected in biological systems.
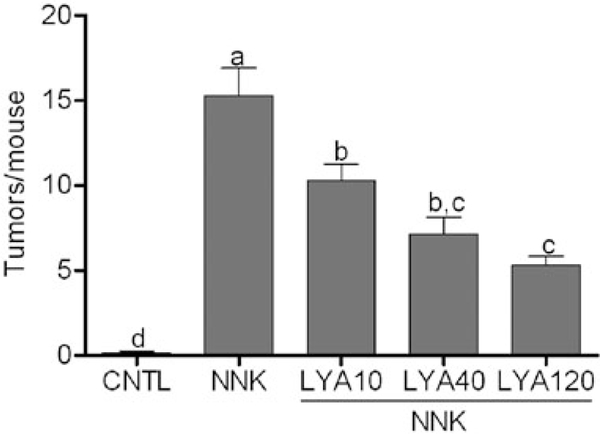
Recently, we investigated the activity of the excentric cleavage product apo-10′-lycopenoic acid on cell proliferation in the following three cell lines: NHBE, a normal human bronchial epithelial cell line; BEAS-2B, an immortalized human bronchial epithelial cell line; and A549 cells, a non-small-cell lung cancer cell, which represent different stages of lung carcinogenesis.91 We showed that apo-10′-lycopenoic acid treatment inhibited cell growth in all three cell lines, albeit with different sensitivity. Three micromolesapo-10′-lycopenoicacidsignificantlyreduced cell numbers by 40 and 60% in NHBE and BEAS-2B, respectively, whereas cell numbers were reduced ~50% in A549 cells treated with 10 μM apo-10′-lycopenoic acid. The growth inhibitory effect was further examined in BEAS-2B and A549 cells using the 3-(4,5dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cell viability was decreased by ~20% in A549 cells while viability decreased ~80% in BEAS-2B cells treated with 0.5 μM apo-10′-lycopenoic acid. The growth inhibitory action of apo-10′-lycopenoic acid was largely due to decreased cell proliferation, as we did not observe any induction of apoptosis. The effects of apo10′-lycopenoic acid on cell cycle distribution were analyzed by fluorescent-activated cell sorting analysis (FACS). Treatment with apo-10′-lycopenoic acid for 48 h significantly decreased A549 cells in S-phase from 31% in cells treated with THF alone to 24% and 21% in cells treated with 3 and 5 μM apo-10′-lycopenoic acid. Accordingly, there was a concomitant increase in the number of cells in G1/G0 phase. These results suggested the effect of apo-10′-lycopenoic acid on cell proliferation was due to effects on cell cycle regulators; thus, we investigated potential cell cycle regulators to identify potential targets of apo-10-lycopenoic acid. Treatment of A549 cells resulted in a dose-dependent decrease in mRNA and protein levels of cyclin E but not cyclin D. Analysis of p21 and p27 mRNA levels revealed no significant effects of apo-10′-lycopenoic acid on mRNA expression. However, there was a significant increase in p21 and p27 protein levels. Similar results were observed in BEAS-2B cells.91 We also observed similar findings in human liver cells. Apo-10′lycopenoic acid dose-dependently inhibited cell growth and induced apoptosis in THLE-2 liver cells by stimulating the cyclin-dependent kinase inhibitor p21 and by reducing activation of Jun N-terminal kinase (JNK) and cyclin D1 gene expression.131 In order to support our in vitro findings, an in vivo study was performed to evaluate the effect of apo-10′-lycopenoic acid on tumor development in the A/J mouse model of lung cancer. A/J mice were preloaded with control diet or diet containing 10, 40, or 120 mg/kg diet of apo-10′lycopenoic acid for 2 weeks before lung tumors were induced by injection of 4-(N-methyl-N – nitrosamino)1-(3-pyridal)-1-butanone (NNK). After 14 weeks on experimental diets, a significant decrease in tumor number (Figure 6) but not tumor incidence was observed in treated animals.91 Interestingly, the plasma levels of apo-10′-lycopenoic acid, which demonstrated the protective effect against lung tumor formation in mice, was much lower than reported plasma lycopene concentrations in humans, suggesting that apo-10’-lycopenoic acid may,at least partially, mediate the chemopreventive activity of lycopene.
Figure 6. Effect of apo-10′-lycopenoic acid supplementation on NNK-induced lung tumor development in A/J mice.
A/J mice were fed control diet or diet supplemented with apo-10′-lycopenoic acid for 16 weeks. Lung tumors were induced by injection of NNK at week 3 of supplementation. Tumor nodules on the surface of mouse lung tissues were counted and recorded as tumor multiplicity (tumors/mouse).
