Abstract
We report an enantioselective coupling between alkynes and indoles. A Rh-hydride catalyst isomerizes alkynes to generate a metal-allyl species that can be trapped with both aromatic and heteroaromatic nucleophiles.
Graphical abstract
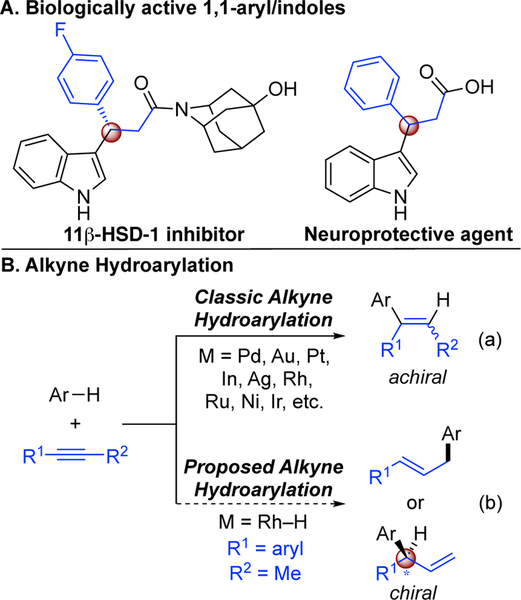
Aryl and heteroaryl rings can be used to increase non-bonding and electrostatic interactions between a small molecule and its macromolecule target.1 Among the top selling therapeutics, more than half contain such aryl structures (Figure 1A).2 Given the relevance of chirality in medicine, inventing enantioselective tools for introducing aromatic nucleophiles warrants pursuit.3 The hydroarylation of alkynes is a modern strategy for functionalizing aryl-structures,4 where two simple functional groups are coupled with high atom economy.5 To date, however, this approach has been limited to generating achiral olefins (Figure 1B, Eq. a). Classic alkyne hydroarylations generate achiral vinylated-arenes via mechanisms that involve alkyne activation with π-acids or arene activation to access aryl-metal species 4d–n In contrast, we imagined using metal-hydride catalysis to couple arenes with alkynes to form allylated products (Figure 1B, Eq. b).6 In this communication, we disclose a regio-and enantioselective alkyne hydroheteroarylation using indoles.7–9
Figure 1.
Inspiration for asymmetric alkyne hydroarylation.
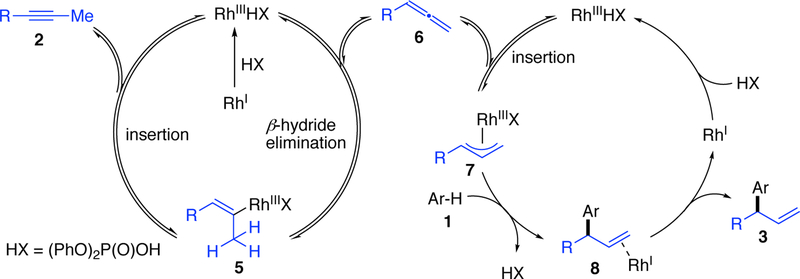
On the basis of previous studies, Rh-hydride catalysts can isomerize alkynes (2) to allenes (6) via a Rh-vinyl species (5) as depicted in Figure 2.10 Subsequent allene insertion into a Rh–H generates a Rh-π-allyl species (7). Various oxygen-,11 sulfur-,12 and nitrogen-based13 nucleophiles have been used to trap 7 and generate carbon-heteroatom bonds with stereocontrol. However, enantioselective C–C bond formation has thus far been only achieved with aldehydes via enamine catalysis.14e We recognized that the key challenge to achieving alkyne hydroarylation would be trapping Rh-π-allyl 7 with an arene 1 (an inherently weaker nucleophile) to generate 3, with high enantio-and regiocontrol. However we were encouraged by Carreira’s Ir-catalyzed polyene cyclization that demonstrates the use of arenes and hetereoarenes as terminating nucleophiles.15
Figure 2.
Proposed Rh-hydride catalyzed alkyne hydroarylation.
