To the Editor:
Recently, on the basis of a case series involving six patients, Sykes et al.1 described the TEMPI syndrome, a novel multisystem disease defined by telangiectasias, erythrocytosis with elevated erythropoietin levels, monoclonal gammopathy, perinephric-fluid collections, and intrapulmonary shunting.
Here we describe a 56-year-old white woman who was referred to the National Institutes of Health (NIH) for participation in our Undiagnosed Diseases Program for evaluation of unexplained secondary erythrocytosis for the preceding 10 years, fatigue, and diffuse pains. After an extensive workup, we found that this patient had clinical features of the TEMPI syndrome,1 and we initiated therapy targeting abnormal plasma cells.
Before the patient’s referral to the NIH, a diagnostic workup showed increasing erythrocytosis, with a hemoglobin level ranging from 18 to 20 g per deciliter (hematocrit, 52 to 58%). The patient required therapeutic phlebotomy every 2 weeks. Serum erythropoietin levels were elevated, at 100 mU per milliliter, and testing for the V617F mutation in the Janus kinase 2 (JAK2) gene was negative. Furthermore, venography of the kidneys, the renin level of the renal veins, and the hemoglobin P50 (the hemoglobin–oxygen affinity) results were normal. To identify a source of ectopic erythropoietin production, the patient underwent extensive imaging that showed only uterine fibroids. In 2007, she underwent a hysterectomy; however, no tumors were detected and the hemoglobin levels remained unchanged postoperatively. In 2010, she had intermittent flank pain, and imaging showed bilateral perinephric-fluid collection. Despite multiple drainage procedures, reaccumulation of fluid occurred each time. Analysis of the fluid showed no malignant cells or infection.
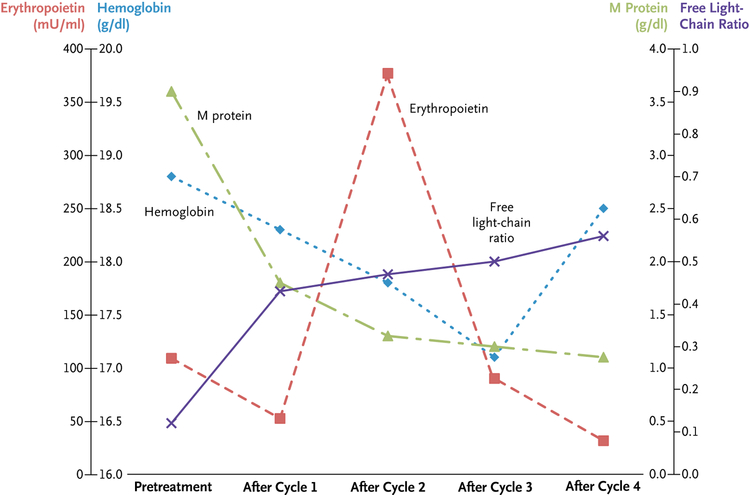
At the NIH, we confirmed the above abnormalities. We also performed a bone marrow biopsy that showed that 10 to 15% of the plasma cells had lambda light-chain restriction (see Fig. 1 in the Supplementary Appendix, available with the full text of this letter at https://NEJM.org), consistent with smoldering myeloma. Serum protein electrophoresis showed an IgG lambda monoclonal (M) protein level of 3.6 g per deciliter. Serum free light chains were skewed with a kappa: lambda free light-chain ratio of 0.12. Given the above findings, we diagnosed the TEMPI syndrome and initiated treatment with subcutaneous bortezomib therapy (at a dose of 1.3 mg per square meter of body-surface area on days 1, 4, 8, and 11 on a 21-day cycle). After four cycles of therapy, we noted the following signs of improvement: the hemoglobin level had decreased slightly from the first to the last data point (Fig. 1) without need for therapeutic phlebotomy, and the levels of erythropoietin and M protein were trending down (the free light-chain ratio normalized after one cycle) (Fig. 1). The perinephric-fluid collection was decreased on the left side and unchanged on the right (see Fig. 2 in the Supplementary Appendix). The telangiectasias remained stable (see Fig. 3 in the Supplementary Appendix). We decided to administer eight cycles of bortezomib.
Figure 1. Hemoglobin, Erythropoietin, and Abnormal Immunoglobulin Serum Levels in Relation to Treatment with a Proteasome Inhibitor.
At baseline, the hemoglobin level was 18.8 g per deciliter, the erythropoietin level was 109 mU per milliliter, the M-protein concentration was 3.6 g per deciliter, and the free light-chain ratio was 0.12. After initiation of bortezomib therapy, the hemoglobin level was decreased slightly to a stable level, and the patient did not require therapeutic phlebotomies. The erythropoietin levels increased initially; however, after four cycles of therapy, they were below baseline levels. The M-protein concentration decreased to 1.1 g per deciliter after four cycles of therapy, and the free light-chain ratio (normal range, 0.26 to 1.65) was normalized after one cycle of therapy.
Given the favorable changes associated with therapy with bortezomib, our findings suggest that plasma cells play a key role in the pathogenesis of the TEMPI syndrome.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Cancer Institute.
References
- 1.Sykes DB, Schroyens W, O’Connell C. The TEMPI syndrome — a novel multisystem disease. N Engl J Med 2011;365: 475–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.