ABSTRACT:
Objectives:
Childhood behavior problems are underidentified in low- and middle-income countries. This study sought to systematically screen for behavior problems among children receiving medical care in Rwanda and investigate factors associated with behavior problems in this cohort.
Methods:
The Pediatric Symptom Checklist (PSC) was translated into Kinyarwanda, following best practices. Children aged 5.9 to 16 years admitted to the inpatient ward of a referral hospital or seen in the outpatient department (OPD) were screened using the PSC. All PSC-positive children and every third PSC-negative child were referred for definitive assessment by a child mental health specialist.
Results:
Among 300 eligible children, 235 were recruited; none refused. PSC scores were positive in 74 of 234 cases (32%, 95% confidence interval 26%–38%); a total of 28 of 74 (40%) PSC-positive children completed mental health assessments. Of these, 16 (57% of those assessed, and 7% of the 235 who were screened) required treatment or further assessment; none of the PSC-negative children did. Screening sensitivity was 100%, and specificity was 71%, with favorable receiver operating characteristics curve and internal consistency. In a multivariate analysis, higher PSC scores were associated with OPD care, central nervous system trauma or infection, and indices of malnutrition and with the use of traditional, complementary, and alternative medicine (TCAM).
Conclusion:
Behavior problems are common among Rwanda children seen in a referral hospital, particularly in the OPD, and are associated with use of TCAM. The Kinyarwanda PSC showed favorable screening characteristics and resulted in some 7% of children accessing needed mental health care.
Index terms: behavior problems, pediatric symptom checklist, complementary and alternative medicine
Growing recognition of the challenge of mental illness in low- and middle-income countries (LMIC) has highlighted the need for global responses to disparities in mental health services.1–3 Connections between mental health, physical health, and economic health are increasingly noted.2,4,5 Awareness of autism and other neurodevelopmental conditions among children in LMIC6–8 has given rise to intervention programs.9 Recognition of early educational influences on long-term cognitive and economic functioning has driven calls for early childhood development and education programs intended to maximize children's cognitive potential.10–12 By contrast, childhood behavior problems, a mainstay of Developmental and Behavioral Pediatrics in high-resource countries, have only recently begun to attract attention in many LMIC.13
This is the case in Rwanda, a country of some 12 million inhabitants in Central and East Africa. To date, there has been no effort in Rwanda to document the extent of childhood behavior problems, nor has their management been a part of pediatric practice or training. Instead, clinical observations suggest that families in Rwanda often follow the advice of religious authorities or traditional healers when dealing with behavior problems, a pattern of care-seeking that has been documented in other African countries.14 Behavior problems may be especially prevalent among children with chronic medical conditions and may in turn affect medical care by reducing adherence to prescribed regimens.15 For these reasons, pediatric clinics and hospitals should be suitable sites for medical professionals to identify behavior problems and provide early intervention.
For the present study, the decision to focus on children in a tertiary care facility was made out of necessity: the first author was a resident in Pediatrics and only had access to children at the main teaching hospital. Pediatricians in Rwanda work in secondary and tertiary care settings and thus have a particular interest in the behaviors of the children seen in those settings. We decided to include both children admitted to the hospital and those seen for medical consultation in the outpatient clinic because the behavior problems of both groups of children were of interest. We did not have any a priori expectation that either group of children would show more behavior problems than the other.
Therefore, the present study had 3 principal aims: (1) to document the prevalence of behavior problems among children seeking medical care at a major referral hospital in Rwanda, (2) to describe the sources of care that parents turn to when dealing with these problems, and (3) to explore the possibility that parents would accept referrals for further evaluation of their children's behavior problems. Secondary aims included exploring demographic and health-related factors potentially associated with behavior problems and describing the sensitivity and specificity of the newly translated version of the questionnaire used to screen for behavior problems in Rwanda.
METHODS
This was a prospective cross-sectional descriptive study with follow-ups conducted at the Centre Hospitalier Universitaire de Kigali (CHUK) hospital, which is the main public referral hospital in the city, and at a public child psychology clinic and a public child psychiatry clinic in Kigali.
