Abstract
Purpose of review
Gut microbiota has the ability to modify the metabolism of wide array of therapeutic drugs. Current treatment modalities used in colorectal cancer have a narrow therapeutic index with a side effects profile that decreases tolerance to these treatments and adversely affects treatment outcome. Harnessing the gut microbiota ability to modify oncotherapeutic drugs metabolism and hence efficacy, could be potentially used to improve treatment outcomes in colorectal cancer patients. This review will shed lights on important findings from recent microbiome interaction studies which would hopefully serve as a useful tool to guide future translative colorectal cancer research.
Recent findings
Recent advances in microbiome studies have revealed an interesting aspect of gut microbes carcinogenic properties in dysbiotic gut environment. Microbiota niche in colorectal cancer can also modify efficacy and toxicity profile of different oncotherapeutic treatment modalities from chemoradiotherapy to immunotherapy. Conversely, each of these treatment modalities has numerous effects on the gastrointestinal flora, causing changes in the gut microbial community that affects host morbidity and mortality.
Summary
Symbiotic gut microbiota is an incredible functioning organ that maintains essential aspects of our homeostasis and immunity. According to the recent body of literature, they also can modify efficacy of many therapeutic drugs including oncotherapy. Considering that unexplainable variable treatment outcomes as well as variable tolerance to treatment have been observed in colorectal cancer patients, studying gut microbiota modulatory effects on oncotherapy might be a feasible approach to explain this phenomenon.
Keywords: Chemotherapy, Radiotherapy, Chemoradiation, Cancer, Oncology, Colon, Microbiome, Microbiota, Pharmacomicrobiomics, Gastrointestinal, Oncotherapy
Introduction
Gut microbiotas are the commensal bacteria and other microorganisms found on the gastrointestinal and colonic epithelia. Gut microbiota is the largest surface area of microbial interaction in the body1. These microbes are essential for the physiology and maintenance of the epithelial barrier. The gut microbial community plays an important role in gut metabolism, immunology and other essential daily functions. These include digestion, vitamin synthesis, disrupting foreign pathogen growth, synthesis and metabolism of choline, bile acids and short chain fatty acid synthesis and metabolism, cortisol regulation and neurotransmitter control of serotonin, dopamine, noradrenaline and GABA2.
There is a great inter-individual diversity of host microbiota. Many factors come into play to create such unique diversity including age, environmental factors like personal habits, antibiotic usage and different disease conditions3,4.
Changes in gut microbiota can disturb the symbiotic relationship between microbiota and the host leading to impaired gut homeostasis. An eventual state of dysbiosis has been linked to colorectal cancer carcinogenesis through different mechanisms. Many studies looked into the procarcinogenic properties of dysbiotic microbiota. An example of bacterial species with procarcinogenic properties includes Streptococcus bovis, Helicobacter pylori, Bacteroides fragilis, Enterococcus faecalis, Clostridium septicum, Fusobacterium spp. and Escherichia coli. The role of microbiota in colorectal carcinogenesis is becoming more evident per multiple studies5.
Studies on host microbial interaction in colorectal cancer has focused mainly on procarcinogenic properties of microbiota and their role in carcinogenesis. One facet of oncomicrobiome field that seems attractive to investigate is the effects of microbiota on different oncotherapeutic treatment modalities used in colorectal cancer including chemotherapy, radiation therapy and immunotherapy.
In recent years, the pharmacomicrobiomics of the correlation between gastrointestinal microbiota and the efficacy of chemotherapy has been studied much further and the critical role of microbiota in the efficacy and toxicity of cancer treatment has been proven1. As we learn more about this topic, it is also important that we understand the relationship between gastrointestinal microbiota and chemoradiotherapy, and the effect of varying gut microbiota on the pharmacokinetics and toxicity of oncotherapy agents.
