Abstract
β-glucosidases [GBA1 (glucocerebrosidase) and GBA2] are ubiquitous essential enzymes. Lysosomal GBA1 and cytosol-facing GBA2 degrade glucosylceramide (GlcCer); GBA1 deficiency causes Gaucher disease, a lysosomal storage disorder characterized by lysosomal accumulation of GlcCer, which is partly converted to glucosylsphingosine (GlcSph). GBA1 and GBA2 also may transfer glucose from GlcCer to cholesterol, yielding glucosylated cholesterol (GlcChol). Here, we aimed to clarify the role of zebrafish Gba2 in glycosphingolipid metabolism during Gba1 deficiency in zebrafish (Danio rerio), which are able to survive total Gba1 deficiency. We developed Gba1 (gba1−/−), Gba2 (gba2−/−), and double (gba1−/−:gba2−/−) zebrafish knockouts using CRISPR/Cas9 and explored the effects of both genetic and pharmacological interventions on GlcCer metabolism in individual larvae. Activity-based probes and quantification of relevant glycolipid metabolites confirmed enzyme deficiency. GlcSph increased in gba1−/− larvae (0.09 pmol/fish) but did not increase more in gba1−/−:gba2−/− larvae. GlcCer was comparable in gba1−/− and WT larvae but increased in gba2−/− and gba1−/−:gba2−/− larvae. Independent of Gba1 status, GlcChol was low in all gba2−/− larvae (0.05 vs. 0.18 pmol/fish in WT). Pharmacologic inactivation of zebrafish Gba1 comparably increased GlcSph. Inhibition of GlcCer synthase (GCS) in Gba1-deficient larvae reduced GlcCer and GlcSph, and concomitant inhibition of GCS and Gba2 with iminosugars also reduced excessive GlcChol. Finally, overexpression of human GBA1 and injection of recombinant GBA1 both decreased GlcSph. We determined that zebrafish larvae offer an attractive model to study glucosidase actions in glycosphingolipid metabolism in vivo, and we identified distinguishing characteristics of zebrafish Gba2 deficiency.
Keywords: Gaucher disease, sphingolipids, lysosphingolipids, lipid metabolism
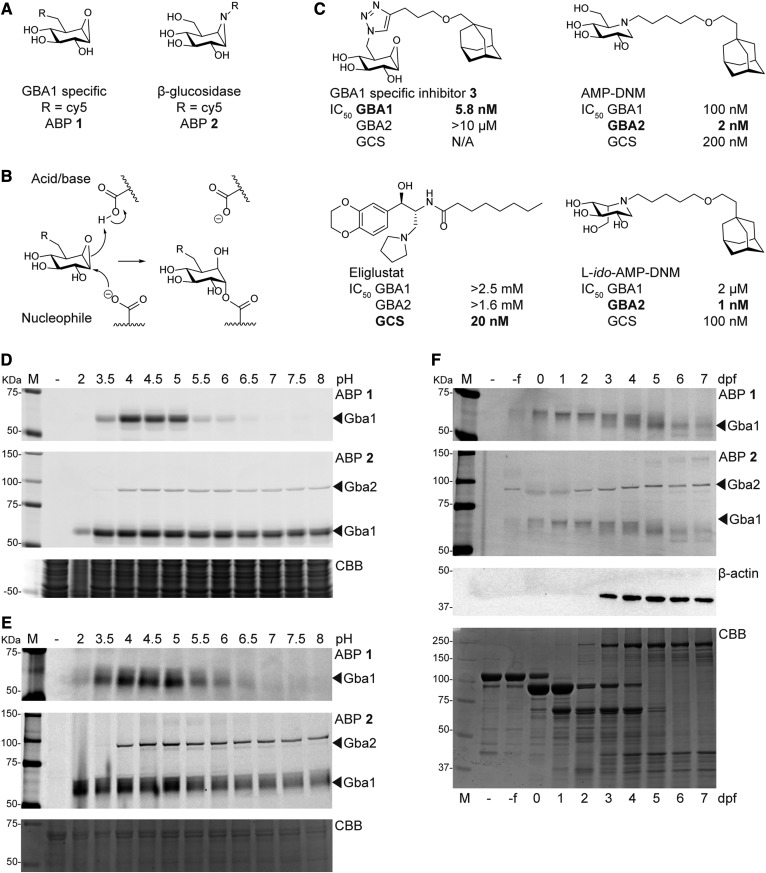
The lysosomal glucocerebrosidase [β-glucocerebrosidase (GBA)1] is a retaining β-glucosidase degrading the glycosphingolipid glucosylceramide (GlcCer) (1). The enzyme receives considerable interest because its deficiency causes Gaucher disease (GD), a recessively inherited lysosomal storage disorder in which GlcCer accumulates in lysosomes, particularly those of tissue macrophages that transform into Gaucher cells (2, 3). GD patients characteristically develop hepatosplenomegaly, leukopenia (especially thrombocytopenia), and abnormalities in coagulation (4). In more severe cases, neuropathology develops, with oculomotor apraxia as a first sign. In its most severe form, complete lack of GBA1 is associated with an acute failure in skin permeability features, causing the so-called collodion baby with severe ichthyosis (2). Individuals that have a genetic defect in GBA1, or carriers of such a mutation, have a markedly increased risk for developing Parkinson’s disease and Lewy-body dementia (5, 6). The molecular mechanisms underlying the complex pathophysiology of GD and the risk imposed by GBA1 abnormalities for α-synucleinopathies are presently unknown. The features and functions of GBA1 are presently extensively investigated. Novel research tools in the field are cell-permeable fluorescent activity-based probes (ABPs) that selectively label retaining β-glucosidases in a mechanism-based manner through covalent binding to the catalytic nucleophile. These allow cross-species visualization of active enzyme molecules in vitro, in situ, and in vivo (7, 8). Cyclophellitol derivative 1 (Fig. 1A), carrying the reporter fluorophore at C8 (cyclophellitol number; corresponding to position C6 in glucose), selectively labels GBA1, arguably because the other human retaining β-glucosidases do not accept the presence of a (bulky) fluorophore at this position (7). Cyclophellitol-aziridine 2 with the fluorophore pointing toward the position occupied by the aglycon of a retaining β-exoglucosidase binds all known cellular human β-glucosidases (GBA1, GBA2, and GBA3) (ABP 2; Fig. 1A, B) (8).
Fig. 1.
Visualization of active Gba1 and Gba2 enzyme in zebrafish. A: Chemical structures of ABPs used in this study: cyclophellitol-epoxide-based ABP 1 (GBA1 specific) and cyclophellitol-aziridine-based ABP 2 (labeling all retaining β-glucosidases). Both ABPs are equipped with a Cy5 fluorophore as reporter. B: Catalytic reaction mechanism of cyclophellitol-based irreversible inhibitors. C: Chemical structures of the GBA1 specific irreversible inhibitor 3 (ME656), iminosugars AMP-DNM and L-ido-AMP-DNM, and eliglustat. IC50 values are given for GBA1, GBA2, and GCS (29, 34). D: Effect of pH on labeling of ZF4 cell homogenate with ABP 1 (100 nM) and ABP 2 (100 nM). E: Effect of pH on labeling of pooled zebrafish homogenate (5 dpf) with ABP 1 (1 μM) and ABP 2 (200 nM). A protein equivalent of one zebrafish was used per condition. F: ABP 1 and ABP 2 labeling of homogenate of oocytes (-f) and developing zebrafish embryos (t = 0–7 dpf). An equivalent of one zebrafish egg or embryo was used per lane. In lane –, sample is denatured prior to ABP addition; Coomassie Brilliant Blue (CBB) staining and β-actin were used as loading controls.
Several corrections for GBA1 deficiency in GD have been developed and novel therapeutic interventions are still being pursued. For almost three decades, nonneuronopathic (type 1) GD has been treated by enzyme replacement therapy, a treatment based on chronic intravenous administration of GBA1 with mannose-terminal N-glycans ensuring targeting to macrophages, the primary GlcCer storage cells (3, 9). An alternative therapeutic approach is substrate reduction therapy (SRT) that aims to reduce the biosynthesis of GlcCer through inhibition of GlcCer synthase (GCS) (10). The first SRT agent developed for GD was N-butyl-deoxynojirimyicin (miglustat) that was registered almost two decades ago for treatment of mild to moderate type 1 GD (11). More recently, an improved inhibitor for GCS, eliglustat (Fig. 1C), has been developed for treatment of type 1 GD patients (12). At present GCS inhibitors with improved brain-permeability have been developed as well as chaperones acting as enzyme stabilizers (3, 13, 14). Moreover, augmentation of GBA1 expression by gene therapy approaches is actively studied in animal models (15).
It has recently been recognized that compensatory mechanisms occur during GBA1 deficiency (3, 16). For example, in GBA1-deficient lysosomes, accumulating GlcCer is partly converted by lysosomal acid ceramidase to its corresponding sphingoid base, glucosylsphingosine (GlcSph) (17). As a result, GlcSph is massively increased in tissues and plasma of GD patients and GBA1-deficient mice (17, 18). Roles for GlcSph in the pathophysiology of GD with respect to organomegaly, osteoporosis, and risks for multiple myeloma, Parkinson’s disease, and reduced cerebral vascularization have been proposed (18–22).
The cytosol-facing GBA2, which metabolizes cytosolic GlcCer (23–26), has recently been shown to have transglucosidase activity as well, and is able to produce glucosylated cholesterol (GlcChol) from GlcCer and cholesterol (27). The role of GBA2 in GD pathophysiology is unclear. Excessive GBA2 activity during deficiency of GBA1 appears detrimental in some aspects. For example, genetically ablating GBA2 in a Gaucher mouse model as well as in Niemann-Pick type C (NPC) mice has been shown to ameliorate symptoms (18, 28). Moreover, pharmacological inhibition of GBA2 by administration of low nanomolar iminosugar derivatives {N-[5-(adamantan-1-ylmethoxy)pentyl]-1-deoxynojirimycin (AMP-DNM) and L-ido-AMP-DNM (29, 30), Fig. 1C} exerts beneficial effects in NPC mice (28).
Zebrafish (Danio rerio) are a popular vertebrate research model because of low-cost maintenance and the ability to produce large clutches of embryos. Zebrafish larvae develop ex utero, are transparent, and pharmacological modulation can be conveniently performed (in 96-well plates) up to 5 days post-fertilization (dpf) (31, 32). Another attractive feature of the zebrafish is the molecular and genetic similarity to mammalian models. For example, the zebrafish genome encodes GlcCer-metabolizing enzymes and their activity can be measured with the same fluorogenic substrates as commonly used for the human and rodent analogs (33). We have recently shown that zebrafish Gba1 and Gba2 react like their human counterparts with available ABPs (33, 34) and exhibit similar inhibitor affinities (33, 35). A complete deficiency of GBA1 causes a fatal skin abnormality in newborn mice and humans (36, 37); however, the introduction of complete Gba1 deficiency in fish is tolerated (38, 39).
The primary goal of our investigation was to study at organismal level the role of Gba2 in glycosphingolipid metabolism during deficiency of Gba1. For this purpose, we used both genetic and pharmacological approaches to modulate Gba1 and Gba2 and subsequently studied the impact on glycosphingolipid levels in individual zebrafish larvae. In Gba1-deficient fish, either genetic or chemically induced, the most remarkable abnormality was the increase in GlcSph, independent of the Gba2 status. The deficiency of Gba2 in zebrafish larvae led to reduction of GlcChol, independent of the Gba1 status. As a secondary goal, we studied the feasibility of pharmacological and genetic interventions. We demonstrated that inhibition of GCS prohibits excessive formation of GlcSph and that concomitant inhibition of Gba2 also prevented excessive GlcChol. Overexpression of human GBA1 in Gba1-deficient larvae reduced GlcSph elevation. The same was observed upon infusion of recombinant human GBA1. Altogether, we demonstrated that zebrafish embryos offer an attractive organismal model to study glycosphingolipid abnormalities in genetic and pharmacological models of Gba1 and Gba2 deficiencies and, moreover, allow the screening of GCS and GBA2 inhibitors regarding corrective effects on lipid abnormalities.
MATERIALS AND METHODS
Zebrafish
WT zebrafish (ABTL) were a mixed lineage of WT AB and WT TL genetic background. Injections to generate CRISPR/Cas9-mediated KO zebrafish were performed in ABTL embryos and adult zebrafish were outcrossed to ABTL WT zebrafish. Gba1 mutant zebrafish were maintained as carriers (heterozygous in gba1 genotype), while gba2 mutant zebrafish were kept and crossed as KO (homozygous in genotype). The gba1−/−:gba2−/− double mutant larvae were continuously generated from adult zebrafish with a gba1+/−: gba2−/− background.