To test this hypothesis, we examined the coupling of various arenes and heteroarenes 1 and 1-phenyl-l-propyne (2a) (Table 1). Successful trapping of the Rh-π-allyl species affords either the branched (3) or the linear regioisomer (4). Using a combination of a Rh-bisphosphine and diphenyl phosphate,11c,14e we observed that arenes and heteroarenes with a wide range of nucleophilicities, based on the Mayr scale (N= 1.33 to 11.63), were successful coupling partners.16, 21 Initial studies using [Rh(COD)C1]2, dppf and diphenyl phosphate showed that the structure of the nucleophile impacted which regioisomer was favored. For example, with benzofuran and 1,3-dimethoxybenzene, we observed the linear isomers as the major product, in accordance with previous studies using Brønsted acid catalysis (>20:1 rr. 29% and 35%, respectively).17 In contrast, 3-ethyl-2,4-dimethyl pyrrole and indole generated the branched isomers upon addition to alkyne 2a (>20:1 rr, 24% and 65%, respectively). On the basis of related studies on alkyne hydroamination, we imagine that regioselectivity can be controlled by tuning the catalyst and acid.13a
Table 1.
Alkyne Hydroarylation using Arenes with a Range of Nucleophilicitiesa
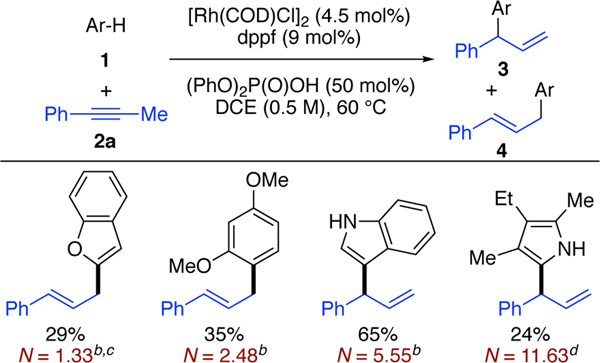 |
1 (0.1 mmol), 2a (0.12 mmol), [Rh(COD)Cl]2 (4.5 mol%), dppf (9.0 mol%), (PhO)2P(O)OH (50 mol%), DCE (0.2 mL), 60 °C,
Nuclcophilicity in DCM.
Nucleophilicity of furan.
Nucleophilicity in MeCN.
Indoles can be site-selectively prenylated at the N, 2-, 3-, 4-, or 7-position via enzymatic or synthetic processes.18 Despite the diverse reactivity of indoles, we observed selective bond formation at the 3-position upon coupling of alkyne 2a and indole to yield 3 as the only regioisomer.
With this promising reactivity demonstrated, we focused on developing an enantioselective coupling using indoles due to the importance of these heterocycles in natural and pharmaceutical products.19 We found that a protocol consisting of [Rh(COD)Cl]2, (R)-Ph-BINAP (L1), and diphenyl phosphate gave the desired branched product (3a) in 5% yield and 20% ee (Table 2).20 In contrast to previous studies where carboxylic acids were used,14a–d more acidic acids (e.g., sulfonic and phosphoric acids) were necessary for reactivity. Increasing the steric bulk of the phosphine substituent improved enantioselectivity (L2, 28% ee and L3, 93% ee). The electron-rich DTBM-BINAP (L3) also dramatically improved the yield to 81% yield. Other biaryl bisphosphine ligands bearing the DTBM-phosphine substituents such as SEGPHOS (L4), GARPHOS (L5), or MeO-BIPHEP (L6) provided similar enantioselectivity but lower reactivity (18–31% yield). With ligand L3, we found that a number of solvents could be used but found that using cyclopentyl methyl ether (CPME) was optimal; 3a was obtained in 92% yield and 91% ee, with lower (2.5 mol%) catalyst loadings.21
Table 2.
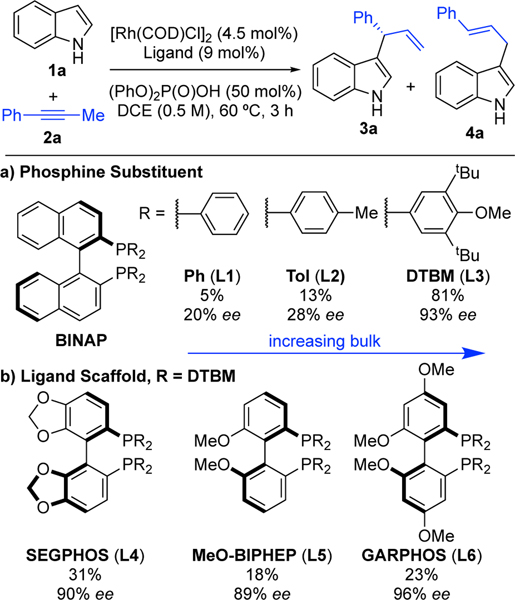 |
1a (0.1 mmol). 2a (0.12 mmol), [Rh(COD)Cl]2 (4.5 mol%), ligand (9.0 mol%), (PhO)2P(O)OH (50 mol%), DCE (0.2 mL), 60 °C, 3 hours.
