Abstract
Osteoarthritis is a chronic degenerative articular disorder. Formation of bone spurs, synovial inflammation, loss of cartilage, and underlying bone restructuring have been reported to be the main pathologic characteristics of osteoarthritis symptoms. The onset and progression of osteoarthritis are attributed to various inflammatory cytokines in joint tissues and fluids that are produced by chondrocytes and/or interact with chondrocytes, as well as to low-grade inflammation in intra-articular tissues. Disruption of the equilibrium between the synthesis and degradation of the cartilage of the joint is the major cause of osteoarthritis. Hence, developing a promising pharmacological tool to restore the equilibrium between the synthesis and degradation of osteoarthritic joint cartilage can be a useful strategy for effectively managing osteoarthritis. In this review, we provide an overview of the research results pertaining to the search for a novel candidate agent for osteoarthritis management via restoration of the equilibrium between cartilage synthesis and degradation. We especially focused on investigations of medicinal plants and natural products derived from them to shed light on the potential pharmacotherapy of osteoarthritis.
Keywords: Osteoarthritis, Pharmacotherapy, Natural products
INTRODUCTION
Osteoarthritis can be defined as a type of articular diseases resulting from the destruction of articular cartilage and subchondral bone. It is the most common degenerative joint disease, especially in elderly people. The joint stiffness and pain have been known to be the most common symptoms of osteoarthritis and inflammation in synovial tissues, the formation of bone spurs, joint cartilage degeneration, and changes in the underlying bone are its pathological characteristics (Mankin, 1982; Aigner and McKenna, 2002). Mechanical stress, injury of the articular structure, inflammation, oxidative stress, and older age were reported to be the etiological factors of osteoarthritis. However, an effective and definitive method for the cure or, at least, management of osteoarthritis has not yet been developed, since the molecular mechanism of the destruction of articular tissues has not been clearly elucidated (Lim et al., 2017; Min et al., 2018; Yoo et al., 2018). To date, the final goal in the management of osteoarthritis is to regulate symptoms including pain, improve the quality of life, and mitigate disability (Blagojevic et al., 2010). Currently, pharmacological management and non-pharmacological management are used for the regulation of osteoarthritis (Table 1). For the non-pharmacological management of osteoarthritis, body weight loss, exercise, and articular surgery are recommended (Anandacoomarasamy and March, 2010). The pharmacological interventions for osteoarthritis include the use of nonsteroidal anti-inflammatory drugs (NSAIDs), symptomatic slow-acting drugs for osteoarthritis, analgesics, putative disease-modifying agents, bone-acting agents, agents for intraarticular injection such as corticosteroids and hyaluronic acid (Cho et al., 2018; Gregori et al., 2018; Hwang et al., 2018). However, these agents are ineffective against the root-cause of osteoarthritis, cause a multitude of severe side effects, and are inadequate for the long-term management of osteoarthritis (Shen and Gatti, 2013; Lee et al., 2017). The onset and progression of osteoarthritis are attributed to various inflammatory cytokines in joint tissues and fluids that are produced by chondrocytes and/or interact with chondrocytes, as well as to low-grade inflammation in intra-articular tissues (Aigner and McKenna, 2002). Disruption of the equilibrium between the synthesis and degradation of the cartilage of the joint is the major cause of osteoarthritis (Mankin, 1982). Thus, developing a promising pharmacological tool to restore the equilibrium between the synthesis and degradation of osteoarthritic joint cartilage can be a useful strategy for effective osteoarthritis management. In this review, we attempted to summarize the results of research for searching a novel candidate agent that could regulate osteoarthritis by restoring the equilibrium between cartilage synthesis and degradation. We particularly focused on studies of medicinal plants and natural products derived from them, in order to shed light on the potential pharmacotherapy of osteoarthritis.
Table 1.
The management of osteoarthritis
| Pharmacological management | Nonsteroidal anti-inflammatory drugs (NSAIDs) Symptomatic slow-acting drugs in osteoarthritis Analgesics Putative disease-modifying agents Bone-acting agents Agents for intraarticular injection including corticosteroids and hyaluronic acid |
| Non-pharmacological management | Body weight loss Taking exercises Articular surgery |
CURRENT CONVENTIONAL PHARMACOTHERAPY FOR THE MANAGEMENT OF OSTEOARTHRITIS
Thus far, NSAIDs, symptomatic slow-acting drugs for osteoarthritis, analgesics, putative disease-modifying agents, bone-acting agents, and agents for intra-articular injection including corticosteroids and hyaluronic acid have been used as pharmacological agents for the management of osteoarthritis. However, it has been reported that these agents are not efficacious against the root-cause of osteoarthritis, cause many severe side effects, and are not adequate for the long-term management of osteoarthritis (Shen and Gatti, 2013; Lee et al., 2017).
NSAIDs
NSAIDs are the most frequently used agents for the management of osteoarthritis. They showed moderate activity against osteoarthritic pain; however, it is recommended that NSAIDs be used intermittently or for a short period. NSAIDs can be classified as cyclooxygenase-2 (COX-2)-selective agents and non-selective agents. COX-2-selective agents are celecoxib, meloxicam, rofecoxib, valdecoxib, polmacoxib, and etoricoxib. Non-selective COX inhibitors are diclofenac, diflunisal, etodolac, flurbiprofen, ibuprofen, indomethacin, ketoprofen, nabumetone, naproxen, oxaprozin, piroxicam, sulindac, tolmetin, azapropazone, carprofen, meclofenamate, tenoxicam, etofenamate, nimesulide, and tiaprofenic acid. Among these, diclofenac, naproxen, celecoxib, rofecoxib, and etoricoxib have been frequently used to manage osteoarthritic pain (Gore et al., 2012).
Symptomatic slow-acting drugs in osteoarthritis
Glucosamine hydrochloride, chondroitin sulfate, diacerein, and glucosamine sulfate are classified as symptomatic slow-acting drugs for osteoarthritis. Prescription-grade chondroitin sulfate and glucosamine sulfate are recommended as first-line agents for the pharmacological management of genicular osteoarthritis. These agents have been reported to show an improvement in physical function and pain in osteoarthritis (Bruyere et al., 2014).
Analgesics
Acetaminophen, tramadol, and opioids including oxycodone are used to control the pain in osteoarthritis during a short period, although these agents are not associated with an improvement in pain in the long term (Hochberg et al., 2012; McAlindon et al., 2014).
Putative disease-modifying agents
Sprifermin, doxycycline, PG-116800 (a matrix metalloproteinase inhibitor), and cindunistat can be classified as putative disease-modifying agents for osteoarthritis. While clinical trials to prove the efficacy of these agents are ongoing, thus far, these agents have not shown significant improvements in structural changes in the joint (Pavelka et al., 2003).
Bone-acting agents
Risedronate, zoledronic acid, strontium ranelate, calcitonin, and vitamin D are classified as bone-acting agents for the regulation of osteoarthritis. They are antiresorptive agents or bone-forming agents. Bone-acting agents showed some potential benefit in the turnover of subchondral bone, although these agents did not show a significant improvement in structural changes of the joint (Baker-LePain and Lane, 2012).
Agents for intra-articular injection
Corticosteroids including triamcinolone, betamethasone, and methylprednisolone and hyaluronic acid are classified as agents for intra-articular injection. Generally, to regulate the acute exacerbation of genicular osteoarthritis, intra-articular injection of corticosteroids is recommended. During the initial 2 to 3 weeks of intervention, intra-articular injection of corticosteroids showed a greater beneficial effect. Furthermore, during follow-up periods of 3 and 6 months, intra-articular injection of hyaluronic acid showed a greater beneficial effect. The combinatorial administration of corticosteroids and hyaluronic acid by intra-articular injection showed a moderate beneficial effect on the pathophysiology of osteoarthritis. However, for long-term pain, intra-articular injection of hyaluronic acid did not show a significant improvement (Bannuru et al., 2009).