Abbreviations: CNTL, non-supplementation plus sham injection; NNK, non-supplementation plus NNK injection; LYA10, LYA40, and LYA120, 10, 40, and 120 mg/kg diet of apo-10′lycopenoic acid supplementation plus NNK injection. Values are presented as means ± SEM, n = 12–14. Groups that do not share a letter are significantly different, P < 0.05. Adapted from Lian et al. (2007).91
It should be pointed out that the potential use of lycopene metabolites, such as apo-10′-lycopenoic acid, as chemopreventive agents against cancers demands careful investigation. The in vivo metabolism of lycopene is complicated, and may be affected by a number of environmental factors, such as oxidative stress induced by cigarette smoking and alcohol consumption. For example, we have shown that high doses of β-carotene in an oxidative environment (such as the lungs of smokers) may result in excess levels of polar metabolites, which can promote carcinogenesis, whereas lower doses of β-carotene have been shown to be protective.132,133 In a very recent study, we observed that high-dose lycopene supplementation in the presence of alcohol ingestion increased hepatic inflammation and TNF-α expression.134 While no apparent adverse effects, such as a decrease in body weight or tissue damage, were observed in our recent study of apo-10’-lycopenoic acid-supplemented, NNK-treated A/J mice,91 an earlier study showed enhancement of benzo[a]pyrene-induced mutagenesis in mouse lung and colon tissues after lycopene supplementation.135 These results suggest that lycopene or lycopene metabolites may, as β-carotene and its metabolites do, enhance carcinogenesis. Further investigation into the dose effects of lycopene, especially in response to smoke exposure and/or alcohol ingestion, as well as a further understanding of the metabolism of apo-10′-lycopenoids on carcinogenesis is needed.
CONCLUSION
To gain a better understanding of the beneficial biological activities of lycopene upon cancer prevention, a greater knowledge of the metabolism of lycopene is needed. In particular, the identification of lycopene metabolites and oxidation products in vivo, the importance of tissue specific lycopene cleavage by CMO1/CMO2, and the potential interaction between lycopene dose and smoking and alcohol ingestion remains a vital step towards a better understanding of lycopene actions. An important question that remains unanswered is whether the effect of lycopene on various cellular functions and signaling pathways is a result of the direct actions of intact lycopene or its derivatives. While evidence is presented in this review to support the latter, more research is clearly needed to identify and characterize additional lycopene metabolites and their biological activities, which will potentially provide invaluable insights into the mechanisms underlying the beneficial effects of lycopene to humans, especially in terms of cancer prevention.
Acknowledgments
Funding. This material is based upon work supported by NIH Grant R01CA104932 and the U.S. Department of Agriculture, Agricultural Research Service, under agreement No. 58–1950-7–707.Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the NIH or the U.S. Department of Agriculture.
Abbreviations:
- ACR
acyclo-retinoic acid
- ARE
antioxidant response element
- CMO1
beta-carotene 15,15′-oxygenase
- CMO2
carotene-9′,10′-oxygenase
- Cx43
connexin 43
- GJC
Gap Junction Communication
- IGF
insulin-like growth factor
- IGFBP
insulin-like growth factor binding protein
- RAR
retinoic acid receptor
- RARE
retinoic acid response element
- RXR
retinoid X receptor
REFERENCES
- 1.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW Jr, Clinton SK. Prostate carcinogenesis in n-methyl-n-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95: 1578–1586. [DOI] [PubMed] [Google Scholar]
- 2.Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW Jr. Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007;67: 836–843. [DOI] [PubMed] [Google Scholar]
- 3.Tomatoes Giovannucci E., tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91: 317–331. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Clinton SK. Tomatoes, lycopene, and prostate cancer. Proc Soc Exp Biol Med. 1998;218: 129–139. [DOI] [PubMed] [Google Scholar]
