Abstract
Pretransplant gut colonization with intrinsically vancomycin-resistant enterococci (iVRE) (Enterococcus gallinarum and Enterococcus casseliflavus) is uncommon and with unknown clinical impact. In a matched-pairs analysis of patients with versus without iVRE colonization (n = 18 in each group), we demonstrated signficantly higher 2-year overall survival (86% [95% confidence interval, 52% to 96%] versus 35% [95% confidence interval, 8% to 65]; P < .01) and lower nonrelapse mortality (P < .01) among colonized patients. Putative metabolomes differentiated iVRE from E. faecalis/faecium and may contribute to a healthier gut microbiome in iVRE-colonized patients.
Keywords: Allogeneic, Biosynthetic, Colonization, Enterococcus, Vancomycin
INTRODUCTION
Gut colonization with vancomycin-resistant enterococci (VRE)—Enterococcus faecium and Enterococcus faecalis—is common. Approximately 15% to 40% of patients are VRE colonized before allogeneic hematopoietic cell transplantation (allo-HCT) and an additional ~10% become colonized within the first 100 days post-HCT [1–3]. We and others have shown that VRE colonization is associated with >3-fold increased risk of post-HCT VRE bloodstream infection [1,2,4] with an attributable mortality rate of ~10% [2,3,5,6]. Enterococcus gallinarum and Enterococcus casseliflavus (with ~75% average nucleotide identity in shared genes) [7] are characterized by susceptibility to teicoplanin and constitutive low-level vancomycin resistance through the chromosomally encoded vanC genotype [8,9]. Although these intrinsically vancomycin-resistant enterococci (iVRE) can be isolated from stool of ~5% of hospitalized patients, clinical infections are rare [10–14] and iVRE bacteremia-related mortality is <2% [15]. Prior use of antibiotics has been reported as a risk factor for iVRE colonization in some, but not all, studies [10,16].
The clinical impact of pre-HCT gut colonization with iVRE is unknown. A total of 98% of all iVRE isolates in a large previous study (hematology/transplant unit) were identified in screening rectal swabs. Of these, 67% were positive at admission and 25% in later samples. Only .4% of patients with a sample positive for iVRE had iVRE bloodstream infection [17]. We report an unexpected association between pre-allo-HCT gut colonization with iVRE and improved overall survival (OS) due to decreased nonrelapse mortality (NRM).
METHODS
Patients and Cultures
We studied allo-HCT recipients with available pre-HCT rectal swab or stool culture results (2011 to 2017). New admissions for allo-HCT were screened for gut VRE colonization once before day 0 and weekly thereafter until discharge. Spectra VRE chromogenic agar medium (Thermo Fisher Scientific, Minneapolis, MN) was used for species-level identification. Cases with ≥1 positive pre-HCT result for iVRE and no positive test for VRE or vancomycin-intermediate enterococci (VIE) were defined as iVRE positive. Patients with no previous positive test for iVRE or VRE/VIE, and with at least 2 negative tests for these, were defined as control patients. Antimicrobial prophylaxis was according to our institutional algorithm and consisted of levofloxacin, acyclovir, and an azole.
Statistical Analysis
In matched-pairs analysis, each iVRE case was matched to a control using the following hierarchical criteria: (1) diagnosis (malignant versus nonmalignant), (2) disease risk (for malignant disorders), (3) donor type, (4) conditioning intensity, and (5) age (±10 years). Neutrophil recovery was defined as the first of the 3 consecutive days with absolute neutrophil count >.5 × 109/μL. Lymphocyte recovery was defined as the first of the 3 consecutive days with absolute lymphocyte count >1.0 × 109/μL. Platelet recovery was defined as the first of the 7 consecutive days with transfused platelet count >20 × 109/μL. We compared patient characteristics across paired groups by McNemar’s test (binary variables), Bowker’s test of symmetry (variables with more than 2 strata), and Prentice-Wilcoxon test (for time-to-event data). The cumulative incidence method was used to estimate relapse, acute grade II to IV or chronic graft-versus-host disease (GVHD), and NRM, treating NRM, death not due to GVHD, and relapse as a competing risk, respectively. In the analysis of relapse and NRM, we excluded patients with nonmalignant disorders. A Gray’s test from a Fine and Gray regression model stratified by matched pairs was used for competing risk analysis. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Biosynthetic Pathway and Phylogenetic Analyses
To explore whether microbial products may explain the observed differences in clinical outcomes, we compared putative biosynthetic gene clusters (BGCs) from relevant strains of Enterococcus. Genomic sequences for Enterococcus strains were downloaded from the NCBI Reference Sequence Database (accessed August 3, 2017). All latest assemblies were obtained for E. casseliflavus (15 records) and E. gallinarum (8 records), while only complete genomes were obtained for E. faecalis (7 records) and E. faecium (11 records; Table 2). BGCs were predicted using antiSMASH v3.0 [18] with the “–inclusive” mode, and secondary metabolite profiles were analyzed for similarity based on BLASTP amino acid identity and overall gene composition, using in-house Python and C code (https://github.com/RRShieldsCutler/iVRE). Similarity networks were generated by comparing high-similarity (>75%) BGCs and visualized with Cytoscape v3.4.0 (http://www.cytoscape.org/).