Recruitment
Between March 2016 and October 2016, children aged 6 to 16 years who were seen in the pediatric outpatient department at the hospital or who were admitted to the hospital inpatient care were recruited sequentially. Children who were admitted to the intensive care unit or who were deemed medically unstable by their treating physician were excluded. Recruitment was carried out by nurses under the supervision of the first author. Informed written consent was given by the caretakers of the study participants, and written assent was sought from children aged 7 years and older. Ethical and scientific approval was obtained from the Institutional Review Board of the College of Medicine and Health Sciences at the University of Rwanda. Research clearance was granted by the Ethical Committee at CHUK.
Baseline Data Collection
Data were collected by the nurses using verbally administered structured questionnaires. The nurses had been trained in data collection by the first author and had demonstrated their proficiency. They administered the Pediatric Symptom Checklist (PSC), a well-validated measure of behavior problems that has been translated for use in many countries.16–19 The PSC comprises 35 items rated as occurring “Never (0),” “Sometimes (1),” or “Often (2).” In keeping with published norms, a total score of 28 or higher was taken as a positive result, indicating a potentially significant behavior problem; subscales for problems of attention, internalizing, and externalizing behaviors were scored according to the published norms; unanswered items were scored zero; responses with 4 or more unanswered items were considered unscorable.20
Child and family demographics were obtained using a form created for this study. Social class was reported using the Rwanda ubudehe classification, where level 1 indicates “can hardly afford basic needs,” level 2 indicates individuals who own or rent a home but “rarely get full time jobs,” level 3 indicates “those who have a job and farmers who go beyond subsistence farming,” and level 4 indicates “those who own large-scale business … as well as public servants” (source: the Government of Rwanda website).
Current diagnosis was abstracted from the medical record. The parent-reported medical history included the total number of hospitalizations and the total days in the hospital within the past year. Parents were asked about any current or past history of central nervous system (CNS) injury or infection or use of antiepileptic medications because these factors were thought to be most relevant to potential behavior problems. Parents were also asked to report other sources of care that they may have used, such as traditional healers or prayers. Current height and weight were abstracted from the medical record. Stunting was defined as height less than the mean for age minus 2 SDs according to the World Health Organization (WHO) Multicentre Growth Reference Study. Underweight was defined as body mass index below the fifth percentile for age by WHO standards.
Scale Translation
Because there was no version of the PSC in Kinyarwanda, the PSC was translated from English into Kinyarwanda following the approved and internationally recommended guidelines for the translation of health-related quality of life measures.21 This process entailed translation into Kinyarwanda, back translation to English, and comparison of the posttranslation English to the original English. The translation task was undertaken by professional translators fluent in both Kinyarwanda (as their mother tongue) and English. The back-translated version was reviewed and approved by a US developmental-behavioral pediatrician (Needlman) and by one of the original creators of the PSC. A Rwanda committee composed of a child psychiatrist and a psychologist, medical doctors, nurses, and the chief translator provided final approval of the translated instrument.
Confirmatory Evaluations
Children with PSC total scores ≥ 28 (henceforth referred to as PSC-positive or as having “behavior problems”), or whose parents expressed significant concern about behavioral health regardless of the PSC score, were referred to a child mental health professional for a confirmatory or “gold standard” assessment. Two experienced professionals provided these assessments, one a child psychiatrist and the other a child psychologist. Both had trained in Rwanda, and one also had trained in Belgium; both adhered to European standards for diagnosis and treatment. The referral to 1 or the other mental health professional was arbitrary based on availability. In addition, a random subsample of children with negative (nonconcern) PSC scores was referred for assessments by the same mental health professionals, who were unaware of the PSC scores.
Analysis
The study used descriptive statistics, independent sample t-tests for evaluation of possible associations between continuous variables, and χ2 tests for categorical variables. Multiple linear regression was used to analyze factors associated with the total PSC score. For the estimation of point prevalence, a 95% confidence interval around the percentage was calculated in the usual manner.22 Cronbach's alpha was used as a measure of internal reliability. Data entry and analysis were executed using Excel and SPSS25, respectively.
RESULTS
Demographics
During the period of data collection, 300 eligible children were seen in the pediatric department, of whom 235 (78%) were recruited for the study. Eligible children included 137 seen in the outpatient department (OPD) and 163 who were admitted to the inpatient (INP) service. Examples of common medical diagnoses among children in the OPD included abdominal pain (5 children), cough (4 children), heart disease (22 children), and HIV (21 children). Among the children admitted to the hospital, common diagnoses included cardiac problems (19 children), HIV with complications (5 children), intestinal obstruction (4 children), malaria with complications (11 children), and malignancies (8 children).