Pharmacomicrobiomics
‘Pharmacomicrobiomics’ is an evolving field that studies the effect of microbiota on drug activity and metabolism. A large number of studies have documented the significant role of gut microbial community on our metabolic capabilities of different therapeutic drugs. The microbial xenobiotic metabolism could be a direct enzymatic effect of the microbiota on drug metabolism or through there indirect impact on host gene expression of enzymes involved in drug metabolism6.
Some of the examples of direct effect of microbiota on drug metabolism include decreased efficacy of digoxin by Eggerthella Lenta and the effect of microbial azoreductase activity on Sulfasalazine metabolism by different microbiota including Bacteroides, Bifidobacterium, Lactobacillus, and Enterococcus. Of the many xenobiotics metabolized by intestinal microbial community include methotrexate, antidiabetic medications like metformin and insulin, cardiovascular medications like nitroglycerine, neurologic medications like clonazepam, risperidone, levodopa and gastrointestinal medications like metronidazole, omeprazole, 5 aminosalicylic acid, among others6.
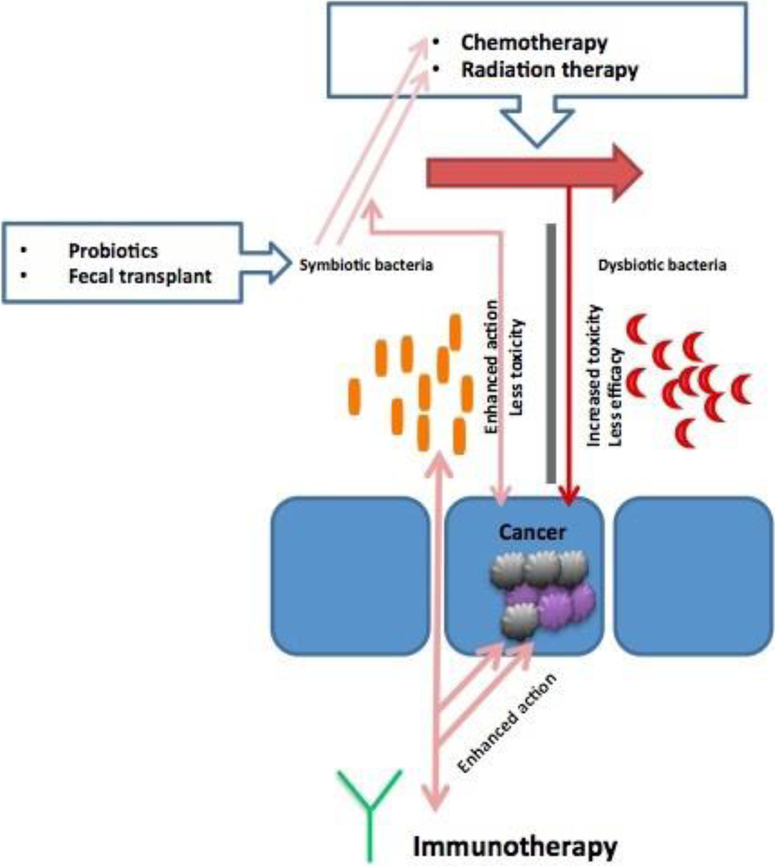
Microbiota also plays a role in the metabolism of oncotherapy agents such as chemotherapy and immunotherapy. Conversely, chemotherapeutic agents influence the growth of gut microbiota through immune modulation7. Moreover, irradiation through its cytotoxic effects and production of radical oxygen species causes epithelial ulcerative lesions and microbial cell damage that leads to a change in gut microbial community. These microbial alterations can then significantly aggravate radiotherapy induced mucositis and worsen the patient’s clinical outcome8. Variations in host gut microbiota have also been correlated with the varied response to Immunotherapeutic agents9. Fig. 1
Figure 1.
The microbiota niche and how it affects chemotherapy, radiation therapy, and immunotherapy. A favorable symbiotic state would enhance the efficacy and reduce toxicity of the listed oncotherapeutic treatment modalities, which is achievable by probiotics administration of fecal transplant. On the other hand, a dysbiotic state would lead to increased toxicity and decreased efficacy of oncotherapy.