- 5.Siler U, Barella L, Spitzer V, et al. Lycopene and vitamin E interfere with autocrine/paracrine loops in the Dunning prostate cancer model. Faseb J. 2004;18: 1019–1021. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Nara E, Kobayashi H, Terao J, Nagao A. Formation of cleavage products by autoxidation of lycopene. Lipids. 2001;36: 191–199. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira AL, Yeum KJ, Russell RM, Krinsky NI, Tang G. Enzymatic and oxidative metabolites of lycopene. J Nutr Biochem. 2003;14: 531–540. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Kotake-Nara E, Ono H, Nagao A. A novel cleavage product formed by autoxidation of lycopene induces apoptosis in HL-60 cells. Free Radic Biol Med. 2003;35: 1653–1663. [DOI] [PubMed] [Google Scholar]
- 9.Aust O, Ale-Agha N, Zhang L, Wollersen H, Sies H, Stahl W. Lycopene oxidation product enhances gap junctional communication. Food Chem Toxicol. 2003;41: 1399–1407. [DOI] [PubMed] [Google Scholar]
- 10.Caris-Veyrat C, Schmid A, Carail M, Bohm V. Cleavage products of lycopene produced by in vitro oxidations: characterization and mechanisms of formation. J Agric Food Chem. 2003;51: 7318–7325. [DOI] [PubMed] [Google Scholar]
- 11.Mernitz H, Wang XD. The bioconversion of carotenoids into vitamin A: implications for cancer prevention In: Loessing IT, ed. Vitamin A: New Research. Hauppauge, New York: Nova Science Publishers, Inc.; 2007:39–57. [Google Scholar]
- 12.Goodman DS, Huang HS. Biosynthesis of vitamin A with rat intestinal enzymes. Science. 1965;149: 879–880. [DOI] [PubMed] [Google Scholar]
- 13.Olson JA, Hayaishi O. The enzymatic cleavage of betacarotene into vitamin A by soluble enzymes of rat liver and intestine. Proc Natl Acad Sci USA. 1965;54: 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving betacarotene to retinal. J Biol Chem. 2000;275: 11915–11920. [DOI] [PubMed] [Google Scholar]
- 15.Wyss A, Wirtz G, Woggon W, et al. Cloning and expression of beta, beta-carotene 15,15′-dioxygenase. Biochem Biophys Res Commun. 2000;271: 334–336. [DOI] [PubMed] [Google Scholar]
- 16.Lindqvist A, Andersson S. Biochemical properties of purified recombinant human beta-carotene 15,15′monooxygenase. J Biol Chem. 2002;277: 23942–23948. [DOI] [PubMed] [Google Scholar]
- 17.Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS. Expression and characterization of a murine enzyme able to cleave beta-carotene. The formation of retinoids. J Biol Chem. 2001;276: 32160–32168. [DOI] [PubMed] [Google Scholar]
- 18.Redmond TM, Gentleman S, Duncan T, et al. Identification, expression, and substrate specificity of a mammalian betacarotene 15,15′-dioxygenase. J Biol Chem. 2001;276: 6560–6565. [DOI] [PubMed] [Google Scholar]
- 19.Wyss A, Wirtz GM, Woggon WD, et al. Expression pattern and localization of beta, beta-carotene 15,15′-dioxygenase in different tissues. Biochem J. 2001;354: 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan W, Jang GF, Haeseleer F, et al. Cloning and characterization of a human beta, beta-carotene-15,15′-dioxygenase that is highly expressed in the retinal pigment epithelium. Genomics. 2001;72: 193–202. [DOI] [PubMed] [Google Scholar]
- 21.Hessel S, Eichinger A, Isken A, et al. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem. 2007;282: 33553–33561. [DOI] [PubMed] [Google Scholar]
- 22.Nagao A, Olson JA. Enzymatic formation of 9-cis, 13-cis, and all-trans retinals from isomers of beta-carotene. Faseb J. 1994;8: 968–973. [DOI] [PubMed] [Google Scholar]
- 23.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9′,10′monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281: 19327–19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boileau AC, Merchen NR, Wasson K, Atkinson CA, Erdman JW Jr. Cis-lycopene is more bioavailable than translycopene in vitro and in vivo in lymph-cannulated ferrets. J Nutr. 1999;129: 1176–1181. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Lian F, Smith DE, Russell RM, Wang XD. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res. 2003;63: 3138–3144. [PubMed] [Google Scholar]
- 26.Liu C, Russell RM, Wang XD. Lycopene supplementation prevents smoke-induced changes in p53, p53 phosphorylation, cell proliferation, and apoptosis in the gastric mucosa of ferrets. J Nutr. 2006;136: 106–111. [DOI] [PubMed] [Google Scholar]