Table 2.
Enterococcus strains used in the genomic pathway analysis
| NCBI ID | Species and strain name |
|---|---|
| NZ_MJEF01000001.1 | Enterococcus casseliflavus strain F1129E107 |
| ENT22 | |
| NZ_ASVX01000001.1 | Enterococcus casseliflavus ATCC 49996 |
| NZ_BCPT01000001.1 | Enterococcus casseliflavus NBRC 100478 |
| NZ_FNFS01000041.1 | Enterococcus casseliflavus strain NLAE-zl-C414 |
| NZ_JXKK01000001.1 | Enterococcus casseliflavus strain DSM 20680 |
| NZ_KB946260.1 | Enterococcus casseliflavus ATCC 49996 |
| NZ_MUBE01000001.1 | Enterococcus casseliflavus strain PAVET15 |
| NZ_KE350225.1 | Enterococcus casseliflavus 14-MB-W-14 |
| NZ_FOMP01000028.1 | Enterococcus casseliflavus strain NLAE-zl-G268 |
| NZ_GG670403.1 | Enterococcus casseliflavus EC30 |
| NZ_GG692832.1 | Enterococcus casseliflavus EC10 |
| NZ_MJEG01000001.1 | Enterococcus casseliflavus strain F1129F 46 ENT23 |
| NZ_GL872323.1 | Enterococcus casseliflavus ATCC 12755 |
| NC_020995.1 | Enterococcus casseliflavus ASM15735v2 |
| NZ_AKCC01000001.1 | Enterococcus casseliflavus EC20 |
| NC_017316.1 | Enterococcus faecalis OG1RF |
| NC_018221.1 | Enterococcus faecalis D32 |
| NC_019770.1 | Enterococcus faecalis str. Symbioflor 1 |
| NZ_CP004081.1 | Enterococcus faecalis DENG1 |
| NZ_CP008816.1 | Enterococcus faecalis ATCC 29212 |
| NZ_CP014949.1 | Enterococcus faecalis LD33 |
| NC_004668.1 | Enterococcus faecalis V583 |
| NC_017960.1 | Enterococcus faecium DO |
| NC_021994.1 | Enterococcus faecium Aus0085 |
| NZ_CP006030.1 | Enterococcus faecium Tl 10 |
| NZ_CP011281.1 | Enterococcus faecium E39 |
| NZ_CP011828.1 | Enterococcus faecium UW8175 |
| NC_020207.1 | Enterococcus faecium NRRL B-2354 |
| NZ_CP012522.1 | Enterococcus faecium 64/3 |
| NZ_CP013009.1 | Enterococcus faecium UW7606x64/3 TC1 |
| NZ_CP013994.1 | Enterococcus faecium 6E6 |
| NZ_LN999844.1 | Enterococcus faecium EFE10021 |
| NC_017022.1 | Enterococcus faecium Aus0004 |
| NZ_CP014067.1 | Enterococcus gallinarum FDAARGOS 163 |
| NZ_JNLR01000001.1 | Enterococcus gallinarum SKF1 |
| NZ_MJED01000001.1 | Enterococcus gallinarum strain F1213F 228 ENT20 |
| NZ_BCQE01000001.1 | Enterococcus gallinarum NBRC 100675 |
| NZ_CM003134.1 | Enterococcus gallinarum A6981 |
| NZ_GG670297.1 | Enterococcus gallinarum EG2 |