SEARCH FOR NOVEL CANDIDATE AGENTS FOR REGULATING OSTEOARTHRITIS FROM MEDICINAL PLANTS AND NATURAL PRODUCTS DERIVED FROM THEM
Inflammation has been reported to be involved in the loss of articular cartilage in osteoarthritis and this mild inflammatory reaction leads to the development of the disease (Kullich et al., 2007). TNF-α and IL-1β, the main catabolic inflammatory cytokines, play a pivotal role in the process of articular cartilage degradation. These cytokines increase the expression and catabolic activity of matrix metalloproteinases (MMPs) on articular cartilage destruction via activation of the NF-κB signaling pathway. Further, the activated NF-κB signaling pathway and other intracellular signaling pathways in concert aggravate the cartilage degeneration (Dean et al., 1989; Birkedal-Hansen et al., 1993). The extracellular matrix present in the joint cartilage regulates the physiological function and metabolism of chondrocytes. Collagen type II and proteoglycans including hyaluronic acid, glycosaminoglycan, and chondroitin sulfate consist of the extracellular matrix. Chondrocyte death (apoptosis), the induction of extracellular matrix degradation, and compromised production of the extracellular matrix might provoke articular cartilage destruction in osteoarthritis (Garnero et al., 2000; Burrage et al., 2006). Based on this information (Table 2), in the present section of this review, we provide an overview of the results of many studies aimed at searching for novel candidate agents for regulating osteoarthritis through control of the equilibrium between the synthesis and degradation of cartilage, especially from medicinal plants and natural products derived from them (Table 3). The medicinal plants and natural products derived from them are listed according to alphabetical order hereon.
Table 2.
The major drug targets of some natural products
| Induction of extracellular matrix degradation by matrix metalloproteinases |
| Compromised production of the extracellular matrix |
| The apoptosis and proliferation of chondrocytes |
| Inflammation and oxidative stress generated in articular tissues |
Table 3.
The list of medicinal plants and natural products showing the effect on the pathophysiology of osteoarthritis
Achyranthes bidentata
Polysaccharides contained in Achyranthes bidentata induced the transition of the G1/S phases of the cell cycle and expression of collagen type II in chondrocytes. They stimulated the expression of CDK6, CDK4, and cyclin D1, promoting the cell cycle and proliferation of chondrocytes (Weng et al., 2014).
Aconitum carmichaelii
Aconitum carmichaelii showed preventive activity against the decrease in bone density and degeneration of cartilage. It stimulated the proliferation of chondrocytes (Tong et al., 2014).
Arnica montana
In a rat model of collagen-induced arthritis (CIA), the total extract of Arnica montana showed an anti-inflammatory effect, in which was reflected by decreased levels of IL-6, NO, IL-1β, TNF-α, and IL-12. The extract also showed an antioxidative effect (Sharma et al., 2016).
Astaxanthin
Astaxanthin, a carotenoid, showed anti-inflammatory and antioxidative effects on cartilage. It suppressed the expression of MMPs including MMP-13, MMP-3, and MMP-1. Astaxanthin also inhibited the phosphorylation of p38 mitogen-activated protein kinase (MAPK) and p44/42 MAPK, and the degradation of inhibitory kappa Bα (IκBα) in interleukin-1β (IL-1β)-stimulated chondrocytes (Chen et al., 2014a).
Apigenin
Apigenin, an anti-inflammatory flavonoid compound, was reported to suppress the gene expression of MMPs including MMP-1, MMP-13, MMP-3, a disintegrin and metalloproteinase with thrombospondin motif-5 (ADAMTS-5), and ADAMTS-4, in primary cultured rabbit chondrocytes. It also decreased the proteolytic activity and secretion of MMP-3. Furthermore, intra-articular injection of apigenin inhibited the in vivo production of MMP-3 protein in the rat knee joint (Park et al., 2016).
Apis mellifera
Venom from the bee species, Apis mellifera, was reported to suppress the expression of MMP-8 and MMP-1 stimulated by TNF-α by affecting the NF-κB signaling pathway. Furthermore, bee venom blocked the TNF-α-induced phosphorylation of ERK1/2, Akt, and JNK (Jeong et al., 2016).
Astragalin
The antioxidative and anti-inflammatory flavonoid compound, kaempferol-3-O-glucopyranoside, also known as astragalin, was reported to inhibit the IL-1β-stimulated activation of NF-κB and MAPK in chondrocytes in patients with osteoarthritis. Astragalin also decreased the production of prostaglandin E2 (PGE2) and nitric oxide (NO) and the expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (Ma et al., 2015).
Aucubin
Aucubin, a natural anti-inflammatory product derived from diverse medicinal plants including Eucommia ulmoides, suppressed the inflammatory response through blocking the phosphorylation and degradation of IκB and the translocation of NF-κB p65 in rat articular chondrocytes stimulated by IL-1β. Furthermore, the compound decreased the production of NO and expression of iNOS, COX-2, and MMPs (Wang et al., 2015).
Baicalein
Baicalein, a natural product derived from Scutellaria baicalensis, has been reported to inhibit the expression of MMP-13 and MMP-3 in human chondrocytes. It also stimulated the production of glycosaminoglycan (GAG) and collagen type II through by affecting the phosphorylation of ERK and p38 (Zhang et al., 2014).
Bauhinia championii
Polysaccharides present in Bauhinia championii were reported to stimulate the proliferation of chondrocytes and promote the transition of the G1/S phases of the cell cycle. These polysaccharides activated the intracellular signaling pathways pivotal in the maintenance of articular cartilage (Li et al., 2013).
Bavachin
Bavachin, a phytoestrogen present in a medicinal plant Psoralea corylifolia, was reported to protect against IL-1β-stimulated cartilage impairment via suppressing the degradation of IκBα and nuclear translocation of NF-κB (Cheng et al., 2010).
Berberine
Berberine, an anti-inflammatory natural product derived from Rhizoma coptidis, has been reported to block the degradation of cartilage and suppress NF-κB signaling pathways in a human chondrosarcoma cell line. Furthermore, it showed a potential chondroprotective effect through inhibiting the apoptosis of chondrocytes and the gene and protein expression of MMP-13, MMP-3, and MMP-1 (Wu et al., 2013; Zhao et al., 2014; Liu et al., 2015; Zhou et al., 2015).
Betulin
Betulin, a natural anti-inflammatory compound isolated from Betulae Cortex, inhibited IL-1β-induced gene expression of MMP-13, MMP-1, and MMP-3 although it stimulated type II collagen gene expression in primary cultured chondrocytes. Furthermore, intra-articular injection of betulin blocked in vivo MMP-3 production in knee joint chondrocytes (Ra et al., 2017).
Biochanin A
Biochanin A contained in red clover was reported to block IL-1β-induced expression of MMPs and restore the compromised expression of TIMP-1 via affecting the NF-κB signaling pathway in chondrocytes (Wu et al., 2014).
Boswellia serrata
A preparation of Boswellia serrata (BS) extract has been reported to inhibit the degeneration of cartilage by MMP-13, MMP-9, and MMP-13 and inflammation in arthritis by suppressing the functions of NO, COX-2, PGE2, and intercellular adhesion molecule-1 (ICAM-1) (Blain et al., 2009; Sengupta et al., 2010). In an animal model of CIA, BS extract showed antioxidative and anti-inflammatory effects (Umar et al., 2014). In a clinical trial, treatment with the extract decreased pain and increased functionality in patients with knee osteoarthritis (Vishal et al., 2011). Boswellic acids, which are natural products isolated from BS, were reported to block anti-inflammatory activity by inhibiting the NF-κB signaling pathway, in experimental models of arthritic inflammation (Khajuria et al., 2008; Ammon, 2010).
Catechins
Catechins, the main polyphenolic compounds present in tea, showed potential anti-arthritic effects, and epigallocatechin-3-gallate, a representative catechin, has been reported to exert chondroprotective activity by inhibiting IL-1β-stimulated expression of IL-8, PGE2, and COX-2, in human synovial fibroblasts (Huang et al., 2010). In an animal model, epigallocatechin-3-gallate decreased the levels of MMP-8, MMP-13, ADAMTS-5, MMP-1, and MMP-3 in articular cartilage (Leong et al., 2014).
