Abstract
Sinomenine (SIN) has been reported its antitumor effects on various types of human cancers, but there is no available information regarding the antitumor effects of SIN and cisplatin on gastric cancer. Here, we examined the antitumor effects of SIN combined with cisplatin on gastric cancer cells as well as the underlying biological mechanisms. CCK-8 assay and Calcusyn 2.0 software analysis, Hoechst 33258 staining and flow cytometry, transwell assay showed that SIN and cisplatin synergistically inhibited growth, induced apoptosis, and suppressed invasion than did either drug alone in gastric cancer cells. Interestingly, no change in the AKT level was found, whereas SIN and cisplatin led to a dramatic decrease in p-AKT level compared with either alone treatment. SIN and cisplatin further decreased the Bcl-2, procaspase-3, and β-catenin, but increased Bax, cleaved dcaspase 3, MMP9, and MMP2 in combined group than in either alone group. Immunofluorescence staining showed again a significant decrease in nucleus β-catenin was found in combined group. These data suggested that SIN sensitizes human gastric cancer cells to cisplatin through negative regulation of PI3K/AKT/Wnt signaling pathway. In conclusion, SIN and cisplatin exerted synergistic antitumor effects in gastric cancer cells and might constitute a promising therapeutic approach for gastric cancer.
Keywords: cisplatin; gastric cancer; PI3K/AKT/Wnt signaling pathways, sinomenine
Introduction
Gastric cancer prevails as one of most common type of malignant tumors affecting humans worldwide [1]. Majority of patients with gastric cancer were usually diagnosed at advanced stage, which negates the surgical treatment [2]. Although the conventional chemotherapy has been commonly used in gastric cancer treatment for decades, the clinical outcomes were far from satisfactory due to drug resistance and cell toxicity [3,4]. Therefore, the application of efficient agents to improve the sensitivity to conventional chemotherapy drugs has become a promising strategy for gastric cancer patients.
Sinomenine (SIN) is a bioactive constituent of sinomenium acutum, famous for its multiple biological activities including anti-inflammation, antiangiogenesids, antiarthritis, and immunosuppression [5–7]. Recently, increasing studies suggested that SIN showed a potential antitumor effects in various types of cancers with low toxicity [8,9]. Aberrant hyperactivation of PI3K/AKT pathway is one of the most common tumor-related signaling pathways; interestingly, PI3k/AKT pathway has been reported to involve in the process of antitumor effects of SIN [10,11]. SIN might also, respectively, sensitize gastric cancer and colon cancer to 5-fluorouracil both in vitro and in vivo [12,13]. These research studies indicated the potentials of SIN in curing human cancers, and it seems to be as a new combination regimen with greater therapeutic effects.
Cisplatin is a well known chemotherapeutic drug for treatment of numerous human cancers. Its molecular mechanisms of action has been related to its ability of interfering with DNA repair mechanisms, causing DNA damage, and subsequently inducing apoptosis in cancer cells [14]. However, single cisplatin chemotherapy is not ideal for the treatment of cancer; drug resistance has been observed in many patients who have relapsed from cisplatin treatment. Hence, increasing research demonstrated its potentials of cisplatin-based combination therapy in treating ovarian cancer [15], gastric cancer [16], esophageal carcinoma [17], lung cancer [18], pancreatic cancer [19], and so on.
To date, there is no available information regarding the antitumor effects of SIN and cisplatin. Here, this study first assesses the antitumor effects of SIN combined with cisplatin on gastric cancer cell lines as well as the underlying biological mechanisms.
Materials and methods
Cell culture, reagents, and antibodies
Three human gastric cancer cells (HGC-27, BGC-823, and SGC-7901) were obtained from the China Center for Type Culture Collection. The human normal gastric epithelial GES-1 cell lines were purchased from iCell Bioscience Inc. (China, Shanghai). The cells were maintained in DMEM/F-12 medium containing 10% fetal bovine serum plus 1% antibiotics (100 IU penicillin and 100 µg/mL streptomycin) in a humidified incubator at 37°C with 5% CO2 atmosphere.
SIN (C19H23NO4·0.3 CHCl3) (Fig. 1a) from Pubchem Compound) was purchased from Sigma (Sigma-Aldrich) and dissolved in 100% dimethylsulfoxide (DMSO) to prepare a 100 mM stock solution for storage at −20°C. Cisplatin was obtained from Sigma and dissolved in normal saline, which was stored at −20°C at a concentration of 4 mg/mL.
Fig. 1.

The structure of sinomenine (SIN) from Pubchem compound.
Primary antibodies against AKT, p-AKT, Bax, Bcl-2, procaspase3, cleaved caspase3 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology. Antibodies against β-catenin, MMP-2, and MMP-9 were purchased from Abcam. The effective working concentration for the above was 1:1000.The secondary antibodies were from LI-COR from Selleckchem, diluted to 1:10 000.
