Abstract
This study aimed to investigate the prevalence/severity of anxiety and depression, and also their correlations with clinical characteristics and survival profiles in acute myeloid leukemia (AML) patients.
In all, 208 AML patients and 200 age and sex-matched healthy controls (HCs) were recruited in this study. Anxiety and depression were assessed by the Hospital Anxiety and Depression Scale (HADS) in AML patients before initiating therapy and in HCs after being enrolled. Treatment response was assessed, and event-free survival (EFS), and also the overall survival (OS) were calculated.
The HADS-anxiety score (P < .001), anxiety prevalence (P < .001), and anxiety severity (P < .001) were increased in AML patients than those in HCs. The HADS-depression score (P < .001), depression prevalence (P < .001), and also depression severity (P < .001) were higher in AML patients compared with HCs. No correlation of anxiety or depression with clinical characteristics was found in AML patients (all P > .05). Moreover, the anxiety (P = .178) and depression (P = .512) rates were similar between complete remission (CR) patients and non-CR patients. Additionally, the EFS was worse in anxiety patients compared with nonanxiety patients (P = .013). The OS was shorter in anxiety patients compared with nonanxiety patients (P = .015) and was also worse in depression patients compared with nondepression patients (P = .007).
Anxiety and depression are much more frequent and severe in AML patients compared to HCs, and both of them predict unfavorable survival profiles in AML patients.
Keywords: acute myeloid leukemia, anxiety, clinical characteristics, depression, survival
1. Introduction
Acute myeloid leukemia (AML), one of the most common acute leukemia, is predicted to be increased in incidence along with the aging of the population because the disease occurs more frequent in the elderly.[1] Although a number of novel drugs have been developed—thanks to the progress in scientific investigations of AML genetics—the overview of AML management does not change much since roughly 3 decades ago.[2] Usually AML treatments include induction chemotherapy and consolidation therapy, and in the latter, hematopoietic stem cell transplantation (HSCT) is currently the only potentially curative treatment; however, HSCT requires a sophisticated risk assessment resulting from a rather high incidence of treatment-related morbidity and mortality; moreover, a matched donor is always challenging to find.[3] Therefore, the prognosis of AML has been improved, but remains poor in recent years, with a 5-year survival rate being approximately under 50% in younger patients and even worse in elderly patients.[4,5]
The substantial mental distress caused by disease burden (such as estimated poor prognosis) has resulted in an increasing prevalence of psychiatric disorders in AML patients with anxiety and depression being the most frequent disorders.[6,7] In fact, in patients with other cancers, anxiety and depression not only present with a pejorative effect on the quality of life, but also have a damaging effect on the treatment efficacy and survival profiles in patients with various cancers, such as nonsmall lung cancer and head and neck cancer.[8–10] Also, anxiety and depression have been reported to have prognostic value in leukemia patients as well; for instance, in chronic leukemia patients, anxiety and depression are correlated with tyrosine kinase inhibitor discontinuation.[11] Nevertheless, there is still no study that has been done to explore the direct prognostic value of anxiety and depression in AML patients.
Thus, this study aimed to investigate the prevalence/severity of anxiety and depression, and also their correlations with clinical characteristics and survival profiles in AML patients.
2. Materials and methods
2.1. Patients
Between January, 2016 and December, 2018, a total of 208 de novo AML patients treated at The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, were recruited in this study. The eligibility criteria included: confirmed diagnosis of primary AML according to the 2008 World Health Organization classification criteria based on the clinical findings, cell morphology, immunophenotyping, cytogenetics, and molecular cytogenetics tests[12]; age above 18 years; no history of mental disease before establishing the diagnosis of AML; being able to complete the Hospital Anxiety and Depression Scale (HADS) assessment independently; and able to be regularly followed up. Also, the following patients were excluded: received any treatment for AML before recruitment; secondary or relapsed AML; suffered from other malignancies; and pregnant or breastfeeding women. In addition, 200 healthy subjects with age and sex matched with AML patients were screened as healthy controls (HCs) cohort on the Department of Health Physical Examination at our hospital. All HCs were able to fulfill the HADS assessment independently, and had neither a history of cancers nor obvious abnormality in general heathy status, which were indicated by medical history, general examinations, and laboratory examinations. The approval for performing the study was obtained from the Institutional Review Board of The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, and the written informed consents were collected from all enrolled subjects before initiation of study.
