Abstract
Dietary plant sterols, such as campesterol and sitosterol, reduce plasma cholesterol concentrations, but any relationship to plaque development and CVD remains unclear. Some epidemiologic studies have suggested that elevated plasma plant sterol concentrations are atherogenic, including the Framingham Offspring Study that identified a positive association between plant sterol concentrations and CVD status. We hypothesized that this suggested atherogenicity relates to the oxidation status of plant sterols (i.e., concentrations of plasma oxyphytosterols). Therefore, in the Framingham Offspring Study cohort, we measured plasma oxyphytosterol concentrations in 144 patients with documented CVD and/or more than 50% carotid stenosis and 383 matched controls. We analyzed plasma oxyphytosterol concentrations by GC/MS/MS and performed conditional logistic regression analysis to determine associations between plasma plant sterol or oxyphytosterol concentrations and CVD status. We found that higher total cholesterol (TC)-standardized campesterol concentrations [odds ratio (OR): 2.36; 95% CI: 1.60, 3.50] and higher sitosterol concentrations (OR: 1.47; 95% CI: 1.09, 1.97) were significantly associated with increased CVD risk, as in the earlier study. However, the sum of absolute oxyphytosterol concentrations (OR: 0.99; 95% CI: 0.81, 1.21) and the sum of TC-standardized oxyphytosterol concentrations (OR: 0.98; 95% CI: 0.80, 1.19) were not associated with an increased CVD risk. Results were comparable for individual absolute and TC-standardized oxycampesterol and oxysitosterol concentrations. Plasma nonoxidized TC-standardized sitosterol and campesterol concentrations showed weak or no correlations with oxyphytosterol concentrations, while all individual plasma concentrations of oxyphytosterol correlated with each other. In conclusion, circulating plasma oxyphytosterols are not associated with CVD risk in the Framingham Offspring Study.
Keywords: plant sterols, cholesterol, lipids, cardiovascular disease risk factors
Plant sterols are natural components of plants and used as functional food ingredients to effectively lower plasma total cholesterol (TC) and especially LDL cholesterol (LDL-C) concentrations (1–3). The daily consumption of 1.5–3 g/d plant sterols lowers plasma LDL-C concentrations by 7% to 12.5% (3). At the same time, plasma plant sterol concentrations modestly increase. The two major plant sterols in the diet as well as in plasma are sitosterol and campesterol. After the daily consumption of plant sterol-enriched foods (on average 1.6 g/d plant sterols; range: 0.3–3.2 g/d), plasma sitosterol concentrations increase on average by 2.24 μmol/l (31%; 95% CI: 26, 37), and those of campesterol increase by 5.00 μmol/l (37%; 95% CI: 29, 45) (4).
In recent years, concerns have been raised about whether these increases might have adverse health effects (5). This discussion relates to at least three arguments. First, patients with phytosterolemia (also known as sitosterolemia), a rare genetic disorder caused by mutations in ABCG5/8 genes, have severely elevated plant sterol concentrations (>50-fold) and frequently develop premature CVD, while cholesterol concentrations are normal or only slightly increased (6). It should be noted, however, that increases in plant sterol concentrations after the consumption of plant sterol-enriched products are only marginal compared with the severely elevated concentrations seen in phytosterolemic patients (4). Second, a genome-wide association study showed that common variants in the ABCG5 gene were associated with both increased plasma plant sterol concentrations and increased coronary artery disease (CAD) risk (7). Finally, some but not all epidemiological studies have reported positive associations between circulating plasma plant sterol concentrations and increased CVD risk (8–10). For example, in the Framingham Offspring Study, plasma total cholesterol (TC)-standardized sitosterol and campesterol concentrations were associated with 1.86- and 2.47-fold increases in CVD risk, respectively (9). However, not all population-based studies have found evidence for an atherogenic role of elevated plant sterol concentrations. For example, in the EPIC-Norfolk Population Study, higher concentrations of plasma plant sterols were not adversely related to CAD (11). In a comprehensive meta-analysis with 17 studies involving 11,182 participants, Genser et al. (12) concluded that no relationship existed between plasma concentrations of sitosterol or campesterol and CVD risk. There was, however, substantial heterogeneity among the studies due to the use of different statistical approaches and study designs and lack of standardized methodology to measure plant sterol concentrations.
Here we hypothesize that discrepancies between studies on the potential atherogenicity of plant sterols may relate to the presence of circulating oxyphytosterols representing the oxidized form of plant sterols. Plant sterols, which are analogues to cholesterol, possess a double bond in their ring structure and are therefore susceptible to oxidation by nonenzymatic processes, such as reactions with reactive oxygen species (7, 13). In animals, inconsistent effects on lesion formation have been reported after the addition of oxyphytosterols to the diet (14–16) or after intraperitoneal application (17). In humans, oxyphytosterols have been identified in aortic valve cusps (18), and we recently showed that plasma oxyphytosterol concentrations are elevated in (pre)diabetic patients compared with healthy controls (19). As plasma oxyphytosterol concentrations increase postprandially after the intake of a plant sterol-enriched meal (20), it is important to investigate whether oxyphytosterols are potentially atherogenic. Therefore, we examined the association between plasma oxyphytosterol concentrations and CVD risk in the Framingham Offspring Study.