- 27.Wu K, Schwartz SJ, Platz EA, et al. Variations in plasma lycopene and specific isomers over time in a cohort of U.S. men. J Nutr. 2003;133: 1930–1936. [DOI] [PubMed] [Google Scholar]
- 28.Wang XD, Tang GW, Fox JG, Krinsky NI, Russell RM. Enzymatic conversion of beta-carotene into beta-apo-carotenals and retinoids by human, monkey, ferret, and rat tissues. Arch Biochem Biophys. 1991;285: 8–16. [DOI] [PubMed] [Google Scholar]
- 29.Tang GW, Wang XD, Russell RM, Krinsky NI. Characterization of beta-apo-13-carotenone and beta-apo-14′-carotenal as enzymatic products of the excentric cleavage of betacarotene. Biochemistry. 1991;30: 9829–9834. [DOI] [PubMed] [Google Scholar]
- 30.Wang XD, Russell RM, Liu C, Stickel F, Smith DE, Krinsky NI. Beta-oxidation in rabbit liver in vitro and in the perfused ferret liver contributes to retinoic acid biosynthesis from beta-apocarotenoic acids. J Biol Chem. 1996;271: 26490–26498. [PubMed] [Google Scholar]
- 31.Kiefer C, Hessel S, Lampert JM, et al. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–14116. [DOI] [PubMed] [Google Scholar]
- 32.Wang XD, Krinsky NI, Marini RP, et al. Intestinal uptake and lymphatic absorption of beta-carotene in ferrets: a model for human beta-carotene metabolism. Am J Physiol. 1992;263: G480–486. [DOI] [PubMed] [Google Scholar]
- 33.Wang XD. Can smoke-exposed ferrets be utilized to unravel the mechanisms of action of lycopene? J Nutr. 2005; 135(Suppl): S2053–S2056. [DOI] [PubMed] [Google Scholar]
- 34.Lindqvist A, He YG, Andersson S. Cell type-specific expression of beta-carotene 9′,10′-monooxygenase in human tissues. J Histochem Cytochem. 2005;53: 1403–1412. [DOI] [PubMed] [Google Scholar]
- 35.Kloer DP, Ruch S, Al-Babili S, Beyer P, Schulz GE. The structure of a retinal-forming carotenoid oxygenase. Science. 2005;308: 267–269. [DOI] [PubMed] [Google Scholar]
- 36.Poliakov E, Gentleman S, Cunningham FX Jr, Miller-Ihli NJ, Redmond TM. Key role of conserved histidines in recombinant mouse beta-carotene 15,15′-monooxygenase-1 activity. J Biol Chem. 2005;280: 29217–29223. [DOI] [PubMed] [Google Scholar]
- 37.Parvin SG, Sivakumar B. Nutritional status affects intestinal carotene cleavage activity and carotene conversion to vitamin A in rats. J Nutr. 2000;130: 573–577. [DOI] [PubMed] [Google Scholar]
- 38.van Vliet T, van Vlissingen MF, van Schaik F, van den Berg H. beta-Carotene absorption and cleavage in rats is affected by the vitamin A concentration of the diet. J Nutr. 1996; 126:499–508. [DOI] [PubMed] [Google Scholar]
- 39.Bachmann H, Desbarats A, Pattison P, et al. Feedback regulation of beta, beta-carotene 15,15′-monooxygenase by retinoic acid in rats and chickens. J Nutr. 2002;132: 3616–3622. [DOI] [PubMed] [Google Scholar]
- 40.Chichili GR, Nohr D, Schaffer M, von Lintig J, Biesalski HK. beta-Carotene conversion into vitamin A in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2005; 46:3562–3569. [DOI] [PubMed] [Google Scholar]
- 41.Boulanger A, McLemore P, Copeland NG, et al. Identification of beta-carotene 15, 15′-monooxygenase as a peroxisome proliferator-activated receptor target gene. Faseb J. 2003;17:1304–1306. [DOI] [PubMed] [Google Scholar]
- 42.Gong X, Tsai SW, Yan B, Rubin LP. Cooperation between MEF2 and PPARgamma in human intestinal beta, betacarotene 15,15′-monooxygenase gene expression. BMC Mol Biol. 2006;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaripheh S, Nara TY, Nakamura MT, Erdman JW Jr. Dietary lycopene downregulates carotenoid 15,15′monooxygenase and PPAR-gamma in selected rat tissues. J Nutr. 2006;136: 932–938. [DOI] [PubMed] [Google Scholar]
- 44.Ziouzenkova O, Orasanu G, Sharlach M, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziouzenkova O, Orasanu G, Sukhova G, et al. Asymmetric cleavage of beta-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator-activated receptor responses. Mol Endocrinol. 2007;21:77–88. [DOI] [PubMed] [Google Scholar]
- 46.Ziouzenkova O, Plutzky J. Retinoid metabolism and nuclear receptor responses: new insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett. 2008;582:32–38. [DOI] [PubMed] [Google Scholar]
- 47.Mein JR, Chongvirihaphan N, Wang XD. The effect of combined antioxidant supplementation (beta-carotene, ascorbic acid and alpha-tocopherol) on the expression of CMO1 and CMO2 in smoke-exposed ferrets. American Association of Cancer Research: Frontiers in Cancer Prevention Research Meeting, Boston, MA; 2006. [Google Scholar]