Cell viability inhibition by sinomenine combined with cisplatin
Cell viability was assessed quantitatively using a Cell Counting Kit-8 (Beyotime) according to the manufactures’ instructions. Cells were seeded in 96-well micro plates at a density of 5 × 103/well, and the medium was replaced the next day by fresh medium containing SIN (0, 50, 100, 200, 400, and 800 μM) and/or cisplatin (0, 0.5, 1, 2, 4, and 8 μg/mL) diluted from the stock solution for 24 hours. Then, 100 μL of the CCK-8 diluted to 1:10, was added to each well, and cells were next incubated for 2 hours. DMEM containing 10% CCK-8 was used as a control. The absorbance at 450 nm was detected using a microplate reader.
Hoechst 33258 staining for apoptosis
Gastric cancer cells in logarithmic growth phase were placed at a final concentration of 5 × 105 cells per well in a six-well plate, which were treated with SIN and/or cisplatin for 24 hours. The cells were subsequently fixed, washed three times with PBS, and stained with Hoechst 33258 (Beyotime). Apoptotic features were assessed by analyzing chromatin condensation and by staining the fragments under an inverted fluorescent microscope (Olympus).
Annexin V/PI staining for apoptosis
PE Annexin V Apoptosis Detection Kit (Biosciences) was used to quantify the percentage of apoptotic cells by flow cytometry. Gastric cancer cells were cultured in the six-plated and exposed to SIN and/or cisplatin for 24 hours as described above. According to the manufacturer’s instructions, adherent cells were harvested and co-stained with 5 μL Annexin V-PE and 5 μL 7-AAD prior to flow cytometric analysis. The density plots show cell populations (live, early apoptotic, necrotic, and late apoptotic or dead cells) according to their fluorescence characteristics.
Cell invasion assay
After treated with SIN and/or cisplatin for 24 hours, the gastric cancer cells were digested and suspended. A total of 100 μL of the cell suspension (8 × 103 cells cultured with serum-free medium) was seeded into the upper chamber of a Transwell insert (polycarbonate membranes with 8 mm pores; Corning) precoated with Matrigel (BD Biosciences) (1:8 diluent of 50 mg/L Matrigel plated in 4°C for 2 hours).
Western blot analysis
HGC-27 cells were grown into a six-well plate for 24 hours, and treated with SIN and/or cisplatin as mentioned above. Total protein was extracted from cells line using radio immunoprecipitation assay buffer mixtured with protease inhibitor for 20 minutes on ice. A total of 40 μg proteins was resolved by 10% SDS-PAGE and then transferred to PVDF membranes (Millipore) using a wet transfer system. Membranes were blocked with 5% nonfat in TBS containing 0.1% Tween-20 for 1 hour at room temperature, and then incubated with various primary antibodies (working concentration, 1: 1000) against AKT, p-AKT, Bcl-2, Bax, cleaved caspase3, procaspase3, β-catenin, MMP-9, MMP-2, and GAPDH overnight at 4°C. After washed with three times TBST, the membranes were incubated with secondary antibody for 1 hour at dark box. Immunoreactive protein bands were visualized with an Odyssey Infrared Imaging System (LI-COR Biosciences).
Immunofluorescence staining for β-catenin
HGC-27 cells were seeded on sterilized glass coverslips layed into six-well plate, and treated with SIN and/or cisplatin for 24 hours. Cell samples were fixed, and then were incubated with primary antibodies against β-catenin (1:100) overnight at 4°C. After that, cells were incubation with the secondary antibody conjugated with rhodamine for 30 minutes, then mounted in medium containing DAPI for 10 minutes at room temperature. Images were obtained under fluorescent microscopy.
Statistical analysis
Statistical analysis was performed using SPSS version 20.0. Data were presented as mean ± SD. The statistical significance of the studies was analyzed using unpaired two-tailed Student t-test. The difference was considered statistically significant at P < 0.05.
Results
Sinomenine and cisplatin synergistically inhibit gastric cancer cells proliferation
To investigate the effect of SIN and/or cisplatin on gastric cancer cells, the inhibitory effect of treatment on cell proliferation was detected by the CCK-8 assay. Gastric cancer cell lines were treated with SIN (0, 50, 100, 200, 400, 800 μM) and/or cisplatin (0, 0.5, 1, 2, 4, 8 μg/mL) for 24 hours. As shown in Fig. 2a, c, and e, SIN and cisplatin can exhibit, respectively, inhibition of gastric cancer cells growth by CCK-8 assay in a concentration-dependent manner. The SIN mean IC50 for HGC-27, SGC-7901, and BGC-803 at 24 hours was 372.60, 284.40, and 279.70 μM; The cisplatin mean IC50 for HGC-27, SGC-7901, and BGC-803 at 24 hours was 3.85, 3.13, and 4.18 μg/mL (Table 1). Interestingly, we also found SIN combined with cisplatin (molar ratio 100: 1) inhibited gastric cancer cells proliferation more effectively than either compound alone did (shown in Fig. 2a, c, and e red line, *P < 0.05). Here, the Calcusyn 2.0 software was further analyzed the combination index. Figure 2b, d, and f showed that combination index value was less than 1 in most concentration ranges, which meant SIN and cisplatin exerted synergistic antiproliferative effect on gastric cancer cells.