| NZ_JXKP01000001.1 | Enterococcus gallinarum strain DSM 24841 |
| NZ_NGMQ01000001.1 | Enterococcus gallinarum strain 2A8 DIV0586 |
RESULTS
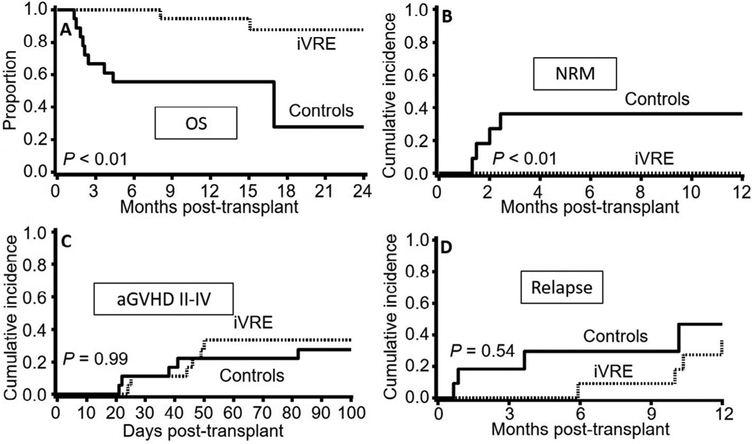
Table 1 shows the characteristics of the iVRE and control groups. The groups were similar in all studied variables. The median follow-up for survivors was 34 months. The iVRE group showed a significantly higher 2-year OS (95% confidence interval [CI]) compared with the control group (86% [95% CI, 52% to 96%] versus 35% [95% CI, 8% to 65%]; P < .01; Figure 1A). This striking difference in OS was due to a significantly lower rate of NRM in the iVRE group (0% versus 36% [95% CI, 8% to 65%]; P < .01; Figure 1B). Acute GVHD II to IV (Figure 1C), relapse (Figure 1D), and 1-year chronic GVHD (17% [95% CI, 0% to 35%] versus 18% [0% to 41%]; P = .44; curves not shown) were similar in the 2 groups. While 9 deaths occurred in the control group (organ failure 45%, relapse 33%, infection 11%, and GVHD 11%), only 2 iVRE patients died, both due to relapse.
Table 1.
Patient characteristics
| iVRE (n = 18) | Control (n = 18) | P | |
|---|---|---|---|
| Age, yr | 10 (1–62) | 11 (1–63) | Matching criterion |
| Male | 10 (65) | 8 (44) | .48 |
| Donor type | Matching criterion | ||
| Matched sibling | 4 (22) | 4 (22) | |
| Matched unrelated | 1 (6) | 1 (6) | |
| Cord blood | 13 (72) | 13 (72) | |
| Reduced-intensity | 4 (22) | 4 (22) | Matching criterion |
| conditioning | |||
| Diagnosis | Matching criterion | ||
| Malignant* | 11 (61) | 11 (61) | |
| Nonmalignant | 7 (39) | 7 (39) | |
| GVHD prophylaxis | .81 | ||
| CsA based | 16 (89) | 15 (83) | |
| Others | 2 (11) | 3 (17) | |
| Disease risk† | Matching criterion | ||
| Standard | 9 (50) | 9 (50) | |
| High | 2 (11) | 2 (11) | |
| Nonmalignant | 7 (39) | 7 (39) | |
| KPS score <90% | 3 (17) | 2 (11) | .65 |
| HCT-CI | .64 | ||
| 0 | 11 (61) | 9 (50) | |
| 1–2 | 2 (11) | 4 (22) | |
| 3 | 5 (28) | 5 (28) | |
Data are presented as median (range) or n (%).
CsA indicates cyclosporine; HCT-CI, Hematopoietic Cell Transplantation Comorbidity Index; KPS, Karnofsky Performance Scale.
Acute leukemia: 10 patients in the iVRE group and 9 in the control group; Myelodysplastic syndromes/myeloproliferative neoplasms: 1 in the iVRE group and 2 in the control group (P = 1.00).