MATERIALS AND METHODS
Study design
As described previously in detail (21), the Framingham Offspring Study is a longitudinal cohort study initiated in 1971 that consisted of the offspring of the Framingham Heart Study and their partners. In total, 5,135 men and women were included, of whom 3,532 participated in the sixth examination cycle (1996–1997). All underwent a standardized medical history and physical examination. Here we elaborate on the findings of Matthan et al. (9), who earlier included 155 cases and 414 matched control participants to examine the relation between cholesterol homeostasis markers, including plasma plant sterols, and CVD risk. Cases were identified as individuals with documented CVD and not taking any lipid-lowering medication (statins, cholestyramin, niacin, or fibrates). For each identified case, three control participants were identified matched for age, sex, BMI, systolic blood pressure, and smoking status. Due to plasma sample availability, we present the details of 144 cases (49 women and 95 men) and 383 control participants (129 women and 254 men). The Institutional Review Boards for Human Research at Tufts University, Tufts Medical Center, and Boston University approved this study.
CVD status and covariate variables
CVD was defined as the occurrence of myocardial infarction, ≥50% carotid stenosis, coronary insufficiency, angina pectoris, cerebrovascular accident, or transient ischemic attack prior to the sixth examination cycle. Hypertension was defined by a diastolic blood pressure ≥90 mmHg or systolic blood pressure ≥140 mmHg or if antihypertensive medication was used. Participants were classified as diabetic patients if their fasting glucose concentration was ≥7.0 mmol/l or if they were taking insulin or oral hypoglycemic medication.
Oxyphytosterol analyses: assay validation
Plasma concentrations of 7α-hydroxy(OH)-campesterol, 7α-OH-sitosterol, 7β-OH-campesterol, 7β-OH-sitosterol, 7-keto-campesterol, and 7-keto-sitosterol were analyzed by GC/MS/MS. We used the method of Husche et al. (22) with minor modifications related to the use of a GC/MS triple quad instead of a GC/MS single quad. The deuterium-labeled internal standards were synthesized according to a previously described method (23). The original volume was reduced from 500 µl to 50 µl, and all reagent amounts were decreased 10-fold except for the dichloromethane, which was kept the same. Butylated hydroxytoluene (5 µl, or 25 mg/ml) was added to the extraction tubes. After adding 10 µl of the internal standard, 50 µl Milli-Q water, 50 µl serum was added, and the tube was placed under a mild stream of nitrogen for 5 min at room temperature. Then, 200 µl of 1 M ethanolic sodium hydroxide was added to the tube and closed with a screwcap with a Teflon layer. The tube was placed in an orbital shaker for 1 h at 284 rpm at room temperature. Thereafter, 100 µl water was added, and the solution was neutralized with 30 µl H3PO4 in water (1:1), vortexed, and checked for a pH <6.5, and then 200 µl 0.9% sodium chloride was added. The rest of the procedure was the same as described previously (22). The current GC/MS triple-quad analysis was validated against the established method by Husche et al. by measuring an independent set of samples at our laboratory. Pearson correlation coefficients were calculated, and good correlations were obtained between both methods (7α-OH-campesterol: r = 0.90; 7α-OH-sitosterol: r = 0.83; 7β-OH-campesterol: r = 0.94; 7β-OH-sitosterol: r = 0.84; and 7-keto-campesterol: r = 0.81). The correlation between the two methodologies was the lowest (r = 0.33) for 7-keto-sitosterol concentrations, for which we do not have an explanation. Most importantly, 7-keto-sitosterol concentrations (as well as all the other oxyphytosterols) for both methods were within the ranges found in literature (24). Plasma oxyphytosterol concentrations are expressed in ng/ml and in nmol/mmol cholesterol after standardization for TC.
Statistical analyses
Plasma triacylglycerol (TAG), plant sterol, and oxyphytosterol concentrations were log-transformed to correct for their skewed distributions, and all data (normal and log-transformed) were standardized to express results on a comparable scale as described previously (9). An independent t-test was performed to compare continuous baseline characteristics between cases and controls and plasma oxyphytosterol concentrations between diabetic and nondiabetic participants. Chi-square tests were performed to compare categorical baseline characteristics between cases and controls. A univariate ANOVA was used to compare plasma oxyphytosterol concentrations between cases and controls, with or without diabetes. Conditional, univariate logistic regression was performed, accounting for the matched nature of the study population, to determine associations between plasma lipids, plant sterols, oxyphytosterols, and CVD status (case or control). In addition, associations between plasma concentrations and CVD status were determined in a multivariate logistic regression model with the following covariates: diastolic blood pressure, LDL-C, HDL-C, TAG, diabetes medication, antihypertensive medication, and the matching variables age, sex, systolic blood pressure, BMI, and smoking status. Conditional logistic regression was performed using proc PHREG with case-control status as the stratifying variable and hazard ratio as the outcome. Pearson correlation coefficients were calculated to determine associations between plasma plant sterols and oxyphytosterol concentrations. All statistical analyses were performed using SAS version 9.4 (SAS institute Inc., Cary, NC), and P < 0.05 was considered to be statistically significant based on two-sided testing.