- 48.Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao DY, Katz NB. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp Biol Med (Maywood). 2002;227:845–851. [DOI] [PubMed] [Google Scholar]
- 49.Lindshield BL, Canene-Adams K, Erdman JW Jr. Lycopenoids: are lycopene metabolites bioactive? Arch Biochem Biophys. 2007;458:136–140. [DOI] [PubMed] [Google Scholar]
- 50.Khachik F, Beecher GR, Goli MB, Lusby WR, Daitch CE. Separation and quantification of carotenoids in human plasma. Methods Enzymol. 1992;213:205–219. [DOI] [PubMed] [Google Scholar]
- 51.Khachik F, Beecher GR, Goli MB, Lusby WR, Smith JC Jr. Separation and identification of carotenoids and their oxidation products in the extracts of human plasma. Anal Chem. 1992;64:2111–2122. [DOI] [PubMed] [Google Scholar]
- 52.Khachik F, Beecher GR, Smith JC Jr. Lutein, lycopene, and their oxidative metabolites in chemoprevention of cancer. J Cell Biochem Suppl. 1995;22:236–246. [DOI] [PubMed] [Google Scholar]
- 53.Khachik F, Spangler CJ, Smith JC Jr, Canfield LM, Steck A, Pfander H. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal Chem. 1997;69:1873–1881. [DOI] [PubMed] [Google Scholar]
- 54.Khachik F, Steck A, Niggli UA, Pfander H. Partial synthesis and structural elucidation of the oxidative metabolites of lycopene identified in tomato paste, tomato juice and human serum. J Agric Food Chem. 1998;46:4874–4884. [Google Scholar]
- 55.Khachik F, Pfander H, Traber B. Proposed mechanisms for the formation of the synthetic and naturally occurring metabolites of lycopene in tomato products and human serum. J Agric Food Chem. 1998;46:4885–4890. [Google Scholar]
- 56.Sicilia T, Bub A, Rechkemmer G, Kraemer K, Hoppe PP, Kulling SE. Novel lycopene metabolites are detectable in plasma of preruminant calves after lycopene supplementation. J Nutr. 2005;135:2616–2621. [DOI] [PubMed] [Google Scholar]
- 57.Gajic M, Zaripheh S, Sun F, Erdman JW Jr. Apo-8’-lycopenal and apo-12’-lycopenal are metabolic products of lycopene in rat liver. J Nutr. 2006;136:1552–1557. [DOI] [PubMed] [Google Scholar]
- 58.Wang XD. Carotenoid oxidative/degradative products and their biological activities In: Krinsky NI, Mayne ST, Sies H, eds. Carotenoids in Health and Disease. New York: Marcel Dekker; 2004:313–335. [Google Scholar]
- 59.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. [DOI] [PubMed] [Google Scholar]
- 60.Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans CA. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996;384:240–242. [DOI] [PubMed] [Google Scholar]
- 61.Conn PF, Schalch W, Truscott TG. The singlet oxygen and carotenoid interaction. J Photochem Photobiol B. 1991;11: 41–47. [DOI] [PubMed] [Google Scholar]
- 62.Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532–538. [DOI] [PubMed] [Google Scholar]
- 63.Bowen P, Chen L, Stacewicz-Sapuntzakis M, et al. Tomato sauce supplementation and prostate cancer: lycopene accumulation and modulation of biomarkers of carcinogenesis. Exp Biol Med (Maywood). 2002;227:886–893. [DOI] [PubMed] [Google Scholar]
- 64.Chen L, Stacewicz-Sapuntzakis M, Duncan C, et al. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J Natl Cancer Inst. 2001;93:1872–1879. [DOI] [PubMed] [Google Scholar]
- 65.Porrini M, Riso P. Lymphocyte lycopene concentration and DNA protection from oxidative damage is increased in women after a short period of tomato consumption. J Nutr. 2000;130:189–192. [DOI] [PubMed] [Google Scholar]
- 66.Riso P, Pinder A, Santangelo A, Porrini M. Does tomato consumption effectively increase the resistance of lymphocyte DNA to oxidative damage? Am J Clin Nutr. 1999;69:712–718. [DOI] [PubMed] [Google Scholar]
- 67.Agarwal S, Rao AV. Tomato lycopene and low density lipoprotein oxidation: a human dietary intervention study. Lipids. 1998;33:981–984. [DOI] [PubMed] [Google Scholar]
- 68.Bub A, Watzl B, Abrahamse L, et al. Moderate intervention with carotenoid-rich vegetable products reduces lipid peroxidation in men. J Nutr. 2000;130:2200–2206. [DOI] [PubMed] [Google Scholar]
- 69.Lian F, Wang XD. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/ antioxidant enzymes in human bronchial epithelial cells. Int J Cancer. 2008;123:1262–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bertram JS. Induction of connexin 43 by carotenoids: functional consequences. Arch Biochem Biophys. 2004;430:120–126. [DOI] [PubMed] [Google Scholar]