Studying microbiota effects and interaction with oncotherapeutic treatment modalities used in colorectal management appears to be a plausible way to investigate the variable treatment outcomes observed in colorectal cancer patients and a potential way to modulate and improve current therapy.
Chemotherapy and microbiota interactions
Gut microbiota play an important role in the metabolism and functionality of medications, including chemotherapeutic agents used in the treatment of colorectal cancer like 5-fluorouracil, oxaliplatin and irinotecan10. Chemotherapy regimens used in colorectal cancer have a narrow therapeutic window with a high toxicity profile more pronounced in the gastrointestinal tract. This toxicity limits their dosing and efficacy. Microbiota can modify the toxicity profile of most chemotherapeutic drugs including drugs used in colorectal cancer through their effects on drug absorption, breakdown and toxicity11.
Microbiota effects on chemotherapy:
Toxicity
A study on rat models showed increased incidence of mucositis among methotrexate users that had lower abundance of anaerobes and Streptococci in their natural flora12.
Muramyl dipeptide of gut microbiota have been found to prevent the mucositis that is a well-known toxicity of doxorubicin, and any changes in the microbiota can increase the risk of these side effects13.
Efficacy and tumor suppression
Some of the effects microbiota exerts on chemotherapy are synergistic and adds up to promote tumor suppression. Oxaliplatin activity is augmented by myeloid cell activation from microbial dysbiosis, cyclophosphamide efficacy is improved by bacterial translocation and the resulting T-helper 17 cell activation, and several available immunotherapeutic agents are facilitated by the T-cell activation caused by gut microbial community14. Mice model studies have shown that natural gut flora supports higher therapeutic efficacy from the use of oxaliplatin, cyclophosphamide and CpG oligodeoxynucleotides immunotherapy than gut microbiota that have been affected by antibiotic therapy and even germ-free intestinal environments7,15.
Microbiota can also negatively impact chemotherapeutic drugs efficacy. An example of microbial interaction that reduces the efficacy of chemotherapy includes metformin-induced bacterial enzyme inhibition which affects 5-FU activity.16 Also, alterations of gut microbiota seen post-antibiotic therapy have been shown to reduce cyclophosphamide and platinum based compounds such as oxaliplatin and cisplatin efficacy on tumors7,17.
Chemotherapy effects on microbiota
Chemotherapy causes an increase in the growth of Bacteroides species, Escherichia species, Enterococcus faecium growth, and reduces the growth of Clostridium IV and Clostridium XIVa, Firmicutes, Bifidobacterium species, Lactobacillus, Veillonella and Faecalibacterium prausnitzii18,19.
5-FU regimens showed increased facultative gram negative bacteria in numerous samples from across the gastrointestinal tract and also showed increased anaerobic bacteria growth in the oral cavity20.
Effects of irinotecan on intestinal microbiota have been studied on rat models. Significant changes were found throughout the gastrointestinal flora, with most extensive changes found in the colon. Increased abundance of Clostridium I, Clostridium XI, Enterobacteriaceae, Escherichia species and Staphylococcus species were found in the jejunum and colon, increased Enterococcus and Serratia species were found in the colon and only increased Escherichia coli was found in the feces21.
Disproportionate expansion of dysbiotic microbiota is seen in the setting of cytotoxic chemotherapy and is associated with a high risk of microbial invasion into the systemic circulation leading to higher morbidity and mortality in colorectal cancer patients22. A type of microbiota called commensal bacteria interacts with TLR and Nuclear Factor kappa B signaling pathways providing a protective effect on intestinal integrity by supporting the development of an innate immune response. Both, the activation of these signaling pathways and the components of innate immunity in the gut are crucial in maintaining barrier function, protecting the gut against injury and fostering mucosal repair. Exposure to chemotherapy impacts the microbial equilibrium, by hampering the growth of commensal bacteria, resulting into negative effect on barrier function, intestinal integrity and repair pathways. As chemotherapy regimens damages the intestinal integrity, it is very possible that the myenteric plexus nerves and peripheral nerve endings become injured as these resides outside the blood-brain barrier. Accordingly, a full understanding of the role chemotherapy-induced intestinal permeability plays in gut-brain axis dysregulation will require further exploratory research trials22.