RESULTS
Baseline characteristics
Due to limited plasma availability, the population for the current study consisted of less participants than the earlier study on plant sterols (144 vs. 155 CVD cases and 383 vs. 414 controls, respectively). Baseline characteristics are shown in Table 1. Cases and controls had a similar age, BMI, systolic blood pressure, and smoking status, as they were matched for these variables. As in the original study population (9), body weight and waist circumference were comparable between cases and controls, while cases had a lower diastolic blood pressure than control participants. Moreover, among cases, more individuals had diabetes (29% vs. 11%) and hypertension (65% vs. 54%). In agreement, the use of diabetic or antihypertensive medication was higher in cases than controls. Despite a slightly different population, concentrations of plasma lipids and plant sterols and associations with CVD status were comparable to those reported previously (9) (supplemental Table S1).
TABLE 1.
Baseline characteristics
| Variables | Cases (n = 144) | Controls (n = 383) | a |
| Age (years) | 67.5 ± 0.7 | 66.3 ± 0.4 | Matched |
| Body weight (kg) | 81.2 ± 1.4 | 83.0 ± 0.8 | 0.25 |
| BMI (kg/m2) | 28.8 ± 0.4 | 28.9 ± 0.3 | Matched |
| Waist circumference (cm) | 101.5 ± 1.0 | 101.5 ± 0.6 | 0.97 |
| SBP (mmHg) | 135.2 ± 1.6 | 135.7 ± 1.0 | Matched |
| DBP (mmHg) | 72.2 ± 0.8 | 76.2 ± 0.5 | <0.0001 |
| Smokers [% (n)] | 15 (22) | 16 (61) | Matched |
| Diabeticsb [% (n)] | 29 (41) | 11 (42) | <0.0001 |
| Hypertensionc [% (n)] | 65 (92) | 54 (205) | 0.0163 |
| Diabetes medication [% (n)] | 16 (23) | 6 (22) | 0.0002 |
| Antihypertensive medication [% (n)] | 53 (75) | 35 (133) | 0.0001 |
Values are means ± SEs unless otherwise indicated. DBP, diastolic blood pressure; SBP, systolic blood pressure.
Based on a two-sided independent t-test or Chi-square test.
Fasting blood glucose ≥7.0 mmol/l or the use of insulin or oral hypoglycemic medication.
SBP ≥140, DBP ≥90, or the use of medication.
Plasma oxyphytosterol concentrations
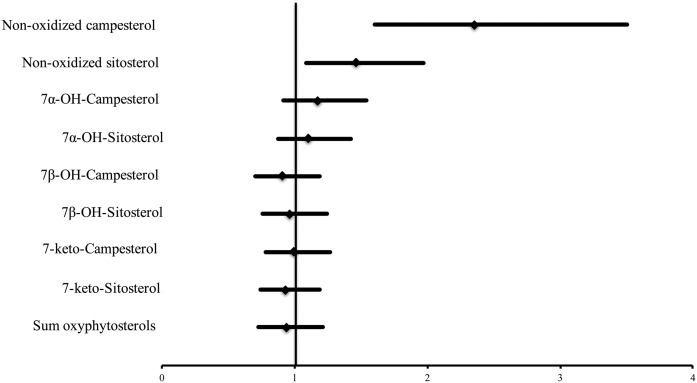
In contrast to the nonoxidized circulating TC-standardized plant sterol concentrations, the univariate analysis showed that none of the oxidized plant sterols was associated with CVD risk. Results were comparable for absolute and TC-standardized oxyphytosterol concentrations (Table 2). The ratios of all individual oxyphytosterol to plant sterol concentrations were inversely correlated with CVD risk (Table 2). Figure 1 shows the multiple adjusted hazard ratios for the nonoxidized and oxidized plant sterol concentrations and CVD risk. Based on the multivariate analysis, higher TC-standardized campesterol [odds ratio (OR): 2.36; 95% CI: 1.60, 3.50] and higher sitosterol concentrations (OR: 1.47; 95% CI: 1.09, 1.97) were significantly associated with an increased CVD risk, whereas the absolute plasma oxyphytosterol concentrations as well as the TC-standardized concentrations were not associated with CVD risk.
TABLE 2.