Yields determined by 1H NMR with 1,2,4,5-tetramethylbenzene as internal standard.
Enantioselectivities determined by chiral SFC.
With this protocol in hand, we explored the hydroheteroarylation of alkyne 2a with various indoles (Table 3). Efficient and selective indole-alkyne coupling occurs with a variety of indole substitution patterns. For example, a methyl group can be incorporated at the N-, 5-, and 7-positions of indole to afford the corresponding allylated indoles with up to 96% yield, >20:1 rr, and 92% ee (3ba, 3ga, 3oa). In comparison, lower ee is observed with 2-methyl indole (3ca, 69% ee). In general, we observe lower enantioselectivity with 2-methyl indole using various aryl-substituted alkynes.21 However, when a phenyl or tert-butyl group is incorporated at the 2-position higher ee is observed (3qa and 3ra, 92% and 86% ee, respectively). Halogenated indoles were successfully coupled with high selectivities (3da, 3ea, 3fa, 3ja, 3na, 3pa). Chemoselective C–C bond formation was observed in the presence of a nucleophilic phenol (3ia) and an electrophilic methyl ester (3ka). A substrate bearing a pinacol borane, a convenient functional handle was transformed smoothly (3la).
Table 3.
Alkyne Hydroheteroarylation with Various Indolesa
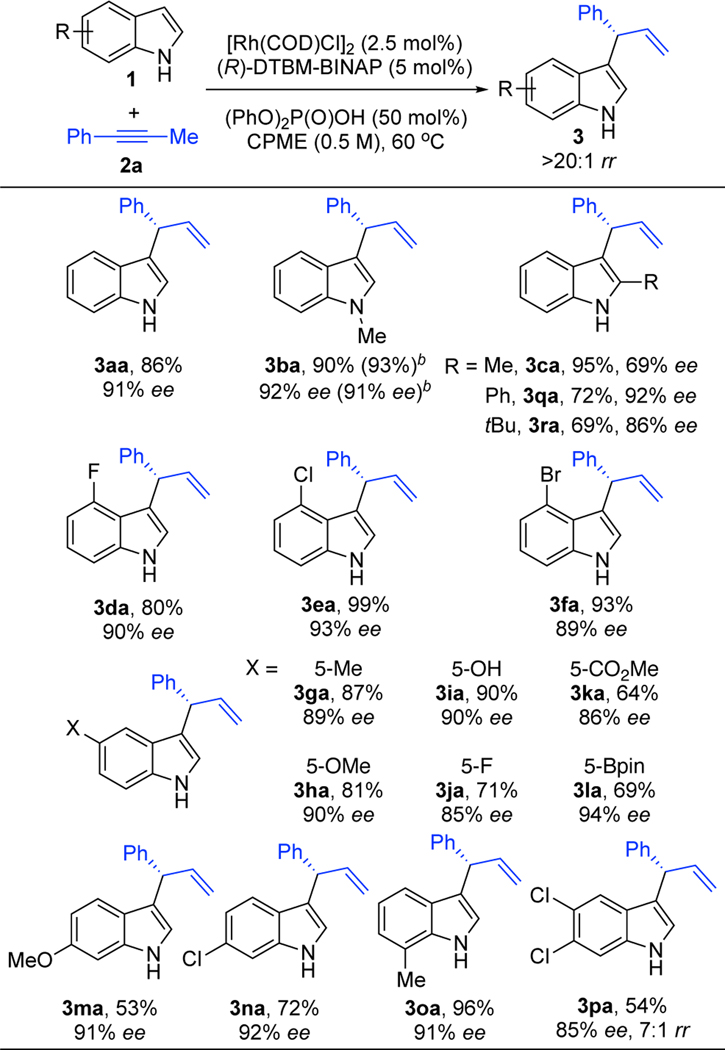 |
1 (0.1 mmol), 2a (0.12 mmol), [Rh(COD)Cl]2 (2.5 mol%), (R)-DTBM-BINAP (5.0 mol%), (PhO)2P(O)OH (50 mol%), CPME (0.2 mL), 60 °C. Isolated yields. rr’s (3:4) determined by 1H NMR analysis of the unpurified reaction mixture. Enantioselectivities determined by chiral SFC.