In the OPD, 97 (71% of those eligible) consented to participate and were recruited; no one refused to give consent; 40 (29%) were not recruited because the staff responsible for recruiting were occupied with other patients. In the INP units, 138 (85% of those eligible) consented to participate and were recruited; no one refused consent; 25 (15%) were not recruited because of staffing issues. One subject was subsequently removed because the Pediatric Symptom Checklist (PSC) was deemed unscorable according to a priori criteria (above), leaving a sample of 137 patients in the OPD group.
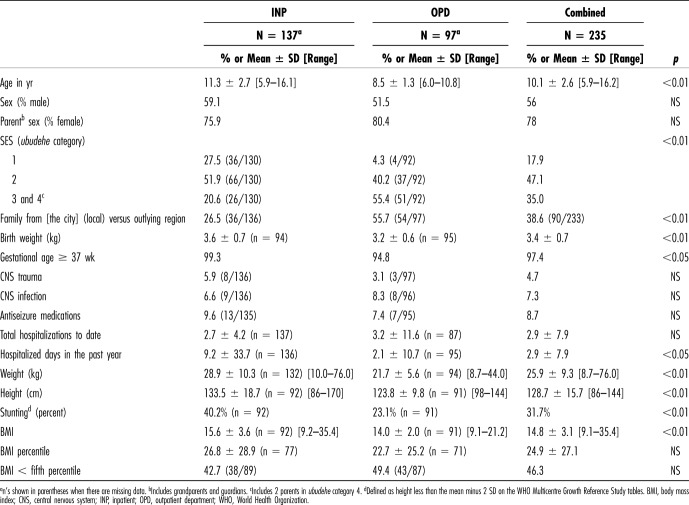
Table 1 presents the demographic and health characteristics for the OPD and INP subsamples and the sample as a whole. Many of the children showed signs of chronic malnutrition: 32% met World Health Organization (WHO) criteria for stunting, and 46% had body mass indexes (BMIs) below the fifth percentile on the WHO charts. Compared with the OPD group, children in the INP cohort were older, larger, and more likely to come from the lower social classes and had spent more days hospitalized in the past year; slightly more of them were born full-term, and they were slightly heavier at birth.
Table 1.
Demographic Characteristics
Screening Test Characteristics
Valid PSC scores were recorded for 234 children, of whom 74 (32%) were PSC-positive (95% confidence interval 26%–38%). On a histogram, PSC scores fell into 2 sharply defined peaks on either side of the cutoff score (Fig. 1). Cronbach's alpha was 0.94 for the full scale, 0.85 for the internalizing subscale, 0.83 for the externalizing subscale, and 0.76 for the attention subscale.
Figure 1.
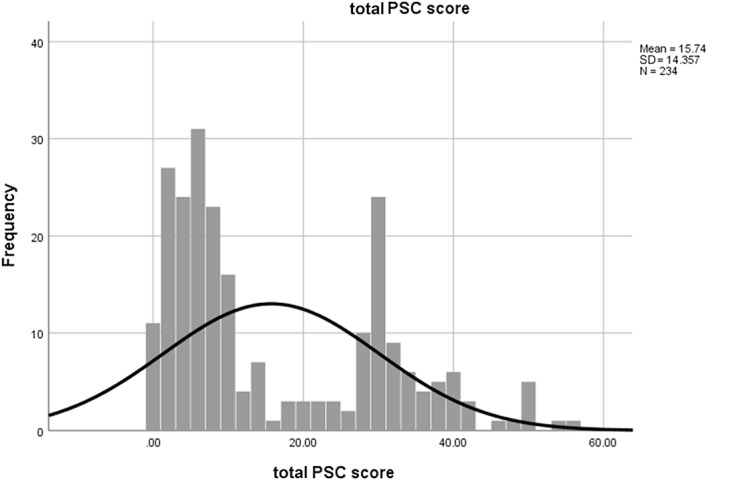
Distribution of PSC total scores. PSC, Pediatric Symptom Checklist.
On the attention problems subscale, high scores (i.e., above the published cutoff) were recorded for 30 children (13%); on the internalizing problems subscale, high scores were recorded for 46 children (20%); and on the externalizing problems subscale, high scores were recorded for 25 children (11%). All children with high subscale scores also had high total PSC scores (≥28), with the exception of 4 children who scored positive for internalizing problems, but negative for total PSC scores. A total of 20 children (9%) were rated high on 2 subscales, and another 6 (3%) were high on all 3 subscales.