Standard indicates malignancies in first or second complete remission or chronic myeloid leukemia-chronic phase; high indicates other malignancies.
Figure 1.
Comparison between patients with pretransplant gut colonization with iVRE versus matched-pair control patients. Patients with iVRE colonization had (A) a significantly higher 2-year OS and (B) a significantly lower 2-year NRM. (C) Acute grade II to IV GVHD and (D) relapse were similar between the groups.
Next, we evaluated whether iVRE colonization was associated with an overall favorable early post-HCT course that preceded lower rates of NRM. Indeed, iVRE patients were discharged approximately 1 week earlier than their matched pairs (4.6 [95% CI, 3.7 to 6.6] weeks versus 5.4 [95% CI, 4.4 to 15.1] weeks; P = .09). We then compared the 2 groups for specific early post-HCT events and milestones. The groups were similar in day 30 cumulative incidence of lymphocyte recovery (22% [95% CI, 4% to 41%] versus 11% [95% CI, 0% to 25%]; P = .19), neutrophil recovery (83% [63% to 96%] versus 78% [95% CI, 57% to 93%]; P = .23), and Clostridium difficile infection (33% in both groups).
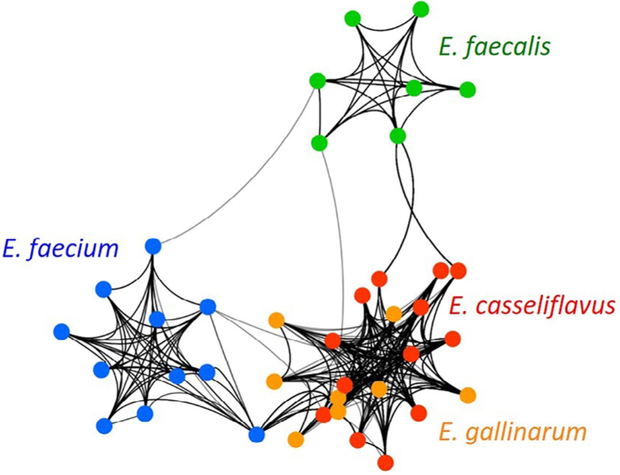
Next, we asked whether iVRE produce distinct secondary metabolites that may confer beneficial effects on the host. Considering the negative impact of gut colonization with VRE on transplant outcomes [1–3], we hypothesized that secondary metabolites encoded by iVRE may be distinctly different from those made by VRE. We tested this hypothesis bioinformatically by analyzing BGCs predicted using antiSMASH v3.0 in all complete genomes of E. faecium/faecalis and all E. gallinarum/casseliflavus genomes available in the NCBI Reference Sequence database. We evaluated pathway similarities between all enterococcal BGCs based on amino acid identity and homologous open reading frame composition. We then used these similarity scores to develop a biosynthetic network where connections between strains are supported exclusively by highly similar BGC profiles. These BGC networks grouped all E. faecalis and E. faecium strains into their own clusters, while strains of iVREs were intermingled in a distinct cluster of their own (Figure 2). Because these patterns are informed strictly by secondary metabolome homology, our bioinformatic result supports the hypothesis that secondary metabolites are a distinguishing characteristic of iVRE versus E. faecalis/faecium.
Figure 2.
Network analysis of predicted BGCs from E. faecium, E. faecalis, E. gallinarum, and E. casseliflavus genomes. Each colored node represents a unique strain and connecting lines indicate at least 75% BGC similarity between the 2 strains connected by the line. The line’s weight is proportional to the number and strength of BGC connections between the 2 strains (i.e., heavier lines indicate a greater number of higher-similarity pathways). Based solely on predicted secondary metabolite profiles, this analysis distinctly clusters iVREs together (overlapping orange and red colors in a single node), while E. faecalis and E. faecium form their own groups (independent nodes colored green and blue, respectively).