Zebrafish were housed and maintained at Leiden University, The Netherlands, according to standard protocols (http://zfin.org). Adult zebrafish were housed at a density of 40–50 adults per tank, on a cycle of 14 h light and 10 h dark and at 28°C. The breeding of fish lines was approved by the local animal welfare committee (Instantie voor Dierwelzijn) of Leiden University and followed the international guidelines specified by the European Union Animal Protection Directive 2010/63/EU. Experiments were performed on embryos and larvae before the free-feeding stage, not falling under animal experimentation law according the European Union Animal Protection Directive 2010/63/EU. Larvae of 6 and 7 dpf were used according to project license AVD1060020184725 held by Dr. R. G. Boot. Embryos and larvae were grown in egg water [60 μg/ml Instant Ocean Sera Marin™ aquarium salts (Sera, Heinsberg, Germany)]. During inhibitor incubations, fish were kept in E2 medium (15 mM NaCl, 0.5 mM KCl, 1 mM MgSO4, 150 μM KH2PO4, 50 μM Na2HPO4, 1 mM CaCl2, 0.7 mM NaHCO3, 0.5 mg/l methylene blue) at 28°C.
Zebrafish cell culture
Zebrafish embryonic fibroblasts [ZF4 cells (40)] were cultured at 28°C with 5% CO2 in DMEM/F12 (Sigma-Aldrich Chemie GmbH, St. Louis, MO) supplemented with 10% (v/v) fetal calf serum, 1% (v/v) Glutamax, and 0.1% (v/v) penicillin/streptomycin. Cells were harvested using trypsin [0.25% (v/v) trypsin in PBS, no EDTA], washed twice with PBS, and cell pellets were stored at −80°C until use.
Chemicals and reagents
ABP 1 (ME569) (33), ABP 2 (JJB367) (8), compound 3 (ME656) (34), AMP-DNM and L-ido-AMP-DNM (30), eliglustat (41), 13C5-sphinganine, 13C5-sphingosine, 13C5-GlcSph, 13C5-lyso-globotriaosylceramide (LysoGb3), C17-lysosphingomyelin (LysoSM), 13C6-GlcChol, and C17-dihydroceramide (27, 42) were synthesized as reported. The standards GlcCer (d18:1/16:0), galactosylceramide (GalCer) (d18:1/16:0), and β-D-galactosyl cholesterol were obtained from Avanti Polar Lipids (Alabaster, AL) and GlcChol from Sigma-Aldrich Chemie GmbH. LC-MS grade methanol, 2-propanol, water, formic acid, and HPLC grade chloroform were purchased from Biosolve (Valkenswaard, The Netherlands). LC-MS grade ammonium formate and sodium hydroxide were from Sigma-Aldrich; butanol and hydrochloric acid were from Merck Millipore (Billerica, MA).
General CRISPR target design
Primers and single guide RNA (sgRNA) sequences were designed with consideration of genomic variation using the known genetic variation of the GRCz10 annotation (ENSDARG00000076058) and purchased from Integrated DNA Technologies (IDT, Leuven, Belgium). The CHOPCHOP web-tool (43, 44) was used with “GN” or “NG” as 5′ requirements. sgRNA sequences are shown in supplemental Table S1.
Generation of sgRNA
For the generation of sgRNA, a cloning-free protocol was adapted (45) using a target-specific oligo (targets 1 and 2 for gba1 and gba2, respectively) and a constant reverse oligo (supplemental Table S1). Target-specific and constant oligos were annealed using a ramp program: 95°C for 5 min, subsequently cooled to 85°C with −2°C/s, cooled from 85°C to 25°C with −0.1°C/s, and hold at 11°C. The annealed oligos were extended using T4 DNA polymerase (Invitrogen, Carlsbad, CA) for 1 h at 11°C. The double strand DNA (dsDNA) template was column purified (Nucleospin PCR and gel clean-up kit; Machinery-Nagel, Düren, Germany) and eluted in 20 μl of RNase-free water. Approximately 200 ng of template were used for the in vitro RNA synthesis in a 5 μl reaction using the MEGAshortscript™ T7 transcription kit (Ambion®, Thermo Fisher Scientific, Waltham, MA) according to manufacturer’s protocol. After overnight incubation at 37°C, dsDNA template was removed by incubation with Turbo DNase (Ambion®, Thermo Fisher Scientific). sgRNA was purified by ammonium acetate/ethanol precipitation. After washing with 75% ethanol, the sgRNA was resuspended in RNase-free water; the concentration was determined (DeNovix DS-11, Wilmington, DE); the integrity of the sgRNA was verified on a 1% agarose gel, and it was stored in 2 μl aliquots at −80°C until use.
Generation of Cas9 mRNA
The pCS2-nCas9n plasmid was generated as previously described (46). The nCas9n plasmid was linearized using NotI (New England Biolabs, Ipswich, MA) and purified (Nucleospin PCR and gel clean-up kit; Machinery-Nagel). The SP6 promoter of the plasmid was used in combination with the mMessage mMachine® SP6 kit (Ambion®, Thermo Fisher Scientific) to synthesize capped Cas9 mRNA. After 3–4 h of incubation at 37°C, dsDNA template was removed by incubation with Turbo DNase (mMessage mMachine® SP6). Cas9 mRNA was purified using RNeasy mini kit (Qiagen, Hilden, Germany). Cas9 mRNA was eluted in RNase-free water; the concentration was determined (DeNovix DS-11); and Cas9 mRNA was stored in 2 μl aliquots at −80°C until use.
Cas9/sgRNA injection
Approximately 1 nl total volume of Cas9 mRNA and sgRNA (200 pg and 150 pg for Cas9 mRNA and sgRNA, respectively) were co-injected into the yolk of one- or two-cell stage embryos. The integrity of Cas9 mRNA and sgRNA was checked afterwards by loading 1 μl of residual injection mix on 1% agarose gel. Embryos were checked regularly for unfertilized and dead embryos and subsequently raised to adulthood to generate founder fish (F0).
Genomic DNA extraction and PCR
Genomic DNA of larvae or fin clips was extracted using QuickExtract™ (EpiCentre®, Madison, WI) by incubation at 65°C for 10 min, followed by incubation at 98°C for 5 min. Samples were vortexed, diluted with water, and centrifuged quickly to spin down all nonprocessed particles that could interfere in the PCR reaction. For rapid screening of genotypes, a high-resolution melt (HRM) analysis was used. The fragment was amplified with IQ SYBR green (Bio-Rad Laboratories, Hercules, CA) and specific forward and reverse primers (300 μM each, supplemental Table S1) using a Bio-Rad CFX96 Touch™ real-time PCR detection system (Bio-Rad Laboratories) and the following conditions: denaturation at 95°C for 3 min, followed by 40 cycles of amplification (95°C for 30 s and 61°C for 30 s), imaging the plate after every extension at 61°C, followed by a hold step at 55°C for 30 s and a melt program from 55°C to 95°C with 0.5°C per step with imaging the plate every step. Melting curve differences were determined using the melting curve RFU values of WT ABTL genomic DNA as reference group. Experimental samples with a shift in melting curve difference with WT were loaded on 2–2.5% agarose gel to confirm the presence of insertions or deletions (indels).
For sequencing, genomic DNA of positive samples from the HRM analysis was amplified (supplemental Table S1) with Phusion high-fidelity DNA polymerase (Thermo Fisher Scientific) according to the manufacturer’s protocol using the following conditions: denaturation at 98°C for 2 min, followed by 35 cycles of amplification (98°C for 10 s, 65°C for 15 s, and 72°C for 20 s) and a final extension at 72°C for 5 min. PCR product was purified (DNA Clean and Concentrator™; Zymo, Irvine, CA) before Sanger sequencing using the sequence primers shown in supplemental Table S1.
Generation of gba1+/−, gba2−/−, and gba1+/−:gba2−/− zebrafish lines
Founder fish were screened by crossing injected zebrafish to WT fish and subsequent screening of the offspring for germ-line-transmitted mutations. Zebrafish with large and out-of-frame mutations were outcrossed to WT and offspring were raised to adulthood. F1 adult zebrafish were fin-clipped and screened for the mutation using HRM analysis. PCR products of positive samples were sequenced, and fish with the desired mutation were maintained.
All F1 heterozygous zebrafish were outcrossed to WT fish at least twice (>F3 heterozygous) before incrossing. Gba1+/−(Δ31 mutation) adult fish were crossed with each other and offspring thereof were used for experiments after genotyping. To generate GBA2-deficient fish, adult F3 gba2+/−(Δ16 mutation) fish with the desired mutation were crossed with each other and screened. Offspring of the F4 homozygous adult gba2−/− fish were used for experiments. For heterozygous gba2 samples, gba2−/+ larvae were obtained by crossing a gba2−/− female with a WT male, while gba2+/− larvae were obtained by crossing a WT female with a gba2−/− male.
To obtain gba1+/−:gba2−/− zebrafish, gba1+/− adult fish were crossed with gba2+/− adult fish and raised to adulthood. Adult fish were screened for the desired mutations as described above. Double heterozygous gba1+/−:gba2+/− fish were crossed with heterozygous gba2+/− fish; adult fish were screened to obtain fish with the desired genotype (gba1+/−:gba2−/−); and the offspring of these fish were used for experiments.
Treatment of fish with inhibitors
Adult fish of WT, gba1+/−, gba2−/−, or gba1+/−:gba2−/− genotype were crossed and developing offspring (8 h postfertilization) were incubated in 100 μl of E2 medium immersed with vehicle [0.1–0.2% (v/v) DMSO] or inhibitor [in 0.1–0.2% (v/v) DMSO]. For embryos of the gba1+/− and gba1+/−:gba2−/− crossings, samples were assigned to their genotype as described above. The Gba1-specific inhibitor 3 [Fig. 1A (34)] was used at a final concentration of 10 μM in all experiments; the iminosugar inhibitors, AMP-DNM (47) and L-ido-AMP-DNM (29), were used at final concentrations of 10 nM, 100 nM, 500 nM, or 10 μM; and eliglustat was used at 200 nM or 1 μM final concentration. At 5 dpf, the larvae were washed three times with E2 medium before genotyping and subsequent labeling with ABPs or extraction of lipids as described above.
Generation of Tol2-mediated transgenesis of hGBA1 zebrafish
The coding sequence of human GBA1 (NCBI code: NM_000157) was amplified using Phusion high-fidelity DNA polymerase with primers described in supplemental Table S1 and subsequently cloned into pDONR using GATEWAY technology (BP reaction; Invitrogen) according to the manufacturer’s instructions. The hGBA1 Tol2 destination vector was obtained by recombining the pDONR-hGBA with a p5E-ubi, p3E-polyA, and pDEST-Tol2-crystalEye, from the Tol2 kit (48) using a LR reaction. The plasmid containing the Tol2 transposase sequence was linearized using NotI and purified (Nucleospin PCR and gel clean-up kit). Capped and polyadenylated Tol2 mRNA was generated using the mMessage mMachine® SP6 kit as described above for Cas9 mRNA. Approximately 1 nl total volume of Tol2 mRNA and pDEST-ubi:hGBA (100 pg and 20 pg for Tol2 mRNA and plasmid, respectively) were co-injected into the yolk of one- or two-cell stage embryos. At 5 dpf, larvae were screened for the expression of cyan fluorescent protein in the lens of the eyes and positive larvae were raised to adulthood. Adult zebrafish were crossed with the gba1+/− carriers, generating ubi:hGBA|Gba1+/− zebrafish, which were crossed and offspring were used for experiments.
Injection of gba1−/− fish with Cerezyme
Gba1+/− adults were crossed and offspring (2 dpf) in the bloodstream (i.e., the vein under the yolk sac directed toward the heart) with 16 or 36 μU rGBA1 [Cerezyme®; 1 nl in 25 mM Kpi (pH 5.2); 1.6–3.6 × 10−5 U/ml; Sanofi Genzyme]. No increased lethality was observed after injections in either the yolk or in the bloodstream. At 5 dpf, the larvae were collected, genotyped, and subsequently labeled with ABPs or lipids were extracted as described above.
Homogenate preparation and protein concentration
ZF4 cell homogenates, oocyte, egg, or whole zebrafish embryo homogenates were prepared in potassium phosphate [KPi lysis buffer; 25 mM K2HPO4-KH2PO4 (pH 6.5) + 0.1% (v/v) Triton X-100 + 25 U/ml Benzonase® nuclease (Merck, Darmstadt, Germany)] by sonication (20% amplitude, 3 s on, 3 s off for 4 cycles) using a Vibra-Cell™ VCX 130 (Sonics, Newtown, CT) while on ice.
Total protein concentration of homogenates was determined using Pierce™ BCA protein assay kit (Thermo Fisher Scientific) and measured using an EMax® plus microplate reader (Molecular Devices, Sunnyvale, CA).