Values in parentheses are for the transformation performed on a 1.0 mmol scale.
Next, we studied the coupling of indole 1a with structurally diverse alkynes (Table 4). Electron-rich alkynes with alkyl or ether substitution undergo efficient and selective coupling with indole (3ab–3ae, 70–88%, >20:1 rr, 82–93% ee). Fluorinated and chlorinated alkynes act as efficient coupling partners (3af and 3ag, 82–93%, >20:1 rr, 88–90% ee). In addition, electron-deficient alkynes with trifluoromethyl substitution undergo hydroarylation with indole to provide 3ai in 97% yield and 92% ee. Chemoselective functionalization occurs even in the presence of electrophilic ethyl ester (3ah). Aromatic and heteroaromatic alkynes (3-thiophene and 1-naphthalene) also undergo hydroarylation (3aj and 3ak). We found that an aromatic or heteroaromatic group on the alkyne is critical for reactivity. For example, an alkyl-substituted alkyne, such as 2-octyne, proved to be unreactive under these conditions (3al).
Table 4.
Hydroheteroarylation of Various Alkynes with Indolea
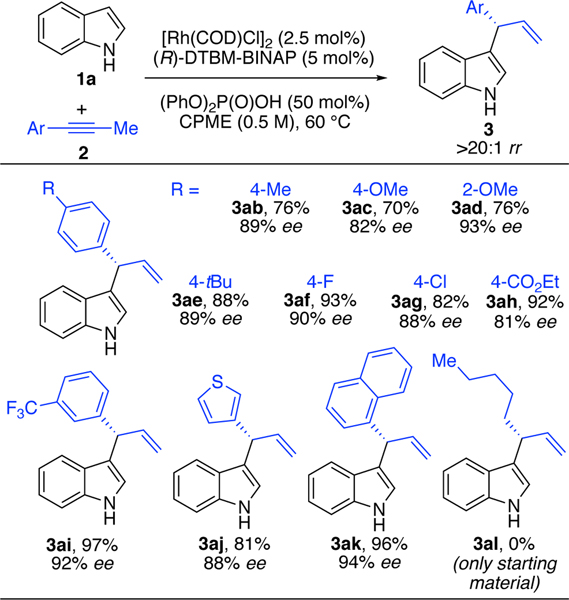 |
1a (0.1 mmol), 2 (0.12 mmol), [Rh(COD)Cl]2 (2.5 mol%), (R)-DTBM-BINAP (5.0 mol%), (PhO)2P(O)OH (50 mol%), CPME (0.2 mL), 60 °C. Isolated yields. rr’s (3:4) determined by 1H NMR analysis of the unpurified reaction mixture. Enantioselectivities determined by chiral SFC.
To support the intermediacy of an allene, we replaced alkyne 2a with phenylallene 6a (Eq. 1).21 Under standard reaction conditions, the desired coupling product 3aa was obtained with similar enantio-and regioselectivity, although in lower yield (33% yield, 91% ee, and >20:1 rr). This result supports the possibility of an allene intermediate. But the diminished yields suggest that high concentrations of allene may be detrimental due to competing decomposition and thus, in situ generation results in better efficiency.11c, 13a
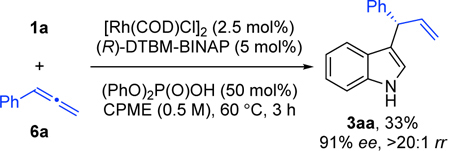 |
(1) |
We have demonstrated a regio-and enantioselective way to hydrofunctionalize alkynes using indoles. The use of Rh-hydride catalysis to isomerize alkynes has enabled access to a complementary hydroheteroarylation motif. Moreover, our study demonstrates the potential of generating C–C bonds under mild conditions using both aromatic and heteroaromatic motifs. Given these promising results, our future studies will focus on enantio-and regioselective coupling using other classes of aromatic nucleophiles.