Among the 74 children rated PSC-positive, 69 (93%) were referred for a mental health assessment. The reason 5 PSC-positive children were not referred was not recorded. Of the 69 referred children, 28 (40%) were subsequently evaluated by either a child psychiatrist or a psychologist. Of the 41 children who were referred but were not assessed, 22 (54%) were unreachable after multiple attempts, 17 (41%) did not attend scheduled appointments, and 2 (5%) gave other reasons (Table 2). Families living in Kigali were more likely to complete assessments than those who traveled from outlying areas (61% vs 39%, p < 0.02).
Table 2.
Outcome of Referrals for Mental Health Assessments
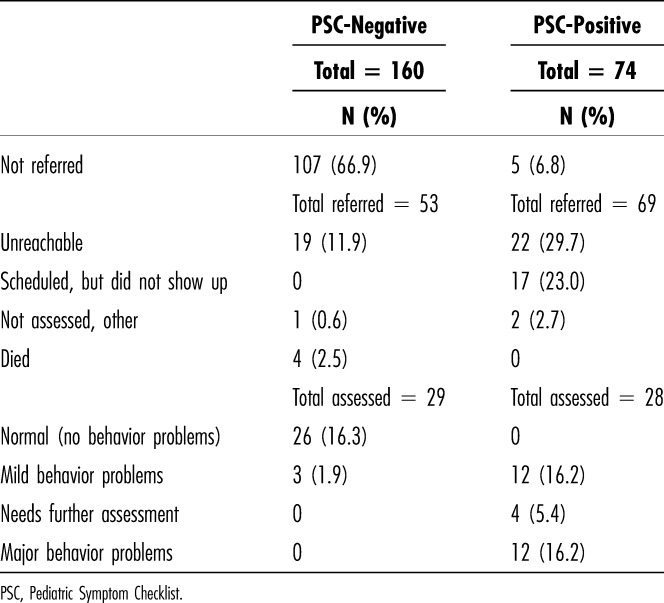
Among the children who were assessed, 12 of 28 (43%) were judged to have either no behavior problems or minor problems requiring advice only, 12 of 28 were judged to have major behavior problems requiring treatment, and 4 of 28 were judged to have problems severe enough to require further mental health assessment, for a true positive rate of 16 of 28 (57%). The true positive rate was similar in the OPD and the INP groups (53% vs 62%, NS).
Among the 160 children rated PSC-negative, 29 were subsequently evaluated by a mental health professional, and all 29 were judged to have minor or no behavior problems. Details of the outcomes of the mental health referrals are presented in Table 2. There was no difference between children rated PSC-positive and those rated PSC-negative in the proportion of referrals that were successfully completed (41% vs 55%, NS).
Taking the mental health assessment as the gold standard, with a judgment of “no problems or minor problems” considered negative, and a judgment of “major problems or needs more assessment” considered positive, the PSC had a sensitivity of 100% (no false negatives) and a specificity of 71% (29 true negative/41 total negatives). Figure 2 shows the receiver operating characteristic curve for the cutoff score of 28, with an area under the curve (AUC) of 0.87; no other cutoff generated a higher AUC.
Figure 2.

ROCs curve, cutoff score 28. ROC, receiver operating characteristic.