Labeling of β-glucosidases with ABPs
ABP 1 (34) was used for specific labeling of Gba1; whereas for concomitant labeling of the three β-glucosidases (Gba1, Gba2, and Gba3), ABP 2 was used (49). A WT homogenate was denatured prior to ABP labeling (lane −) to account for aspecific adhesion.
ZF4 cell homogenate (10 μl, 30 μg protein) was preincubated with 5 μl 300 mM McIlvaine buffer (pH 2–8) for 5 min on ice before addition of ABP 1 [5 μl; 400 nM in MQ with 2% (v/v) DMSO, final concentration of 100 nM and 0.5% (v/v) DMSO] or ABP 2 [5 μl; 400 nM in MQ, 2% (v/v) DMSO, final concentration of 100 nM and 0.5% (v/v) DMSO]. Samples were incubated for 30 min at 28°C before addition of 5× Laemmli sample buffer [5 μl; 25% (v/v) 1.25 M Tris-HCL (pH 6.8), 50% (v/v) 100% glycerol, 10% (w/v) sodium dodecyl sulfate (SDS), 8% (w/v) DTT, and 0.1% (w/v) bromophenol blue] and boiled for 5 min at 98°C.
Zebrafish homogenate (10 μl, 1 zebrafish per incubation) was preincubated with 5 μl 300 mM McIlvaine (pH 2–8) for 5 min on ice before addition of ABP 1 or ABP 2 [5 μl ABP 1; 4 μM in MQ with 2% (v/v) DMSO, final concentration of 1 μM and 0.5% (v/v) DMSO or 5 μl ABP 2; 800 nM in MQ with 2% (v/v) DMSO, final concentration of 200 nM and 0.5% (v/v) DMSO]. Samples were incubated for 30 min at 28°C before addition of 5× Laemmli sample buffer (5 μl), and samples were briefly boiled. Homogenates of zebrafish in different developmental stages (10 μl, 1 egg or embryo per lane) were incubated with 10 μl ABP 1 [2 μM in 150 mM McIlvaine (pH 4) with 1% (v/v) DMSO, final concentration of 1 μM with 0.5% (v/v) DMSO] or ABP 2 [200 nM in 150 mM McIlvaine (pH 6) with 1% (v/v) DMSO, final concentration of 200 nM and 0.5% (v/v) DMSO] and incubated for 30 min at 28°C. After incubation, 5× Laemmli sample buffer (5 μl) was added and samples were boiled for 5 min at 98°C.
For characterization of the gba1, gba2, and gba1:gba2 double KO, individual zebrafish 5 dpf larvae with different genotypes were homogenized in 30 μl of KPi buffer using a Dounce homogenizer (10 s; Pellet pestle motor, Kimble® Kontes). Zebrafish homogenate (10 μl, one-third of a zebrafish) was incubated with ABP 1 and ABP 2 as described for the different developmental stages. Larvae were lysed in 20 μl Kpi in the experiments with Gba1 KO, inhibitor 3-treated WT, larvae overexpressing human GBA1 or injected with Cerezyme. Homogenate (one-half of a zebrafish) was used to label active Gba1 with ABP 1 as described above, while human GBA1 was labeled using the optimal conditions for human GBA1 [2 μM in 150 mM McIlvaine (pH 5.2) with 0,1% (v/v) Triton X-100 and 0.2% (w/v) sodium taurocholate, and 1% (v/v) DMSO, final concentration of 1 μM with 0.5% (v/v) DMSO] (7).
Gel electrophoresis and fluorescence scanning
ABP-labeled protein samples were separated by electrophoresis on 8% (w/v) SDS-PAGE gel for 2 h at 90 V before scanning the fluorescence of the wet-slab gel with a Typhoon FLA 9500 [GE Healthcare, Chicago, IL; Cy5 (635 nm λEX, 665 nm λEM), 750 V, pixel size 100 μm].
Western blot and total protein staining
β-Actin as loading control was visualized by Western blot using primary rabbit anti-actin antibody (ab8227; Abcam, Cambridge, UK) at 1:2,000 and donkey anti-rabbit HRP-linked secondary antibody (Bio-Rad Laboratories) at 1:5,000. Chemiluminescence was visualized using a ChemiDocMP imager (Bio-Rad Laboratories) in chemiluminescence settings with an exposure of 1 min. Total protein loading was visualized using Coomassie brilliant blue G250 and scanned on the ChemiDocMP imager.
(Glyco)sphingolipid analysis
Neutral (glyco)sphingolipids, (glyco)sphingoid bases, and hexosylcholesterol (HexChol) were extracted from the same individual zebrafish using an acidic Bligh and Dyer procedure [1:1:0.9 chloroform:methanol:100 mM formate buffer (pH 3.1)] according to methods described before (27, 42, 50). To an individual zebrafish was added 20 μl of internal standard mixture (0.1 pmol/μl of 13C5-sphinganine, 13C5-sphingosine, 13C5-GlcSph, 13C5-lysoGb3, and C17-lysoSM in methanol), 20 μl of C17-dihydroceramide (20 pmol/μl in methanol), 20 μl of 13C6-GlcChol (0.1 pmol/μl in methanol) followed by methanol and chloroform (2:1, v/v). After brief mixing, the samples were left at room temperature for 1 h with occasional stirring and 3× 1 min sonication in a bath sonifier (VWR ultrasonic cleaner USC). Samples were centrifuged for 10 min at 15,700 g to spin down precipitated proteins. The supernatant was transferred to a clean tube, while excess organic solvent was evaporated and genomic DNA was extracted from the remaining material to validate the genotype. Chloroform and 100 mM formate buffer (pH 3.1) were added to the supernatant, to a final ratio of 1:1:0.9 methanol:chloroform:formate buffer, to induce separation of phases. The upper phase was used for analysis of lyso(glyco)sphingolipids and the lower phase for analysis of neutral (glyco)sphingolipids and HexChol. After centrifugation, the upper phase was transferred to a clean tube and the lower phase (chloroform phase) was extracted an additional time with methanol and formate buffer. Pooled upper phases were concentrated at 45°C in an Eppendorf Concentrator Plus and a butanol/water (1:1, v/v) extraction was performed. The upper phase (butanol phase) was transferred to a clean tube and concentrated. Lipids were dissolved in 100 μl of methanol, stirred, sonicated for 30 s in a bath sonifier, and centrifuged. The supernatant was transferred to a vial for subsequent LC-MS/MS analysis.
The remaining lower chloroform phase was transferred to a clean tube and the interphase was washed with chloroform. The pooled lower chloroform phases were split, whereby one part was used to analyze HexChol and the part for analysis of neutral glycosphingolipids was transferred to a Pyrex tube and dried at 45°C under a gentle stream of nitrogen. Deacylation was performed by adding of 500 μl of sodium hydroxide (0.1 M NaOH in methanol) using a microwave-assisted saponification method (50). The samples were cooled and neutralized by adding hydrogen chloride (50 μl of 1 M HCl in methanol) and dried, followed by butanol/water extraction and preparation for LC-MS/MS as described above. For determination of HexChol (27), the other half was concentrated; a butanol/water extraction was performed; and samples were prepared for LC-MS/MS analysis as described above.
Glycosphingolipid analysis was performed from three independent crossings, and incubations, extractions, and measurements using biological replicates as described in the Results section.
For hydrophilic interaction liquid chromatography (HILIC) separation, different individual larvae (n = 3–9) were extracted as described above and lipids were resuspended in acetonitrile:methanol (9:1, v/v) prior to transfer to LC-MS vials.
LC-MS/MS
Measurements were performed using a Waters UPLC-Xevo-TQS micro instrument (Waters Corporation, Milford, MA) in positive mode using an ESI source. For measurements of (glyco)sphingoid bases, deacylated neutral (glyco)sphingolipids, and HexChol, a BEH C18 column (2.1 × 50 mm with 1.7 μm particle size; Waters Corporation) was used with eluents and LC-MS/MS programs as described previously for (glyco)sphingoid bases (42) and HexChol (27), respectively. A BEH HILIC column (2.1 × 100 mm with 1.7 μm particle size; Waters Corporation) was used at 30°C for the separation of lipids with glucosyl and galactosyl moiety. In general, eluent A contained 10 mM ammonium formate in acetonitrile/water (97:3, v/v) and 0.01% (v/v) formic acid, and eluent B consisted of 10 mM ammonium formate in acetonitrile/water (75:15, v/v) and 0.01% (v/v) formic acid. Lyso- and deacylated glycosphingolipids were eluted in 18 min with a flow of 0.4 ml/min using the following program: 85% A from 0 to 2 min, 85–70% A from 2 to 2.5 min, 70% A from 2.5 to 5.5 min, 70–60% A from 5.5 to 6 min, 60% A from 6 to 8 min, 60–0% A from 8 to 8.5 min, 0–85% A from 8.5 to 9.5 min, and re-equilibration of the column with 85% A from 10 to 18 min. HexChol was eluted in 18 min with a flow of 0.25 ml/min using the following program: 100% A from 0 to 3 min, 100–0% A from 3 to 3.5 min, 0% A from 3.5 to 4.5 min, 0–100% A from 4.5 to 5 min, and re-equilibration with 100% A from 5 to 18 min. Data were analyzed with MassLynx 4.1 software (Waters Corporation).
Statistics
Statistical analyses were performed using GraphPad Prism (v7.00; GraphPad Software) and values are presented as mean ± SD. Lipid data were analyzed by one-way ANOVA using Dunnett’s or Tukey’s test as described in the Results section (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
RESULTS
Gba1 and Gba2 of zebrafish: detection with fluorescent ABPs
Zebrafish have one ortholog of human lysosomal GBA1 (38), and zebrafish Gba1 (UniProt accession P04062) shows 58% identity and 73% similarity to the human GBA1 enzyme. The zebrafish Gba1 protein consists of 518 amino acids with a predicted mass of 58 kDa. The zebrafish ortholog (UniProt accession E7F5W0) of human nonlysosomal β-glucosidase shows 66% identity and 79% similarity to human GBA2 and a predicted mass of 96 kDa (35).
We labeled a homogenate of zebrafish embryonic fibroblasts (ZF4 cell line) (40) and a homogenate of pooled WT zebrafish larvae (5 dpf) with ABPs at different pH (Fig. 1D, E). ABP 1, a Cy5 fluorescent cyclophellitol-epoxide targeting specifically Gba1, labeled a protein with an apparent molecular mass around 60 kDa in the zebrafish homogenates, most favorable at pH 4.0 (Fig. 1D, top panel). The observed molecular mass coincides with that of the glycosylated zebrafish ortholog of Gba1, and the optimal labeling at acidic pH is consistent with the pH optimum reported for Gba1 (7). Zebrafish homogenates incubated with fluorescent ABP 2, the fluorescent cyclophellitol-aziridine that labels all retaining β-glucosidases (8), revealed that, besides Gba1, a protein with an apparent molecular mass of about 95 kDa was also detected, coinciding with the predicted molecular mass of zebrafish Gba2 (Fig. 1D, E).
Next, we labeled homogenates of developing zebrafish embryos, from unfertilized oocytes to 7 dpf zebrafish larvae (-f to 7 dpf, Fig. 1F). Active Gba1 and Gba2 molecules were already detected in both oocytes and fertilized eggs, suggesting the presence of active β-glucosidases in the yolk of the developing embryo deposited by the adult zebrafish female. The intensity of ABP-labeled Gba2 increased over time (in days), while Gba1 intensity reduced in the developing embryo. Of note, although the presence of very abundant yolk proteins, predominantly isoforms of the phospholipoglycoprotein vitellogenin (51), influenced the apparent molecular mass of the ABP-labeled enzyme, it did not influence target engagement of the ABP.
Gba1−/− larvae accumulate GlcSph
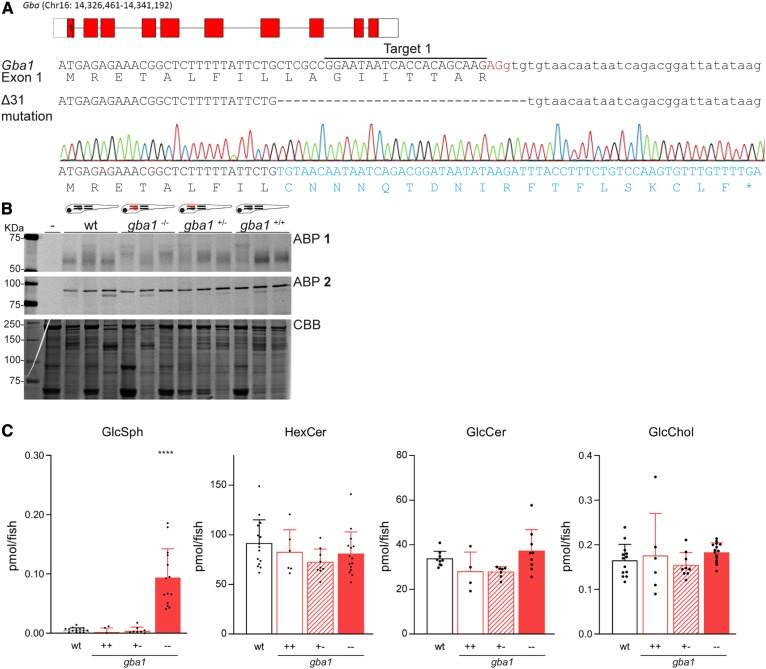
To obtain a gba1 mutant zebrafish, an appropriate sgRNA sequence was selected in the first exon of gba1 (target 1, Fig. 2A, upper and middle panel). Injection of Cas9 mRNA and sgRNA into the one-cell stage of WT embryos generated a founder fish with a germ-line transmitted deletion of 31 bp in the splice-site region of exon 1 (gba1Δ31, Fig. 2A, lower panel). This founder was subsequently used to generate a heterozygous gba1+/− zebrafish line without malformations (supplemental Fig. S2). Homozygous gba1−/− mutant larvae were obtained following crossing of the adult gba1+/− carriers and characterization of offspring by genotyping.