Supplementary Material
ACKNOWLEDGMENT
Funding provided by UC Irvine and the National Institutes of Health (GM105938). We are grateful to Eli Lilly for a Grantee Award. F.A.C. is grateful for an NSF Graduate Research Fellowship.
Footnotes
ASSOCIATED CONTENT
Supporting Information.
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and spectral data for all new compounds (PDF)
No competing financial interests have been declared.
REFERENCES
- (1).Dalvia D; Sajiv N; Kang P; Loi C-M Influence of Aromatic Rings on ADME Properties of Drugs, In Metabolism, Pharmo-kinetics and Toxicity of Functional Group; Royal Society of Chemistry: Cambridge, U.K., 2010; pp. 275–327. [Google Scholar]
- (2).(a) McGrath NA; Brichacek M; Njardarson JT J. Chem. Educ 2010, 87, 1348; [Google Scholar]; (b) Taylor RD; MacCoss M; Lawson AD G. J. Med. Chem 2014, 57, 5845. [DOI] [PubMed] [Google Scholar]
- (3).(a) For select reviews, see: Hayashi T; Yamasaki K Chem. Rev 2003, 103, 2829; [DOI] [PubMed] [Google Scholar]; (b) Schmidt F; Stemmler RT; Rudolph J; Bolm C Chem. Soc. Rev 2006, 35, 454; [DOI] [PubMed] [Google Scholar]; (c) Alexakis A; Backvall JE; Krause N; Pamies O; Dieguez M Chem. Rev 2008, 108, 2796; [DOI] [PubMed] [Google Scholar]; (d) Harutyunyan SR; den Hartog T; Geurts K; Minnaard AJ; Feringa BL Chem. Rev 2008, 108, 2824; [DOI] [PubMed] [Google Scholar]; (e) Poulsen TB; Jorgenson KA Chem. Rev 2008, 108, 2903; [DOI] [PubMed] [Google Scholar]; (f) Cherney AH; Kadunce NT; Reisman SE Chem. Rev 2015, 115, 9587; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Tian P; Dong H-Q; Lin G-Q ACS Catal 2012, 2, 95. [Google Scholar]
- (4).(a) For select reviews, see: Nevado C; Echavarren AM Synthesis 2005, 2, 167; [Google Scholar]; (b) Kitamura T Eur. J. Org. Chem 2009, 1111; [Google Scholar]; (c) Ackermann L Acc. Chem. Res 2014, 47, 281 For select examples of alkyne hydroarylation that generate an internal olefin, see: [DOI] [PubMed] [Google Scholar]; (d) Fallon BJ; Derat E; Amatore M; Aubert C; Chemla F; Ferreira F; Perez-Luna A; Petit M; J. Am. Chem. Soc 2015, 137, 2448; [DOI] [PubMed] [Google Scholar]; (e) Liu Z; Derosa J; Engle KM J. Am. Chem. Soc 2016, 138, 13076; [DOI] [PubMed] [Google Scholar]; (f) Ding D; Mou T; Feng M; Jiang XJ Am. Chem. Soc 2016, 138, 5218; [DOI] [PubMed] [Google Scholar]; (g) Kumar NYP; Bechtoldt A; Raghuvanshi K; Ackermann L Angew. Chem. Int. Ed 2016, 55, 6929; For select examples of alkyne hydroheteroarylation with indoles to generate vinylated products, see: [DOI] [PubMed] [Google Scholar]; (h) Lu W; Jia C; Kitamura T; Fujiwara Y Org. Lett 2000, 2, 2927; [DOI] [PubMed] [Google Scholar]; (i) Nakao Y; Kanyiva KS; Oda S; Hiyama TJ Am. Chem. Soc 2006, 128, 8146; [DOI] [PubMed] [Google Scholar]; (j) Bhaskar G; Saikumar C; Perumal PT; Tet. Lett 2010, 51, 3141; [Google Scholar]; (k) Samala S; Mandadapu AK; Saifuddin M; Kundu BJ Org. Chem 2013, 78, 6769; [DOI] [PubMed] [Google Scholar]; (l) Ferrer C; Echavarren AM Angew. Chem. Int. Ed 2006, 45, 1105; [DOI] [PubMed] [Google Scholar]; (m) Ding Z; Yoshikai N Angew. Chem. Int. Ed 2012, 51, 4698; [DOI] [PubMed] [Google Scholar]; (n) Lu Q; Greßies S; Klauck FJR; Glorius F Angew. Chem. Int. Ed 2017, 56, 6660. [DOI] [PubMed] [Google Scholar]