Fig. 2.

Effects of sinomenine (SIN) or/and cisplatin on gastric cancer cells viability were evaluated by CCK-8. (a), (c), and (e) HGC-27, SGC-7901, and BGC-823 cell lines were treated with SIN (0, 50, 100, 200, 400, 800 μM) and/or cisplatin (0, 0.5, 1, 2, 4, 8 μg/mL) for 24 hours, respectively. (b), (d), and (f) Combination index curve for HGC-27, SGC-7901, and BGC-823 cell lines was treated as above. The combination index values were determined using the Calcusyn 2.0 software. Combination index = 1, cumulative effect; combination index < 1, synergistic effect; and combination index > 1, antagonistic effect.
Table 1.
Summary of IC50 values of sinomenine or cisplatin in gastric cancer cells.

Additionally, the results in Fig. 3 showed SIN has no obvious inhibitory effect on human normal gastric epithelial GES-1 cell lines in the SIN concentration range from 50 to 400 μM, and the cell viability at SIN 800 and 1000 μM was 86.7 and 76.2%. All the above results indicated SIN has no significant inhibitory effect on GES-1 cells within a certain range, but SIN in this range can exert synergistic antiproliferative effect on gastric cancer cells with cisplatin.
Fig. 3.
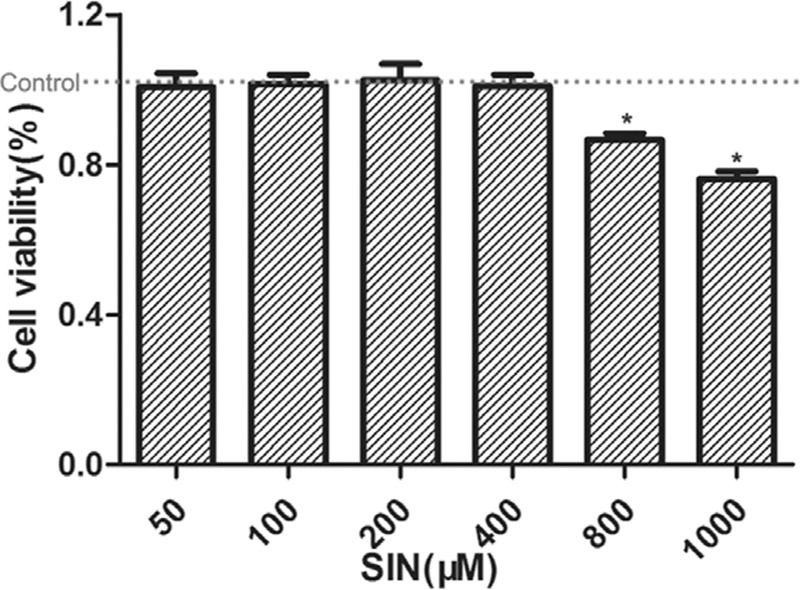
Effects of sinomenine (SIN) on human normal gastric epithelial GES-1 cell lines by CCK-8. The cell viability in control was noted by red line. *P < 0.05 vs control.
Apoptosis induced by sinomenine and cisplatin
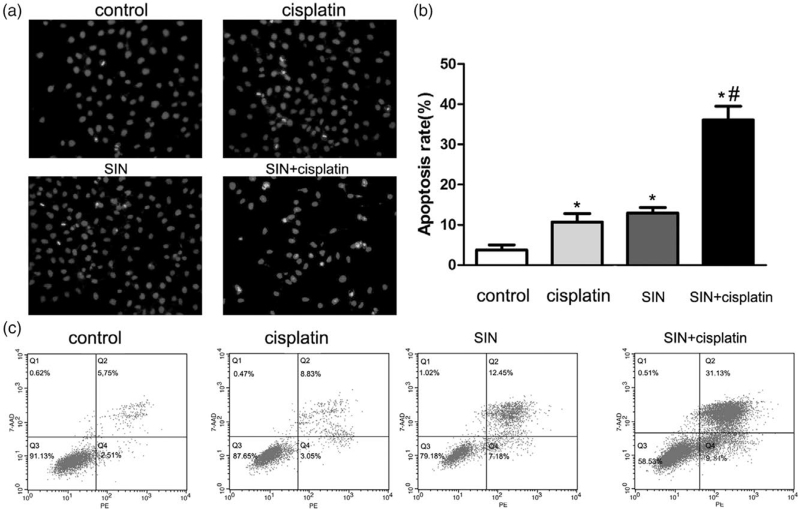
To investigate whether the inhibitory effect of SIN and cisplatin is related to the induction of apoptosis, Hoechst 33258 staining was performed. Normal-blue fluorescence was found in normal cells, whereas condensed nuclei and karyorrhexis with bright-blue fluorescence were found in a1poptosis cells. As shown in Fig. 4a, more bright-blue fluorescent nuclei were found in SIN (100 μM) and/or cisplatin(1 μg/mL) group than in control. The percentages of apoptotic cells in treated groups (cisplatin 1 μg/mL, SIN 100 μM and combination) were 10.73 ± 2.93%, 12.97 ± 1.89%, and 36.1 ± 4.76%, respectively (Fig. 4b, P < 0.05), which revealed that SIN sensitizes gastric cancer cells to cisplatin-induced apoptosis.