Fig. 2.
CRISPR/Cas9-mediated disruption of Gba1 in zebrafish. A: Top panel: Schematic representation of the gba1 gene on chromosome 16. Middle panel: DNA sequence of the exon1-intron1 boundary of gba1 with the exon in upper case and intron in lower case, the sgRNA target sequence underlined, the PAM site in red, and the protein sequence shown below. Lower panel: The 31 bp deletion (Δ31), obtained from the sequence trace of an homozygous gba1Δ31 larvae, is located in the splice-region with the altered predicted translated protein sequence given in blue and leads to a premature stop codon (*). B: ABP labeling of homogenate of individual zebrafish larvae at 5 dpf (WT, gba1−/−, gba1+/−, or gba1+/+ from incross; n = 3) with ABP 1 (top panel) or ABP 2 (middle panel). In lane –, sample is denatured prior to ABP addition; CBB staining was used as loading control. C: GlcSph, HexCer, GlcCer, and GlcChol levels were determined of individual zebrafish larvae in picomoles per fish; WT (n = 15), gba1+/+ (n = 6), gba1+/− (n = 9), and gba1−/− (n = 15) for GlcSph, HexCer, and GlcChol; and WT (n = 9), gba1+/+ (n = 4), gba1+/− (n = 7), and gba1−/− (n = 9) for GlcCer. Data are depicted as mean ± SD and analyzed using one-way ANOVA (Dunnett’s test) with WT as control group with ****P < 0.0001.
To validate the gba1−/− fish (with Δ31 mutation), their Gba1 status was examined by labeling with ABP 1. Comparison of WT, heterozygous gba1+/−, and gba1−/− KO larvae labeled with ABP 1 revealed a reduction of the ∼60 kDa Gba1 in the gba1−/− 5 dpf larvae (Fig. 2B). Some residual labeled protein at 60 kDa was observed in the homozygous gba1−/− larvae, argued as the deposition of maternal Gba1 enzyme from the heterozygous female (52).
MS, as such, does not distinguish between lipids with a glucose or galactose moiety. We used HILIC to separate glucosyl- and galactosyl-containing lipids of additional larvae and observed that accumulated HexSph is solely GlcSph in 5 dpf larvae, while aberrant HexChol is solely GlcChol. In the case of hexosylceramide (HexCer), about 30% can be attributed to GalCer in 5 dpf WT zebrafish larvae, whereby GalCer levels do not change upon genetic or pharmacological modulation, and the vast majority being GlcCer, showing aberrant levels upon modulation.
To establish whether Gba1 is truly impaired in gba1−/− fish, their glycosphingolipid content was determined. In the 5 dpf gba1−/− larvae, total HexCer, i.e., GlcCer and/or GalCer, and GlcChol were not significantly increased (Fig. 2C). In the course of the experiments, we used HILIC column chromatography to measure sphingolipids with glucose and galactose moieties in a separate set of zebrafish larvae. This revealed that in the studied 5 dpf larvae, more than 70% of HexCer is GlcCer (supplemental Fig. S5). This lipid, like total HexCer, did not significantly accumulate in gba1−/− zebrafish larvae. Likewise, HILIC separation revealed that solely GlcSph accumulates in the gba1−/− larvae (Fig. 2C, supplemental Fig. S5). No significant difference was detected for other (glyco)sphingolipids such as sphinganine, sphingosine, dihydroceramide, ceramide, GalCer, and dihexosylceramide (supplemental Fig. S6).
Next, 5 dpf larvae were dissected into head and body regions and glycosphingolipid levels were determined (supplemental Fig. S7). GlcSph and GlcCer levels were significantly increased in both regions. GlcChol was also detected in the brain region in comparable levels to the body region.
To conclude, the prominent accumulation of GlcSph in the mutant fish resembles the marked increase of GlcSph in Gba1-deficient patients and mice (53). Thus, we introduced a functional deficiency in lysosomal Gba1 activity in the gba1−/− fish, promoting active conversion of accumulating GlcCer to GlcSph.
Deficiency in Gba2 results in a prominent decrease in GlcChol
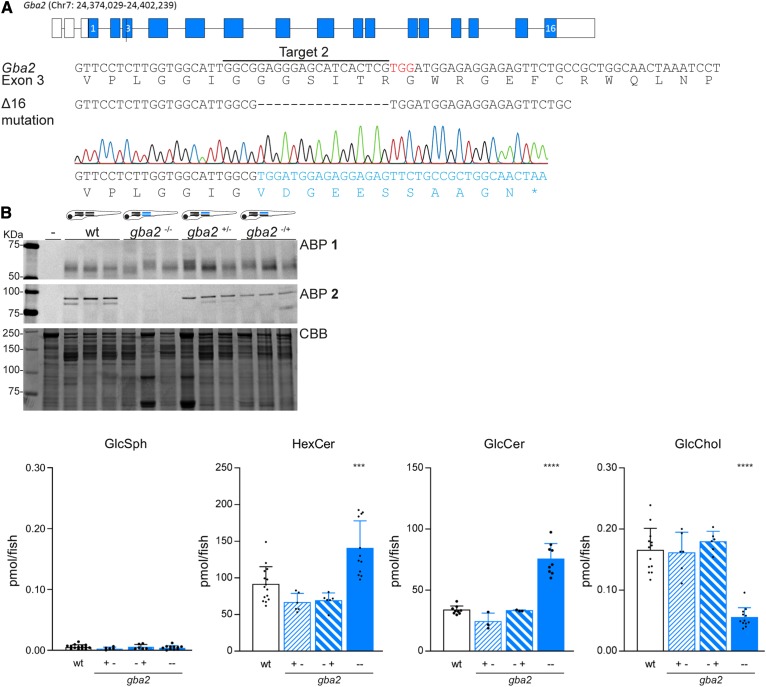
Next, we similarly generated a Gba2-deficient fish. An appropriate sgRNA sequence (target 2, Fig. 3A, upper and middle panel) was selected in the third exon of the gba2 gene, and subsequent rounds of screening and crossing resulted in a zebrafish with a 16 bp deletion in exon 3 of gba2. This deletion created a premature stop codon (Fig. 3A, lower panel). Adult homozygous gba2−/− zebrafish showed no malformations (supplemental Fig. S2) or aberrant behavior. Moreover, adult homozygous gba2−/− zebrafish produced regular sized clutches with normally developing larvae until the free-feeding stage of 5 dpf, suggesting that the male gba2−/− fish are fertile in contrast to some strains of GBA2 KO mice (24, 54).
Fig. 3.
CRISPR/Cas9-mediated disruption of Gba2 in zebrafish. A: Top panel: Schematic representation of gba2 gene on chromosome 7. Middle panels: DNA sequence of exon 3 of gba2 with the sgRNA target sequence underlined, the PAM site in red, and the protein sequence shown below. Lower panel: The 16 bp deletion (Δ16), as obtained from the sequence trace, introduces a premature stop codon (*) in the altered predicted translated protein sequence, given in blue. B: ABP labeling of homogenate of individual zebrafish larvae at 5 dpf (WT, gba2−/−, and both heterozygous gba2+/− options, n = 3) with ABP 1 (top panel) or ABP 2 (middle panel). In lane –, sample is denatured prior to ABP addition; CBB staining was used as loading control. C: GlcSph, HexCer, GlcCer, and GlcChol levels were determined of individual zebrafish larvae in picomoles per fish; WT (n = 15), gba2+/− (n = 6), gba2−/+ (n = 6), and gba2−/− (n = 12) for GlcSph, HexCer, and GlcChol; and WT (n = 9), gba2+/− (n = 3), gba2−/+ (n = 3), and gba2−/− (n = 9) for GlcCer. Data are depicted as mean ± SD and analyzed using one-way ANOVA (Dunnett’s test) with WT as control group with ***P < 0.001 and ****P < 0.0001.
To validate GBA2 deficiency in the gba2−/− fish (with Δ16 mutation), enzyme status was examined by ABP 2 labeling. Homogenates of zebrafish larvae of different gba2 genotypes were incubated with GBA1-specific ABP 1 and broad-spectrum ABP 2. Homozygous gba2−/− fish showed complete absence of ABP-labeled enzyme at 90 kDa, while heterozygous offspring from two different crossings exhibited residual ABP-labeled Gba2 enzyme (Fig. 3B).
The lipid composition of gba2−/− larvae (5 dpf) showed an increase in HexCer, predominately GlcCer (Fig. 3C). Additionally, these larvae exhibited a very prominent decrease of HexChol (Fig. 3C). As observed for GlcSph, HILIC separation revealed that GlcChol is the predominant form of HexChol (>95%) in 5 dpf zebrafish larvae (supplemental Fig. S8). The gba2−/− fish showed no increase in GlcSph and no significant differences were found for other (glyco)sphingolipids such as sphinganine, sphingosine, dihydroceramide, ceramide, GalCer, and dihexosylceramide (Fig. 3C, supplemental Fig. S6). The GlcChol reduction was not observed in WT and heterozygous gba2+/− larvae. The marked reduction in GlcChol in the gba2−/− zebrafish larvae is similar to that observed in Gba2-deficient mice (27). This suggests that zebrafish Gba2 also acts as a transglucosylase similar to rodent and human Gba2, generating GlcChol from GlcCer and cholesterol.
Aberrant GlcSph, GlcCer, and GlcChol levels in gba1−/−:gba2−/− larvae
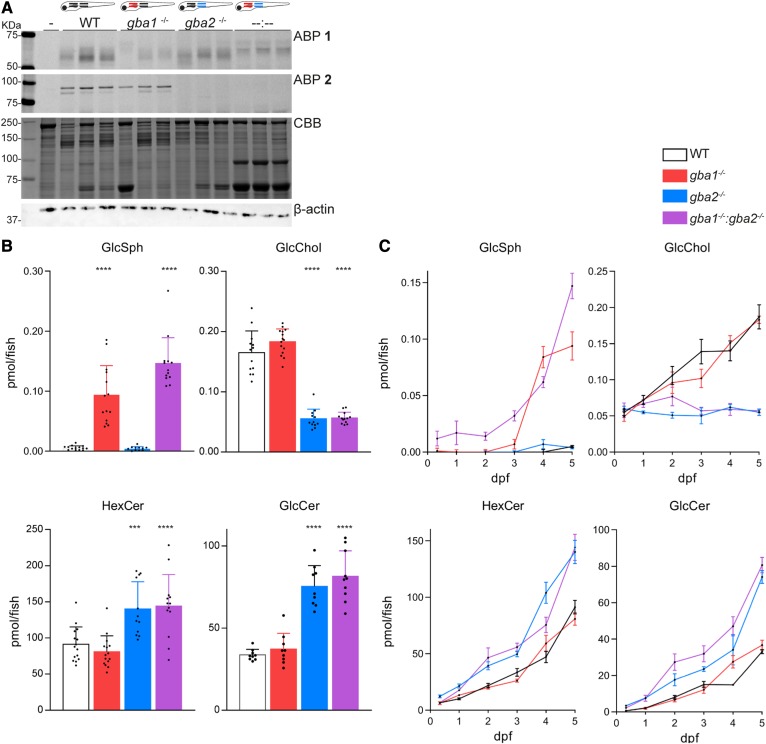
The gba1+/−:gba2−/− adult carrier zebrafish, used to produce gba1−/−:gba2−/− larvae, did not show malformations (supplemental Fig. S2) and gave regular sized clutches. To examine the gba1−/−:gba2−/− double KO zebrafish, we employed the same ABP labeling as described above. As in the respective Gba1 and Gba2 single KO, no Gba2 enzyme was visualized on gel, while a residual ABP-labeled enzyme with a molecular mass comparable to Gba1 was present in the double KO 5 dpf larvae (Fig. 4A).