Probiotics and supplementation
Intestinal mucositis is one of the most common side effects of (5-FU)-based chemotherapy, including FOLFOX (5-FU, leucovorin and oxaliplatin) and FOLFIRI (5FU, leucovorin and irinotecan), which is commonly used as the standard of care front and second line regimens in the management of colorectal cancer.
Several studies demonstrated that administration of probiotics was able to mitigate chemotherapy induced intestinal mucositis. Chen et al demonstrated that administration of oral Lactobacillus probiotic (Lcr35) suppresses FOLFOX-induced mucosal injury in mouse models of colorectal cancer23.
Another studied example is the increased incidence of mucositis in patients treated with irinotecan and have a high abundance of bacteria with β-glucuronidase activity. In mouse models, oral supplementation of competing natural flora bacteria and 5-fluorouracil reduced the host mortality significantly24. A regimen of amoxapine and ciprofloxacin to reduce bacterial β-glucuronidase activity have also been able to reduce the incidence and morbidity of irinotecan associated mucositis25.
One randomized controlled trial has shown a significant reduction in the incidence of severe diarrhea in patients on 5-FU based chemotherapy regimens when they were given probiotic supplementation26.
Based on the grounds that probiotics are able to attenuate chemotherapy induced intestinal mucositis, current management of colorectal cancer patients undergoing chemotherapy should consider probiotic supplementation. Prospective larger controlled trials are needed to validate this clinical approach.
Radiotherapy and microbiota
Effects of microbiota on radiotherapy have been investigated in recent studies which have found that changes in gut microbiota are an important causative factor in the adverse effects of radiation enteropathy11.
The altered oral microbes in nasopharyngeal carcinoma patients have been correlated to more severe radiation induced mucositis.27 Studies have reported a decrease in Firmicutes species, increase in Fusobacterium species, and an increase in unclassified bacteria19. Streptococcus mitis increased significantly in nasopharyngeal carcinoma (NPC) patients after irradiation27.
Numerous studies have proved that radiotherapy causes major changes in gut microbial community28–30. Radiotherapy reduces the variation in gastrointestinal and colonic microbiota20. This improved uniformity was seen amongst gastrointestinal and gynecologic cancer patients who have diarrhea post-irradiation and those who don’t28,30. Patients who have radiation induced diarrhea have a greater change in the gut microbiota community than their peers and hence, gut microbiota are an essential protective factor in post-irradiation diarrhea30,31. Those who experienced diarrhea were found to have increased Bacteroides, Dialister, Veillonella and unclassified bacterial species and reduced Clostridium XI and XVIII, Faecalibacterium, Oscillibacter, Parabacteroides and Prevotella19,30. There is also evidence to suggest that patients undergoing radiation therapy have a high incidence of Clostridium difficile infection with a high mortality32. Modulation of colonic microbiota to protect against radiation induced colitis and diarrhea provides a great window of opportunity for future research in the colorectal cancer population.
Radiation-microbe interactions have also been proven to have positive effects on oncotherapy. Myeloablative radiation therapy causes translocation of host intestinal microbes that have been useful in boosting cytotoxic T cell responses in patients with metastatic melanoma and hematopoietic stem cell transplants33.
Radiation induced dysbiosis has been proven to contribute to colitis in mouse models. Colitis was localized to the area irradiated with maximum changes observed six weeks post-irradiation. Marked upregulation of proinflammatory cytokines such as IL-1β was noted. The post-irradiation microbial community was proven to cause greater bowel damage and colitis29. Numerous Gram-negative bacteria, such as facultative Proteobacteria anaerobes increase significantly as mucositis worsens. Toxic bacterial components are expected to play a role in the aggravation of mucositis. Mild mucositis patients were found to have more diverse microbiota and lower Actinobacillus growth, while severe mucositis patients had higher Streptococcus growth. Changes in oral microbial community correlated with the progression and aggravation of radiotherapy-induced mucositis in the nasopharyngeal carcinoma (NPC) patients.