Celastrol
In primary human osteoarthritic chondrocytes, celastrol, a natural product inhibiting heat shock protein (HSP) 90β, showed a suppressive effect on IL-1β-stimulated expression of MMP-13, MMP-3, MMP-1, COX-2, and iNOS-2 (Ding et al., 2013).
Clematis chinensis
In an animal model of osteoarthritis established by the intra-articular injection of monosodium iodoacetate, a saponin fraction isolated from Clematis chinensis, showed an inhibitory effect against cartilage damage and destruction of the joint by suppressing the decomposition of the extracellular matrix and chondrocyte injury (Wu et al., 2010).
Crocin
Crocin, a natural compound derived from Crocus sativus, was reported to block both the expression of MMP-13, MMP-3, and MMP-11 by inhibiting the NF-κB signaling pathway in articular chondrocytes and the degeneration of cartilage in vivo (Ding et al., 2010).
Curcumin
Curcumin, a natural compound isolated from Curcuma longa (CL), is a known anti-inflammatory agent and regulates diverse inflammatory statuses including osteoarthritis. The compound showed ameliorative effects on inflammation of the joint in an animal model of arthritis (Nonose et al., 2014). A preparation of CL total extract exerted an inhibitory effect against periarticular tissue damage and joint inflammation in an in vivo arthritis model via inhibiting the NF-κB signaling pathway (Funk et al., 2006).
Delphinidin
In osteoarthritic chondrocytes, delphinidin, an antioxidative anthocyanidin found in various vegetables and fruits, blocked the expression of COX-2 and PGE2. Delphinidin also suppressed IL-1β-induced NF-κB signaling by affecting IRAK-1 phosphorylation (Haseeb et al., 2013).
Eucommia ulmoides
An aqueous extract of Eucommia ulmoides showed antiosteoarthritic effects, based on histopathological examination of articular tissues and inhibitory regulation of serum and synovial fluid levels of MMP-13, MMP-3, and MMP-1 (Lu et al., 2013; Xie et al., 2015).
Ferulic acid
A natural product present in Angelica sinensis, ferulic acid, showed the potency of an anti-osteoarthritic agent by blocking the hydrogen peroxide (H2O2)-induced expression of MMP-13 and MMP-1 in chondrocytes (Chen et al., 2010).
GCSB-5
GCSB-5 is a standardized extract from a mixture of six herbs including Saposhnikovia divaricata, Achyranthes japonica, Acanthopanax sessiliflorus, Cibotium barometz, Glycine max, and Eucommia ulmoides, developed in South Korea for the regulation of osteoarthritis in knee joint (Cho et al., 2016). In an animal model of osteoarthritis established using monosodium iodoacetate, intra-articular injection of GCSB-5 blocked the production of anti-type II collagen antibody and PGE2, regulating the balance of cytokines and inflammatory mediators (Kim et al., 2016). In a clinical trial, the safety and efficacy of GCSB-5 were comparable to those of celecoxib, a selective COX-2 inhibitor, in the treatment of knee joint osteoarthritis (Park et al., 2013).
Gentiopicroside
Gentiopicroside derived from Gentiana macrophylla suppressed the IL-1β-stimulated expression of MMPs and the phosphorylation of JNK, ERK, and p38, in murine articular chondrocytes. Furthermore, it promoted type II collagen production (Zhao et al., 2015).
Ginsenosides
Ginsenosides isolated from Panax ginseng showed various biological effects. Ginsenoside Rb1, a subtype of ginsenosides, suppressed the levels of MMP-13 and MMP-1, NO, iNOS, IL-1β, and TNF-α, and promoted the expression of type II collagen (Kim et al., 2012; Cheng et al., 2013). Ginsenosides Rg1, Rg3, Rg5, Rk1, Rf, Rd, Rc, and F4 were reported to exert chondroprotective effect (Huang et al., 2014; Lee et al., 2014).
Harpagophytum procumbens
Harpagophytum procumbens has been utilized as a folk medicine for managing musculoskeletal degenerative diseases including osteoarthritis. The total extract of Harpagophytum procumbens exerted chondroprotective effects via blocking the activity of MMPs and elastase and the production of inflammation mediators including IL-1β and TNF-α (Fiebich et al., 2001). In a clinical trial, diverse HP extracts showed ameliorative effects on limited movement and pain in patients with osteoarthritis of the hip and knee (Chantre et al., 2000; Chrubasik et al., 2002; Wegener and Lupke, 2003).
Honokiol
Honokiol, a major natural compound isolated from Magnolia officinalis, blocked IL-1β-stimulated expression of MMP-13, IL-6, iNOS, NO, COX-2, and PGE2 via the NF-κB signaling pathway (Chen et al., 2014b).
Icariin
Icariin, a compound derived from Epimedium pubescens, was reported to inhibit the IL-1β-stimulated expression of MMP-13 in chondrocytes. Furthermore, it enhanced extracellular matrix synthesis and showed chondroprotective effects (Li et al., 2012).
Luteolin
Luteolin, a flavonoid compound derived from Lonicerae flos, blocked IL-1β-stimulated gene expression, secretion, and enzyme activity of MMP-3 in cultured articular chondrocytes. It inhibited the gene expression levels of ADAMTS-4, MMP-13, MMP-1, and ADAMTS-5, and affected the in vivo production of MMP-3 protein in the rat knee joint (Kang et al., 2014).
Monotropein
Monotropein, a compound present in Morinda officinalis, was reported to block IL-1β-stimulated expression of MMP-13 and MMP-3 in chondrocytes (Wang et al., 2014).
Morin
Morin, a flavonoid compound, has been reported to exert anti-inflammatory, antioxidative, and anticancer effects. Morin blocked IL-1β-stimulated expression of MMP-13 and MMP-3 and promoted the expression of TIMP-1 via suppression of the phosphorylation of ERK1/2 and p38 (Chen et al., 2012).
Oleanolic acid
Oleanolic acid, a triterpenoid compound present in various fruit and vegetables, promoted type II collagen gene expression and blocked the gene expression of ADAMTS-5, MMP-1, MMP-13, ADAMTS-4, and MMP-3. Furthermore, it decreased in vitro enzyme activity and in vivo production of MMP-3 (Kang et al., 2017).
Panax notoginseng
A preparation of Panax notoginseng (PN) extract suppressed the production of IL-1, iNOS, TNF-α, and MMP-13 in vitro (Chang et al., 2007).
PG201
PG201, a multi-component standardized extract of medicinal plants for managing the symptoms of osteoarthritis, showed a protective effect on the cartilage in an animal model of collagenase-induced arthritis (Shin et al., 2003; Park et al., 2005). It also showed a significant effect on osteoarthritis in a clinical trial (Ha et al., 2016).
Phellodendron amurense
Phellodendron amurense, a medicinal plant with immunostimulatory and anti-inflammatory properties, was reported to protect articular cartilage via blocking IL-1β-stimulated type II collagen degradation and proteoglycan release (Kim et al., 2011).
Pinocembrin
Pinocembrin contained in propolis showed a suppressive effect on MMP-13 and MMP-3 expression through affecting the NF-κB signaling pathway in human chondrocytes (Zhang et al., 2015).
Piperine
Piperine, a natural compound present in black pepper (Piper nigrum), has been reported to exert a suppressive effect on IL-1β-induced elevated levels of MMPs, COX-2, NO, PGE2, and iNOS through NF-κB signaling (Ying et al., 2013).
Prunetin
Prunetin, a natural product found in Glycyrrhiza glabra, inhibited the in vivo production of MMP-3 stimulated by IL-1β. It also blocked the gene expression, secretion, and enzyme activity of MMP-3 in primary cultured rabbit chondrocytes (Nam et al., 2016).