Fig. 4.
Biochemical evaluation of gba1, gba2, and double gba1:gba2 KO zebrafish larvae. A: ABP labeling of homogenate of individual zebrafish larvae at 5 dpf (WT, gba1−/−, gba2−/−, and gba1−/−:gba2−/−, n = 3) with ABP 1 (top panel) or ABP 2 (middle panel). In lane –, the sample is denatured prior to ABP addition; CBB staining and β-actin were used as loading control (lower panels). B: GlcSph, HexCer, GlcCer, and GlcChol levels were determined of individual 5 dpf zebrafish larvae in picomoles per fish; WT (n = 15), gba1−/− (n = 15), gba2−/− (n = 12), and gba1−/−:gba2−/− (n = 13) for GlcSph, HexCer, and GlcChol; and WT (n = 9), gba1−/− (n = 9), gba2−/− (n = 9), and gba1−/−:gba2−/− (n = 10) for GlcCer. Data are depicted as mean ± SD and analyzed by one-way ANOVA (Dunnett’s test) with WT as control group. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. C: Developing zebrafish embryos were harvested at different ages and GlcSph, HexCer, GlcCer, and GlcChol levels were determined in picomoles per fish [8 h post fertilization (n = 4–5), 1 dpf (n = 3–5), 2 dpf (n = 3–5), 3 dpf (n = 3–5), 4 dpf (n = 3–6)]. Data on relevant lipid levels of 5 dpf larvae are obtained from C. Data are depicted as mean ± SEM
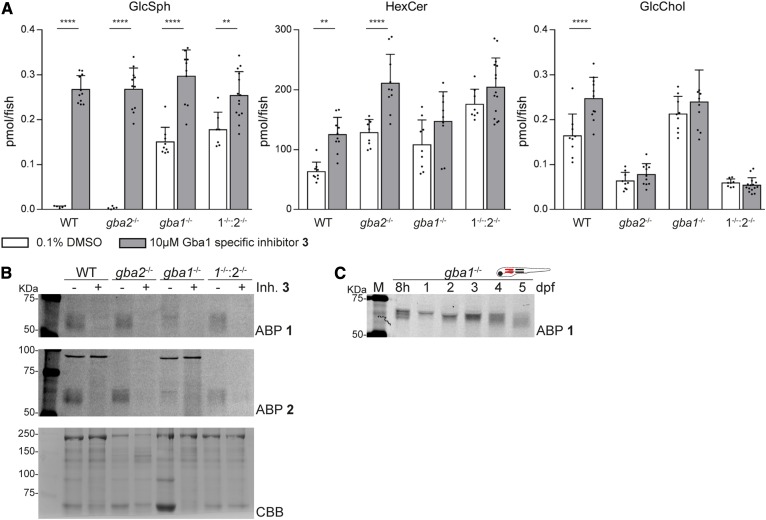
(Glyco)sphingolipid analysis of gba1−/−:gba2−/− larvae showed increased HexCer levels, predominantly GlcCer, compared with single gba1−/− larvae but similar to that of single gba2−/− larvae (Fig. 4B). Moreover, in the double KO larvae, GlcChol was significantly decreased, similar to that observed in gba2−/− larvae. Compared with WT larvae, a significant accumulation of GlcSph in gba1−/−:gba2−/− larvae was detected. GlcSph levels in double KO fish tended to be somewhat higher than in the gba1−/− larvae (Fig. 4B), although developing zebrafish showed considerable variation in rapidly accumulating GlcSph.
Next, we analyzed the age dependence of glycosphingolipid changes in developing embryos, from 8 hours postfertilization (hpf) to 5 dpf (Fig. 4C). The elevation of GlcSph in the gba1−/− and gba1−/−:gba2−/− embryos was detectable from 3 dpf onwards. HexCer (predominantly GlcCer) was also found to increase with age, accumulating more rapidly in the gba2−/− and gba1−/−:gba2−/− embryos. GlcChol increased with age in WT and gba1−/− embryos, but remained low (around 0.05 pmol/fish) in the Gba2-deficient fish. The gradual increase with age of GlcChol suggests that it is formed in the developing embryos. In other words, its origin is unlikely to be entirely the yolk. Other glycosphingolipids (GalCer and dihexosylceramide) also increased with the age of the embryo independent of genotype (supplemental Fig. S9).
Additional accumulation of GlcSph in chemically induced Gba1-deficient larvae
Adamantyl-cyclophellitol 3, a recently designed highly specific inhibitor of GBA1, was used to investigate the effect of acute pharmacological induction of Gba1 deficiency (34). Incubation of WT larvae with 10 μM of 3 for 5 days led to a significant increase in GlcSph in the developing larvae (Fig. 5A) and also in their surrounding water (supplemental Fig. S10). Incubation of gba2−/− larvae increased GlcSph comparable to WT, which indicates that the increase of GlcSph is independent of the Gba2 status (Fig. 5A). Incubation of gba1−/− and gba1−/−:gba2−/− larvae with the Gba1-specific inhibitor led to a significant increase in GlcSph compared with vehicle-treated gba1−/− or gba1−/−:gba2−/− larvae (Fig. 5A). GlcChol was only significantly increased in WT treated larvae, while HexCer was increased in all genotypes incubated with 3 (Fig. 5A, supplemental Fig. S11). A potential explanation is the additional inhibition of maternal Gba1 by compound 3 immediately after fertilization, thereby generating a completely Gba1-deficient fish larvae. Indeed, ABP labeling of vehicle-treated gba1−/− backgrounds visualized a 60 kDa protein (Fig. 5B), while ABP-labeled Gba1 was not visible in any of the zebrafish pretreated with inhibitor 3 (Fig. 5B, supplemental Fig. S4B). Of note, ABP-labeled Gba1 was present in Gba1−/− embryos of all ages (Fig. 5C). Together, the lipid and ABP data suggest the presence of maternal Gba1 enzyme, which can be inhibited by compound 3.
Fig. 5.
Chemical inactivation of Gba1 shows full gba1 deficiency with increased GlcSph levels. WT, gba2−/−, gba1−/−, and gba1−/−:gba2−/− embryos were treated with vehicle [0.1% (v/v) DMSO] or inhibitor 3 (10 μM) for 5 days and relevant lipid levels were determined of individual larvae in picomoles per fish (n = 7-15) (A) or active β-glucosidase enzyme was visualized with ABP 1 (top panel) or ABP 2 (middle panel) (B); CBB staining was used as loading control (lower panel). C: Gba1−/− embryos were harvested at different ages and active Gba1 was visualized with ABP 1. Data of GlcSph, HexCer, and GlcChol are depicted as mean ± SD and analyzed using one-way ANOVA (Tukey’s test). *P < 0.05, **P < 0.001, ***P < 0.001, and ****P < 0.0001.
Lipid corrections by inhibition of GCS and Gba2
Next, we studied the feasibility of pharmacological intervention and correction of glycosphingolipid abnormalities of Gba1-deficient zebrafish larvae by SRT using treatment with reported GCS inhibitors as well as concomitant GCS and GBA2 inhibitors.
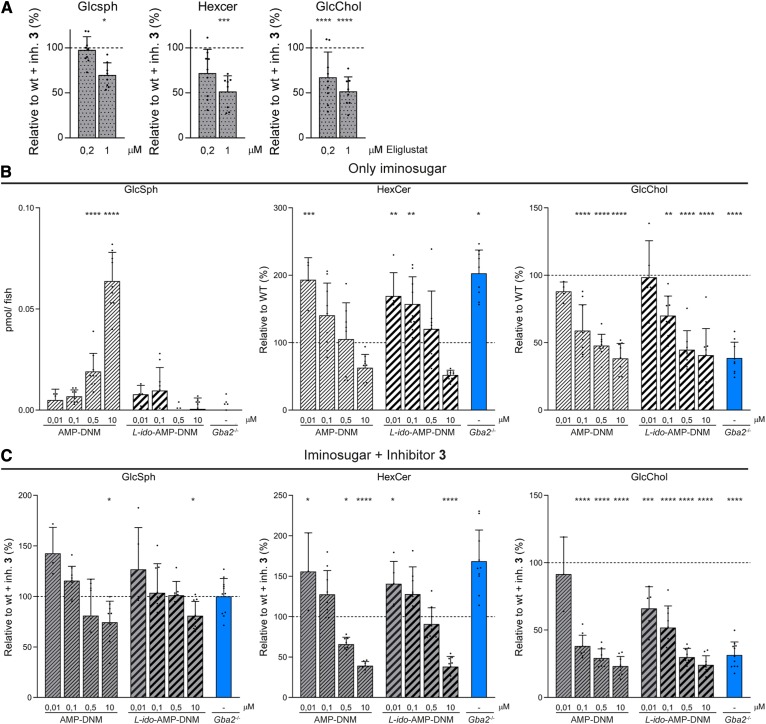
WT zebrafish embryos were simultaneously incubated with the Gba1-specific inhibitor 3 and the potent specific GCS inhibitor, eliglustat, to study GCS inhibition in a Gba1-deficient background (Fig. 1C). Incubation with 1 μM of eliglustat for 5 days led to a decrease in HexCer and the derived lipids GlcSph and HexChol (Fig. 6A). As for characterization of the genetic KO larvae, the reduction in HexCer levels was prominently due to a decrease in GlcCer levels, based on our HILIC method performed on additional larvae (supplemental Fig. S12).
Fig. 6.
Pharmacological inhibition of Gba1, Gba2, and Gcs. A: WT embryos were treated simultaneously with inhibitor 3 (inh. 3) and eliglustat. Lipid levels were determined of individual larvae (n = 8–9). Ratios of GlcSph, HexCer, and GlcChol are depicted relative to WT embryos incubated with inhibitor 1 only (100% line). B: WT embryos were incubated for 5 days with different concentrations of AMP-DNM (striped bars) or L-ido-AMP-DNM (thicker striped bars) and lipid levels were determined of individual larvae (n = 5–9). Data of GlcSph are depicted in picomoles per larvae, while ratios of HexCer and GlcChol are depicted relative to vehicle-treated WT (100% line). C: WT embryos were treated with inhibitor 3 (10 μM) and different concentrations of AMP-DNM (striped bars) or L-ido-AMP-DNM (thicker striped bars) and lipid levels were determined of individual larvae (n = 5–9). Data of GlcSph, HexCer, and GlcChol are depicted relative to inhibitor 3-treated WT (100% line). Inhibitor 3-treated gba2−/− (blue bar) is used as control for pharmacological Gba1 inhibition in a full genetic Gba2-deficient background. Data are depicted as mean ± SD and analyzed by one-way ANOVA (Dunnett’s test) with WT as control group. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
The iminosugars, AMP-DNM and L-ido-AMP-DNM, have been reported as low nanomolar GBA2 inhibitors, though they also inhibit GCS and GBA1 at a higher concentration (Fig. 1C) (29). Because the reported potencies are in vitro and in situ IC50, we first recapitulated the enzyme-specific inhibition of AMP-DNM and L-ido-AMP-DNM in whole WT zebrafish larvae. The embryos were incubated with different concentrations of AMP-DNM (10 nM, 100 nM, 500 nM, and 10 μM) and L-ido-AMP-DNM (10 nM, 100 nM, 500 nM, and 10 μM), and GlcSph, HexCer, and GlcChol levels were analyzed as ratios relative to vehicle-treated WT (Fig. 6B). At the lowest concentration of 10 nM, both iminosugars already caused an increase in HexCer, but no prominent decrease in GlcChol yet. At the higher concentration of 100 nM, both iminosugars caused almost complete in vivo inhibition of Gba2, as reflected by decreasing GlcChol and simultaneously increasing HexCer (Fig. 6B). At very high concentrations of AMP-DNM and L-ido-AMP-DNM (500 nM and 10 μM), levels of HexCer, predominantly GlcCer, decreased, indicating that GCS was inhibited as well (Fig. 6B, supplemental Fig. S13). A significant increase in GlcSph was observed at high AMP-DNM concentrations (10 μM, Fig. 6B), but not in the case of L-ido-AMP-DNM, which is known to hardly inhibit GBA1.
Next, we analyzed the potential of the iminosugars to pharmacologically correct the glycosphingolipid abnormalities in Gba1-deficient zebrafish larvae. WT embryos were simultaneously incubated with 3 and different concentrations of AMP-DNM or L-ido-AMP-DNM, and glycosphingolipid levels were analyzed as a ratio relative to control Gba1-deficient zebrafish larvae incubated with 3 alone. Incubation with 100 nM of AMP-DNM and L-ido-AMP-DNM resulted in reduction of GlcChol compared with control (Fig. 6C), indicating Gba2 inhibition. At this concentration, no reduction of GlcSph was found, indicating that pharmacological inhibition of Gba2 in a Gba1-deficient background fails to correct the accumulation of GlcSph at the developmental stage of 5 dpf. Using high concentrations of AMP-DNM and L-ido-AMP-DNM (10 μM, Fig. 6C), both Gba2 and GCS were inhibited, as indicated by the reduction of GlcChol and HexCer, mainly GlcCer levels (Fig. 6C, supplemental Fig. S12). At these high concentrations, significant reduction in GlcSph also became apparent.