Risk Factors for Behavior Problems
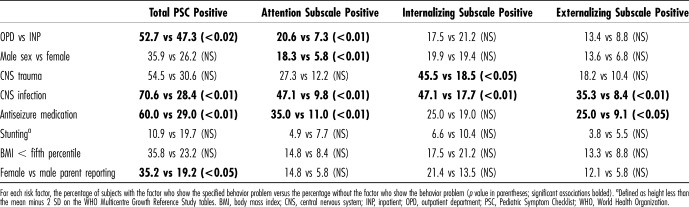
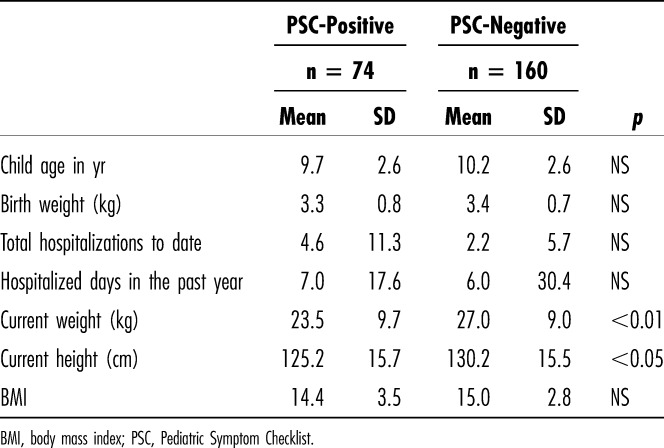
Pediatric Symptom Checklist total scores ≥ 28 were more common in children seen in the OPD and in children with histories of central nervous system (CNS) trauma (rr 1.8), CNS infection (rr 2.5), and use of antiseizure medications (rr 2.1) (Table 3). Male gender was associated with increased attention problems, but not with the PSC total score nor with other subscales. Reporting by the mother (as opposed to the father) was associated with increased PSC scores (Table 3). Neither stunting (by WHO norms) nor underweight was associated with PSC-positive scores (Table 3), although PSC-positive children were shorter and weighed less (Table 4). We did not find associations between the PSC-positive score and child age, birth weight, total hospitalizations, or hospital days in the past year, nor was social class associated with behavior problems (for this analysis, ubudehe class 3 and class 4 were combined because only 2 families fell into class 4).
Table 3.
Risk Factors Associated With Behavior Problems
Table 4.
Associations Between PSC-Positive Scores and Continuous Variables
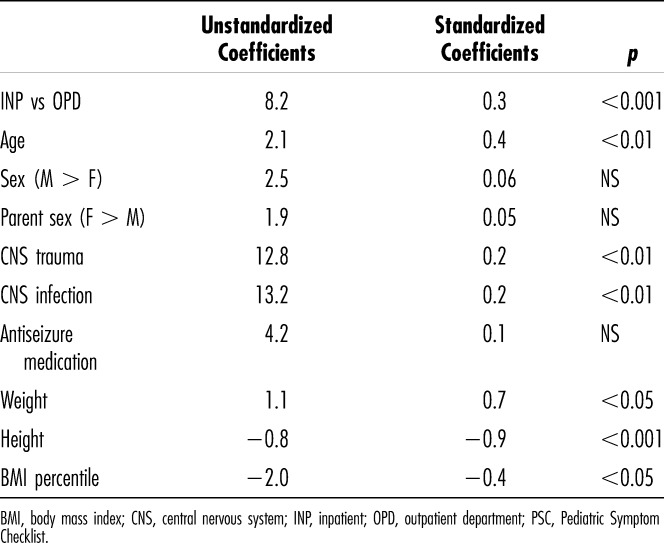
To account for potential confounding factors, a multiple linear regression analysis was performed with the PSC total score as the dependent variable. In this analysis, CNS trauma and infection remained significantly associated with behavior problems, but seizure medications did not. The clinical setting remained significant, with children seen in the OPD receiving higher total PSC scores. Weight, height, and BMI (considered as continuous variables) were also significantly associated with PSC scores. Lower height and lower BMI were associated with increased behavior problems, as anticipated for markers of malnutrition. However, higher weight was also associated with higher PSC scores (Table 5). Adding family location (Kigali vs outlying region) did not change the results.
Table 5.
Multiple Linear Regression, With PSC Total Score as Dependent Variable
Traditional, Complementary, and Alternative Medicine
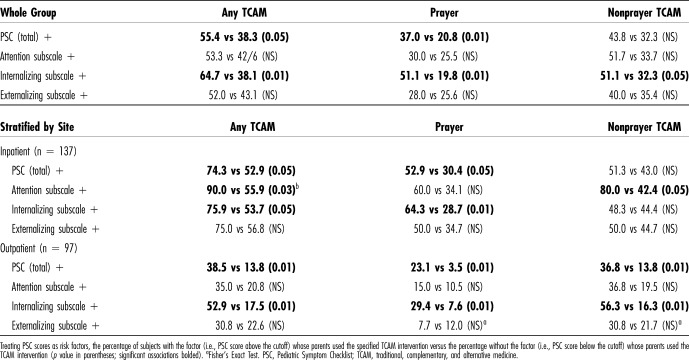
Among the study participants as a whole, 44% (103/234) reported using traditional, complementary, and alternative medicine (TCAM) for their children, chiefly herbal and other medications (35%), prayer (26%), and traditional healers and practices (9%). Table 6 shows TCAM practices including any TCAM, prayer, and nonprayer practices associated with the presence of the total PSC score and subscales. For example, 55% of parents reporting PSC-positive problems reported using TCAM, compared with 39% of parents not reporting PSC-positive problems (relative risk 1.4, p < 0.05). The presence of internalizing problems (in contrast to attention problems and externalizing problems) was associated with both prayer and nonprayer TCAM.