Resveratrol
Resveratrol, a well-known natural product derived from diverse plants including grapes, has been reported to suppress the expression of iNOS, COX-2, TNF-α, and IL-1β by blocking the NF-κB signaling pathway (Wang et al., 2012). It was reported that resveratrol blocked the gene expression and secretion of MMP-3 in rabbit chondrocytes. Furthermore, it suppressed IL-1β-stimulated gene expression of various MMPs via blocking of the phosphorylation of inhibitory kappa B kinase (IKK), phosphorylation and degradation of inhibitory kappa Bα (IκBα), and phosphorylation and nuclear translocation of NF-κB p65 in human chondrocytes (Kang et al., 2018).
Schisandrae Fructus
The ethanol extract of Schisandrae Fructus showed chondroprotective activity and inhibited the expression of COX-2, MMPs, and iNOS via suppressing the phosphorylation of JNK, p38, and ERK1/2, and NF-κB signaling, in human chondrocytes (Jeong et al., 2015).
Sinomenine
Sinomenine, a natural product derived from Sinomenium acutum, has been reported to decrease MMP-13 expression and glycosaminoglycan (GAG) release. It also increased TIMP-1 activity, thereby suppressing apoptosis of cells and fragmentation of DNA in chondrocytes (Ju et al., 2010a).
SKI306X
Kim et al. (2005) reported that SKI306X blocked the degradation of the matrix by suppressing the gene expression, secretion and enzyme activity of MMPs, in rabbit articular cartilage. In a double-blind, controlled clinical trial, SKI 306X, a standardized extract of a mixture of three medicinal plants including Trichosanthes kirilowii, Prunella vulgaris, and Clematis mandshurica, showed a pain-relieving effect without significant adverse effects, in knee osteoarthritis patients (Jung et al., 2001). In another clinical trial, SKI306X showed a protective effect on the cartilage in patients with knee osteoarthritis (Kim et al., 2017).
Symphytum officinalis
Topical administration of a preparation of Symphytum officinalis extract could regulate pain and articular mobility in knee osteoarthritis (Grube et al., 2007).
Tetramethylpyrazine
Tetramethylpyrazine present in Ligusticum wallichii has been reported to suppress the apoptosis of chondrocytes and the expression of iNOS, MMP-13, COX-2, and MMP-3 and promote the expression of collagen type II and TIMP-1 (Ju et al., 2010b; Liang et al., 2014).
Tetrandrine
Tetrandrine isolated from Stephania tetrandra has been reported to exert the chondroprotective activity via blocking IL-1β-stimulated expression of MMPs and β-catenin signaling and promoting the expression of TIMP-1, in vitro and in vivo (Zhou et al., 2013). It also blocks the expression of PGE2, IL-6, TNF-α, IL-1β, and NO via blocking NF-κB signaling in articular inflammation (Gao et al., 2016).
Withania somnifera
A preparation of the total extract of Withania somnifera, a medicinal plant used as a folk remedy for alleviating osteoarthritis, exerted a potential protective effect on the degradation of articular tissues by inhibiting collagenase activity (Ganesan et al., 2011). In a clinical trial, Withania somnifera extract showed a significant pain-relieving effect on osteoarthritic knee joint (Ramakanth et al., 2016). Withaferin A, the major active natural compound in Withania somnifera, showed anti-inflammatory action by controlling TNF-α, the paw volume, lipid peroxidation, and lysosomal enzymes, in an animal model of arthritis (Sabina et al., 2008).
Willow bark
Willow bark has long been utilized as a folk remedy for managing pain. A preparation of willow bark extract showed an inhibitory effect on the development of oxidative stress and production of proinflammatory cytokines in an animal model of arthritis (Sharma et al., 2011). This effect may be dependent on the blocking of monocyte activation by suppressing the activity of COX-2 and TNF-α (Bonaterra et al., 2010). In a few clinical trials, willow bark extract decreased the pain in osteoarthritis patients (Schmid et al., 2001; Beer and Wegener, 2008; Uehleke et al., 2013).
Wogonin
Wogonin, a flavonoid compound showing anti-inflammatory activity, exerted chondroprotective effects in vitro and in vivo. In cultured articular chondrocytes, wogonin promoted the expression of collagen type II and suppressed MMP expression. Furthermore, intra-articular injection of wogonin blocked the gene expression, production, and activity of MMP-3, and in vivo production of MMP-3 (Park et al., 2015).
Zingiber officinale
Ginger, Zingiber officinale, has been reported to show anti-inflammatory effects by elevating the serum level of corticosterone and blocking COX and lipoxygenase (LOX) (Grzanna et al., 2005; van Breemen et al., 2011). A preparation of Zingiber officinale extract controlled pain in patients with osteoarthritis (Altman and Marcussen, 2001).
CONCLUSION AND FUTURE DIRECTION FOR OSTEOARTHRITIS RESEARCH
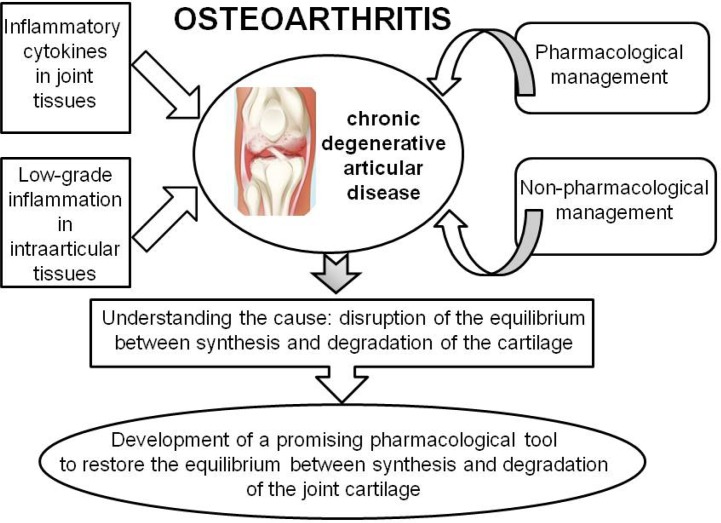
As mentioned previously, the use of agents for the conventional pharmacological management of osteoarthritis alone cannot address the root-cause of osteoarthritis. Furthermore, these agents show diverse and severe side effects and are not adequate for long-term management of osteoarthritis. On the other hand, a majority of natural products have shown inhibitory effects on proinflammatory cytokine-induced expression and catabolic activity of MMPs in articular cartilage via activation of the NF-κB signaling pathway. They showed suppressive effects on the apoptosis of chondrocytes, induction of extracellular matrix degradation, and decrease in the production of the extracellular matrix, in articular cartilage. However, there is no front-line candidate natural product and/or medicinal plant to reverse or prevent the development of the signs and symptoms of osteoarthritis, despite the many experimental and clinical studies conducted thus far (Fig. 1). Therefore, it is timely to develop an optimal candidate through optimization of the chemical structures of natural products showing the strongest anti-inflammatory, anti-apoptotic, and anti-catabolic activities, to restore the equilibrium between the synthesis and degradation of articular cartilage. Additionally, after joint injury, fibrotic cartilage is generated instead of the normal hyaline cartilage. Thus, it is ideal to develop a novel anti-fibrotic and anti-inflammatory candidate molecule that would facilitate the synthesis of the normal hyaline cartilage in the process of regeneration of articular cartilage.
Fig. 1.
Overview of pathophysiology and management of osteoarthritis and strategy for the development of a promising pharmacological tool. The onset and progression of osteoarthritis, a chronic degenerative articular disorder, are attributed to various inflammatory cytokines in joint tissues and fluids that are produced by chondrocytes and/or interact with chondrocytes, as well as to low-grade inflammation in intraarticular tissues. Disruption of the equilibrium between synthesis and degradation of the cartilage of the joint is the major cause of osteoarthritis. Developing a promising pharmacological tool to restore the equilibrium between synthesis and degradation of osteoarthritic joint cartilage can be a useful strategy for the effective management of osteoarthritis.
Acknowledgments
This research was supported by NRF-2014R1A6A1029617 and NRF-2017R1C1B1005126, Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education.
Footnotes
CONFLICT OF INTEREST
The authors have declared that there is no conflict of interest.