2.2. Collection of baseline data
The baseline data of all the AML patients were recorded after enrollment, which included the age, sex, French-American-British (FAB) classification, cytogenetics and molecular cytogenetics, risk stratification, and also white blood cell (WBC) level. The FAB classification was assessed according to the criteria developed by the FAB Cooperative Group[13]; the cytogenetics and molecular cytogenetics were examined by conventional cytogenetic and molecular analyses or fluorescence in situ hybridization; and the risk stratification was evaluated in accordance with the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology of AML (Version 2.2014).
2.3. Assessment of anxiety and depression
Anxiety and depression of AML patients were assessed before the initiation of any therapy, with the use of HADS. Before HADS assessment, patients were given a guide on how to fill out the HADS questionnaires; then AML patients were required to fulfill the HADS questionnaires independently. Apart from this, anxiety and depression of HCs were evaluated after they signed the informed consents, which were assessed by HADS as well. Both the HADS-anxiety (HADS-A) subscale and the HADS-depression (HADS-D) subscale consisted of 7 questions that were scored from 0 to 3 points individually, resulting in 0 to 21 points, and the evaluation of anxiety and depression severity were based on the HADS-A and HADS-D score: 0 to 7, no anxiety/depression; 8 to 10, mild anxiety/depression; 11 to 14, moderate anxiety/depression; 15 to 21, severe anxiety/depression.[14]
2.4. Follow-up
After the assessment of whether a patient was considered a candidate for intensive induction chemotherapy, appropriate regimens (eg, “7 + 3” regimens) were administered for induction therapy as AML guidelines recommended. After induction therapy, treatment response was assessed, and the complete remission (CR) was defined as follows (all criteria need to be fulfilled): bone marrow blasts <5%; absence of circulating blasts and blasts with Auer rods; absence of extramedullary disease; absolute neutrophil count >1.0 × 109/L (1000/μL); platelet count >100 × 109/L (100,000/μL). In addition, all patients were consecutively followed up till December 31, 2018, with a median follow-up duration of 17.0 months, and the event-free survival (EFS), and also the overall survival (OS) were calculated as follows: EFS, from the date of entry into the study to the date of induction treatment failure, relapse from CR, or death; patients without any of these events were censored on the date that they were lastly examined; OS, from the date of entry into the study to the date of death; patients who were alive at the last follow-up were censored on the date that they were last known to be alive.
2.5. Statistical analysis
All data processing and analysis were performed by the use of SPSS 21.0 statistical software (IBM, Chicago, IL) and GraphPad Prism 7.02 software (GraphPad Software Inc., San Diego, CA). The continuous variable was presented as mean value ± standard deviation (SD), and the categorized variable was displayed as count (percentage). Comparison for the continuous variable was determined by the Student t test, and the comparison for the categorized variable was examined by the chi-square test or Wilcoxon rank-sum test as appropriate. Kaplan-Meier curves were plotted to illuminate the EFS and OS profiles, and the log-rank test was used to analyze the difference of EFS and OS between groups. A 2-sided P value of less than .05 was applied as the threshold for significance.