Fig. 4.
Apoptosis induced by sinomenine (SIN) (100 μM) and/or cisplatin (1 μg/mL)for 24 hours on HGC-27 cell lines. (a) Apoptosis was evaluated by Hoechst 33258 straining. Normal-blue fluorescence was found in normal cells, whereas condensed nuclei and karyorrhexis with bright-blue fluorescence were found in apoptosis cells. Original magnification: 200×. (b) Quantitative analysis of apoptotic cells rate in each group in (a). Bar graph for the apoptosis rate in cisplatin, SIN, combination of SIN and cisplatin. *P < 0.05 versus control cells; #P < 0.05 versus 1 μg/mL cisplatin alone cells. (c) Detection of apoptosis rate of gastric cancer cells by Annexin V-PE/7-AAD staining. All the above data are mean ± SD from the average of three experiments.
Apoptosis induced by SIN and cisplatin was further detected by Annexin PE/7-AAD staining. As shown in Fig. 4c, the percentages of apoptotic cells (Q2 and Q4) in treated groups (cisplatin 1 μg/mL, SIN 100 μM, and combination ) were 11.88, 19.63, and 40.97%, respectively (Fig. 4c). Above results suggested that the combined treatment had a stronger apoptotic effect than either individual treatment did.
Sinomenine and cisplatin synergistically suppress gastric cancer cells invasion
A transwell assay was performed, showing the invasion abilities of gastric cancer cells. As shown in Fig. 5, invasive cell counts were significantly decreased in those treated with SIN and/or cisplatin compared with control group (Fig. 5a), invasive cell counts in treated groups (cisplatin 1 μg/mL, SIN 100 μM, and combination) were 22.33 ± 3.30, 16 ± 4.32 and 6.33 ± 1.70, respectively (Fig. 5b, P < 0.05), which indicated that SIN and cisplatin synergistically inhibit gastric cancer cells invasion.
Fig. 5.

Effects of sinomenine (SIN) or/and cisplatin on gastric cancer cells invasion were evaluated by Transwell assay with Matrigel. HGC-27 cell lines were treated with SIN and/or cisplatin for 24 hours as above. (a) The number of cells that invaded through the membrane precoated with Matrigel was counted under a light microscope with 200C× magnification. (b) Bar graph of the relative number of invading cells in (a). *P < 0.05 versus control cells; #P < 0.05 versus 1 μg/mL cisplatin alone cells.
Sinomenine enhanced cisplatin-induced antitumor effects on gastric cancer cells via suppressing PI3K/AKT/Wnt signaling pathways
To explore the synergistic mechanism of SIN and cisplatin on the proliferation, apoptosis and invasion of gastric cancer cells, the level of key proteins was further measured by Western blot (Fig. 6a–d). The cells were treated with cisplatin and/or SIN for 24 hours as above. As shown in Fig. 6a, no change in the AKT level was found, whereas SIN and cisplatin led to a dramatic decrease in the p-AKT level compared with either alone treatment. Figure 6a also showed SIN and/or cisplatin increased Bax expression and decreased Bcl-2 expression in the cells, decreasing the Bcl-2/Bax ratio. The decrease in the Bcl-2/Bax ratio was greater in the combined group than that in either alone group (Fig. 6b, P < 0.05). The levels of apoptotic proteins cleaved caspase-3 increased significantly in treatment groups compared with the control group, increasing the cleaved caspase-3/procaspase-3 ratio. The increase in the cleavedcaspase-3/procaspase-3 ratio was much higher in combined group than in either alone group (Fig. 6c, P < 0.05). The above results suggested SIN treatment further potentiated cisplatin-induced apoptotic and proapoptotic protein changes via PI3K/AKT pathways.