Microbial profiling can be a useful step in the prediction, screening and evaluation of radiotherapy-induced enteropathy in colorectal cancer patients to predict susceptible patient groups. Microbial transplants can also be a useful step in the prevention of adverse effects and the reduction of radiotherapy related morbidity34,27.
Immunotherapy and microbiota
Immunotherapy now has numerous approved agents to treat variety of malignancies including colorectal cancer35. As we are learning further about the natural anti-tumor host immune response, we are looking for potential ways to enhance and potentiate this response.
The response to immunotherapy has been shown to have a variable outcome based on the gastrointestinal and colonic microbiota. Some of the microbial interactions are helpful in boosting the immunotherapy efficacy. Several available immunotherapeutic agents have been shown to be facilitated by the T-cell activation caused by gut microbial community14. In mice models and human subjects, the CTLA-4 blocker Ipilimumab has been shown to affect the growth of Bacteroides fragilis, Bacteroides thetaiotaomicron and Burkholderiales. Ipilimumab’s observed effect on microbiota is synergistic and helps control tumor growth36.
Numerous studies looked into microbiota effects on PD-1 blockade therapy in melanoma patients. Patients with abundant concentrations of Akkermansia muciniphila, Ruminococcaceae family, Bifidobacterium longum, Collinsella aerofaciens and Enterococcus faecium showed a higher clinical response to anti-PD-1 therapy and those with altered gut microbiota from chronic antibiotic usage or other gut altering events, failed to respond to anti-PD-1 treatment37,38.
The microbiome-immunotherapy interaction can also influence medication toxicity. In mouse models, mice on methotrexate therapy showed higher incidence of mucositis if they had recent antibiotic intervention. However, this can be reversed when Toll-like receptor 2 ligand is administered39.
One approach to boost the antitumor immunity through modulation of microbiome is oral and fecal microbial transplantation. This could potentially improve patient response to therapy and clinical outcome. Immune profiling can be a helpful step in evaluating patients to assure high therapeutic response9,40.
Oral Bifidobacterium was useful in improving anti-PD L1 response in rat models. This combination of therapy was nearly 100% effective in tumor control41. In CpG oligodeoxynucleotides immunotherapy, better responses to treatment have been seen in patients with Alistipes shahii in their intestinal microbial community than patients with microbial dysbiosis post-antibiotics or even a germ-free intestinal environment. This enhanced efficacy can be achieved in all patients by bacterial profiling and Alistipes shahii supplementation7,15. On assessment of microbial profiles, it was found that patients on anti-CTLA-4 treatments who had higher Bacteroides species in their gut microbiota also exhibited an increased chance of resistance to colitis. Hence, when Bacteroides species were supplemented in mouse models, an increased protection to colitis was seen amongst those patients39.
Microbiota can be potentially harnessed to boost the anti-tumor immune response and immunotherapeutic drug efficacy in colorectal cancer patients, but especially in non-responder patients who did not show any clinical benefits on immunotherapy.
Conclusion
In conclusion, this review highlighted some of the important aspects of microbiota diverse interactions with the current treatment modalities used in colorectal cancer from chemoradiotherapy to immunotherapy. The current body of evidence supports that their effects can range from achieving a better tolerance and an optimal efficacious dose of chemotherapy without obstacles of toxicity to reducing radiotherapy unwanted side effects to boosting efficacy of the antitumor immune response initiated by immunotherapy. Implementing microbiota in the current scheme of therapy in colorectal cancer seems promising in improving oncotherapy efficacy and related morbidity and mortality as well as adopting a more personalized approach that takes into account the great inter-individual diversity of host microbiota. As most of the current studies available are done on mice or rat models, future studies on humans would be the next step in our progress towards developing a better understanding of the relationship between gut microbiome and oncotherapeutic modalities in colorectal cancer.