Table 6.
Outpatients: Association of High PSC Scores With TCAM Use
Traditional, complementary, and alternative medicine practices were also associated with the site of care: TCAM was significantly more prevalent among INP children than those seen in the OPD (58% vs 24%, p < 0.001). To control for possible confounding by this INP/OPD factor, subjects were stratified by the site of care. Within both INP and OPD groups, PSC-positive scores remained associated with TCAM use, largely because of internalizing problems in both INP and OPD groups, and attention problems in the INP group (Table 6).
There was a trend linking TCAM use with poorer adherence to the recommendation for a mental health assessment, with 66% of TCAM-using parents adhering to the recommendation, compared with 88% of non–TCAM-using parents; this difference did not however reach statistical significance in our sample (p = 0.13). Restricting the analysis to subjects who were PSC-positive did not change this result.
DISCUSSION
In this series of 235 physically ill children consecutively recruited in the outpatient department (OPD) and inpatient (INP) unit of an academic medical center in Rwanda, 31% screened positive for clinically significant behavior problems on the Pediatric Symptom Checklist (PSC), a widely used screening instrument translated into Kinyarwanda for this study. Assessments by a child psychiatrist or child psychologist were completed on 28 PSC-positive children, resulting in 16 children who were judged in need of further mental health services. Thus, from a practical perspective, the screening identified and connected to services some 7% of children (16/235) whose mental health needs might otherwise have been overlooked by the medical system. (The clinical outcome of those services—what benefits the children and families ultimately experienced—is beyond the scope of the present study.) At the same time, 41 (59%) of the 69 PSC-positive children who were referred for assessments failed to complete them for a variety of reasons, reflecting the real-world limitations of a strategy based on referrals to specialists.
The Kinyarwanda PSC showed favorable internal reliability and screening test characteristics, with an estimated sensitivity of 100% and specificity of 71%. The distribution of scores and receiver operating characteristic analysis support the published cutoff score of ≥28. Several items on the PSC, such as “complains of aches and pain” and “tires easily,” describe many children with chronic and acute illness so that the PSC could be misleading when used with sick and hospitalized children. Nevertheless, the true positive rate of 57% documented in our study indicates that even among physically ill children, the PSC identified children with actual mental health problems.
Factors independently associated with higher PSC scores included central nervous system (CNS) trauma and infection, and shorter stature. Decreased linear growth is an indicator of chronic nutrient deficiencies that adversely affect brain development and thus might conceivably result in behavior problems. Lower body mass index, an indicator of more acute malnutrition, was also associated with higher PSC scores. The positive association between PSC scores and child weight is harder to rationalize. Our study was mainly intended to document the prevalence of behavior problems among children receiving care in a tertiary care setting. Unpacking the complex interactions among social and medical factors in the causality of behavior problems in children growing up in a developing country lies beyond the scope of this study.
As expected, many of the parents also used traditional, complementary, and alternative medicine (TCAM) interventions, including prayers, herbal medications, and services from traditional healers. TCAM use was more common among hospitalized children, who were presumably sicker, and TCAM use was higher among children with significant behavior problems in both the OPD and INP settings. These data suggest that parents may have been turning to TCAM practices for help with their children's behavior problems (although the connection between TCAM and behavior problems was not queried explicitly in the questionnaire). This would make sense inasmuch as standard medical training and practice in Rwanda have not focused on behavior problems, and therefore, many doctors practicing standard (i.e., Western, biomedical) medicine are not prepared to manage these problems.
These findings represent the first systematic exploration of behavior problems among children in Rwanda. Our results, obtained among physically ill children seen in a tertiary care hospital, cannot be taken as an estimate of the prevalence of behavior problems in the country as a whole. For this, future studies would need data collected in local health centers, schools, or other community settings. The development of a Kinyarwanda PSC with good internal reliability and favorable screening characteristics, which we report here, is a necessary first step toward obtaining these data.