Fig. 6.
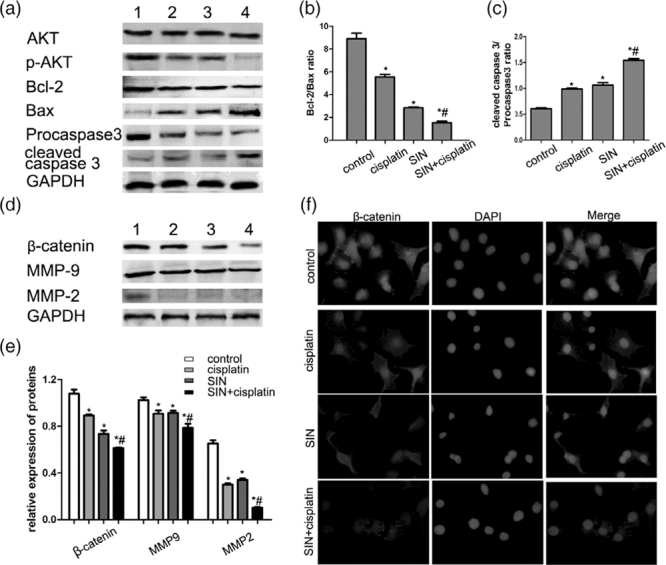
Sinomenine (SIN) and cisplatin regulated the PI3K/AKT/Wnt signaling pathways in gastric cancer. HGC-27 cell lines were treated with SIN and/or cisplatin for 24 hours as above. (a) Effects of SIN and cisplatin on the PI3L/AKT pathway were analyzed by Western blotting. (b) Analysis of the Bcl-2/Bax ratio in (a). (c) Analysis of the cleaved caspase 3/procaspase 3 ratio in (a). (d) Effects of SIN and cisplatin on the Wnt/β-catenin pathways were analyzed by Western blotting. (e) Quantitative analysis of proteins in (d). (f) Immunofluorescence staining of β-catenin expression in HGC-27 cells. B-catenin proteins were stained with rhodamine (red) and nuclei were stained with DAPI (blue). All the experiments are carried out three times.*P < 0.05 versus control cells; #P < 0.05 versus 1 μg/mL cisplatin alone cells.
Wnt/β-catenin signaling pathway was closely involved in the differentiation, invasion, and metastasis of carcinoma. Hence, we detected whether SIN and cisplatin medicates the viability of this key pathway. Figure 6d showed a decrease level in β-catenin, and its downstream targeted proteins MMP-9 and MMP-2 in combined group than either alone group. Figure 6e presented a quantitative analysis of proteins in (Fig. 6d), showing a significant difference in the β-catenin, MMP9, and MMP2 proteins in treated groups (P < 0.05, respectively). Interestingly, the expression and location of β-catenin were also measured by immunofluorescence staining (Fig. 6f). Cells in the control group showed accumulated β-catenin staining in cytoplasm and nucleus. However, cisplatin led to a fewer fluorescence staining of β-catenin compared with the control group, which was strengthened by SIN. A significant decrease in nucleus β-catenin was found in the combined group than in SIN or cisplatin alone (Fig. 6e). All the above results indicated SIN and cisplatin synergistically suppress gastric cancer cells biological behaviors by medicating the inactivation of PI3K/AKT/Wnt signaling pathways.
Discussion
Gastric cancer is the mainly gastrointestinal malignant tumor. Recent cancer statistics have shown an increasing trend on the incidence and mortality rate of gastric cancer, with estimated 27 510 new cases and 11 140 deaths [20]. Majority of patients often present with advanced stage because gastric cancer is always asymptomatic in its early stages, which affirms chemotherapy regimens represent the primary treatment option for gastric cancer. However, the overall outcome remains poor mainly due to drug resistance. It is vital to develop novel natural agents and combination of regimens to improve therapy for advanced gastric cancer.
To overcome drug resistance, cisplatin has commonly used in combination with other drugs in treating numerous human cancers [14]. Up to now, more and more studies have reported that the antitumor effect of SIN [11,21]. Previous studies has identified SIN augments 5-FU induced apoptosis on gastrointestinal tumors both in vivo and in vitro [13,22]. However, the specific mechanism is still unclear, and a few studies focus on the antitumor effects of SIN and cisplatin. In this research, we found SIN and cisplatin synergistically inhibit gastric cancer cells biological behaviors through inactivating PI3K/AKT/Wnt signaling pathways.