Footnotes
Conflict of Interest
Azhar Saeed, Fariha Faruk Eshrat, Shahid Umar, and Anwaar Saeed declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Rizkallah MR, Saad R, Aziz RK. The Human Microbiome Project, personalized medicine and the birth of pharmacomicrobiomics. Curr Pharmacogenomics Pers Med. 2010;8:182–193. [Google Scholar]
- 2.Evans JM, Morris LS, Marchesi JR. The gut microbiome: the role of a virtual organ in the endocrinology of the host. J Endocrinol. 2013;218:R37–R47. [DOI] [PubMed] [Google Scholar]
- 3.Claesson MJ, Cusack S, Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci. 2011;108:4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwabe RF and Jobin C (2013) The microbiome and cancer Nat Rev Cancer 13 800 10.1038/nrc3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagnière J, Raisch J, Veziant J, et al. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol. 2016. January 14;22(2):501–18.• Comprehensive review article highlighting the impact of Gut microbiota on colon cancer development and outcome.
- 6.Haiser HJ & Turnbaugh PJ Developing a metagenomic view of xenobiotic metabolism. Pharmacol. Res 69, 21–31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdak-Rothkamm S, Rothkamm K (2018) Radiation-induced bystander and systemic effects serve as a unifying model system for genotoxic stress responses. Reviews in Mutation Research 778(4) 13–22 [DOI] [PubMed] [Google Scholar]
- 9.Zitvogel L, Galluzzi L, Viaud S, et al. Cancer and the gut microbiota: An unexpected link. Sci Transl Med. 2015;7:1–10. doi: 10.1126/scitranslmed.3010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015. November 5;1:15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira MR, Muls A, and Dearnaley DP, et al. (2014) Microbiota and radiation-induced bowel toxicity: lessons from inflammatory bowel disease for the radiation oncologist Lancet Oncol 15(3) e139–e147 10.1016/S1470-2045(13)70504-7. [DOI] [PubMed] [Google Scholar]
- 12.Montassier E, Batard E, and Massart S, et al. (2014) 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation Microb Ecol 67 690–699. [DOI] [PubMed] [Google Scholar]
- 13.Nigro G, Rossi R, Commere PH, et al. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe 15, 792–798 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Daillère R, Vétizou M, and Waldschmitt N, et al. (2016) Enterococcus hirae and barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects Immunity 45 931–943 10.1016/j.immuni.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 15.Iida N, Dzutsev A, Stewart CA, et al. (2013). Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970. 10.1126/science.1240527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott TA, Quintaneiro LM, Norvaisas P, et al. Host-microbe co-metabolism dictates cancer drug efficacy in C. elegans. Cell. 2017;169:442–456.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwielehner J, Lassl C, Hippe B, et al. (2011). Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS ONE 6:e28654 10.1371/journal.pone.0028654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stringer AM, Gibson RJ, Logan RM, et al. (2007). Chemotherapy-induced diarrhea is associated with changes in the luminal environment in the DA rat. Exp. Biol. Med. (Maywood) 232, 96–106. 10.3181/00379727-207-2320096 [DOI] [PubMed] [Google Scholar]
- 19.Manichanh C, Varela E, Martinez C, et al. (2008). The gut microbiota predispose to the pathophysiology of acute post-radiotherapy diarrhea. Am. J. Gastroenterol 103, 1754–1761. 10.1111/j.1572-0241.2008.01868.x [DOI] [PubMed] [Google Scholar]
- 20.Stringer AM, Gibson RJ, Bowen JM, et al. (2009). Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int. J. Exp. Pathol 90, 489–499. 10.1111/j.1365-2613.2009.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin XB, Dieleman LA, Ketabi A, et al. (2012). Irinotecan (CPT-11) Irinotecan (CPT-11)chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS ONE 7:e39764 10.1371/journal.pone.0039764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajic JE, et al. (2018). From the Bottom-Up: Chemotherapy and Gut-Brain Axis Dysregulation. Front Behav Neurosci. 2018; 12: 104.doi: 10.3389/fnbeh.2018.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CW, Liu CY, Lee HC, et al. Lactobacillus casei Variety rhamnosus Probiotic Preventively Attenuates 5-Fluorouracil/Oxaliplatin-Induced Intestinal Injury in a Syngeneic Colorectal Cancer Model. Front Microbiol. 2018. May 15;9:983.• An animal model study validating the positive protective impact of modulating the gut microbiome in the setting of 5FU based chemotherapy. Attenuation of intestinal injury seen as a result of Probiotic supplementation.