3. Results
3.1. Baseline characteristics
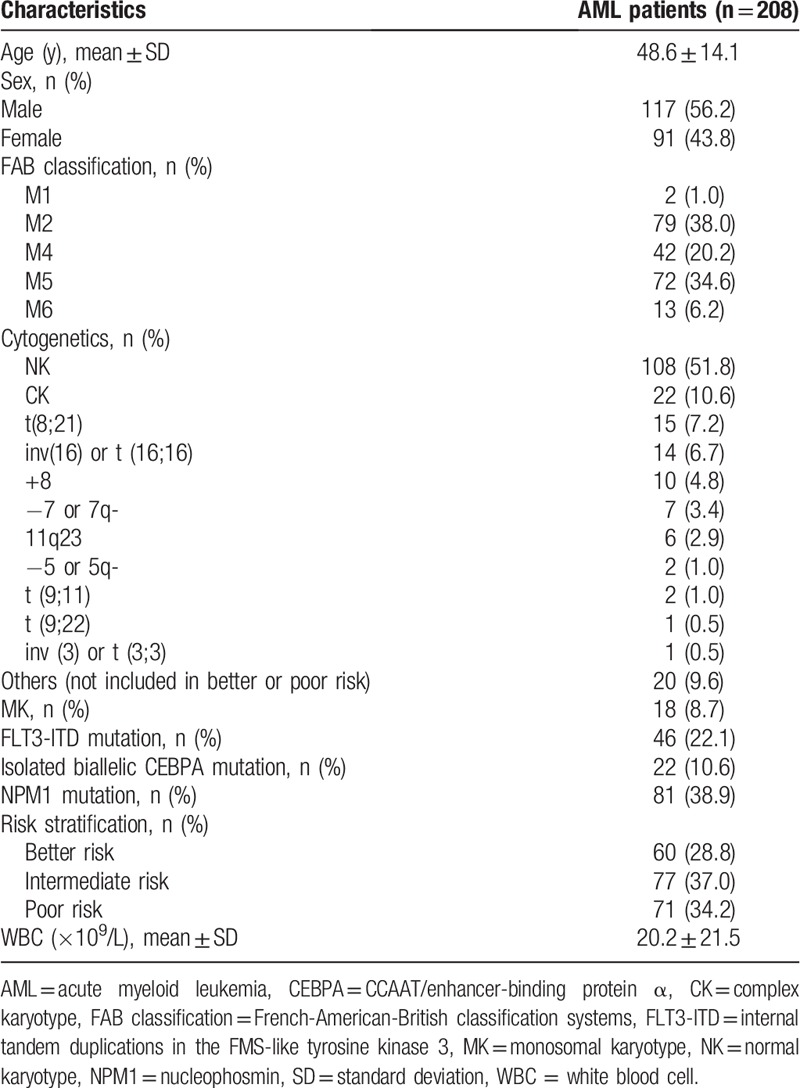
The mean age of AML patients was 48.6 ± 14.1 years, in which there were 117 (56.2%) males, and also 91 (43.8%) females (Table 1). The numbers of patients with FAB classification of M1, M2, M4, M5, and M6 were 2 (1.0%), 79 (38.0%), 42 (20.2%), 72 (34.6%), and 13 (6.2%), respectively. In addition, there were 18 (8.7%) patients who had monosomal karyotype (MK). Also, the numbers of patients who had internal tandem duplications in the FMS-like tyrosine kinase 3 (FLT3-ITD) mutation, isolated biallelic CCAAT/enhancer-binding protein α (CEBPA) mutation, and also nucleophosmin (NPM1) mutation were 46 (22.1%), 22 (10.6%), and 81 (38.9%), respectively. The numbers of patients with risk stratification of better risk, intermediate risk, and poor risk were 60 (28.8%), 77 (37.0%), and 71 (34.2%), respectively. And the mean value of WBC level was 20.2 ± 21.5 × 109/L. The detailed information of other baseline characteristics in AML patients was displayed in Table 1.
Table 1.
Baseline characteristics of AML patients.
3.2. Comparison of anxiety and depression between AML patients and HCs
The HADS-A score (P < .001) (Fig. 1A), anxiety prevalence (P < .001) (Fig. 1B), and anxiety severity (P < .001) (Fig. 1C) were all greatly increased in AML patients than those in HCs. And the HADS-D score (P < .001) (Fig. 1D), depression prevalence (P < .001) (Fig. 1E), and also depression severity (P < .001) (Fig. 1F) were also dramatically elevated in AML patients compared with HCs. These data indicated that anxiety and depression were much more prevalent and severe in AML patients compared with the healthy cohort.
Figure 1.