Absolute and TC-standardized oxyphytosterol concentrations in plasma
| Variables | Cases (n = 144) | Controls (n = 383) | Hazard Ratioa per 1 SD | P |
| Oxyphytosterols (nmol/l) | ||||
| 7α-OH-campesterol | 1.32 ± 0.07 | 1.25 ± 0.04 | 1.08 (0.88, 1.33) | 0.46 |
| 7α-OH-sitosterol | 0.88 ± 0.06 | 0.85 ± 0.02 | 1.07 (0.88, 1.31) | 0.50 |
| 7β-OH-campesterol | 1.30 ± 0.05 | 1.29 ± 0.03 | 0.97 (0.80, 1.19) | 0.77 |
| 7β-OH-sitosterol | 1.42 ± 0.05 | 1.42 ± 0.03 | 1.02 (0.83, 1.24) | 0.85 |
| 7-ketocampesterol | 3.28 ± 0.14 | 3.30 ± 0.09 | 1.01 (0.83, 1.24) | 0.89 |
| 7-ketositosterol | 13.8 ± 0.48 | 13.9 ± 0.25 | 1.00 (0.82, 1.21) | 0.96 |
| Sum oxyphytosterolsb | 22.0 ± 0.68 | 22.0 ± 0.35 | 0.99 (0.81, 1.21) | 0.92 |
| Oxyphytosterols (nmol/mmol TC) | ||||
| 7α-OH-campesterol | 0.25 ± 0.01 | 0.24 ± 0.01 | 1.07 (0.87, 1.32) | 0.51 |
| 7α-OH-sitosterol | 0.17 ± 0.01 | 0.16 ± 0.00 | 1.06 (0.87, 1.29) | 0.59 |
| 7β-OH-campesterol | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.96 (0.79, 1.18) | 0.71 |
| 7β-OH-sitosterol | 0.28 ± 0.01 | 0.27 ± 0.01 | 1.01 (0.83, 1.23) | 0.94 |
| 7-ketocampesterol | 0.63 ± 0.03 | 0.63 ± 0.02 | 1.01 (0.83, 1.23) | 0.95 |
| 7-ketositosterol | 2.68 ± 0.11 | 2.67 ± 0.05 | 0.99 (0.81, 1.20) | 0.89 |
| Sum oxyphytosterolsb | 4.26 ± 0.15 | 4.22 ± 0.07 | 0.98 (0.80, 1.19) | 0.83 |
| Oxidation statusc | ||||
| 7α-OH-campesterol | 1.25 ± 0.08 | 1.39 ± 0.05 | 0.77 (0.61, 0.96) | 0.02 |
| 7α-OH-sitosterol | 1.21 ± 0.09 | 1.29 ± 0.05 | 0.81 (0.65, 1.01) | 0.07 |
| 7β-OH-campesterol | 1.23 ± 0.06 | 1.45 ± 0.05 | 0.67 (0.53, 0.85) | 0.001 |
| 7β-OH-sitosterol | 1.91 ± 0.09 | 2.14 ± 0.06 | 0.74 (0.58, 0.93) | 0.01 |
| 7-ketocampesterol | 3.19 ± 0.19 | 3.72 ± 0.13 | 0.78 (0.63, 0.97) | 0.03 |
| 7-ketositosterol | 19.12 ± 1.11 | 21.49 ± 0.78 | 0.78 (0.63, 0.97) | 0.02 |
Values are means ± SEs unless otherwise indicated. Log transformation was applied for all oxyphytosterols.
Based on univariate conditional logistical regression (i.e., matched case-control analysis). Values in parentheses are 95% CIs.
Sum of 7α-OH-phytosterols, 7β-OH-phytosterols, and 7-ketophytosterols.
Calculated as ((oxyphytosterol × 100)/plant sterol)% × 102.
Fig. 1.
Hazard ratios (lower and upper limits) for plant sterol concentrations (umol/mmol chol) and oxyphytosterol concentrations (nmol/mmol chol) and CVD risk in a multivariate conditional logistic regression model. Hazard ratios are adjusted for age, sex, BMI, systolic blood pressure, and smoking status (matching variables) and diastolic blood pressure, LDL-C, HDL-C, TAG, diabetes medication, and antihypertensive medication (covariates).
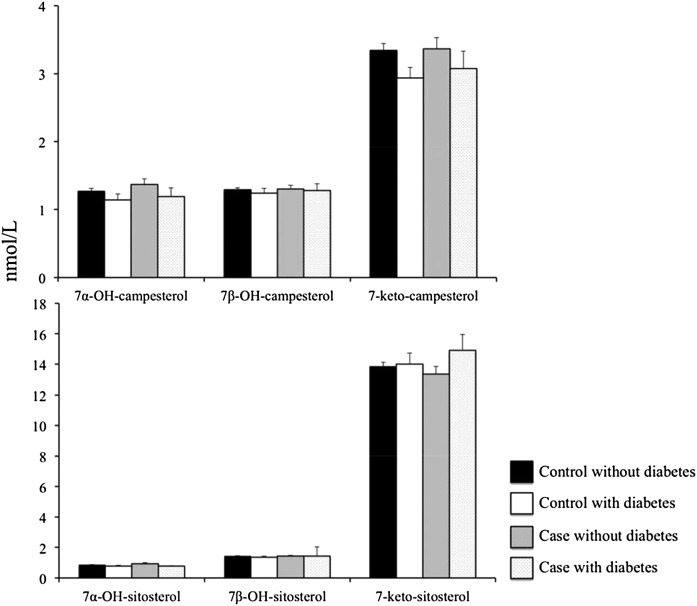
CVD risk markers and oxyphytosterol concentrations were also compared between individuals with and without diabetes. Plasma TC concentrations were comparable, but diabetic individuals had lower LDL-C concentrations (3.16 ± 0.89 vs. 3.37 ± 0.82 mmol/l; P < 0.05), lower HDL-C concentrations (1.10 ± 0.34 vs. 1.27 ± 0.40 mmol/l; P < 0.01), and higher TAG concentrations (1.84 ± 0.63 vs. 1.36 ± 0.52 mmol/l; P < 0.0001). Absolute oxyphytosterol and TC-standardized concentrations were comparable between individuals with and without diabetes (data not shown). When categorizing the population on the basis of diabetes status as well as on CVD status, oxyphytosterol concentrations (sum and individual isoforms) were comparable between each of the categories (cases with diabetes, cases without diabetes, controls with diabetes, and controls without diabetes) (Fig. 2).