- 71.Trosko JE, Chang CC, Upham B, Wilson M. Epigenetic toxicology as toxicant-induced changes in intracellular signalling leading to altered gap junctional intercellular communication. Toxicol Lett. 1998;102–103:71–78. [DOI] [PubMed] [Google Scholar]
- 72.King TJ, Bertram JS. Connexins as targets for cancer chemoprevention and chemotherapy. Biochim Biophys Acta. 2005;1719:146–160. [DOI] [PubMed] [Google Scholar]
- 73.Hossain MZ, Wilkens LR, Mehta PP, Loewenstein W, Bertram JS. Enhancement of gap junctional communication by retinoids correlates with their ability to inhibit neoplastic transformation. Carcinogenesis. 1989;10:1743–1748. [DOI] [PubMed] [Google Scholar]
- 74.Zhang LX, Cooney RV, Bertram JS. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: relationship to their cancer chemopreventive action. Carcinogenesis. 1991;12:2109–2114. [DOI] [PubMed] [Google Scholar]
- 75.Bertram JS, Pung A, Churley M, Kappock TJt, Wilkins LR, Cooney RV. Diverse carotenoids protect against chemically induced neoplastic transformation. Carcinogenesis. 1991; 12:671–678. [DOI] [PubMed] [Google Scholar]
- 76.Rogers M, Berestecky JM, Hossain MZ, et al. Retinoid-enhanced gap junctional communication is achieved by increased levels of connexin 43 mRNA and protein. Mol Carcinog. 1990;3:335–343. [DOI] [PubMed] [Google Scholar]
- 77.Zhang LX, Cooney RV, Bertram JS. Carotenoids up-regulate connexin43 gene expression independent of their provitamin A or antioxidant properties. Cancer Res. 1992;52:5707–5712. [PubMed] [Google Scholar]
- 78.Stahl W, von Laar J, Martin HD, Emmerich T, Sies H. Stimulation of gap junctional communication: comparison of acyclo-retinoic acid and lycopene. Arch Biochem Biophys. 2000;373:271–274. [DOI] [PubMed] [Google Scholar]
- 79.Hix LM, Vine AL, Lockwood SF, Bertram JS. Retinoids and carotenoids as cancer chemopreventive agents: role of upregulated gap junctional communication In: Packer L, Obermueller-Jevic U, Kraemer K, Sies H, eds. Carotenoids and Retinoids: Molecular Aspects and Health Issues. Champaigne, Illinois: AOCS Press; 2005:182–203. [Google Scholar]
- 80.Chambon P A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–954. [PubMed] [Google Scholar]
- 81.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. [DOI] [PubMed] [Google Scholar]
- 82.Napoli JL, Race KR. Biogenesis of retinoic acid from betacarotene. Differences between the metabolism of betacarotene and retinal. J Biol Chem. 1988;263:17372–17377. [PubMed] [Google Scholar]
- 83.Wang XD, Krinsky NI, Benotti PN, Russell RM. Biosynthesis of 9-cis-retinoic acid from 9-cis-beta-carotene in human intestinal mucosa in vitro. Arch Biochem Biophys. 1994;313:150–155. [DOI] [PubMed] [Google Scholar]
- 84.Lian F, Hu KQ, Russell RM, Wang XD. Beta-cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor beta expression. Int J Cancer. 2006;119:2084–2089. [DOI] [PubMed] [Google Scholar]
- 85.Prakash P, Liu C, Hu KQ, Krinsky NI, Russell RM, Wang XD. Beta-carotene and beta-apo-14’-carotenoic acid prevent the reduction of retinoic acid receptor beta in benzo[a]pyrene-treated normal human bronchial epithelial cells. J Nutr. 2004;134:667–673. [DOI] [PubMed] [Google Scholar]
- 86.Ben-Dor A, Nahum A, Danilenko M, et al. Effects of acycloretinoic acid and lycopene on activation of the retinoic acid receptor and proliferation of mammary cancer cells. Arch Biochem Biophys. 2001;391:295–302. [DOI] [PubMed] [Google Scholar]
- 87.Araki H, Shidoji Y, Yamada Y, Moriwaki H, Muto Y. Retinoid agonist activities of synthetic geranyl geranoic acid derivatives. Biochem Biophys Res Commun. 1995;209:66–72. [DOI] [PubMed] [Google Scholar]
- 88.Muto Y, Moriwaki H, Omori M. In vitro binding affinity of novel synthetic polyprenoids (polyprenoic acids) to cellular retinoid-binding proteins. Gann. 1981;72:974–977. [PubMed] [Google Scholar]
- 89.Suzui M, Masuda M, Lim JT, Albanese C, Pestell RG, Weinstein IB. Growth inhibition of human hepatoma cells by acyclic retinoid is associated with induction of p21(CIP1) and inhibition of expression of cyclin D1. Cancer Res. 2002;62:3997–4006. [PubMed] [Google Scholar]