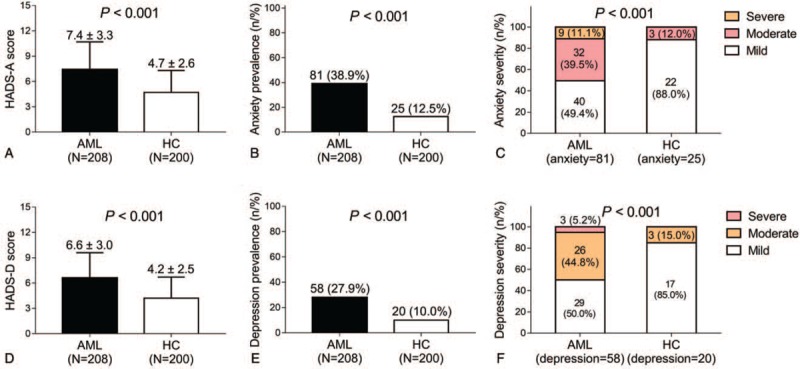
Anxiety and depression in AML patients and HCs. The HADS-A score (A), anxiety prevalence (B), anxiety severity (C), HADS-D score (D), depression prevalence (E), and depression (F) between AML patients and HCs are shown. Comparison between 2 groups was determined by Student t test, chi-square test, or Wilcoxon rank-sum test. P value of less than .05 was applied as the threshold for significance. AML = acute myeloid leukemia, HADS-A = Hospital Anxiety and Depression Scale—anxiety, HADS-D = Hospital Anxiety and Depression Scale—depression, HCs = healthy controls.
3.3. Correlations of anxiety and depression with clinical characteristics in AML patients
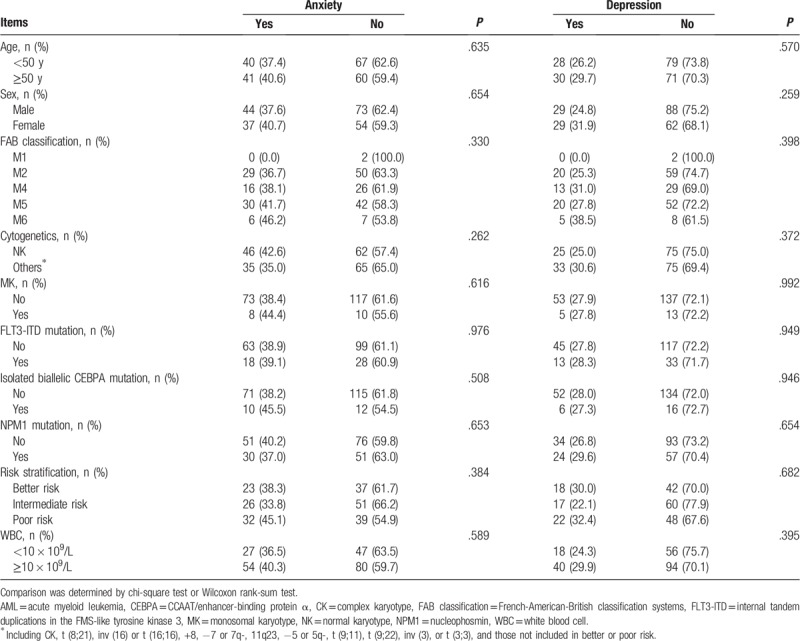
Anxiety was not correlated with age (P = .635), sex (P = .654), FAB classification (P = .330), cytogenetics (P = .262), MK (P = .616), FLT3-ITD mutation (P = .976), isolated biallelic CEBPA mutation (P = .508), NPM1 mutation (P = .653), risk stratification (P = .384), or WBC level (P = .589) in AML patients (Table 2). In addition, depression was not associated with age (P = .570), sex (P = .259), FAB classification (P = .398), cytogenetics (P = .372), MK (P = .992), FLT3-ITD mutation (P = .949), isolated biallelic CEBPA mutation (P = .946), NPM1 mutation (P = .654), risk stratification (P = .682), or WBC level (P = .395) in AML patients, either.
Table 2.
Correlation of anxiety or depression with clinical characteristics in AML patients.
3.4. Correlations of anxiety and depression with treatment response
After the induction treatment, the number of patients who achieved CR was 164 (78.8%) (CR patients); the remaining 44 (21.2%) patients did not achieve CR (non-CR patients) (Fig. 2A). Also, the induction death rate was 7.7%. Moreover, the anxiety rate (P = .178) (Fig. 2B) and depression rate (P = .512) (Fig. 2C) were of no difference between CR patients and non-CR patients, though they presented with a tread to be decreased in CR patients than non-CR patients.