Our findings are roughly concordant with previous studies. Behavior problems have been documented previously in African children with serious medical illness. In Botswana, among HIV+ children aged 8 to 16 years seen at 2 public hospitals, 20% scored above a cutoff of 20 points on the Setswana version of the PSC.23 In Uganda, 19% of children suffering from severe cerebral malaria showed clinical behavior problems on the Strengths and Difficulties Questionnaire, compared with 15% of children with anemic malaria and 7% of a community comparison group.24 In other continents, rates of behavior problems have varied, depending on the population studied. Among low-income children in Turkey, a positive rate of nearly 40% was recorded using the PSC-17.25 Among Brazilian schoolchildren, the PSC-positive rate was 14%.18 In the United States, a study of some 21,000 children found a PSC-positive rate of 13% among school-age children20; a previous study among low-income US children documented a positive rate of 22%.26 By contrast, our study failed to show an association between social class and parent-reported behavior problems.
Limitations
Findings from the present study, conducted in a single referral hospital, cannot be generalized to children in other medical settings, nor to children in the community. Although the study design called for consecutive recruitment, staffing issues resulted in some children being missed; it is not clear how this problem might have biased the results in 1 direction or another. The interviews were conducted by clinical staff (chiefly nurses) in the OPD and INP units; thus, interviewers differed according to the site.
For this study, we took assessments by 2 doctoral-level mental health professionals as the “gold standard” against which to judge the PSC results. However, we did not ask the consultants to report to us the diagnoses they made, but only their degree of concern about each patient. The mental health assessments did not use standardized research protocols; the time elapsed between referral and assessment was not recorded, and although an attempt was made to keep the experts unaware of the PSC findings, there was no way to prevent patients from conveying this information and possibly biasing the assessment.
Readers in developed countries might also wonder to what extent professional judgments about mental health and illness translate across geographic and cultural boundaries. This question is beyond the scope of our study, other than to note that professional training in Rwanda has been brought from Europe and is therefore comparable with training in the United States. Notwithstanding these limitations, the mental health assessments used in our study represented the highest quality care currently available in Rwanda and therefore can lay claim to “real-world” validity.
Clinical Implications
This study suggests that screening for behavior problems using the Kinyarwanda version of the PSC may have utility in Rwanda, although significant barriers remain in both identifying and treating behavior problems. We have documented both the potential clinical benefits and limitations of a management strategy based on referral to mental health experts. The present study adds to the body of work documenting clinically important behavior problems in children in low- and middle-income countries (LMIC) and suggests links between the development of behavior problems and potentially preventable nutritional problems and CNS injury and infection.
In Rwanda, parents whose children display behavior problems seek help not only from doctors but also from prayer and traditional medications and healers. One likely reason is the shortage of professionals trained in modern mental health modalities. It is also possible that traditional treatments may be more acceptable to some families, and some might prefer to use both modalities together. Potential risks of each type of therapy, and potential benefits, need to be considered. Future studies will need to explore what the optimal roles of Western mental health therapies and traditional and faith-based approaches will be for Rwanda. A first step in this direction would be to document the extent of behavioral health concerns in the general population. The treatment of serious medical conditions will remain the top priority for tertiary care hospitals in the developing world. However, the present study contributes to the understanding that many sick children in LMIC have psychological and behavioral needs that may be amenable to treatment after the most acute medical needs have been addressed.
ACKNOWLEDGMENTS
Thanks to Mme Agnes Manirafasha, PhD, and Tanya Rogo, MD; and special thanks to my colleagues at CHUK and the team of nurses with whom we shared the difficult work of giving care to sick children.
Footnotes
Disclosure: The authors declare no conflict of interest.