- 90.Muto Y, Moriwaki H, Ninomiya M, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561–1567. [DOI] [PubMed] [Google Scholar]
- 91.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10′lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 2007;28:1567–1574. [DOI] [PubMed] [Google Scholar]
- 92.Freemantle SJ, Spinella MJ, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003;22:7305–7315. [DOI] [PubMed] [Google Scholar]
- 93.Mandlekar S, Hong JL, Kong AN. Modulation of metabolic enzymes by dietary phytochemicals: a review of mechanisms underlying beneficial versus unfavorable effects. Curr Drug Metab. 2006;7:661–675. [DOI] [PubMed] [Google Scholar]
- 94.Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–268. [DOI] [PubMed] [Google Scholar]
- 95.Talalay P Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. [DOI] [PubMed] [Google Scholar]
- 96.Talalay P, Dinkova-Kostova AT, Holtzclaw WD. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv Enzyme Regul. 2003;43:121–134. [DOI] [PubMed] [Google Scholar]
- 97.Giudice A, Montella M. Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. Bioessays. 2006;28:169–181. [DOI] [PubMed] [Google Scholar]
- 98.Ramos-Gomez M, Kwak MK, Dolan PM, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gradelet S, Astorg P, Leclerc J, Chevalier J, Vernevaut MF, Siess MH. Effects of canthaxanthin, astaxanthin, lycopene and lutein on liver xenobiotic-metabolizing enzymes in the rat. Xenobiotica. 1996;26:49–63. [DOI] [PubMed] [Google Scholar]
- 100.Breinholt V, Lauridsen ST, Daneshvar B, Jakobsen J. Dose-response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett. 2000;154: 201–210. [DOI] [PubMed] [Google Scholar]
- 101.Ben-Dor A, Steiner M, Gheber L, et al. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther. 2005;4:177–186. [PubMed] [Google Scholar]
- 102.Bhuvaneswari V, Velmurugan B, Balasenthil S, Ramachandran CR, Nagini S. Chemopreventive efficacy of lycopene on 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Fitoterapia. 2001;72: 865–874. [DOI] [PubMed] [Google Scholar]
- 103.Karas M, Amir H, Fishman D, et al. Lycopene interferes with cell cycle progression and insulin-like growth factor I signaling in mammary cancer cells. Nutr Cancer. 2000;36:101–111. [DOI] [PubMed] [Google Scholar]
- 104.Levy J, Bosin E, Feldman B, et al. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutr Cancer. 1995;24: 257–266. [DOI] [PubMed] [Google Scholar]
- 105.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. [DOI] [PubMed] [Google Scholar]
- 106.Clemmons DR, Busby WH, Arai T, et al. Role of insulin-like growth factor binding proteins in the control of IGF actions. Prog Growth Factor Res. 1995;6:357–366. [DOI] [PubMed] [Google Scholar]
- 107.Jerome L, Shiry L, Leyland-Jones B. Deregulation of the IGF axis in cancer: epidemiological evidence and potential therapeutic interventions. Endocr Relat Cancer. 2003;10: 561–578. [DOI] [PubMed] [Google Scholar]
- 108.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. [DOI] [PubMed] [Google Scholar]
- 109.Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. [DOI] [PubMed] [Google Scholar]
- 110.Ma J, Pollak MN, Giovannucci E, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–625. [DOI] [PubMed] [Google Scholar]
- 111.Arab L, Steck-Scott S, Bowen P. Participation of lycopene and beta-carotene in carcinogenesis: defenders, aggressors, or passive bystanders? Epidemiol Rev. 2001;23:211–230. [DOI] [PubMed] [Google Scholar]
- 112.Clinton SK, Emenhiser C, Schwartz SJ, et al. cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev. 1996;5:823–833. [PubMed] [Google Scholar]
- 113.Giovannucci E Insulin-like growth factor-I and binding protein-3 and risk of cancer. Horm Res. 1999;51(Suppl 3): 34–41. [DOI] [PubMed] [Google Scholar]
- 114.Giovannucci E A review of epidemiologic studies of tomatoes, lycopene, and prostate cancer. Exp Biol Med (Maywood). 2002;227:852–859. [DOI] [PubMed] [Google Scholar]
- 115.Karas M, Danilenko M, Fishman D, LeRoith D, Levy J, Sharoni Y. Membrane-associated insulin-like growth factor-binding protein-3 inhibits insulin-like growth factor-Iinduced insulin-like growth factor-I receptor signaling in ishikawa endometrial cancer cells. J Biol Chem. 1997;272: 16514–16520. [DOI] [PubMed] [Google Scholar]