DISCUSSION
We observed a markedly lower NRM, translating into improved OS, among patients colonized pre-HCT with iVRE. Four potential explanations are considered: (1) colonization with iVRE may be associated with non–microbiota-related factors that influence mortality. For example, iVRE colonization may be more common among patients with a certain diet or environmental exposure influencing NRM (iVRE is found in food, water, plants, soil, and animal gut) [19]. The prevalence of iVRE colonization in hospitalized patients is as high as 25% in Ethiopia [20] and 35% in Brazil [21], highlighting the role of environmental factors. (2) iVRE may release metabolites with trophic effects on enterocytes, resulting in enhanced gut barrier integrity. Gut barrier-enhancing effects are a known property of short-chain fatty acids such as butyrate, produced mainly by Clostridia during fermentation of undigested carbohydrates [22,23]. (3) iVRE metabolites may actively contribute to establishing a “healthier” microbiome that protects against various events early post-transplant (as demonstrated by shorter hospitalization in our iVRE patients). This may result from beneficial microbial metabolites that modulate the host immune system, or induce colonization resistance against enteric pathogens (e.g., via production of certain bacteriocins). A metabolite-mediated effect is supported by our observation that putative secondary metabolomes in iVRE are more conserved and distinct from those in E. faecalis/faecium. Unique metabolic pathways in iVRE are exemplified by inulin fermentation and genes for acetoin dehydrogenase (converting acetoin to acetaldehyde and acetyl coenzyme A), both restricted to E. casseliflavus [7]. (4) iVRE signature may be a consequence of a “healthier” microbiome. Ultimately, distinguishing among these possible explanations and testing whether the observed association in this study is directly causal will likely require preclinical microbiota transplantation experiments.
Microbiota niche competition may be important in iVRE colonization. An experimental study was performed in 1997 to identify the effect of oral vancomycin on gut microbial ecology. Twenty healthy volunteers received oral cefuroxime for 1 week, and 10 of these individuals subsequently received oral vancomycin for 1 week. Vancomycin administration resulted in a rapid emergence of E. gallinarum/casseliflavus [24]. Metabolomic studies of iVRE would be informative in identifying unique metabolites with potential effects on barrier function or host immunity. These metabolites may be targets for known drugs and identify avenues for novel drug discovery. Potential clinical implications include use of probiotics, metabolite supplementation, or drugs that mimic the effects of beneficial metabolites on signaling pathways in intestinal and mucosal immune cells. Harnessing the microbiota and adopting their beneficial products could thereby improve transplant outcomes.
Despite matching for potential relevant confounders, our study is inherently limited by its retrospective design. Furthermore, our strict matching for clinically important variables restricted our sample size. Due to the rarity of iVRE colonization, a prospective study is likely unfeasible. Nonetheless, studies with larger numbers of patients seem necessary to validate our results. A large proportion of patients in our study were cord blood transplant recipients, children, and patients with nonmalignant disorders. Although these were among factors on which matching occurred, the generalizability of our results to other populations needs further investigation. As an example, a cord blood–specific interplay between the reconstituting immune system and microbiota might have contributed to the difference in outcomes in this study. Finally, our bioinformatics approach is limited by the availability of relevant microbial genomes and the predicted nature of the pathways. These methods will become more powerful as more clinical strains are sequenced. Microbial gene expression and biochemical characterization are necessary to evaluate metabolite expression patterns during colonization.