REFERENCES
- Aigner T, McKenna L. Molecular pathology and pathobiology of osteoarthritic cartilage. Cell Mol Life Sci. 2002;59:5–18. doi: 10.1007/s00018-002-8400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman RD, Marcussen KC. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001;44:2531–2538. doi: 10.1002/1529-0131(200111)44:11<2531::AID-ART433>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ammon HPT. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine. 2010;17:862–867. doi: 10.1016/j.phymed.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Anandacoomarasamy A, March L. Current evidence for osteoarthritis treatments. Ther Adv Musculoskelet Dis. 2010;2:17–28. doi: 10.1177/1759720X09359889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-LePain JC, Lane NE. Role of bone architecture and anatomy in osteoarthritis. Bone. 2012;51:197–203. doi: 10.1016/j.bone.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61:1704–1711. doi: 10.1002/art.24925. [DOI] [PubMed] [Google Scholar]
- Beer AM, Wegener T. Willow bark extract (Salicis cortex) for gonarthrosis and coxarthrosis—results of a cohort study with a control group. Phytomedicine. 2008;15:907–913. doi: 10.1016/j.phymed.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthr Cartil. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Blain EJ, Ali AY, Duance VC. Boswellia frereana (frankincense) suppresses cytokine-induced matrix metalloproteinase expression and production of pro-inflammatory molecules in articular cartilage. Phyther Res. 2009;24:905–912. doi: 10.1002/ptr.3055. [DOI] [PubMed] [Google Scholar]
- Bonaterra GA, Heinrich EU, Kelber O, Weiser D, Metz J, Kinscherf R. Anti-inflammatory effects of the willow bark extract STW 33-I (Proaktiv®) in LPS-activated human monocytes and differentiated macrophages. Phytomedicine. 2010;17:1106–1113. doi: 10.1016/j.phymed.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Bruyere O, Cooper C, Pelletier JP, Branco J, Luisa Brandi M, Guillemin F, Hochberg MC, Kanis JA, Kvien TK, Martel-Pelletier J, Rizzoli R, Silverman S, Reginster JY. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Semin Arthritis Rheum. 2014;44:253–263. doi: 10.1016/j.semarthrit.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- Chang SH, Choi Y, Park JA, Jung DS, Shin J, Yang JH, Ko SY, Kim SW, Kim JK. Anti-inflammatory effects of BT-201, an n-butanol extract of Panax notoginseng, observed in vitro and in a collagen-induced arthritis model. Clin Nutr. 2007;26:785–791. doi: 10.1016/j.clnu.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Chantre P, Cappelaere A, Leblan D, Al E. Efficacy and tolerance of Harpagophytum procumbens versus diacerhein in treatment of osteoarthritis. Phytomedicine. 2000;7:177–183. doi: 10.1016/S0944-7113(00)80001-X. [DOI] [PubMed] [Google Scholar]
- Chen MP, Yang SH, Chou CH, Yang KC, Wu CC, Cheng YH, Lin FH. The chondroprotective effects of ferulic acid on hydrogen peroxide-stimulated chondrocytes: inhibition of hydrogen peroxide-induced proinflammatory cytokines and metalloproteinase gene expression at the mRNA level. Inflamm Res. 2010;59:587–595. doi: 10.1007/s00011-010-0165-9. [DOI] [PubMed] [Google Scholar]
- Chen WP, Hu PF, Bao JP, Wu LD. Morin exerts antiosteoarthritic properties: an in vitro and in vivo study. Exp Biol Med. 2012;237:380–386. doi: 10.1258/ebm.2011.011271. [DOI] [PubMed] [Google Scholar]
- Chen WP, Xiong Y, Shi YX, Hu PF, Bao JP, Wu LD. Astaxanthin reduces matrix metalloproteinase expression in human chondrocytes. Int Immunopharmacol. 2014a;19:174–177. doi: 10.1016/j.intimp.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Tsai KS, Chan DC, Lan KC, Chen CF, Yang RS, Liu SH. Honokiol, a low molecularweight natural product, prevents inflammatory response and cartilage matrix degradation in human osteoarthritis chondrocytes. J Orthop Res. 2014b;32:573–580. doi: 10.1002/jor.22577. [DOI] [PubMed] [Google Scholar]
- Cheng CC, Chen YH, Chang WL, Yang SP, Chang DM, Lai JH, Ho LJ. Phytoestrogen bavachin mediates anti-inflammation targeting IκB kinase-IκBα-NF-κB signaling path-way in chondrocytes in vitro. Eur J Pharmacol. 2010;636:181–188. doi: 10.1016/j.ejphar.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Cheng W, Wu D, Zuo Q, Wang Z, Fan W. Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation and apoptosis in human articular chondrocytes. Int Orthop. 2013;37:2065–2070. doi: 10.1007/s00264-013-1990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HK, Kim SY, Choi MJ, Baek SO, Kwak SG, Ahn SH. The effect of GCSB-5, a new herbal medicine, on changes in pain behavior and neuroglial activation in a rat model of lumbar disc herniation. J Korean Neurosurg Soc. 2016;59:98–105. doi: 10.3340/jkns.2016.59.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Gupta S, Cho HS, Park MS, Mok SJ, Han I, Kim HS. Role of nonsteroidal anti-inflammatory drug in treatment of extra-abdominal desmoid tumors. Clin Orthop Surg. 2018;10:225–233. doi: 10.4055/cios.2018.10.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrubasik S, Thanner J, Kunzel O, Conradt C, Black A, Pollak S. Comparison of outcome measures during treatment with the proprietary Harpagophytum extract Doloteffin in patients with pain in the lower back, knee or hip. Phytomedicine. 2002;9:181–194. doi: 10.1078/0944-7113-00140. [DOI] [PubMed] [Google Scholar]
- Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989;84:678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding QH, Cheng Y, Chen WP, Zhong HM, Wang XH. Celastrol, an inhibitor of heat shock protein 90β potently suppresses the expression of matrix metalloproteinases, inducible nitric oxide synthase and cyclooxygenase-2 in primary human osteoarthritic chondrocytes. Eur J Pharmacol. 2013;708:1–7. doi: 10.1016/j.ejphar.2013.01.057. [DOI] [PubMed] [Google Scholar]
- Ding Q, Zhong H, Qi Y, Cheng Y, Li W, Yan S, Wang X. Anti-arthritic effects of crocin in interleukin-1β-treated articular chondrocytes and cartilage in a rabbit osteoarthritic model. Inflamm Res. 2010;62:17–25. doi: 10.1007/s00011-012-0546-3. [DOI] [PubMed] [Google Scholar]
- Fiebich B, Heinrich M, Hiller K, Kammerer N. Inhibition of TNF-α synthesis in LPS-stimulated primary human monocytes by Harpagophytum extract SteiHap 69. Phytomedicine. 2001;8:28–30. doi: 10.1078/0944-7113-00002. [DOI] [PubMed] [Google Scholar]
- Funk JL, Frye JB, Oyarzo JN, Kuscuoglu N, Wilson J, McCaffrey G, Stafford G, Chen G, Lantz RC, Jolad SD, Sólyom AM, Kiela PR, Timmermann BN. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452–3464. doi: 10.1002/art.22180. [DOI] [PubMed] [Google Scholar]
- Ganesan K, Sehgal PK, Mandal AB, Sayeed S. Protective effect of Withania somnifera and Cardiospermum halicacabum extracts against collagenolytic degradation of collagen. Appl Biochem Biotechnol. 2011;165:1075–1091. doi: 10.1007/s12010-011-9326-8. [DOI] [PubMed] [Google Scholar]
- Gao LN, Feng QS, Zhang XF, Wang QS, Cui YL. Tetrandrine suppresses articular inflammatory response by inhibiting pro-inflammatory factors via NF-κB inactivation. J Orthop Res. 2016;34:1557–1568. doi: 10.1002/jor.23155. [DOI] [PubMed] [Google Scholar]