Fig. 2.
Oxyphytosterol concentrations in control subjects without diabetes (n = 341), control subjects with diabetes (n = 42), cases without diabetes (n = 102), and cases with diabetes (n = 42). Values are presented as means ± SEs.
Correlations between nonoxidized and oxidized plant sterols
Plasma nonoxidized TC-standardized sitosterol concentrations showed weak correlations with 7α-OH-sitosterol (r = 0.12; P < 0.01), 7β-OH-sitosterol (r = 0.17; P < 0.001), and 7-keto-sitosterol concentrations (r = 0.13; P < 0.01). Plasma nonoxidized TC-standardized campesterol concentrations did not correlate with 7β-OH-campesterol or 7-keto-campesterol, and only a weak correlation with 7α-OH-campesterol was present (r = 0.14; P < 0.01). Furthermore, all individual plasma concentrations of oxyphytosterol correlated with each other (Table 3).
TABLE 3.
Correlation between individual oxyphytosterol and oxycholesterol concentrations
| Parameter | Parameter | Correlation | P |
| 7α-OH-campesterol | 7β-OH-campesterol | 0.628 | <0.0001 |
| 7-ketocampesterol | 0.412 | <0.0001 | |
| 7β-OH-campesterol | 7-ketocampesterol | 0.421 | <0.0001 |
| 7α-OH-sitosterol | 7β-OH-sitosterol | 0.564 | <0.0001 |
| 7-ketositosterol | 0.410 | <0.0001 | |
| 7β-OH-sitosterol | 7-ketositosterol | 0.500 | <0.0001 |
Correlations are based on the whole group and are not separated by case/control status on log-transformed TC-standardized oxyphytosterol concentrations.
DISCUSSION
Here we report the results of a multivariate-adjusted approach showing that absolute plasma oxyphytosterol as well as TC-standardized oxyphytosterol concentrations were not associated with CVD risk, defined as documented CVD and/or ≥50% carotid stenosis, in participants of the Framingham Offspring Study. This indicates that the presence of circulating oxyphytosterols is most likely not an underlying reason for the existing controversy around the potential atherogenicity of elevated plasma plant sterol concentrations (12). Our data are partly in agreement with Fuhrmann et al. (25), who did not find an association between absolute and TC-standardized oxyphytosterol concentrations, except for a positive association for 7α-OH-campesterol, with cardiovascular events in subjects admitted for elective coronary angiography. While information regarding oxyphytosterol metabolism and their effects on human health is scarce, data from in vitro and animal studies have shown that oxyphytosterols might be atherogenic (5, 24). Despite their low plasma concentrations in humans, oxyphytosterols are a factor 103 lower compared with plant sterols and have been identified in aortic valve cusps of CAD patients (18). Moreover, (pre)diabetics have been shown to have higher plasma oxyphytosterol concentrations compared with healthy controls (19). However, in the current study we could not demonstrate an association between plasma oxyphytosterol concentrations and CVD risk, and we found weak correlations between circulating TC-standardized plasma oxyphytosterol concentrations and their respective nonoxidized plant sterol precursors. While absolute and TC-standardized oxyphytosterol concentrations were not related to CVD risk, the ratio of individual oxyphytosterols to plant sterols was inversely correlated with CVD risk, suggesting that a higher oxidation status protects against CVD. However, because in this cohort plant sterol concentrations were positively associated with CVD while no associations were observed between oxidized plant sterols and CVD risk, this surprising finding for the oxyphytosterol to plant sterol ratio most likely relates to the positive relation of increased plant sterol concentrations with CVD risk. Moreover, this ratio actually demonstrates the lack of correlation between plant sterols and oxyphytosterols in plasma, that is, an increase in plant sterols without a concomitant increase in oxyphytosterols. This then raises the question as to where the oxyphytosterols circulating in plasma actually come from. In fact, the actual question of interest relates to whether (aortic) tissue concentrations of oxyphytosterols correlate with CVD while plasma oxyphytosterols may not necessarily reflect tissue concentrations. For nonoxidized plant sterol concentrations, it is known that plasma and tissue concentrations correlate (26). However, Schött et al. (27) measured oxyphytosterol concentrations in plasma and aortic valve cusps in patients undergoing elective aortic valve replacement and showed only weak correlations between oxyphytosterol concentrations in plasma and aortic tissue. We also could not previously demonstrate correlations between oxyphytosterol concentrations in plasma with their concentrations in red blood cells or platelets (19). These findings suggest that plasma oxyphytosterol concentrations may not reflect tissue concentrations. It might be possible that the lack of correlation between plasma and tissue concentrations of oxyphytosterols is due to the fact that oxyphytosterols are produced within tissues and, as a spillover mechanism, are secreted back into the circulation (28). However, this suggestion is based only on the lack of correlation between plasma and tissue concentrations in aortic valve cusps and blood cells. For future research, it would be interesting to assess whether correlations exist between oxyphytosterols in plasma and in more metabolically active tissues, such as endothelial cells or hepatocytes, and between oxyphytosterols in these tissues and CVD risk. In summary, plasma might not be the correct compartment for assessing the potential atherogenicity of oxyphytosterols, and our results should therefore be interpreted with caution.