ACKNOWLEDGMENTS
Financial disclosure: The authors have nothing to disclose.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
REFERENCES
- 1.Hefazi M, Damlaj M, Alkhateeb HB, et al. Vancomycin-resistant Enterococcus colonization and bloodstream infection: prevalence, risk factors, and the impact on early outcomes after allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. Transpl Infect Dis. 2016;18:913–920. [DOI] [PubMed] [Google Scholar]
- 2.Weinstock DM, Conlon M, Iovino C, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2007;13:615–621. [DOI] [PubMed] [Google Scholar]
- 3.Kamboj M, Chung D, Seo SK, et al. The changing epidemiology of vancomycin-resistant Enterococcus (VRE) bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Biol Blood Marrow Transplant. 2010;16:1576–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vydra J, Shanley RM, George I, et al. Enterococcal bacteremia is associated with increased risk of mortality in recipients of allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55:764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avery R, Kalaycio M, Pohlman B, et al. Early vancomycin-resistant enterococcus (VRE) bacteremia after allogeneic bone marrow transplantation is associated with a rapidly deteriorating clinical course. Bone Marrow Transplant. 2005;35:497–499. [DOI] [PubMed] [Google Scholar]
- 6.Almyroudis NG, Fuller A, Jakubowski A, et al. Pre- and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2005;7:11–17. [DOI] [PubMed] [Google Scholar]
- 7.Palmer KL, Godfrey P, Griggs A, et al. Comparative genomics of enterococci: variation in Enterococcus faecalis, clade structure in E. faecium, and defining characteristics of E. gallinarum and E. casseliflavus. MBio. 2012;3:e00318–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent S, Knight RG, Green M, Sahm DF, Shlaes DM. Vancomycin susceptibility and identification of motile enterococci. J Clin Microbiol. 1991;29:2335–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leclercq R, Dutka-Malen S, Duval J, Courvalin P. Vancomycin resistance gene vanC is specific to Enterococcus gallinarum. Antimicrob Agents Chemother. 1992;36:2005–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toye B, Shymanski J, Bobrowska M, Woods W, Ramotar K. Clinical and epidemiological significance of enterococci intrinsically resistant to vancomycin (possessing the vanC genotype). J Clin Microbiol. 1997;35:3166–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid KC, Cockerill FR, Patel R. Clinical and epidemiological features of Enterococcus casseliflavus/flavescens and enterococcus gallinarum bacteremia: a report of 20 cases. Clin Infect Dis. 2001;32:1540–1546. [DOI] [PubMed] [Google Scholar]
- 12.Ruoff KL, de la Maza L, Murtagh MJ, Spargo JD, Ferraro MJ. Species identities of enterococci isolated from clinical specimens. J Clin Microbiol. 1990;28:435–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson JE, Sweeney AH, Simms M, et al. An analysis of 110 serious enterococcal infections. Epidemiology, antibiotic susceptibility, and outcome. Medicine (Baltimore). 1995;74:191–200. [DOI] [PubMed] [Google Scholar]
- 14.Gordon S, Swenson JM, Hill BC, et al. Antimicrobial susceptibility patterns of common and unusual species of enterococci causing infections in the United States. Enterococcal Study Group. J Clin Microbiol. 1992;30:2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi S, Lee S, Kim TH, et al. Clinical features and outcomes of bacteremia caused by Enterococcus casseliflavus and Enterococcus gallinarum: analysis of 56 cases. Clin Infect Dis. 2004;38:53–61. [DOI] [PubMed] [Google Scholar]
- 16.Batistão DW, Gontijo-Filho PP, Conceição N, de Oliveira AG, Ribas RM. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz. 2012;107:57–63. [DOI] [PubMed] [Google Scholar]
- 17.Tschudin Sutter S, Frei R, Dangel M, Gratwohl A, Bonten M, Widmer AF. Not all patients with vancomycin–resistant enterococci need to be isolated. Clin Infect Dis. 2010;51:678–683. [DOI] [PubMed] [Google Scholar]
- 18.Weber T, Blin K, Duddela S, et al. antiSMASH 3.0–a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015;43:W237–W243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebreton F, Willems RJL, Gilmore MS. Enterococcus Diversity, Origins in Nature, and Gut Colonization Boston, MA: Massachusetts Eye and Ear Infirmary; 2014. [PubMed] [Google Scholar]
- 20.Abamecha A, Wondafrash B, Abdissa A. Antimicrobial resistance profile of Enterococcus species isolated from intestinal tracts of hospitalized patients in Jimma, Ethiopia: BMC Res Notes. 2015;8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mondino SSB, Castro ACD, Mondino PJJ, Carvalho Mda GS, Silva KMF, Teixeira LM. Phenotypic and genotypic characterization of clinical and intestinal enterococci isolated from inpatients and outpatients in 2 Brazilian hospitals. Microb Drug Resist. 2003;9:167–174. [DOI] [PubMed] [Google Scholar]
- 22.Peng L, Li Z-R, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edlund C, Barkholt L, Olsson-Liljequist B, Nord CE. Effect of vancomycin on intestinal flora of patients who previously received antimicrobial therapy. Clin Infect Dis. 1997;25:729–732. [DOI] [PubMed] [Google Scholar]