SIN was an active alkaloid isolated from China natural plant Caulis sinomenii, which has been widely used in the treatment of acute arthritis and rheumatoid arthritis (RA) clinically for SIN possessing anti-inflammatory and immunoregulatory properties [23,24]. It has also been reported SIN might be even more favorable than NSAIDs for the RA patients [25]. Interestingly, the SIN-hyaluronic acid conjugate seemed to be an effective therapeutic means for the treatment of knee osteoarthritis [26]. A recent research also showed SIN could decrease the expression of CD14/TLR4 and intracellular free calcium level, activate JAK2/STAT3 pathway to inhibit inflammatory response through α7nAChR in macrophages [27]. Besides that, SIN has also been widely used in a variety of cancers including prostate cancer [28], esophageal squamous cell carcinoma [29], renal carcinoma [11], and breast cancer [30]. These research studies indicated the promising potentials of SIN in suppressing the proliferation and metastasis of cancers.
Research studies have also shown SIN inhibited proliferation of SGC-7901 gastric adenocarcinoma cells and tremendously suppress the growth of LoVo cells compared to 5-FU alone group [8,13]. A similar conclusion was drawn in our research that SIN exhibited inhibition of three gastric cancer cell lines growth in a concentration-dependent manner. Our results also revealed SIN and cisplatin exerted synergistic antiproliferative effect on gastric cancer cells. Meanwhile, we found SIN has no significant inhibitory effect on human normal gastric epithelial GES-1 cell lines within an effective concentration range, which indicated the relatively low toxicity of SIN (Fig. 3). We further detected SIN augments cisplatin-induced apoptosis in gastric cancer cells, which was assessed by Hoechst 33258 staining and Annexin V/PI staining, as the percentage of apoptosis was higher in gastric cancer cells treated with combined treatment group than with either agent alone. In addition, research has shown SIN inhibits the migration and invasion of PC3/KYSE150/KYSE450/Het-1A cells [28,30], which approved our results SIN and cisplatin synergistically suppress gastric cancer cells invasion, as the invasive cell counts were average 6.33 in SIN+ciaplstin group (P < 0.05). Above evidences approved this first finding that SIN treatment in combination with cisplatin has stronger anti-tumor effects on gastric cancer cells than either agent alone.
Western-blot analysis was performed to further clarify the synergistic mechanism of SIN and cisplatin on the proliferation, apoptosis, and invasion of gastric cancer cells. Research has clarified that PI3K/AKT pathway is overactivated in more pathway components and in more tumor types than any other signaling pathways involved in numerous cancer [31]. In this research, SIN functioned as a growth inhibitor of PI3K/AKT signaling pathways, as SIN led to an obvious decrease in the p-AKT level compared with the control group, and SIN in combination with cisplatin resulted in a dramatic decrease in the p-AKT level compared with either agent alone. Researchers have demonstrated activated AKT phosphorylates a large number of substrates controlling cellular functions, including cell survival, growth, metabolism, tumorigenesis, and metastasis [32]. Hence, we further detected the downstream targets of PI3K/AKT pathway.
In this study, SIN and cisplatin caused an increase in the levels of apoptotic proteins Bax and cleaved caspase-3, and a decrease in Bcl-2, procaspase-3, which decreases the Bcl-2/Bax ratio and increases the cleaved caspase-3/procaspase-3 ratio. These results further approved the findings by Hoechst 33258 staining and Annexin V/PI staining. Nowadays, two major apoptotic pathways have been well characterized: the cell death receptor-mediated extrinsic pathway and the mitochondria-mediated intrinsic pathway. It has been reported Bcl-2 family proteins were important components of mitochondrial permeability transition pore (mPTP), and the structure and permeability of mPTP will be changed as Bcl-2/Bax protein ratio is reduced [33]. The mitochondrial outer membrane permeabilization then caused the release of Cyt C and AIF and then activation of caspase-9 and caspase-3/-7 subsequently [34]. The above evidences revealed the combination of SIN and cisplatin could induce gastric cancer cells intrinsic apoptosis through PI3K/AKT pathways.
We also found a decrease level of β-catenin and its downstream targeted proteins MMP-9 and MMP-2 in the combined group than either alone group, further approving our results from transwell assay (Fig. 5). A pivotal process of cancer invasion and metastasis is the proteolytic degradation of the extracellular matrix by MMPs, especially MMP-2 and MMP-9 [35]. Previous research presented accumulation and activation of β-catenin in cytoplasm is the critical process of Wnt/β-catenin signaling pathway. It has also been reported GSK-3β was a common point of intersection between PI3K/AKT and Wnt/β-catenin signaling pathways [36,37]. Many research studies have also reported that MMP-2 and MMP-9 expression were mediated by the PI3K/Akt pathway [38,39]. These evidences and observations above contribute to indicating combined treatment of SIN and cisplatin synergistically suppress gastric cancer cells invasion through negative regulation of PI3K/AKT/Wnt signaling pathway.