Thus, both eliglustat and the iminosugars at higher dose were able to pharmacologically correct glycosphingolipid abnormalities in Gba1-deficient whole zebrafish larvae.
Rescue of Gba1 deficiency by expression or injection of human GBA1
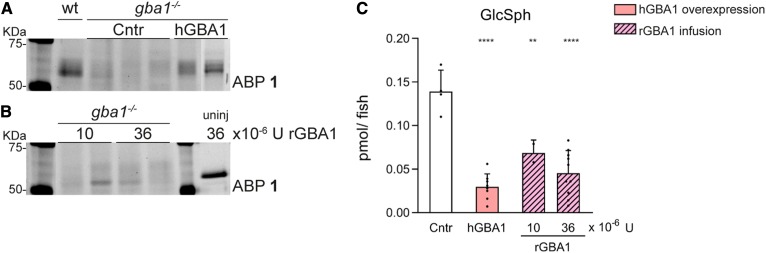
Finally, we studied correction of abnormal glycosphingolipid metabolism by introducing human GBA1 to Gb1-deficient zebrafish embryos. Two approaches were used: overexpression of human GBA1 using the Tol2 transposase method (48) and injection of recombinant GBA1 enzyme (Cerezyme®) in the bloodstream of 2 dpf gba1−/− zebrafish embryos. The presence of active GBA1 was detected by labeling with ABP 1 (Fig. 7A). Overexpression in the zebrafish resulted in the presence of human GBA1 with heterogeneous molecular mass, indicating differently glycosylated forms. Infusion of rGBA1 into the embryos led to the presence of one distinct band labeled by the ABP 1 (Fig. 7A). In the latter experiments, variation among individual injected embryos was noted. The significant decrease in GlcSph indicates that both overexpression and infusion of human GBA1 functionally correct the absence of zebrafish Gba1 (Fig. 7B).
Fig. 7.
Glycosphingolipid correction by introduction of human GBA1. A: Human GBA1 was stably overexpressed in the gba1−/− zebrafish background using the ubiquitin promoter. Zebrafish Gba1 and human GBA1 were visualized with ABP 1. B: Recombinant GBA1 was introduced by injection in the bloodstream of 2 dpf zebrafish with 10 or 36 × 10−6 U rGBA1 (Cerezyme®) and visualized with ABP 1. An equivalent of 36 × 10−6 U rGBA1 was labeled and used as control on gel. C: GlcSph levels were determined in picomoles per fish of uninjected control gba1−/− zebrafish, gba1−/− zebrafish stably overexpressing hGBA1, and gba1−/− zebrafish infused with rGBA1. Data are depicted as mean ± SD and analyzed by one-way ANOVA (Dunnett’s test) with WT as control group. **P < 0.01 and ****P < 0.0001.
In conclusion, glycosphingolipid abnormalities can be corrected by pharmacological and genetic intervention and corrections can be detected in individual zebrafish samples.
DISCUSSION
The primary goal of our investigation was to study GlcCer metabolism during deficiency of the lysosomal Gba1 in a whole organismal model. For this purpose, we selected developing zebrafish larvae until 5 dpf, an attractive model to investigate genetic disorders and to study related biochemical abnormalities and pharmacological or genetic correction of the disease. We particularly focused on the potential role of cytosol-facing Gba2 in compensatory GlcCer metabolism during inadequate Gba1 activity. To generate a deficiency of Gba1 and/or Gba2 in zebrafish, we used two different approaches: CRISPR/Cas9-mediated KO of the Gba1 gene (gba1−/− fish) as well as chemical inactivation using specific inhibitors. A Cy5 fluorescent ABP, labeling active Gba1 enzyme molecules through covalent binding to the catalytic nucleophile (7), was used to confirm the genetic KO of Gba1 in gba1−/− fish as well as its complete inactivation by selective Gba1 inhibitor 3 (34). Of note, ABP labeling of active Gba1 in gba1−/− and gba1−/−gba2−/− fish in the developing zebrafish embryo pointed to the presence of maternal Gba1. Apparently, maternal Gba1 enzyme is deposited by the heterozygous gba1+/− mother in the yolk of the embryo, a phenomenon described earlier for other lysosomal enzymes as well (52).
The viability of gba1−/− and gba1−/−:gba2−/− fish deserves notice (38). In humans and mice, a complete deficiency of Gba1 is not compatible with terrestrial life due to altered skin permeability causing transepidermal water loss (37, 55). Recently the abundant presence of active GBA1 in the stratum corneum of human skin has been visualized by labeling with a specific fluorescent ABP and zymography (56). Fortuitously, different properties of fish skin and habitat allow generation of animals with a Gba1 deficiency.
We previously developed mass spectrometric methods, using identical 13C-encoded standards, to sensitively quantify the key lipids of interest during GBA1 deficiency: the primary storage lipid GlcCer and the secondary metabolites GlcSph and GlcChol (27, 42). MS, as such, does not distinguish between lipids with a glucose or galactose moiety. We used HILIC to separate glucosyl- and galactosyl-containing lipids of additional larvae and observed that accumulated HexSph is solely GlcSph in 5 dpf larvae, while aberrant HexChol is solely GlcChol. In the case of HexCer, about 30% can be attributed to GalCer and the remaining 70% to GlcCer in 5 dpf WT zebrafish larvae. GlcCer showed altered levels upon genetic or pharmacological modulation while GalCer levels did not change.
The observed abnormalities in GlcCer and its metabolites, GlcSph and GlcChol, in 5 dpf Gba1-deficient larvae in the absence or presence of Gba2 warrant discussion. Total HexCer and the HILIC-separated GlcCer were found to be not significantly abnormal in the gba1−/− larvae. Apparently, accumulating GlcCer in gba1−/− larvae can be alternatively metabolized to GlcSph by acid ceramidase or the presence of maternal enzyme in yolk offers somehow degradative capacity. In contrast, deficiency of Gba2 in the 5 dpf larvae does have a major impact on GlcCer levels. The gba2−/− larvae showed a clearly elevated HexCer level (2-fold), which is attributed to an increase in GlcCer levels and potentially accumulates at the cytosolic side of membranes. Combined Gba1 and Gba2 deficiency does not lead to more prominent HexCer accumulation than deficiency of Gba2 alone.
We assessed the marked elevation of GlcSph, which is from accumulating GlcCer in lysosomes by acid ceramidase (17). The abnormality is exploited for diagnostic purposes and monitoring of GD patients regarding disease progression and correction by therapy (19, 53, 57–59). Prominent accumulation of GlcSph develops in zebrafish with the gba1−/− background starting around 2–3 dpf, but not in WT or gba2−/− zebrafish larvae. Elevation of GlcSph is also rapidly induced by exposing larvae to the Gba1 suicide inhibitor 3, independent of their gba1 and gba2 genotype. Thus, our data suggest that Gba2 status does not markedly influence GlcSph levels during Gba1 deficiency. This suggests that either the Gba2 activity toward GlcSph is insufficient to significantly reduce GlcSph accumulation formed in 5 dpf zebrafish or that GlcSph insufficiently reaches Gba2. Of note, in mice with induced Gba1 deficiency in the white blood cell lineage, Mistry et al. (18) observed increased GlcSph that was not changed by combined Gba2 deficiency, similar to our findings with zebrafish embryos.
The occurrence of GlcChol abnormalities in zebrafish impaired in Gba1 and/or Gba2 was assessed. The existence of GlcChol has been noted in chicken and mammalian tissues, while the glucosylated sterol was shown to be metabolized by GBA1 as well as GBA2 (27, 60, 61). Evidence has been presented for mice that GBA1 largely degrades GlcChol to glucose and cholesterol. Contrary to this, GBA2 forms GlcChol from GlcCer and cholesterol by transglucosylase activity (27). In case of extreme intralysosomal cholesterol accumulation, as in NPC or chemically induced by U18666A, lysosomal GBA1 actively generates GlcChol via transglucosylation (27). GlcChol levels tended to be elevated in gba1−/− and chemically induced Gba1-deficient larvae, in line with Gba1 involvement in GlcChol turnover. While a low level of GlcChol was detected in Gba2-deficient embryos at 8 hpf (0.05 pmol/fish), similar to WT embryos, GlcChol levels did not increase with age in the Gba2-deficient fish, in contrast to WT embryos. This illustrates the contribution of Gba2 to GlcChol biosynthesis in zebrafish, similarly to earlier observations in mice (27). Taken together, this suggests that sterol glucoside metabolism by Gba1 and Gba2 in the zebrafish is similar to that observed in humans and mice (supplemental Table S2) (27, 62). Given the observed abnormalities in GlcChol during abnormal GlcCer metabolism, its physiological significance seems intriguing as well as the role of Gba1 and Gba2 in the molecular function of GlcChol.
The iminosugar, miglustat (N-butyl-deoxynojirimycin), a registered oral agent to treat mild type 1 GD, markedly inhibits GBA2 activity at the administered dose (100 mg three times daily) (47). A very large number of type 1 GD patients have been treated with miglustat for more than a decade without major side effects except for intestinal complaints due to inhibition of intestinal glycosidases (63). Apparently, in these individuals, GBA2 inhibition has no overt detrimental consequences. Moreover, concurrent deficiency of GBA2 in a mouse model with induced deficiency of GBA1 in the white blood cell lineage has been reported to exert positive effects, such as improvements in visceral, hematologic, and skeletal symptoms (18). A beneficial effect of GBA2 deficiency has also been observed for NPC mice, consistent with the use of the GBA2 inhibitor, miglustat, in treatment of this disorder (28). In sharp contrast to all these positive findings regarding reduced GBA2 activity during GBA1 deficiency, loss of function of GBA2 is reported to be associated with hereditary spastic paraplegia and cerebellar ataxia in humans (64–66). A very recent study reported that some GBA2-KO mice display a strong locomotor defect, while other animals, with the same mutation but in a different background, show only mild alterations of the gait pattern and no signs of cerebellar defects (54). It thus appears that the outcome of GBA2 deficiency may be subtly influenced by yet poorly understood factors. The recent notion that GBA2 has substrates beyond GlcCer, such as GlcChol, and potentially other glucosylated metabolites, may ultimately lead to an explanation for the presently puzzling heterogeneity in outcome of GBA2 deficiency. In supplemental Table S2, an overview is presented of reported glycosphingolipid abnormalities in different Gba1-, Gba2-, and Gba1:Gba2-deficient animals (zebrafish, mice, and humans). Consistently, Gba1 deficiency is associated with elevation of GlcSph and Gba2 deficiency with reduced GlcChol levels.
As the final part of our investigation, we evaluated the feasibility of reducing GlcCer synthesis by inhibition of GCS with potent cell-permeable inhibitors. The GCS-specific inhibitor, eliglustat, registered for SRT of type 1 GD, led to the expected reduction of HexCer and concomitant decrease of GlcChol in 5 dpf zebrafish. A slight, but significant, decrease in GlcSph was also observed. The iminosugar, L-ido-AMP-DNM, inhibiting GCS and GBA2 at high doses, also led to reductions of GlcSph and GlcChol in Gba1-deficient larvae. Thus, developing zebrafish embryos offer an organismal model to screen GCS and GBA2 inhibitors and to assess their corrective effect on lipid abnormalities. Also, enzyme replacement therapy was feasible in zebrafish larvae and showed GlcSph correction after injection of recombinant GBA1 with mannose-terminal N-glycans in the bloodstream of 2 dpf zebrafish embryos.
Finally, genetic overexpression of human GBA1 in the zebrafish Gba1-deficient background ameliorated GlcSph accumulation. This indicates that the zebrafish model is able to synthesize human GBA protein; zebrafish Limp2 is able to transport it to the lysosome; and the human GBA1 is functionally active in the lysosome shown by the significant reduction of GlcSph, all at the zebrafish optimal temperature of 28.5°C.