- Garnero P, Rousseau JC, Delmas PD. Molecular basis and clinical use of biochemical markers of bone, cartilage, and synovium in joint diseases. Arthritis Rheum. 2000;43:953–968. doi: 10.1002/1529-0131(200005)43:5<953::AID-ANR1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Use and costs of prescription medications and alternative treatments in patients with osteoarthritis and chronic low back pain in community-based settings. Pain Pract. 2012;12:550–560. doi: 10.1111/j.1533-2500.2012.00532.x. [DOI] [PubMed] [Google Scholar]
- Gregori D, Giacovelli G, Minto C, Barbetta B, Gualtieri F, Azzolina D, Vaghi P, Rovati LC. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564–2579. doi: 10.1001/jama.2018.19319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube B, Grünwald J, Krug L, Staiger C. Efficacy of a comfrey root (Symphyti offic. radix) extract ointment in the treatment of patients with painful osteoarthritis of the knee: results of a double-blind, randomised, bicenter, placebo-controlled trial. Phytomedicine. 2007;14:2–10. doi: 10.1016/j.phymed.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Grzanna R, Lindmark L, Frondoza CG. Ginger--an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8:125–132. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- Ha CW, Park YB, Min BW, Han SB, Lee JH, Won YY, Park YS. Prospective, randomized, double-blinded, double-dummy and multicenter phase IV clinical study comparing the efficacy and safety of PG201 (Layla) and SKI306X in patients with osteoarthritis. J Ethnopharmacol. 2016;181:1–7. doi: 10.1016/j.jep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- Haseeb A, Chen D, Haqqi TM. Delphinidin inhibits IL-1β-induced activation of NF-κB by modulating the phosphorylation of IRAK-1Ser376 in human articular chondrocytes. Rheumatology. 2013;52:998–1008. doi: 10.1093/rheumatology/kes363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- Huang GS, Tseng CY, Lee CH, Su SL, Lee HS. Effects of (−)-epigallocatechin-3-gallate on cyclooxygenase 2, PGE2, and IL-8 expression induced by IL-1β in human synovial fibroblast. Rheumatol Int. 2010;30:1197–1203. doi: 10.1007/s00296-009-1128-8. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wu D, Fan W. Protection of ginsenoside Rg1 on chondrocyte from IL-1β-induced mitochondria-activated apoptosis through PI3K/Akt signaling. Mol Cell Biochem. 2014;392:249–257. doi: 10.1007/s11010-014-2035-1. [DOI] [PubMed] [Google Scholar]
- Hwang CJ, Chung SS, Lee KY, Lee JH, Moon SH, Kim JH, Cho KJ, Ahn JS, Kim DS, Park YS, Park HJ. Analgesic efficacy and safety of prolonged–release oxycodone/naloxone in Korean patients with chronic pain from spinal disorders. Clin Orthop Surg. 2018;10:33–40. doi: 10.4055/cios.2018.10.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee HH, Choi EO, Lee KW, Kim KY, Kim SG, Hong SH, Kim GY, Park C, Kim HK, Choi YW, Choi YH. Schisandrae Fructus inhibits IL-1β-induced matrix metalloproteinases and inflammatory mediators production in SW1353 human chondrocytes by suppressing NF-κB and MAPK activation. Drug Dev Res. 2015;76:474–483. doi: 10.1002/ddr.21283. [DOI] [PubMed] [Google Scholar]
- Jeong YJ, Shin JM, Bae YS, Cho HJ, Park KK, Choe JY, Han SM, Moon SK, Kim WJ, Choi YH, Kim CH, Chang HW, Chang YC. Melittin has a chondroprotective effect by inhibiting MMP-1 and MMP-8 expressions via blocking NF-κB and AP-1 signaling pathway in chondrocytes. Int Immunopharmacol. 2016;25:400–405. doi: 10.1016/j.intimp.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Ju XD, Deng M, Ao YF, Yu CL, Wang JQ, Yu JK, Cui GQ, Hu YL. Protective effect of sinomenine on cartilage degradation and chondrocytes apoptosis. Yakugaku Zasshi. 2010a;130:1053–1060. doi: 10.1248/yakushi.130.1053. [DOI] [PubMed] [Google Scholar]
- Ju XD, Deng M, Ao YF, Yu CL, Wang JQ, Yu JK, Cui GQ, Hu YL. The protective effect of tetramethylpyrazine on cartilage explants and chondrocytes. J Ethnopharmacol. 2010b;132:414–420. doi: 10.1016/j.jep.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Jung YB, Roh KJ, Jung JA, Jung K, Yoo H, Cho YB, Kwak WJ, Kim DK, Kim KH, Han CK. Effect of SKI 306X, a new herbal anti-arthritic agent, in patients with osteoarthritis of the knee: a double-blind placebo controlled study. Am J Chin Med. 2001;29:485–491. doi: 10.1142/S0192415X01000502. [DOI] [PubMed] [Google Scholar]
- Kang BJ, Ryu J, Lee CJ, Hwang SC. Luteolin inhibits the activity, secretion and gene expression of MMP-3 in cultured articular chondrocytes and production of MMP-3 in the rat knee. Biomol Ther (Seoul) 2014;22:239–245. doi: 10.4062/biomolther.2014.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DG, Lee HJ, Kim KT, Hwang SC, Lee CJ, Park JS. Effect of oleanolic acid on the activity, secretion and gene expression of matrix metalloproteinase-3 in articular chondrocytes in vitro and the production of matrix metalloproteinase-3 in vivo. Korean J Physiol Pharmacol. 2017;21:197–204. doi: 10.4196/kjpp.2017.21.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DG, Lee HJ, Lee CJ, Park JS. Inhibition of the expression of matrix metalloproteinases in articular chondrocytes by resveratrol through affecting nuclear factor-kappa B signaling pathway. Biomol Ther (Seoul) 2018;26:560–567. doi: 10.4062/biomolther.2018.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajuria A, Gupta A, Suden P, Singh S, Malik F, Singh J, Gupta BD, Suri KA, Srinivas VK, Ella K, Qazi GN. Immunomodulatory activity of biopolymeric fraction BOS 2000 from Boswellia serrata. Phyther Res. 2008;22:340–348. doi: 10.1002/ptr.2320. [DOI] [PubMed] [Google Scholar]
- Kim JH, Huh JE, Baek YH, Lee JD, Choi DY, Park DS. Effect of Phellodendron amurense in protecting human osteoarthritic cartilage and chondrocytes. J Ethnopharmacol. 2011;134:234–242. doi: 10.1016/j.jep.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Kim JH, Ryu KH, Jung KW, Han CK, Kwak WJ, Cho YB. SKI306X suppresses cartilage destruction and inhibits the production of matrix metalloproteinase in rabbit joint cartilage explant culture. J Pharmacol Sci. 2005;98:298–306. doi: 10.1254/jphs.FPJ04058X. [DOI] [PubMed] [Google Scholar]
- Kim JI, Choi JY, Kim KG, Lee MC. Efficacy of JOINS on cartilage protection in knee osteoarthritis: prospective randomized controlled trial. Knee Surg Relat Res. 2017;29:217–224. doi: 10.5792/ksrr.17.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Na JY, Song KB, Choi DS, Kim JH, Kwon YB, Kwon J. Protective effect of ginsenoside Rb1 on hydrogen peroxide-induced oxidative stress in rat articular chondrocytes. J Ginseng Res. 2012;36:161–168. doi: 10.5142/jgr.2012.36.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WK, Chung HJ, Pyee Y, Choi TJ, Park HJ, Hong JY, Shin JS, Lee JH, Ha IH, Lee SK. Effects of intra-articular SHINBARO treatment on monosodium iodoacetateinduced osteoarthritis in rats. Chin Med. 2016;11:17. doi: 10.1186/s13020-016-0089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullich W, Fagerer N, Schwann H. Effect of the NSAID nimesulide on the radical scavenger glutathione S-transferase in patients with osteoarthritis of the knee. Curr Med Res Opin. 2007;23:1981–1986. doi: 10.1185/030079907X223486. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lim H, Shehzad O, Kim YS, Kim HP. Ginsenosides from Korean red ginseng inhibit matrix metalloproteinase-13 expression in articular chondrocytes and prevent cartilage degradation. Eur J Pharmacol. 2014;724:145–151. doi: 10.1016/j.ejphar.2013.12.035. [DOI] [PubMed] [Google Scholar]