Figure 2.
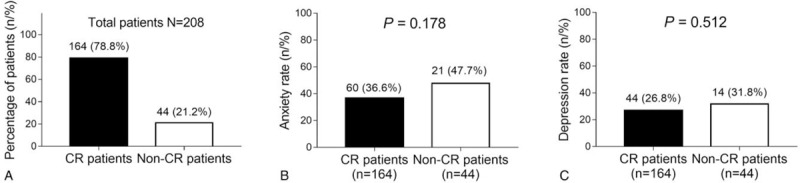
Correlations of anxiety and depression with treatment response. The percentage of CR patients and non-CR patients (A), anxiety rate between CR patients and non-CR patients (B), and depression rate between CR patients and non-CR patients (C) are shown. Comparison between 2 groups was determined by chi-square test. P value of less than .05 was applied as the threshold for significance. CR = complete remission.
3.5. Correlations of anxiety and depression with survival profiles
In terms of survival profiles, the EFS was less prolonged in anxiety patients compared with nonanxiety patients (P = .013) (Fig. 3A), whereas the EFS seemed to be worse in depression patients compared with nondepression patients, but showed no statistical significance (P = .052) (Fig. 3B). In addition, the median EFS in anxiety patients and nonanxiety patients were 13.0 (7.9–18.1) months and 18.0 (15.6–24.4) months, and the median EFS in depression patients and nondepression patients were 14 (6.0–22.0) months and 16.0 (11.6–20.4) months, respectively. As for OS, it was worse in anxiety patients compared with nonanxiety patients (P = .015) (Fig. 4A) and was shorter in depression patients compared with nondepression patients as well (P = .007) (Fig. 4B). Apart from this, the median OS in anxiety patients and nonanxiety patients were 24.0 (17.4–30.5) months and 34.0 (29.0–39.0) months, and the median OS in depression patients and nondepression patients were 23 (16.8–29.2) months and 33.0 (28.4–37.6) months, respectively. These results indicated that anxiety and depression were associated with worse survival profiles in AML patients.
Figure 3.
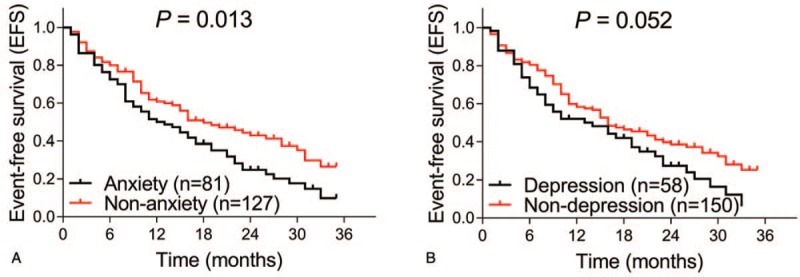
Correlations of anxiety and depression with EFS. The EFS between anxiety patients and nonanxiety patients (A), and between depression and nondepression patients (B) are shown. Kaplan-Meier curves were plotted to illuminate the EFS, and the log-rank test was used to analyze the difference of EFS between groups. P value of less than .05 was applied as the threshold for significance. EFS = event-free survival.
Figure 4.
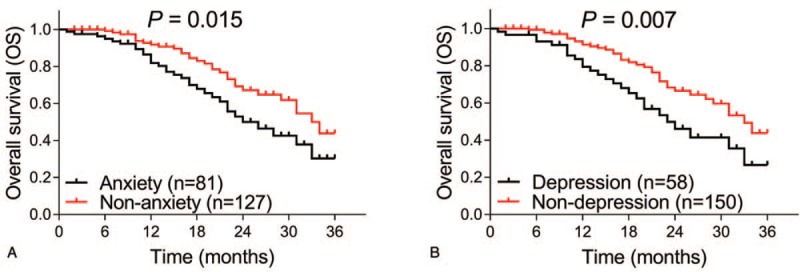
Correlations of anxiety and depression with OS. The OS between anxiety patients and nonanxiety patients (A), and between depression and nondepression patients (B) are shown. Kaplan-Meier curves were plotted to illuminate the OS, and the log-rank test was used to analyze the difference of OS between groups. P value of less than .05 was applied as the threshold for significance. OS = overall survival.