The inconsistency between elevated plasma plant sterol concentrations and CVD risk as observed in numerous (observational) studies remains to be explained. In this respect, it has been postulated that increased cholesterol absorption rates, indicated by elevated TC-standardized plant sterol concentrations used as surrogate markers for cholesterol absorption, could be a possible explanation. Indeed, high cholesterol absorption and low cholesterol synthesis rates have been associated with increased CVD risk in patients referred to angiography (29) and with increased all-cause and CVD mortality rates in participants of the Ludwigshafen Risk and Cardiovascular Health Study (30). However, lower cholesterol absorption rates, estimated by TC-standardized plant sterol concentrations, have also been associated with an increased CVD risk (31, 32). Furthermore, cholesterol absorption rates are supposed to be typically low in individuals with metabolic syndrome and type 2 diabetes, and these patients are overall at increased risk to develop CVD (33). Thus, evidence on the relation between cholesterol absorption, as measured by TC-standardized plant sterol concentrations, and CVD risk, explaining the potential atherogenicity of plasma plant sterols, is inconclusive. In any case, the use of TC-standardized plant sterols concentrations as surrogate markers for cholesterol absorption has been validated only in healthy subjects, and caution is warranted when applied to other populations, such as CVD patients (34, 35).
In the current study, we investigated whether plasma oxyphytosterol concentrations were higher in diabetic versus nondiabetic individuals, as diabetic patients are in general characterized by elevated oxidative stress, such as higher oxycholesterol and malonyldialdehyde concentrations and lower trolox equivalent antioxidant capacity values (36–38), and have a higher risk of CVD overall. Indeed, we previously demonstrated that plasma oxyphytosterol concentrations were significantly higher in individuals with impaired glucose tolerance or type 2 diabetes compared with healthy controls (19). In the current study, however, plasma oxyphytosterol concentrations were not significantly different between diabetic and nondiabetic individuals, irrespective of their CVD status. It could be speculated, however, that the control participants in this Framingham cohort might not be as healthy as the healthy population included in an earlier study (19) because they were matched to the cases on the basis of their age, sex, BMI, systolic blood pressure, and smoking status.
In conclusion, this study showed that circulating plasma oxyphytosterol concentrations are not associated with CVD risk in the Framingham Offspring Study participants. Further research is needed to investigate whether this is also true for tissue oxyphytosterol concentrations and CVD risk.
Supplementary Material
Acknowledgments
The authors thank the Framingham Heart Study staff for providing access to the data sets. The FHS is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (contract no. N01-HC-25 195).
Footnotes
Abbreviations:
- CAD
- coronary artery disease
- HDL-C
- HDL cholesterol
- LDL-C
- LDL cholesterol
- OR
- odds ratio
- TAG
- triacylglycerol
- TC
- total cholesterol
This work was supported by Dutch Organization for Scientific Research TOP Grant 91208006. R.T.R. and E.A.T. were employed by Unilever R&D at the time the study was conducted. Unilever (before divesting its spreads business now operating under the name Upfield™) marketed food products with added plant sterols. None of the other authors have any conflicts of interest to declare. This manuscript was not prepared in collaboration with Framingham Heart Study (FHS) investigators and does not necessarily reflect the opinions or views of the FHS, Boston University, or the National Heart, Lung, and Blood Institute.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Demonty I., Ras R. T., van der Knaap H. C., Duchateau G. S., Meijer L., Zock P. L., Geleijnse J. M., and Trautwein E. A.. 2009. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J. Nutr. 139: 271–284. [DOI] [PubMed] [Google Scholar]
- 2.Musa-Veloso K., Poon T. H., Elliot J. A., and Chung C.. 2011. A comparison of the LDL-cholesterol lowering efficacy of plant stanols and plant sterols over a continuous dose range: results of a meta-analysis of randomized, placebo-controlled trials. Prostaglandins Leukot. Essent. Fatty Acids. 85: 9–28. [DOI] [PubMed] [Google Scholar]
- 3.Ras R. T., Geleijnse J. M., and Trautwein E. A.. 2014. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: a meta-analysis of randomised controlled studies. Br. J. Nutr. 112: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ras R. T., Hiemstra H., Lin Y., Vermeer M. A., Duchateau G. S., and Trautwein E. A.. 2013. Consumption of plant sterol-enriched foods and effects on plasma plant sterol concentrations–a meta-analysis of randomized controlled studies. Atherosclerosis. 230: 336–346. [DOI] [PubMed] [Google Scholar]