- (5).Trost BM Science 1991, 254, 1471. [DOI] [PubMed] [Google Scholar]
- (6).(a) For select reviews on allylic alkylation, see: Trost BM; Van Vranken DL Chem. Rev 1996, 96, 395; [DOI] [PubMed] [Google Scholar]; (b) Falciola CA; Alexakis A Eur. J. Org. Chem 2008, 3765; [Google Scholar]; (c) Zhuo C-X; Zheng C; You S-L Acc. Chem Res 2014, 47, 2558; [DOI] [PubMed] [Google Scholar]; (d) Butt NA; Zhang W Chem. Soc. Rev 2015, 44, 7929; [DOI] [PubMed] [Google Scholar]; (e) Hethcox JC; Shockley SE; Stoltz BM ACS Catal 2016, 6, 6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) For select examples of Ir-catalyzed indole allylic alkylation, see: Liu W-B; He H; Dai L-X; You S-L Org. Lett 2008, 10, 1815; [DOI] [PubMed] [Google Scholar]; (b) Huang L; Dai L-X; You S-LJ Am. Chem. Soc 2016, 138, 5793. [DOI] [PubMed] [Google Scholar]
- (8).(a) For select examples of Ru-catalyzed indole allylic alkylation, see: Zaitsev AB; Gruber S; Plüss PA; Pregosin PS; Veiros LF; Wörle MJ Am. Chem. Soc 2008, 130, 11604; [DOI] [PubMed] [Google Scholar]; (b) Sundararaju B; Achard M; Demerseman B; Toupet L; Sharma GVM; Bruneau C Angew. Chem. Int. Ed 2010, 49, 2782. [DOI] [PubMed] [Google Scholar]
- (9).(a) For select examples of Pd-catalyzed indole allylic alkylation, see: Kimura M; Futamata M; Mukai R; Tamaru YJ Am. Chem. Soc 2005, 127, 4592; [DOI] [PubMed] [Google Scholar]; (b) Gao R-D; Liu C; Dai L-X; Zhang W; You S-L Org. Lett 2014, 16, 3919; [DOI] [PubMed] [Google Scholar]; (c) Zhang Z-X; Chen S-C; Jiao L Angew. Chem. Int. Ed 2016, 55, 8090; [DOI] [PubMed] [Google Scholar]; (d) Panda S; Ready JM J. Am. Chem Soc 2017, 139, 6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) For a review, see: Koschker P; Breit B Acc. Chem. Res 2016, 49, 1524 For select examples using Pd, see: [DOI] [PubMed] [Google Scholar]; (b) Kadota I; Shibuya A; Gyoung YS; Yamamoto YJ Am. Chem. Soc 1998, 120, 10262; [Google Scholar]; (c) Gao Z Wu Z; Fang X; Lin A; Yao H Org. Lett 2016, 18, 3906; [DOI] [PubMed] [Google Scholar]; (d) Yang C; Zhang K; Wu Z; Yao H; Lin A Org. Lett 2016, 18, 5332 For select examples of metal-catalyzed alkyne isomerization followed by trapping with electrophiles, see: [DOI] [PubMed] [Google Scholar]; (e) Obora Y; Hatanaka S; Ishii Y Org. Lett 2009, 11, 3510; [DOI] [PubMed] [Google Scholar]; (f) Park BY; Nguyen KD; Chaulagain MR; Komanduri V; Krische MJ J. Am. Chem. Soc 2014, 136, 11902; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Liang T; Nguyen KD; Zhang WD; Krische MJ J. Am. Chem. Soc 2015, 137, 3161; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Chen Q-A; Cruz FA; Dong VM J. Am. Chem. Soc 2015, 137, 3157; [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Liang T; Zhang W; Chen T-Y; Nguyen KD; Krische MJ J. Am. Chem. Soc 2015, 137, 13066; [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Liang T; Zhang W; Krische MJ J. Am. Chem. Soc 2015, 137, 16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).(a) For examples of C–O bond formation, see: Lumbroso A; Koschker P; Vautravers NR; Breit BJ Am. Chem. Soc 2011, 133, 2386; [DOI] [PubMed] [Google Scholar]; (b) Koschker P; Kähny M; Breit BJ Am. Chem. Soc 2015, 137, 3131; [DOI] [PubMed] [Google Scholar]; (c) Liu Z; Breit B Angew. Chem. Int. Ed 2016, 55, 8440. [DOI] [PubMed] [Google Scholar]