Our present study has focused on the role of Gba2 in glycosphingolipid metabolism during deficiency of Gba1. In the future, it will be of great interest to study the possible physiological implications of lipid abnormalities in mutant fish at adult age. It will be of interest to establish whether lipid-laden macrophages also play an important role in the pathophysiology in zebrafish with deficient Gba1, as in GD. Of interest is also a careful analysis of the skin properties of Gba1-deficient zebrafish given the severe abnormalities observed in mice and humans completely lacking GBA1 (36, 37). Finally, investigation of the male gonads of Gba1-deficient zebrafish is warranted, given the reported abnormalities in this tissue of GBA2-deficient rodents (24, 67). Earlier investigations by Zancan et al. (39) and Keatinge et al. (38) reported impaired bone ossification and microglial activation prior to α-synuclein-independent neuronal cell death in Gba1-deficient zebrafish, respectively. Our mutant zebrafish with combined β-glucosidase deficiencies offer models to obtain further insight in the potential modulating role of Gba2 during Gba1 deficiency. This is of great interest, particularly because current treatments of GBA1-deficient GD patients may impact on GBA2. For example, the registered drug miglustat is a nanomolar GBA2 inhibitor (47). The recently approved drug eliglustat, a potent inhibitor of GCS, is used for SRT of GD (68, 69). This agent, by lowering GlcCer, will likely concomitantly reduce the transglucosylase activity of GBA2 (28). At present, it is entirely unclear whether reduction of Gba2 activity during Gba1 deficiency is harmful, beneficial, or without consequence.
In conclusion, in zebrafish larvae, the inactivation of Gba1 leads to a prominent increase in GlcSph, recently proposed to promote α-synuclein aggregation characteristic of Parkinson’s disease (21), and a modest increase of GlcChol. Deficiency of Gba2 causes a modest increase in GlcCer levels and a prominent reduction in GlcChol. These findings are reminiscent to findings made in cultured cells as well as mice and humans treated with iminosugars known to inhibit GBA2 (28, 70). Gba2 inactivation during Gba1 deficiency in zebrafish embryos exhibited little impact on GlcSph levels but reduced GlcChol levels. Abnormalities in GlcSph and GlcChol levels in zebrafish can be corrected with iminosugar derivatives with inhibitory activity toward GCS and Gba2 concomitantly. Regarding the pathophysiology of GD, models of GBA1 deficiency in mice are intrinsically more informative than models in zebrafish. The general physiology of mice is far closer to that of humans. However, the use of zebrafish offers some practical advantages, such as the ease with which multiple genetic traits can be modified and the role of genetic modifiers can be subsequently studied. It is planned by us to introduce in fish with a Gba1-deficient background other traits (e.g., acid ceramidase deficiency that should prevent formation of GlcSph). In conclusion, zebrafish offer an organismal model to assess lipid abnormalities caused by Gba1 deficiency, the impact of Gba2, and the feasibility of pharmacological intervention.
Supplementary Material
Acknowledgments
The authors thank Dr. J. Bing for synthesizing the ABP 2 used in this study and Arwin Groenewoud for sharing the ZF4 cells and plasmids of the Tol2 kit.
Footnotes
Abbreviations:
- ABP
- activity-based probe
- AMP-DNM
- N-[5-(adamantan-1-ylmethoxy)pentyl]-1-deoxynojirimycin
- dpf
- days post-fertilization
- dsDNA
- double strand DNA
- GalCer
- galactosylceramide
- GBA1
- glucocerebrosidase
- GBA2
- β-glucosidase 2
- GCS
- glucosylceramide synthase
- GD
- Gaucher disease
- GlcCer
- glucosylceramide
- GlcChol
- glucosylated cholesterol
- GlcSph
- glucosylsphingosine
- HexCer
- hexosylceramide
- HexChol
- hexosylcholesterol
- HILIC
- hydrophilic interaction liquid chromatography
- hpf
- hours postfertilization
- HRM
- high-resolution melt
- NPC
- Niemann-Pick type C
- sgRNA
- single guide RNA
- SRT
- substrate reduction therapy
This work was supported by the Netherlands Organisation for Scientific Research (NWO-CW, ChemThem Grant to J.M.F.G.A. and H.S.O.). The authors declare that they have no conflicts of interest with the contents of this article.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Brady R. O., Kanfer J. N., Bradley R. M., and Shapiro D.. 1966. Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher’s disease. J. Clin. Invest. 45: 1112–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler E., and Grabowski G. A.. 2001. Glucosylceramide lipidosis-Gaucher disease. In The Metabolic and Molecular Bases of Inherited Disease. C. R. Scriver, A. L. Beaudet, W. S. Sly, et al., editors. McGraw-Hill, New York. 3635–3668. [Google Scholar]
- 3.Ferraz M. J., Kallemeijn W. W., Mirzaian M., Herrera Moro D., Marques A., Wisse P., Boot R. G., Willems L. I., Overkleeft H. S., and Aerts J. M.. 2014. Gaucher disease and Fabry disease: new markers and insights in pathophysiology for two distinct glycosphingolipidoses. Biochim. Biophys. Acta. 1841: 811–825. [DOI] [PubMed] [Google Scholar]
- 4.Hollak C. E., Levi M., Berends F., Aerts J. M., and van Oers M. H.. 1997. Coagulation abnormalities in type 1 Gaucher disease are due to low-grade activation and can be partly restored by enzyme supplementation therapy. Br. J. Haematol. 96: 470–476. [DOI] [PubMed] [Google Scholar]
- 5.Sidransky E., Nalls M. A., Aasly J. O., Aharon-Peretz J., Annesi G., Barbosa E. R., Bar-Shira A., Berg D., Bras J., Brice A., et al. . 2009. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 361: 1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuang D., Leverenz J. B., Lopez O. L., Hamilton R. L., Bennett D. A., Schneider J. A., Buchman A. S., Larson E. B., Crane P. K., Kaye J. A., et al. . 2012. GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology. 79: 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witte M. D., Kallemeijn W. W., Aten J., Li K. Y., Strijland A., Donker-Koopman W. E., van den Nieuwendijk A. M., Bleijlevens B., Kramer G., Florea B. I., et al. . 2010. Ultrasensitive in situ visualization of active glucocerebrosidase molecules. Nat. Chem. Biol. 6: 907–913. [DOI] [PubMed] [Google Scholar]
- 8.Kallemeijn W. W., Li K. Y., Witte M. D., Marques A. R., Aten J., Scheij S., Jiang J., Willems L. I., Voorn-Brouwer T. M., van Roomen C. P., et al. . 2012. Novel activity-based probes for broad-spectrum profiling of retaining beta-exoglucosidases in situ and in vivo. Angew. Chem. Int. Ed. Engl. 51: 12529–12533. [DOI] [PubMed] [Google Scholar]
- 9.Barton N. W., Furbish F. S., Murray G. J., Garfield M., and Brady R. O.. 1990. Therapeutic response to intravenous infusions of glucocerebrosidase in a patient with Gaucher disease. Proc. Natl. Acad. Sci. USA. 87: 1913–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platt F. M., Jeyakumar M., Andersson U., Priestman D. A., Dwek R. A., Butters T. D., Cox T. M., Lachmann R. H., Hollak C., Aerts J. M., et al. . 2001. Inhibition of substrate synthesis as a strategy for glycolipid lysosomal storage disease therapy. J. Inherit. Metab. Dis. 24: 275–290. [DOI] [PubMed] [Google Scholar]
- 11.Cox T., Lachmann R., Hollak C., Aerts J., van Weely S., Hrebicek M., Platt F., Butters T., Dwek R., Moyses C., et al. . 2000. Novel oral treatment of Gaucher’s disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 355: 1481–1485. [DOI] [PubMed] [Google Scholar]
- 12.Shayman J. A. 2010. Eliglustat tartrate: glucosylceramide synthase inhibitor treatment of type 1 Gaucher disease. Drugs Future. 35: 613–620. [PMC free article] [PubMed] [Google Scholar]
- 13.Jung O., Patnaik S., Marugan J., Sidransky E., and Westbroek W.. 2016. Progress and potential of non-inhibitory small molecule chaperones for the treatment of Gaucher disease and its implications for Parkinson disease. Expert Rev. Proteomics. 13: 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shayman J. A. 2018. Targeting glucosylceramide synthesis in the treatment of rare and common renal disease. Semin. Nephrol. 38: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahl M., Doyle A., Olsson K., Mansson J. E., Marques A. R. A., Mirzaian M., Aerts J. M., Ehinger M., Rothe M., Modlich U., et al. . 2015. Lentiviral gene therapy using cellular promoters cures type 1 Gaucher disease in mice. Mol. Ther. 23: 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aerts J. M., Ferraz M. J., Mirzaian M., Gaspar P., Oussoren S. V., Wisse P., Kuo C-L., Lelieveld L. T., Kytidou K., Hazeu M. D., et al. . 2017. Lysosomal Storage Diseases. For Better or Worse: Adapting to Defective Lysosomal Glycosphingolipid Breakdown. In eLS. John Wiley & Sons, Ltd., Chichester, UK. 1–13. [Google Scholar]
- 17.Ferraz M. J., Marques A. R., Appelman M. D., Verhoek M., Strijland A., Mirzaian M., Scheij S., Ouairy C. M., Lahav D., Wisse P., et al. . 2016. Lysosomal glycosphingolipid catabolism by acid ceramidase: formation of glycosphingoid bases during deficiency of glycosidases. FEBS Lett. 590: 716–725. [DOI] [PubMed] [Google Scholar]
- 18.Mistry P. K., Liu J., Sun L., Chuang W. L., Yuen T., Yang R., Lu P., Zhang K., Li J., Keutzer J., et al. . 2014. Glucocerebrosidase 2 gene deletion rescues type 1 Gaucher disease. Proc. Natl. Acad. Sci. USA. 111: 4934–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukas J., Cozma C., Yang F., Kramp G., Meyer A., Nesslauer A. M., Eichler S., Bottcher T., Witt M., Brauer A. U., et al. . 2017. Glucosylsphingosine causes hematological and visceral changes in mice-evidence for a pathophysiological role in Gaucher disease. Int. J. Mol. Sci. 18: E2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair S., Branagan A. R., Liu J., Boddupalli C. S., Mistry P. K., and Dhodapkar M. V.. 2016. Clonal immunoglobulin against lysolipids in the origin of myeloma. N. Engl. J. Med. 374: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taguchi Y. V., Liu J., Ruan J., Pacheco J., Zhang X., Abbasi J., Keutzer J., Mistry P. K., and Chandra S. S.. 2017. Glucosylsphingosine promotes alpha-synuclein pathology in mutant GBA-associated Parkinson’s disease. J. Neurosci. 37: 9617–9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith N. J., Fuller M., Saville J. T., and Cox T. M.. 2018. Reduced cerebral vascularization in experimental neuronopathic Gaucher disease. J. Pathol. 244: 120–128. [DOI] [PubMed] [Google Scholar]
- 23.van Weely S., Brandsma M., Strijland A., Tager J. M., and Aerts J. M.. 1993. Demonstration of the existence of a second, non-lysosomal glucocerebrosidase that is not deficient in Gaucher disease. Biochim. Biophys. Acta. 1181: 55–62. [DOI] [PubMed] [Google Scholar]
- 24.Yildiz Y., Matern H., Thompson B., Allegood J. C., Warren R. L., Ramirez D. M., Hammer R. E., Hamra F. K., Matern S., and Russell D. W.. 2006. Mutation of beta-glucosidase 2 causes glycolipid storage disease and impaired male fertility. J. Clin. Invest. 116: 2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boot R. G., Verhoek M., Donker-Koopman W., Strijland A., van Marle J., Overkleeft H. S., Wennekes T., and Aerts J. M.. 2007. Identification of the non-lysosomal glucosylceramidase as beta-glucosidase 2. J. Biol. Chem. 282: 1305–1312. [DOI] [PubMed] [Google Scholar]
- 26.Körschen H. G., Yildiz Y., Raju D. N., Schonauer S., Bonigk W., Jansen V., Kremmer E., Kaupp U. B., and Wachten D.. 2013. The non-lysosomal beta-glucosidase GBA2 is a non-integral membrane-associated protein at the endoplasmic reticulum (ER) and Golgi. J. Biol. Chem. 288: 3381–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marques A. R., Mirzaian M., Akiyama H., Wisse P., Ferraz M. J., Gaspar P., Ghauharali-van der Vlugt K., Meijer R., Giraldo P., Alfonso P., et al. . 2016. Glucosylated cholesterol in mammalian cells and tissues: formation and degradation by multiple cellular beta-glucosidases. J. Lipid Res. 57: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marques A. R., Aten J., Ottenhoff R., van Roomen C. P., Herrera Moro D., Claessen N., Vinueza Veloz M. F., Zhou K., Lin Z., Mirzaian M., et al. . 2015. Reducing GBA2 activity ameliorates neuropathology in Niemann-Pick type C mice. PLoS One. 10: e0135889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wennekes T., Meijer A. J., Groen A. K., Boot R. G., Groener J. E., van Eijk M., Ottenhoff R., Bijl N., Ghauharali K., Song H., et al. . 2010. Dual-action lipophilic iminosugar improves glycemic control in obese rodents by reduction of visceral glycosphingolipids and buffering of carbohydrate assimilation. J. Med. Chem. 53: 689–698. [DOI] [PubMed] [Google Scholar]