- 5.Vanmierlo T., Husche C., Schott H. F., Pettersson H., and Lutjohann D.. 2013. Plant sterol oxidation products–analogs to cholesterol oxidation products from plant origin? Biochimie. 95: 464–472. [DOI] [PubMed] [Google Scholar]
- 6.Ajagbe B. O., Othman R. A., and Myrie S. B.. 2015. Plant sterols, stanols, and sitosterolemia. J. AOAC Int. 98: 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teupser D., Baber R., Ceglarek U., Scholz M., Illig T., Gieger C., Holdt L. M., Leichtle A., Greiser K. H., Huster D., et al. . 2010. Genetic regulation of serum phytosterol levels and risk of coronary artery disease. Circ Cardiovasc Genet. 3: 331–339. [DOI] [PubMed] [Google Scholar]
- 8.Assmann G., Cullen P., Erbey J., Ramey D. R., Kannenberg F., and Schulte H.. 2006. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case-control analysis of the Prospective Cardiovascular Munster (PROCAM) study. Nutr. Metab. Cardiovasc. Dis. 16: 13–21. [DOI] [PubMed] [Google Scholar]
- 9.Matthan N. R., Pencina M., LaRocque J. M., Jacques P. F., D’Agostino R. B., Schaefer E. J., and Lichtenstein A. H.. 2009. Alterations in cholesterol absorption/synthesis markers characterize Framingham offspring study participants with CHD. J. Lipid Res. 50: 1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajaratnam R. A., Gylling H., and Miettinen T. A.. 2000. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J. Am. Coll. Cardiol. 35: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 11.Pinedo S., Vissers M. N., von Bergmann K., Elharchaoui K., Lutjohann D., Luben R., Wareham N. J., Kastelein J. J., Khaw K. T., and Boekholdt S. M.. 2007. Plasma levels of plant sterols and the risk of coronary artery disease: the prospective EPIC-Norfolk Population Study. J. Lipid Res. 48: 139–144. [DOI] [PubMed] [Google Scholar]
- 12.Genser B., Silbernagel G., De Backer G., Bruckert E., Carmena R., Chapman M. J., Deanfield J., Descamps O. S., Rietzschel E. R., Dias K. C., et al. . 2012. Plant sterols and cardiovascular disease: a systematic review and meta-analysis. Eur. Heart J. 33: 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hovenkamp E., Demonty I., Plat J., Lutjohann D., Mensink R. P., and Trautwein E. A.. 2008. Biological effects of oxidized phytosterols: a review of the current knowledge. Prog. Lipid Res. 47: 37–49. [DOI] [PubMed] [Google Scholar]
- 14.Liang Y. T., Wong W. T., Guan L., Tian X. Y., Ma K. Y., Huang Y., and Chen Z. Y.. 2011. Effect of phytosterols and their oxidation products on lipoprotein profiles and vascular function in hamster fed a high cholesterol diet. Atherosclerosis. 219: 124–133. [DOI] [PubMed] [Google Scholar]
- 15.Plat J., Theuwissen E., Husche C., Lutjohann D., Gijbels M. J., Jeurissen M., Shiri-Sverdlov R., van der Made I., and Mensink R. P.. 2014. Oxidised plant sterols as well as oxycholesterol increase the proportion of severe atherosclerotic lesions in female LDL receptor+/− mice. Br. J. Nutr. 111: 64–70. [DOI] [PubMed] [Google Scholar]
- 16.Tomoyori H., Kawata Y., Higuchi T., Ichi I., Sato H., Sato M., Ikeda I., and Imaizumi K.. 2004. Phytosterol oxidation products are absorbed in the intestinal lymphatics in rats but do not accelerate atherosclerosis in apolipoprotein E-deficient mice. J. Nutr. 134: 1690–1696. [DOI] [PubMed] [Google Scholar]
- 17.Weingärtner O., Husche C., Schott H. F., Speer T., Bohm M., Miller C. M., McCarthy F., Plat J., Lutjohann D., and Laufs U.. 2015. Vascular effects of oxysterols and oxyphytosterols in apoE −/− mice. Atherosclerosis. 240: 73–79. [DOI] [PubMed] [Google Scholar]
- 18.Luister A., Schott H. F., Husche C., Schafers H. J., Bohm M., Plat J., Graber S., Lutjohann D., Laufs U., and Weingartner O.. 2015. Increased plant sterol deposition in vascular tissue characterizes patients with severe aortic stenosis and concomitant coronary artery disease. Steroids. 99: 272–280. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner S., Mensink R. P., Haenen G. R., Bast A., Binder C. J., Bekers O., Husche C., Lutjohann D., and Plat J.. 2017. The effects of vitamin E or lipoic acid supplementation on oxyphytosterols in subjects with elevated oxidative stress: a randomized trial. Sci. Rep. 7: 15288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumgartner S., Mensink R. P., Konings M., Schott H. F., Friedrichs S., Husche C., Lutjohann D., and Plat J.. 2015. Postprandial plasma oxyphytosterol concentrations after consumption of plant sterol or stanol enriched mixed meals in healthy subjects. Steroids. 99: 281–286. [DOI] [PubMed] [Google Scholar]