- (12).For an example of C–S bond formation, see: Xu K; Kha-kyzadeh V; Bury T; Breit B; J. Am. Chem. Soc 2014, 136, 16124. [DOI] [PubMed] [Google Scholar]
- (13).(a) For examples of C–N bond formation, see: Chen Q-A; Chen Z; Dong VM J. Am Chem Soc 2015, 137, 8392; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Haydl AM; Hilpert LJ; Breit B Chem. Eur. J. 2016, 22, 6547. [DOI] [PubMed] [Google Scholar]
- (14).(a) For examples of C–C bond formation, see: Beck TM; Breit B Org. Lett 2016, 18, 124; [DOI] [PubMed] [Google Scholar]; (b) Cruz FA; Chen Z; Kurtoic SI; Dong VM Chem. Commun 2016, 52, 5836; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Li C; Grugel C; Breit B Chem. Commun 2016, 52, 5840; [DOI] [PubMed] [Google Scholar]; (d) Beck TM; Breit B Eur. J. Org. Chem 2016, 5839; [Google Scholar]; (e) Cruz FA; Dong VM J. Am Chem. Soc, 2017, 139, 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Schafroth MA; Sarlah D; Krautwald S; Carreira EM J. Am Chem. Soc 2012, 134, 20276. [DOI] [PubMed] [Google Scholar]
- (16).Mayr H; Ofial AR J. Phys. Org. Chem 2008, 21, 584. [Google Scholar]
- (17).Bras JL; Muzart J Tetrahedron 2007, 63, 7942. [Google Scholar]
- (18).(a) For select reviews, see: Williams RM; Stocking EM; Sanz-Cerveza JF Top. Curr. Chem 2000, 209, 97; [Google Scholar]; (b) Li S-M Nat. Prod. Rep 2010, 27, 57; [DOI] [PubMed] [Google Scholar]; (c) Lindel T; Marsch N; Adla SK Top. Curr. Chem 2012, 309, 67 For select examples, see: [DOI] [PubMed] [Google Scholar]; (d) Kranen E; Steffan N; Maas R; Li S-M; Jose J ChemCatChem 2011, 3, 1200; [Google Scholar]; (e) Schwarzer DD; Gritsch PJ; Gaich T Angew. Chem. Int. Ed 2012, 51, 11514; [DOI] [PubMed] [Google Scholar]; (f) Wollinsky B; Ludwig L; Hamacher A; Yu X; Kassack MU; Li S-M Bioorg. Med. Chem. Lett 2012, 22, 3866; [DOI] [PubMed] [Google Scholar]; (g) Yu X; Liu Y; Xie X; Zheng X-D; Li S-MJ Biol. Chem 2012, 287, 1371; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Johnson KF; Zeeland RV; Stanley LM Org. Lett 2013, 15, 2798; [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Ruchti J; Carreira EM J. Am. Chem. Soc 2014, 136, 16756; [DOI] [PubMed] [Google Scholar]; (j) Müller JM; Stark CB W. Angew. Chem. Int. Ed 2016, 55, 4798. [DOI] [PubMed] [Google Scholar]
- (19).(a) Joule JA; Mills K Heterocyclic Chemistry, 4th ed., Blackwell Science: Oxford, 2000. [Google Scholar]; (b) Faulkner DJ Nat. Prod. Rep 2002, 19, 1. [DOI] [PubMed] [Google Scholar]; (c) O’Connor SE; Maresh JJ Nat. Prod. Rep 2006, 23, 532; [DOI] [PubMed] [Google Scholar]; (d) Goetz AE; Silberstein AL; Corsello MA; Garg NK J. Am. Chem. Soc 2014, 136, 3036; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Moreno J; Picazo E; Morrill JA; Smith JM; Garg NK J. Am. Chem. Soc 2016, 138, 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Absolute configuration was determined by comparison of optical rotation, see SI.
- (21).See SI for further experimental details, including other sub-strates evaluated, solvent evaluation, and use of a deuterated alkyne.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.