- Lee M, Yoo J, Kim JG, Kyung HS, Bin SI, Kang SB, Choi CH, Moon YW, Kim YM, Han SB, In Y, Choi CH, Kim J, Lee BK, Cho S. A randomized, multicenter, phase III trial to evaluate the efficacy and safety of polmacoxib compared with celecoxib and placebo for patients with osteoarthritis. Clin Orthop Surg. 2017;9:439–457. doi: 10.4055/cios.2017.9.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong DJ, Choudhury M, Hanstein R, Hirsh DM, Kim SJ, Majeska RJ, Schaffler MB, Hardin JA, Spray DC, Goldring MB, Cobelli NJ, Sun HB. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse posttraumatic osteoarthritis model. Arthritis Res Ther. 2014;16:508. doi: 10.1186/s13075-014-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Li D, Yuan T, Zhang X, Xiao Y, Wang R, Fan Y, Zhang X. Icariin: a potential promoting compound for cartilage tissue engineering. Osteoarthr Cartil. 2012;20:1647–1656. doi: 10.1016/j.joca.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Li H, Li X, Liu G, Chen J, Weng X, Liu F, Xu H, Liu X, Ye H. Bauhinia championi (Benth.) Benth. polysaccharides upregulate Wnt/β-catenin signaling in chondrocytes. Int J Mol Med. 2013;32:1329–1336. doi: 10.3892/ijmm.2013.1527. [DOI] [PubMed] [Google Scholar]
- Liang QQ, Ding DF, Xi ZJ, Chen Y, Li CG, Liu SF, Lu S, Zhao YJ, Shi Q, Wang YJ. Protective effect of ligustrazine on lumbar intervertebral disc degeneration of rats induced by prolonged upright posture. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/508461. 508461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HA, Song EK, Seon JK, Park KS, Shin YJ, Yang HY. Causes of aseptic persistent pain after total knee arthroplasty. Clin Orthop Surg. 2017;9:50–56. doi: 10.4055/cios.2017.9.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SC, Lee HP, Hung CY, Tsai CH, Li TM, Tang CH. Berberine attenuates CCN2-induced IL-1β expression and prevents cartilage degradation in a rat model of osteoarthritis. Toxicol Appl Pharmacol. 2015;289:20–29. doi: 10.1016/j.taap.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Lu H, Jiang J, Xie G, Liu W, Yan G. Effects of an aqueous extract of Eucommia on articular cartilage in a rat model of osteoarthritis of the knee. Exp Ther Med. 2013;6:684–688. doi: 10.3892/etm.2013.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Piao T, Wang Y, Liu J. Astragalin inhibits IL-1β-induced inflammatory mediators production in human osteoarthritis chondrocyte by inhibiting NF-κB and MAPK activation. Int Immunopharmacol. 2015;25:83–87. doi: 10.1016/j.intimp.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Mankin HJ. The response for articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–466. doi: 10.2106/00004623-198264030-00022. [DOI] [PubMed] [Google Scholar]
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, Kwoh K, Lohmander S, Rannou F, Roos EM, Underwood M. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Min BW, Kang CS, Lee KJ, Bae KC, Cho CH, Choi JH, Sohn HJ, Sin HK. Radiographic progression of osteoarthritis after rotational acetabular osteotomy: minimum 10-year follow-up outcome according to the Tönnis grade. Clin Orthop Surg. 2018;10:299–306. doi: 10.4055/cios.2018.10.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam DC, Kim BK, Lee HJ, Shin HD, Lee CJ, Hwang SC. Effects of prunetin on the proteolytic activity, secretion and gene expression of MMP-3 in vitro and production of MMP-3 in vivo. Korean J Physiol Pharmacol. 2016;20:221–228. doi: 10.4196/kjpp.2016.20.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonose N, Pereira JA, Machado PRM, Rodrigues MR, Sato DT, Martinez CAR. Oral administration of curcumin (Curcuma longa) can attenuate the neutrophil inflammatory response in zymosan-induced arthritis in rats. Acta Cir Bras. 2014;9:727–734. doi: 10.1590/S0102-86502014001800006. [DOI] [PubMed] [Google Scholar]
- Park JS, Lee HJ, Lee DY, Jo HS, Jeong JH, Kim DH, Nam DC, Lee CJ, Hwang SC. Chondroprotective effects of wogonin in experimental models of osteoarthritis in vitro and in vivo. Biomol Ther (Seoul) 2015;23:442–448. doi: 10.4062/biomolther.2015.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Kim DK, Shin HD, Lee HJ, Jo HS, Jeong JH, Choi YL, Lee CJ, Hwang SC. Apigenin regulates interleukin-1β-induced production of matrix metalloproteinase both in the knee joint of rat and in primary cultured articular chondrocytes. Biomol Ther (Seoul) 2016;24:163–170. doi: 10.4062/biomolther.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KC, Park EJ, Kim ER, Kim Y, Chung SH, Cho BW, Kim S, Jin M. Therapeutic effects of PG201, an ethanol extract from herbs, through cartilage protection on collagenase-induced arthritis in rabbits. Biochem Biophys Res Commun. 2005;331:1469–1477. doi: 10.1016/j.bbrc.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Park YG, Ha CW, Han CD, Bin SI, Kim HC, Jung YB, Lim HC. A prospective, randomized, double-blind, multi-center comparative study on the safety and efficacy of Celecoxib and GCSB-5, dried extracts of six herbs, for the treatment of osteoarthritis of knee joint. J Ethnopharmacol. 2013;149:816–824. doi: 10.1016/j.jep.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Pavelka K, Bruyere O, Rovati LC, Olejarova M, Giacovelli G, Reginster JY. Relief in mild-to-moderate pain is not a confounder in joint space narrowing assessment of full extension knee radiographs in recent osteoarthritis structure-modifying drug trials. Osteoarthr Cartil. 2003;11:730–737. doi: 10.1016/S1063-4584(03)00166-3. [DOI] [PubMed] [Google Scholar]
- Ra HJ, Lee HJ, Jo HS, Nam DC, Lee YB, Kang BH, Moon DK, Kim DH, Lee CJ, Hwang SC. Betulin suppressed interleukin-1β-induced gene expression, secretion and proteolytic activity of matrix metalloproteinase in cultured articular chondrocytes and production of matrix metalloproteinase in the knee joint of rat. Korean J Physiol Pharmacol. 2017;21:19–26. doi: 10.4196/kjpp.2017.21.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakanth GS, Uday Kumar C, Kishan PV, Usharani P. A randomized, double blind placebo controlled study of efficacy and tolerability of Withaina somnifera extracts in knee joint pain. J Ayurveda Integr Med. 2016;7:151–157. doi: 10.1016/j.jaim.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabina EP, Chandal S, Rasool MK. Inhibition of monosodium urate crystal-induced inflammation by withaferin A. J Pharm Pharm Sci. 2008;11:46–55. doi: 10.18433/j35k58. [DOI] [PubMed] [Google Scholar]
- Schmid B, Lüdtke R, Selbmann HK, Kötter I, Tschirdewahn B, Schaffner W, Heide L. Efficacy and tolerability of a standardized willow bark extract in patients with osteoarthritis: Randomized placebo-controlled, double blind clinical trial. Phytother Res. 2001;15:344–350. doi: 10.1002/ptr.981. [DOI] [PubMed] [Google Scholar]