- 30.Ghisaidoobe A. T., van den Berg R. J., Butt S. S., Strijland A., Donker-Koopman W. E., Scheij S., van den Nieuwendijk A. M., Koomen G. J., van Loevezijn A., Leemhuis M., et al. . 2014. Identification and development of biphenyl substituted iminosugars as improved dual glucosylceramide synthase/neutral glucosylceramidase inhibitors. J. Med. Chem. 57: 9096–9104. [DOI] [PubMed] [Google Scholar]
- 31.Ordas A., Raterink R. J., Cunningham F., Jansen H. J., Wiweger M. I., Jong-Raadsen S., Bos S., Bates R. H., Barros D., Meijer A. H., et al. . 2015. Testing tuberculosis drug efficacy in a zebrafish high-throughput translational medicine screen. Antimicrob. Agents Chemother. 59: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rennekamp A. J., and Peterson R. T.. 2015. 15 years of zebrafish chemical screening. Curr. Opin. Chem. Biol. 24: 58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo C. L., Kallemeijn W. W., Lelieveld L. T., Mirzaian M., Zoutendijk I., Vardi A., Futerman A. H., Meijer A. H., Spaink H. P., Overkleeft H. S., et al. . 2019. In vivo inactivation of glycosidases by conduritol B epoxide and cyclophellitol as revealed by activity-based protein profiling. FEBS J. 286: 584–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Artola M., Kuo C. L., Lelieveld L. T., Rowland R. J., van der Marel G. A., Codee J. D. C., Boot R. G., Davies G. J., Aerts J., and Overkleeft H. S.. 2019. Functionalized cyclophellitols are selective glucocerebrosidase inhibitors and induce a bona fide neuropathic Gaucher model in zebrafish. J. Am. Chem. Soc. 141: 4214–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sultana S., Truong N. Y., Vieira D. B., Wigger J. G., Forrester A. M., Veinotte C. J., Berman J. N., and van der Spoel A. C.. 2016. Characterization of the zebrafish homolog of beta-glucosidase 2: a target of the drug miglustat. Zebrafish. 13: 177–187. [DOI] [PubMed] [Google Scholar]
- 36.Sidransky E., Sherer D. M., and Ginns E. I.. 1992. Gaucher disease in the neonate: a distinct Gaucher phenotype is analogous to a mouse model created by targeted disruption of the glucocerebrosidase gene. Pediatr. Res. 32: 494–498. [DOI] [PubMed] [Google Scholar]
- 37.Holleran W. M., Ginns E. I., Menon G. K., Grundmann J. U., Fartasch M., McKinney C. E., Elias P. M., and Sidransky E.. 1994. Consequences of beta-glucocerebrosidase deficiency in epidermis. Ultrastructure and permeability barrier alterations in Gaucher disease. J. Clin. Invest. 93: 1756–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keatinge M., Bui H., Menke A., Chen Y. C., Sokol A. M., Bai Q., Ellett F., Da Costa M., Burke D., Gegg M., et al. . 2015. Glucocerebrosidase 1 deficient Danio rerio mirror key pathological aspects of human Gaucher disease and provide evidence of early microglial activation preceding alpha-synuclein-independent neuronal cell death. Hum. Mol. Genet. 24: 6640–6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zancan I., Bellesso S., Costa R., Salvalaio M., Stroppiano M., Hammond C., Argenton F., Filocamo M., and Moro E.. 2015. Glucocerebrosidase deficiency in zebrafish affects primary bone ossification through increased oxidative stress and reduced Wnt/beta-catenin signaling. Hum. Mol. Genet. 24: 1280–1294. [DOI] [PubMed] [Google Scholar]
- 40.Driever W., and Rangini Z.. 1993. Characterization of a cell line derived from zebrafish (Brachydanio rerio) embryos. In Vitro Cell. Dev. Biol. Anim. 29A: 749–754. [DOI] [PubMed] [Google Scholar]
- 41.McEachern K. A., Fung J., Komarnitsky S., Siegel C. S., Chuang W. L., Hutto E., Shayman J. A., Grabowski G. A., Aerts J. M., Cheng S. H., et al. . 2007. A specific and potent inhibitor of glucosylceramide synthase for substrate inhibition therapy of Gaucher disease. Mol. Genet. Metab. 91: 259–267. [DOI] [PubMed] [Google Scholar]
- 42.Mirzaian M., Wisse P., Ferraz M. J., Marques A. R. A., Gaspar P., Oussoren S. V., Kytidou K., Codee J. D. C., van der Marel G., Overkleeft H. S., et al. . 2017. Simultaneous quantitation of sphingoid bases by UPLC-ESI-MS/MS with identical (13)C-encoded internal standards. Clin. Chim. Acta. 466: 178–184. [DOI] [PubMed] [Google Scholar]
- 43.Montague T. G., Cruz J. M., Gagnon J. A., Church G. M., and Valen E.. 2014. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 42: W401–W407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Labun K., Montague T. G., Gagnon J. A., Thyme S. B., and Valen E.. 2016. CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 44: W272–W276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gagnon J. A., Valen E., Thyme S. B., Huang P., Akhmetova L., Pauli A., Montague T. G., Zimmerman S., Richter C., and Schier A. F.. 2014. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS One. 9: e98186 [Erratum. 2014. PLoS One. 9: e106396.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jao L. E., Wente S. R., and Chen W.. 2013. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA. 110: 13904–13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Overkleeft H. S., Renkema G. H., Neele J., Vianello P., Hung I. O., Strijland A., van der Burg A. M., Koomen G. J., Pandit U. K., and Aerts J. M.. 1998. Generation of specific deoxynojirimycin-type inhibitors of the non-lysosomal glucosylceramidase. J. Biol. Chem. 273: 26522–26527. [DOI] [PubMed] [Google Scholar]
- 48.Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., and Chien C. B.. 2007. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236: 3088–3099. [DOI] [PubMed] [Google Scholar]
- 49.Schröder S. P., van de Sande J. W., Kallemeijn W. W., Kuo C. L., Artola M., van Rooden E. J., Jiang J., Beenakker T. J. M., Florea B. I., Offen W. A., et al. . 2017. Towards broad spectrum activity-based glycosidase probes: synthesis and evaluation of deoxygenated cyclophellitol aziridines. Chem. Commun. (Camb.). 53: 12528–12531. [DOI] [PubMed] [Google Scholar]
- 50.Groener J. E., Poorthuis B. J., Kuiper S., Helmond M. T., Hollak C. E., and Aerts J. M.. 2007. HPLC for simultaneous quantification of total ceramide, glucosylceramide, and ceramide trihexoside concentrations in plasma. Clin. Chem. 53: 742–747. [DOI] [PubMed] [Google Scholar]
- 51.Link V., Shevchenko A., and Heisenberg C. P.. 2006. Proteomics of early zebrafish embryos. BMC Dev. Biol. 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan X., Klein M., Flanagan-Steet H. R., and Steet R.. 2010. Selective yolk deposition and mannose phosphorylation of lysosomal glycosidases in zebrafish. J. Biol. Chem. 285: 32946–32953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dekker N., van Dussen L., Hollak C. E., Overkleeft H., Scheij S., Ghauharali K., van Breemen M. J., Ferraz M. J., Groener J. E., Maas M., et al. . 2011. Elevated plasma glucosylsphingosine in Gaucher disease: relation to phenotype, storage cell markers, and therapeutic response. Blood. 118: e118–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woeste M. A., Stern S., Raju D. N., Grahn E., Dittmann D., Gutbrod K., Dormann P., Hansen J. N., Schonauer S., Marx C. E., et al. . 2019. Species-specific differences in nonlysosomal glucosylceramidase GBA2 function underlie locomotor dysfunction arising from loss-of-function mutations. J. Biol. Chem. 294: 3853–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farfel-Becker T., Vitner E. B., and Futerman A. H.. 2011. Animal models for Gaucher disease research. Dis. Model. Mech. 4: 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Smeden J., Dijkhoff I. M., Helder R. W. J., Al-Khakany H., Boer D. E. C., Schreuder A., Kallemeijn W. W., Absalah S., Overkleeft H. S., Aerts J., et al. . 2017. In situ visualization of glucocerebrosidase in human skin tissue: zymography versus activity-based probe labeling. J. Lipid Res. 58: 2299–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rolfs A., Giese A. K., Grittner U., Mascher D., Elstein D., Zimran A., Bottcher T., Lukas J., Hubner R., Golnitz U., et al. . 2013. Glucosylsphingosine is a highly sensitive and specific biomarker for primary diagnostic and follow-up monitoring in Gaucher disease in a non-Jewish, Caucasian cohort of Gaucher disease patients. PLoS One. 8: e79732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mirzaian M., Wisse P., Ferraz M. J., Gold H., Donker-Koopman W. E., Verhoek M., Overkleeft H. S., Boot R. G., Kramer G., Dekker N., et al. . 2015. Mass spectrometric quantification of glucosylsphingosine in plasma and urine of type 1 Gaucher patients using an isotope standard. Blood Cells Mol. Dis. 54: 307–314. [DOI] [PubMed] [Google Scholar]
- 59.Arkadir D., Dinur T., Revel-Vilk S., Becker Cohen M., Cozma C., Hovakimyan M., Eichler S., Rolfs A., and Zimran A.. 2018. Glucosylsphingosine is a reliable response biomarker in Gaucher disease. Am. J. Hematol. 93: E140–E142. [DOI] [PubMed] [Google Scholar]
- 60.Wertz P. W., Stover P. M., Abraham W., and Downing D. T.. 1986. Lipids of chicken epidermis. J. Lipid Res. 27: 427–435. [PubMed] [Google Scholar]
- 61.Akiyama H., Nakajima K., Itoh Y., Sayano T., Ohashi Y., Yamaguchi Y., Greimel P., and Hirabayashi Y.. 2016. Aglycon diversity of brain sterylglucosides: structure determination of cholesteryl- and sitosterylglucoside. J. Lipid Res. 57: 2061–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akiyama H., and Hirabayashi Y.. 2017. A novel function for glucocerebrosidase as a regulator of sterylglucoside metabolism. Biochim. Biophys. Acta Gen. Subj. 1861: 2507–2514. [DOI] [PubMed] [Google Scholar]
- 63.Giraldo P., Andrade-Campos M., Alfonso P., Irun P., Atutxa K., Acedo A., Barez A., Blanes M., Diaz-Morant V., Fernandez-Galan M. A., et al. . 2018. Twelve years of experience with miglustat in the treatment of type 1 Gaucher disease: the Spanish ZAGAL project. Blood Cells Mol. Dis. 68: 173–179. [DOI] [PubMed] [Google Scholar]
- 64.Hammer M. B., Eleuch-Fayache G., Schottlaender L. V., Nehdi H., Gibbs J. R., Arepalli S. K., Chong S. B., Hernandez D. G., Sailer A., Liu G., et al. . 2013. Mutations in GBA2 cause autosomal-recessive cerebellar ataxia with spasticity. Am. J. Hum. Genet. 92: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin E., Schule R., Smets K., Rastetter A., Boukhris A., Loureiro J. L., Gonzalez M. A., Mundwiller E., Deconinck T., Wessner M., et al. . 2013. Loss of function of glucocerebrosidase GBA2 is responsible for motor neuron defects in hereditary spastic paraplegia. Am. J. Hum. Genet. 92: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woeste M. A., and Wachten D.. 2017. The enigmatic role of GBA2 in controlling locomotor function. Front. Mol. Neurosci. 10: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raju D., Schonauer S., Hamzeh H., Flynn K. C., Bradke F., Vom Dorp K., Dormann P., Yildiz Y., Trotschel C., Poetsch A., et al. . 2015. Accumulation of glucosylceramide in the absence of the beta-glucosidase GBA2 alters cytoskeletal dynamics. PLoS Genet. 11: e1005063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cox T. M., Drelichman G., Cravo R., Balwani M., Burrow T. A., Martins A. M., Lukina E., Rosenbloom B., Goker-Alpan O., Watman N., et al. . 2017. Eliglustat maintains long-term clinical stability in patients with Gaucher disease type 1 stabilized on enzyme therapy. Blood. 129: 2375–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mistry P. K., Lukina E., Ben Turkia H., Shankar S. P., Baris H., Ghosn M., Mehta A., Packman S., Pastores G., Petakov M., et al. . 2017. Outcomes after 18 months of eliglustat therapy in treatment-naive adults with Gaucher disease type 1: the phase 3 ENGAGE trial. Am. J. Hematol. 92: 1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ashe K. M., Bangari D., Li L., Cabrera-Salazar M. A., Bercury S. D., Nietupski J. B., Cooper C. G., Aerts J. M., Lee E. R., Copeland D. P., et al. . 2011. Iminosugar-based inhibitors of glucosylceramide synthase increase brain glycosphingolipids and survival in a mouse model of Sandhoff disease. PLoS One. 6: e21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.