- 21.Feinleib M., Kannel W. B., Garrison R. J., McNamara P. M., and Castelli W. P.. 1975. The Framingham Offspring Study. Design and preliminary data. Prev. Med. 4: 518–525. [DOI] [PubMed] [Google Scholar]
- 22.Husche C., Weingartner O., Pettersson H., Vanmierlo T., Bohm M., Laufs U., and Lutjohann D.. 2011. Validation of an isotope dilution gas chromatography-mass spectrometry method for analysis of 7-oxygenated campesterol and sitosterol in human serum. Chem. Phys. Lipids. 164: 425–431. [DOI] [PubMed] [Google Scholar]
- 23.Plat J., Brzezinka H., Lutjohann D., Mensink R. P., and von Bergmann K.. 2001. Oxidized plant sterols in human serum and lipid infusions as measured by combined gas-liquid chromatography-mass spectrometry. J. Lipid Res. 42: 2030–2038. [PubMed] [Google Scholar]
- 24.Wang M. M., and Lu B. Y.. 2018. How do oxyphytosterols affect human health? Trends Food Sci. Technol. 79: 148–159. [Google Scholar]
- 25.Fuhrmann A., Weingartner O., Meyer S., Cremers B., Seiler-Mussler S., Schott H. F., Kerksiek A., Friedrichs S., Ulbricht U., Zawada A. M., et al. . 2018. Plasma levels of the oxyphytosterol 7alpha-hydroxycampesterol are associated with cardiovascular events. Atherosclerosis. 279: 17–22. [DOI] [PubMed] [Google Scholar]
- 26.Weingärtner O., Lutjohann D., Ji S., Weisshoff N., List F., Sudhop T., von Bergmann K., Gertz K., Konig J., Schafers H. J., et al. . 2008. Vascular effects of diet supplementation with plant sterols. J. Am. Coll. Cardiol. 51: 1553–1561. [DOI] [PubMed] [Google Scholar]
- 27.Schott H. F., Luister A., Husche C., Schafers H. J., Bohm M., Plat J., Lutjohann D., Laufs U., and Weingartner O.. 2014. The relationships of phytosterols and oxyphytosterols in plasma and aortic valve cusps in patients with severe aortic stenosis. Biochem. Biophys. Res. Commun. 446: 805–810. [DOI] [PubMed] [Google Scholar]
- 28.Schött H. F., Baumgartner S., Husche C., Luister A., Friedrichs S., Miller C. M., McCarthy F. O., Plat J., Laufs U., Weingartner O., et al. . 2017. Oxidation of sitosterol and transport of its 7-oxygenated products from different tissues in humans and ApoE knockout mice. J. Steroid Biochem. Mol. Biol. 169: 145–151. [DOI] [PubMed] [Google Scholar]
- 29.Weingärtner O., Weingartner N., Scheller B., Lutjohann D., Graber S., Schafers H. J., Bohm M., and Laufs U.. 2009. Alterations in cholesterol homeostasis are associated with coronary heart disease in patients with aortic stenosis. Coron. Artery Dis. 20: 376–382. [DOI] [PubMed] [Google Scholar]
- 30.Silbernagel G., Fauler G., Hoffmann M. M., Lutjohann D., Winkelmann B. R., Boehm B. O., and Marz W.. 2010. The associations of cholesterol metabolism and plasma plant sterols with all-cause and cardiovascular mortality. J. Lipid Res. 51: 2384–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fassbender K., Lutjohann D., Dik M. G., Bremmer M., Konig J., Walter S., Liu Y., Letiembre M., von Bergmann K., and Jonker C.. 2008. Moderately elevated plant sterol levels are associated with reduced cardiovascular risk–the LASA study. Atherosclerosis. 196: 283–288. [DOI] [PubMed] [Google Scholar]
- 32.Strandberg T. E., Gylling H., Tilvis R. S., and Miettinen T. A.. 2010. Serum plant and other noncholesterol sterols, cholesterol metabolism and 22-year mortality among middle-aged men. Atherosclerosis. 210: 282–287. [DOI] [PubMed] [Google Scholar]
- 33.Kassi E., Pervanidou P., Kaltsas G., and Chrousos G.. 2011. Metabolic syndrome: definitions and controversies. BMC Med. 9: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackay D. S., and Jones P. J.. 2012. Plasma noncholesterol sterols: current uses, potential and need for standardization. Curr. Opin. Lipidol. 23: 241–247. [DOI] [PubMed] [Google Scholar]
- 35.Miettinen T. A., Gylling H., and Nissinen M. J.. 2011. The role of serum non-cholesterol sterols as surrogate markers of absolute cholesterol synthesis and absorption. Nutr. Metab. Cardiovasc. Dis. 21: 765–769. [DOI] [PubMed] [Google Scholar]
- 36.Arca M., Natoli S., Micheletta F., Riggi S., Di Angelantonio E., Montali A., Antonini T. M., Antonini R., Diczfalusy U., and Iuliano L.. 2007. Increased plasma levels of oxysterols, in vivo markers of oxidative stress, in patients with familial combined hyperlipidemia: reduction during atorvastatin and fenofibrate therapy. Free Radic. Biol. Med. 42: 698–705. [DOI] [PubMed] [Google Scholar]
- 37.Baumgartner S., Mensink R. P., den Hartog G., Bast A., Bekers O., Husche C., Lutjohann D., and Plat J.. 2013. Oxyphytosterol formation in humans: identification of high vs. low oxidizers. Biochem. Pharmacol. 86: 19–25. [DOI] [PubMed] [Google Scholar]
- 38.Del Rio D., Stewart A. J., and Pellegrini N.. 2005. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 15: 316–328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.