- 24.Din MO, Danino T, and Prindle A, et al. (2016) Synchronized cycles of bacterial lysis for in vivo delivery Nature 536 81–85 10.1038/nature18930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kodawara T, Higashi T, and Negoro Y, et al. (2016) The inhibitory effect of ciprofloxacin on the β-glucuronidase-mediated deconjugation of the irinotecan metabolite SN-38-G Basic Clin Pharmacol Toxicol 118 333–337 10.1111/bcpt.12511 [DOI] [PubMed] [Google Scholar]
- 26.Osterlund P, Ruotsalainen T, Korpela R, et al. (2007) Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study Br J Cancer 97(8) 1028–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu XX, Yang XJ, Chao YL, et al. (2017). The Potential Effect of Oral Microbiota in the Prediction of Mucositis During Radiotherapy for Nasopharyngeal Carcinoma. EBioMedicine. 2017 April;18:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nam YD; Kim HJ; Seo JG; et al. Impact of Pelvic Radiotherapy on Gut Microbiota of Gynecological Cancer Patients Revealed by Massive Pyrosequencing. PLoS ONE 2013, 8, e82659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerassy-Vainberg S; Blatt A; Danin-Poleg Y; et al. Radiation Induces Proinflammatory Dysbiosis: Transmission of Inflammatory Susceptibility by Host Cytokine Induction. Gut 2018, 67, 97–107.• This study illustrates the impact of radiation on shifting the microbial niche toward dysbiosis. This was shown to highly correlating with radiation induced intestinal inflammation.
- 30.Wang A; Ling Z; Yang Z; et al. Gut Microbial Dysbiosis May Predict Diarrhea and Fatigue in Patients Undergoing Pelvic Cancer Radiotherapy: A Pilot Study. PLoS ONE 2015, 10, e0126312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montassier E, Batard E, Massart S, et al. (2014). 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation. Microb. Ecol 67, 690–699. 10.1007/s00248-013-0355-4 [DOI] [PubMed] [Google Scholar]
- 32.Neemann K, Freifeld A (2017) Clostridium difficile-Associated Diarrhea in the Oncology Patient Journal of Oncology Practice 13(1) 25–30 10.1200/JOP.2016.018614 [DOI] [PubMed] [Google Scholar]
- 33.Dudley ME et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol 26, 5233–5239 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira MR, Muls A, Dearnaley DP, Andreyev HJ. (2014). Microbiota and radiation-induced bowel toxicity: lessons from inflammatory bowel disease for the radiation oncologist. Lancet Oncol. doi: [DOI] [PubMed] [Google Scholar]
- 35.Maus MV, Fraietta JA, Levine BL, et al. Adoptive Immunotherapy for Cancer or Viruses. Annu Rev Immunol. 2014. 10.1146/annurev-immunol-032713-120136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vétizou M, Pitt JM, Daillère R, et al. (2015). Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084. 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706.• This study provides backing evidence that quality and quantity of the gut microbiome impact responses to check point inhibitors.
- 38.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frank M, Hennenberg EM, and Eyking A, et al. (2015) Europe PMC Funders Group Europe PMC Funders Author Manuscripts TLR signaling modulates side effects of anticancer therapy in the small intestine J Immunol 194 1983–1995 10.4049/jimmunol.1402481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sivan A, Corrales L, Hubert N, et al. (2015). Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089. 10.1126/science.aac4255 [DOI] [PMC free article] [PubMed] [Google Scholar]