Conclusion
In conclusion, our study suggests that SIN sensitizes gastric cancer cells to cisplatin via negative regulation of PI3K/AKT/Wnt signaling pathway. This study suggests the potential usefulness of SIN, a promising chemosensitized antitumor agent, in the management of gastric cancer. However, some points remain unclear: how does SIN with cisplatin induce the decrease in levels of p-AKT? We should ask whether other important factors participate in SIN sensitizing gastric cancer cells to cisplatin. Additionally, studies are also necessary to demonstrate the synergistic anti-tumor effects of SIN and cisplatin in vivo. These issues should be addressed in the future.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer Groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 20173524–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu J, Gao J, Lu Z, Li Y, Shen L. Serum levels of TUBB3 correlate with clinical outcome in Chinese patients with advanced gastric cancer receiving first-line paclitaxel plus capecitabine. Med Oncol 2012293029–3034 [DOI] [PubMed] [Google Scholar]
- 3.Ohtsu A. Chemotherapy for metastatic gastric cancer: past, present, and future. J Gastroenterol 200843256–264 [DOI] [PubMed] [Google Scholar]
- 4.Seo JH, Jeong ES, Lee KS, Heo SH, Jeong DG, Choi YK. Lentivirus-mediated shrna targeting of cyclin D1 enhances the chemosensitivity of human gastric cancer to 5-fluorouracil. Int J Oncol 2013432007–2014 [DOI] [PubMed] [Google Scholar]
- 5.Zhao XX, Peng C, Zhang H, Qin LP. Sinomenium acutum: a review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharm Biol 2012501053–1061 [DOI] [PubMed] [Google Scholar]
- 6.Teng P, Liu HL, Zhang L, Feng LL, Huai Y, Deng ZS, et al. Synthesis and biological evaluation of novel sinomenine derivatives as anti-inflammatory agents. Eur J Med Chem 20125063–74 [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Li XK. Immunosuppressive and anti-inflammatory activities of sinomenine. Int Immunopharmacol 201111373–376 [DOI] [PubMed] [Google Scholar]
- 8.Lv Y, Li C, Li S, Hao Z. Sinomenine inhibits proliferation of SGC-7901 gastric adenocarcinoma cells via suppression of cyclooxygenase-2 expression. Oncol Lett 20112741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L, Luan H, Liu Q, Jiang T, Liang H, Dong X, Shang H. Activation of PI3K/akt and ERK signaling pathways antagonized sinomenine-induced lung cancer cell apoptosis. Mol Med Rep 201251256–1260 [DOI] [PubMed] [Google Scholar]
- 10.Hubbard PA, Moody CL, Murali R. Allosteric modulation of ras and the PI3K/AKT/mtor pathway: emerging therapeutic opportunities. Front Physiol. 2014;5:478. doi: 10.3389/fphys.2014.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng F, Ma YX, Liang L, Zhang P, Feng J. The pro-apoptosis effect of sinomenine in renal carcinoma via inducing autophagy through inactivating PI3K/AKT/mtor pathway. Biomed Pharmacother 2018971269–1274 [DOI] [PubMed] [Google Scholar]
- 12.Liao F, Yang Z, Lu X, Guo X, Dong W. Sinomenine sensitizes gastric cancer cells to 5-fluorouracil in vitro and in vivo. Oncol Lett 201361604–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang JX, Yang ZR, Wu DD, Song J, Guo XF, Wang J, Dong WG. Suppressive effect of sinomenine combined with 5-fluorouracil on colon carcinoma cell growth. Asian Pac J Cancer Prev 2014156737–6743 [DOI] [PubMed] [Google Scholar]
- 14.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 2014740364–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson AJ, Saskowski J, Barham W, Yull F, Khabele D. Thymoquinone enhances cisplatin-response through direct tumor effects in a syngeneic mouse model of ovarian cancer. J Ovarian Res. 2015;8:46. doi: 10.1186/s13048-015-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J, Hu X, Li J, Wu D, Lan Q, Wang Q, et al. Enhancing conventional chemotherapy drug cisplatin-induced anti-tumor effects on human gastric cancer cells both in vitro and in vivo by thymoquinone targeting PTEN gene. Oncotarget 2017885926–85939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X, Ma J, Vikash V, Li J, Wu D, Liu Y, et al. Thymoquinone augments cisplatin-induced apoptosis on esophageal carcinoma through mitigating the activation of JAK2/STAT3 pathway. Dig Dis Sci 201863126–134 [DOI] [PubMed] [Google Scholar]