See the Video Abstract at www.jdbp.org
REFERENCES
- 1.Raviola G, Becker AE, Farmer P. A global scope for global health—including mental health. Lancet. 2011;378:1613–1615. [DOI] [PubMed] [Google Scholar]
- 2.Mental health care—the economic imperative. Lancet. 2011;378:1440. [DOI] [PubMed] [Google Scholar]
- 3.Prince M, Patel V, Saxena S, et al. No health without mental health. Lancet. 2007;370:859–877. [DOI] [PubMed] [Google Scholar]
- 4.Baron EC, Rathod SD, Hanlon C, et al. Impact of district mental health care plans on symptom severity and functioning of patients with priority mental health conditions: the Programme for Improving Mental Health Care (PRIME) cohort protocol. BMC Psychiatry. 2018;18:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel V, Boyce N, Collins PY, et al. A renewed agenda for global mental health. Lancet. 2011;378:1441–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong AY, Hsia Y, Chan EW, et al. The variation of psychopharmacological prescription rates for people with autism spectrum disorder (ASD) in 30 countries. Autism Res. 2014;7:543–554. [DOI] [PubMed] [Google Scholar]
- 7.Bakare MO, Munir KM. Autism spectrum disorders (ASD) in Africa: a perspective. Afr J Psychiatry (Johannesbg). 2011;14:208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mankoski RE, Collins M, Ndosi NK, et al. Etiologies of autism in a case-series from Tanzania. J Autism Dev Disord. 2006;36:1039–1051. [DOI] [PubMed] [Google Scholar]
- 9.Engle PL, Black MM, Behrman JR, et al. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369:229–242. [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson M, Yasamy MT, Emerson E, et al. Setting global research priorities for developmental disabilities, including intellectual disabilities and autism. J Intellect Disabil Res. 2014;58:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grantham-McGregor S, Cheung YB, Cueto S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koura KG, Boivin MJ, Davidson LL, et al. Usefulness of child development assessments for low-resource settings in francophone Africa. J Dev Behav Pediatr. 2013;34:486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan B, Avan BI. Qualitative adaptation of child behaviour problem instruments in a developing-country setting. East Mediterr Health J. 2014;20:450–458. [PubMed] [Google Scholar]
- 14.Oshikoya KA, Senbanjo IO, Njokanma OF, et al. Use of complementary and alternative medicines for children with chronic health conditions in Lagos, Nigeria. BMC Complement Altern Med. 2008;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cadman D, Boyle M, Szatmari P, et al. Chronic illness, disability, and mental and social well-being: findings of the Ontario Child Health Study. Pediatrics. 1987;79:805–813. [PubMed] [Google Scholar]
- 16.Murphy JM, Bergmann P, Chiang C, et al. The PSC-17: subscale scores, reliability, and factor structure in a new national sample. Pediatrics. 2016;138:e20160038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowenthal E, Lawler K, Harari N, et al. Validation of the Pediatric Symptom Checklist in HIV-infected Batswana. J Child Adolesc Ment Health. 2011;23:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muzzolon SRB, Cat MNL, Santos LHC. Evaluation of the Pediatric Symptom Checklist as a screening tool for the identification of emotional and psychosocial problems. Rev Paul Pediatr. 2013;31:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginige P, Tennakoon SU, Wijesinghe WH, et al. Prevalence of behavioral and emotional problems among seven to eleven year old children in selected schools in Kandy District, Sri Lanka. J Affect Disord. 2014;167:167–170. [DOI] [PubMed] [Google Scholar]
- 20.Jellinek MS, Murphy JM, Little M, et al. Use of the Pediatric Symptom Checklist to screen for psychosocial problems in pediatric primary care: a national feasibility study. Arch Pediatr Adolesc Med. 1999;153:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Épidemiology. 1993;46:1417–1432. [DOI] [PubMed] [Google Scholar]
- 22.Colton T. Statistics in Medicine. Boston: Little, Brown and Company; 1974. [Google Scholar]
- 23.Lowenthal E, Lawler K, Harari N, et al. Rapid psychosocial function screening test identified treatment failure in HIV+ African youth. AIDS Care. 2012;24:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Idro R, Kakooza-Mwesige A, Asea B, et al. Cerebral malaria is associated with long-term mental health disorders: a cross sectional survey of a long-term cohort. Malar J. 2016;15:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erdogan S, Ozturk M. Psychometric evaluation of the Turkish version of the Pediatric Symptom Checklist-17 for detecting psychosocial problems in low-income children. J Clin Nurs. 2011;20:2591–2599. [DOI] [PubMed] [Google Scholar]
- 26.Murphy JM, Reede J, Jellinek MS, et al. Screening for psychosocial dysfunction in inner-city children: further validation of the Pediatric Symptom Checklist. J Am Acad Child Adolesc Psychiatry. 1992;31:1105–1111. [DOI] [PubMed] [Google Scholar]