- 116.Nahum A, Zeller L, Danilenko M, et al. Lycopene inhibition of IGF-induced cancer cell growth depends on the level of cyclin D1. Eur J Nutr. 2006;45:275–282. [DOI] [PubMed] [Google Scholar]
- 117.Mucci LA, Tamimi R, Lagiou P, et al. Are dietary influences on the risk of prostate cancer mediated through the insulin-like growth factor system? BJU Int. 2001;87:814–820. [DOI] [PubMed] [Google Scholar]
- 118.Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:852–861. [PubMed] [Google Scholar]
- 119.Vrieling A, Voskuil DW, Bonfrer JM, et al. Lycopene supplementation elevates circulating insulin-like growth factor binding protein-1 and −2 concentrations in persons at greater risk of colorectal cancer. Am J Clin Nutr. 2007; 86:1456–1462. [DOI] [PubMed] [Google Scholar]
- 120.Nahum A, Hirsch K, Danilenko M, et al. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27(Kip1) in the cyclin E-cdk2 complexes. Oncogene. 2001;20:3428–3436. [DOI] [PubMed] [Google Scholar]
- 121.Salman H, Bergman M, Djaldetti M, Bessler H. Lycopene affects proliferation and apoptosis of four malignant cell lines. Biomed Pharmacother. 2007;61:366–369. [DOI] [PubMed] [Google Scholar]
- 122.Livny O, Kaplan I, Reifen R, Polak-Charcon S, Madar Z, Schwartz B. Lycopene inhibits proliferation and enhances gap-junction communication of KB-1 human oral tumor cells. J Nutr. 2002;132:3754–3759. [DOI] [PubMed] [Google Scholar]
- 123.Obermuller-Jevic UC, Olano-Martin E, Corbacho AM, et al. Lycopene inhibits the growth of normal human prostate epithelial cells in vitro. J Nutr. 2003;133:3356–3360. [DOI] [PubMed] [Google Scholar]
- 124.Huang CS, Liao JW, Hu ML. Lycopene inhibits experimental metastasis of human hepatoma SK-Hep-1 cells in athymic nude mice. J Nutr. 2008;138:538–543. [DOI] [PubMed] [Google Scholar]
- 125.Hwang ES, Bowen PE. Cell cycle arrest and induction of apoptosis by lycopene in LNCaP human prostate cancer cells. J Med Food. 2004;7:284–289. [DOI] [PubMed] [Google Scholar]
- 126.Hantz HL, Young LF, Martin KR. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp Biol Med (Maywood). 2005;230:171–179. [DOI] [PubMed] [Google Scholar]
- 127.Palozza P, Sheriff A, Serini S, et al. Lycopene induces apoptosis in immortalized fibroblasts exposed to tobacco smoke condensate through arresting cell cycle and downregulating cyclin D1, pAKT and pBad. Apoptosis. 2005;10: 1445–1456. [DOI] [PubMed] [Google Scholar]
- 128.Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A. Carotenoids affect proliferation of human prostate cancer cells. J Nutr. 2001;131:3303–3306. [DOI] [PubMed] [Google Scholar]
- 129.Nara E, Hayashi H, Kotake M, Miyashita K, Nagao A. Acyclic carotenoids and their oxidation mixtures inhibit the growth of HL-60 human promyelocytic leukemia cells. Nutr Cancer. 2001;39:273–283. [DOI] [PubMed] [Google Scholar]
- 130.Kotake-Nara E, Kim SJ, Kobori M, Miyashita K, Nagao A. Acyclo-retinoic acid induces apoptosis in human prostate cancer cells. Anticancer Res. 2002;22:689–695. [PubMed] [Google Scholar]
- 131.Hu KQ, Wang Y, Russell RM, Wang XD, eds. Apo-10′-Lycopenoic acid functions as a peroxisome proliferator-activated receptor-gamma activator and inhibits liver cell growth in vitro and in vivo OB/OB mice treated with diethylnitrosamine In: Hashimoto H, ed. Carotenoid Science; No. 12. Osaka, Japan: International Carotenoid Society; 2008. [Google Scholar]
- 132.Liu C, Wang XD, Bronson RT, Smith DE, Krinsky NI, Russell RM. Effects of physiological versus pharmacological beta-carotene supplementation on cell proliferation and histopathological changes in the lungs of cigarette smoke-exposed ferrets. Carcinogenesis. 2000;21:2245–2253. [DOI] [PubMed] [Google Scholar]
- 133.Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell M. Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst. 1999;91:60–66. [DOI] [PubMed] [Google Scholar]
- 134.Veeramachaneni S, Ausman LM, Choi SW, Russell RM, Wang XD. High dose lycopene supplementation increases hepatic cytochrome P4502E1 protein and inflammation in alcohol-fed rats. J Nutr. 2008;138:1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guttenplan JB, Chen M, Kosinska W, Thompson S, Zhao Z, Cohen LA. Effects of a lycopene-rich diet on spontaneous and benzo[a]pyrene-induced mutagenesis in prostate, colon and lungs of the lacZ mouse. Cancer Lett. 2001;164: 1–6. [DOI] [PubMed] [Google Scholar]