- Sengupta K, Krishnaraju AV, Vishal AA, Mishra A, Trimurtulu G, Sarma KVS, Raychaudhuri SK, Raychaudhuri SP. Comparative efficacy and tolerability of 5-Loxin and Aflapin against osteoarthritis of the knee: a double blind, randomized, placebo controlled clinical study. Int J Med Sci. 2010;7:366–377. doi: 10.7150/ijms.7.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Arif M, Nirala RK, Gupta R, Thakur SC. Cumulative therapeutic effects of phytochemicals in Arnica montana flower extract alleviated collagen-induced arthritis: inhibition of both pro-inflammatory mediators and oxidative stress. J Sci Food Agric. 2016;96:1500–1510. doi: 10.1002/jsfa.7252. [DOI] [PubMed] [Google Scholar]
- Sharma S, Sahu D, Das HR, Sharma D. Amelioration of collagen-induced arthritis by Salix nigra bark extract via suppression of pro-inflammatory cytokines and oxidative stress. Food Chem Toxicol. 2011;49:3395–3406. doi: 10.1016/j.fct.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Shen X, Gatti R. The safety and efficacy of intra-articular dual molecular weighted hyaluronic acid in the treatment of knee osteoarthritis: the I.D.E.H.A. study. Orthop Rev (Pavia) 2013;5:e33. doi: 10.4081/or.2013.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SS, Jin M, Jung HJ, Kim B, Jeon H, Choi JJ, Kim JM, Cho BW, Chung SH, Lee YW, Song YW, Kim S. Suppressive effects of PG201, an ethanol extract from herbs, on collagen-induced arthritis in mice. Rheumatology (Oxford) 2003;42:665–672. doi: 10.1093/rheumatology/keg209. [DOI] [PubMed] [Google Scholar]
- Tong P, Xu S, Cao G, Jin W, Guo Y, Cheng Y, Jin H, Shan L, Xiao L. Chondroprotective activity of a detoxicated traditional Chinese medicine (Fuzi) of Aconitum carmichaeli Debx against severe-stage osteoarthritis model induced by mono-iodoacetate. J Ethnopharmacol. 2014;151:740–744. doi: 10.1016/j.jep.2013.11.048. [DOI] [PubMed] [Google Scholar]
- Uehleke B, Müller J, Stange R, Kelber O, Melzer J. Willow bark extract STW 33-I in the long-term treatment of outpatients with rheumatic pain mainly osteoarthritis or back pain. Phytomedicine. 2013;20:980–984. doi: 10.1016/j.phymed.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Umar S, Umar K, Sarwar AHMG, Khan A, Ahmad N, Ahmad S, Katiyar CK, Husain SA, Khan HA. Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine. 2014;21:847–856. doi: 10.1016/j.phymed.2014.02.001. [DOI] [PubMed] [Google Scholar]
- van Breemen RB, Tao Y, Li W. Cyclooxygenase-2 inhibitors in ginger (Zingiber officinale) Fitoterapia. 2011;82:38–43. doi: 10.1016/j.fitote.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishal AA, Mishra A, Raychaudhuri SP. A double blind, randomized, placebo controlled clinical study evaluates the early efficacy of aflapin in subjects with osteoarthritis of knee. Int J Med Sci. 2011;8:615–622. doi: 10.7150/ijms.8.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wu L, Li L, Chen S. Monotropein exerts protective effects against IL-1β-induced apoptosis and catabolic responses on osteoarthritis chondrocytes. Int Immunopharmacol. 2014;23:575–580. doi: 10.1016/j.intimp.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Wang J, Gao JS, Chen JW, Li F, Tian J. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol Int. 2012;32:1541–1548. doi: 10.1007/s00296-010-1720-y. [DOI] [PubMed] [Google Scholar]
- Wang SN, Xie GP, Qin CH, Chen YR, Zhang KR, Li X, Wu Q, Dong WQ, Yang J, Yu B. Aucubin prevents interleukin-1 beta induced inflammation and cartilage matri degradation via inhibition of NF-κB signaling pathway in rat articular chondrocytes. Int Immunopharmacol. 2015;24:408–415. doi: 10.1016/j.intimp.2014.12.029. [DOI] [PubMed] [Google Scholar]
- Wegener T, Lupke N. Treatment of patients with arthrosis of hip or knee with an aqueous extract of devil’s claw (Harpagophytum procumbens DC.) Phytother Res. 2003;17:1165–1172. doi: 10.1002/ptr.1322. [DOI] [PubMed] [Google Scholar]
- Weng X, Lin P, Liu F, Chen J, Li H, Huang L, Zhen C, Xu H, Liu X, Ye H, Li X. Achyranthes bidentata polysaccharides activate the Wnt/β-catenin signaling pathway to promote chondrocyte proliferation. Int J Mol Med. 2014;34:1045–1050. doi: 10.3892/ijmm.2014.1869. [DOI] [PubMed] [Google Scholar]
- Wu CM, Li TM, Tan TW, Fong YC, Tang CH. Berberine reduces the metastasis of chondrosarcoma by modulating the α v β 3 integrin and the PKC δ, c-Src, and AP-1 signaling pathways. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/423164. 423164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DQ, Zhong HM, Ding QH, Ba L. Protective effects of biochanin A on articular cartilage: in vitro and in vivo studies. BMC Complement Altern Med. 2014;14:444. doi: 10.1186/1472-6882-14-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Xu X, Dai Y, Xia L. Therapeutic effect of the saponin fraction from Clematis chinensis osbeck roots on osteoarthritis induced by monosodium iodoacetate through protecting articular cartilage. Phyther Res. 2010;24:538–546. doi: 10.1002/ptr.2977. [DOI] [PubMed] [Google Scholar]
- Xie GP, Jiang N, Wang SN, Qi RZ, Wang L, Zhao PR, Liang L, Yu B. Eucommia ulmoides Oliv. bark aqueous extract inhibits osteoarthritis in a rat model of osteoarthritis. J Ethnopharmacol. 2015;162:148–154. doi: 10.1016/j.jep.2014.12.061. [DOI] [PubMed] [Google Scholar]
- Ying X, Chen X, Cheng S, Shen Y, Peng L, Xu H. Piperine inhibits IL-1β-induced expression of inflammatory mediators in human osteoarthritis chondrocyte. Int Immunopharmacol. 2013;17:293–299. doi: 10.1016/j.intimp.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Oh HC, Park SH, Kim JK, Kim SH. Does obesity affect clinical and radiological outcomes in minimally invasive total knee arthroplasty? Minimum 5-year follow-up of minimally invasive tka in obese patients. Clin Orthop Surg. 2018;10:315–321. doi: 10.4055/cios.2018.10.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Huang B, Xiong C, Yue Z. Pinocembrin inhibits matrix metalloproteinase expression in chondrocytes. IUBMB Life. 2015;67:36–41. doi: 10.1002/iub.1343. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhu Y, Chen X, Zhang Y, Zhang Y, Jia Y, Wang H, Liu Y, Xiao L. Baicalein ameliorates inflammatory-related apoptotic and catabolic phenotypes in human chondrocytes. Int Immunopharmacol. 2014;21:301–308. doi: 10.1016/j.intimp.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhang T, Xia C, Shi L, Wang S, Zheng X, Hu T, Zhang B. Berberine ameliorates cartilage degeneration in interleukin-1β-stimulated rat chondrocytes and in a rat model of osteoarthritis via Akt signaling. J Cell Mol Med. 2014;18:283–292. doi: 10.1111/jcmm.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ye J, Wu GT, Peng XJ, Xia PF, Ren Y. Gentiopicroside prevents interleukin-1 beta induced inflammation response in rat articular chondrocyte. J Ethnopharmacol. 2015;172:100–107. doi: 10.1016/j.jep.2015.06.031. [DOI] [PubMed] [Google Scholar]
- Zhou X, Li W, Jiang L, Bao J, Tao L, Li J, Wu L. Tetrandrine inhibits the Wnt/β-catenin signalling pathway and alleviates osteoarthritis: an in vitro and in vivo study. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/809579. 809579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liu SQ, Yu L, He B, Wu SH, Zhao Q, Xia SQ, Mei HJ. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis. 2015;20:1187–1199. doi: 10.1007/s10495-015-1152-y. [DOI] [PubMed] [Google Scholar]