- 18.Xu XM, Zhang Y, Qu D, Liu HB, Gu X, Jiao GY, Zhao L. Combined anticancer activity of osthole and cisplatin in NCI-H460 lung cancer cells in vitro. Exp Ther Med 20135707–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apostolou P, Toloudi M, Chatziioannou M, Ioannou E, Knocke DR, Nester J, et al. Anvirzel™ in combination with cisplatin in breast, colon, lung, prostate, melanoma and pancreatic cancer cell lines. BMC Pharmacol Toxicol. 2013;14:18. doi: 10.1186/2050-6511-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019697–34 [DOI] [PubMed] [Google Scholar]
- 21.Song L, Liu D, Zhao Y, He J, Kang H, Dai Z, et al. Sinomenine inhibits breast cancer cell invasion and migration by suppressing NF-κb activation mediated by IL-4/mir-324-5p/CUEDC2 axis. Biochem Biophys Res Commun 2015464705–710 [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Yang ZR, Dong WG, Zhang JX, Guo XF, Song J, Qiu S. Cooperative inhibitory effect of sinomenine combined with 5-fluorouracil on esophageal carcinoma. World J Gastroenterol 2013198292–8300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JY, Kim KJ, Kim J, Choi SU, Kim SH, Ryu SY. Anti-osteoclastogenic effects of isoquinoline alkaloids from the rhizome extract of sinomenium acutum. Arch Pharm Res 201639713–720 [DOI] [PubMed] [Google Scholar]
- 24.Huang L, Li T, Zhou H, Qiu P, Wu J, Liu L. Sinomenine potentiates degranulation of RBL-2H3 basophils via up-regulation of phospholipase A2 phosphorylation by annexin A1 cleavage and ERK phosphorylation without influencing on calcium mobilization. Int Immunopharmacol 201528945–951 [DOI] [PubMed] [Google Scholar]
- 25.Xu M, Liu L, Qi C, Deng B, Cai X. Sinomenine versus nsaids for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Planta Med 2008741423–1429 [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Shao H, Fang S, Cheng Y, Ling P, Chen J. Evaluation of pharmacokinetics and pharmaco-dynamics of sinomenine-hyaluronic acid conjugate after intra-articular administration for osteoarthritis treatment. Drug Des Devel Ther 201913657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu RL, Zhi YK, Yi L, Luo JF, Li J, Bai SS, et al. Sinomenine regulates CD14/TLR4, JAK2/STAT3 pathway and calcium signal via α7nachr to inhibit inflammation in LPS-stimulated macrophages. Immunopharmacol Immunotoxicol 201941172–177 [DOI] [PubMed] [Google Scholar]
- 28.Xu F, Li Q, Wang Z, Cao X. Sinomenine inhibits proliferation, migration, invasion and promotes apoptosis of prostate cancer cells by regulation of mir-23a. Biomed Pharmacother. 2019;112:108592. doi: 10.1016/j.biopha.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 29.Guanen Q, Junjie S, Baolin W, Chaoyang W, Yajuan Y, Jing L, et al. Mir-214 promotes cell meastasis and inhibites apoptosis of esophageal squamous cell carcinoma via PI3K/AKT/mtor signaling pathway. Biomed Pharmacother 2018105350–361 [DOI] [PubMed] [Google Scholar]
- 30.Song L, Liu D, Zhao Y, He J, Kang H, Dai Z, et al. Sinomenine reduces growth and metastasis of breast cancer cells and improves the survival of tumor-bearing mice through suppressing the shh pathway. Biomed Pharmacother 201898687–693 [DOI] [PubMed] [Google Scholar]
- 31.Guo H, German P, Bai S, Barnes S, Guo W, Qi X, et al. The PI3K/AKT pathway and renal cell carcinoma. J Genet Genomics 201542343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 20071291261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birkinshaw RW, Czabotar PE. The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. Semin Cell Dev Biol 201772152–162 [DOI] [PubMed] [Google Scholar]
- 34.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 2004305626–629 [DOI] [PubMed] [Google Scholar]
- 35.Banday MZ, Sameer AS, Mir AH, Mokhdomi TA, Chowdri NA, Haq E. Matrix metalloproteinase (MMP) -2, -7 and -9 promoter polymorphisms in colorectal cancer in ethnic kashmiri population - a case-control study and a mini review. Gene 201658981–89 [DOI] [PubMed] [Google Scholar]
- 36.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/akt and apoptosis: size matters. Oncogene 2003228983–8998 [DOI] [PubMed] [Google Scholar]
- 37.Bonvini P, Hwang SG, El-Gamil M, Robbins P, Kim JS, Trepel J, Neckers L. Nuclear beta-catenin displays GSK-3beta- and APC-independent proteasome sensitivity in melanoma cells. Biochim Biophys Acta 20001495308–318 [DOI] [PubMed] [Google Scholar]
- 38.Kennedy SG, Wagner AJ, Conzen SD, Jordán J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 199711701–713 [DOI] [PubMed] [Google Scholar]
- 39.Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, et al. Tissue invasion and metastasis: molecular, biological and clinical perspectives. Semin Cancer Biol 201535SupplS244–S275 [DOI] [PubMed] [Google Scholar]