4. Discussion
The importance of anxiety and depression have been increasingly implicated in leukemia patients, which not only drives increasing attention to the mental health need but also leads to a paradigm shift in the management of leukemia.[6,7,15] However, correlations of anxiety and depression with clinical characteristics and survival profiles in AML patients have rarely been studied. In this study, we investigated the prevalence and severity of anxiety and depression in AML patients, and the correlations of anxiety, and also depression with clinical characteristics and survival profiles in AML patients, which elucidated that: anxiety and depression were much more prevalent and severe in AML patients compared with HCs; anxiety and depression predicted worse EFS and OS in AML patients.
Living with AML makes the patients have to face multiple serious symptoms, adverse events caused by treatment, a poor survival estimation, and heavy medically financial burden, resulting in substantial emotional stress, such as anxiety, depression, fear, and hopelessness.[16] However, the investigation of psychiatric status in AML patients is very insufficient. A recent longitudinal study reports that the rate of depression and anxiety symptoms in older AML patients receiving intensive chemotherapy and nonintensive chemotherapy is 33.3% and 30.0%, respectively.[7] A cross-sectional study evaluating the quality of life in AML patients reports that 2 out of 18 patients have anxiety assessed by HAD-A, and 1 out of 18 patients have depression assessed by HAD-D.[17] Another prospective cohort study reveals that the depression prevalence in older adult AML patients receiving induction chemotherapy is 38.9%, and the other psychosocial and physical damages, and also their prevalence in elderly AML patients are cognitive impairment (31.5%), distress (53.7%), impairment in activities of daily living (48.2%), impairment in physical performance (53.7%), and comorbidity (46.3%).[18] These prior studies all indicate that the prevalence of anxiety and depression in AML patients are numerically high; however, these studies present several concerns: most of the studies are conducted in the elderly AML patients; lack of control group/cohort; included samples are relatively small. Thus, to address these issues, we conducted this current study which enrolled large-sample-size adult AML patients and healthy volunteers as controls. We discovered that anxiety and depression were much more frequent and severe in AML patients compared with HCs, which might result from the distress in AML patients that was caused by the following reasons: the burden of the disease itself, such as the AML symptoms including fatigue, weakness, shortness of breath, and insufficient physical function, etc[16]; and other burdens that was caused by AML, such as the fear of death, financial cost, family burden, and so on.
The most common types of mood disorders include anxiety and depression; these mood disorders are reported to play a role in clinical outcome prediction in patients with cancers; nevertheless, quite few studies have been conducted to assess the prognostic value of anxiety and depression in patients with AML.[19–21] A previous prospective study reveals that the elderly AML patient who is complicated with depressive symptoms exhibits increased reduction in Short Physical Performance Battery score from follow-up to baseline compared with those who were not complicated with depressive symptoms.[22] Moreover, a cohort study elucidates that symptoms of anxiety and depression are correlated with damaged general life satisfaction in AML survivors postallogeneic stem cell transplantation.[23] Moreover, a study aiming at evaluating the prognostic role of geriatric assessment in elderly AML patients shows that patients with impaired cognitive function and physical function have worse OS, and after adjustment of age, sex, Eastern Cooperative Oncology Group score, cytogenetic risk group, myelodysplastic syndrome, and hemoglobin; these 2 factors are still associated with unfavorable OS.[24] These previous studies are similar but distinctive compared with our study: the patient cohorts are different, these previous studies are conducted in the elderly AML patients or AML patients post-transplant, whereas our study was conducted in adult de novo AML patients; none of those studies have explicitly evaluated the prognostic value of anxiety and depression in AML patients, they mostly focus on physical function, cognitive function, or anxiety/depressive symptoms; they also mainly focus on investigating the predictive factors for quality of life but not clinical outcomes; the number of the included patients is relatively small. In our study, we investigated the prognostic value of anxiety and depression for predicting survival profiles in adult de novo AML patients with large samples, and found that patients with anxiety had worse EFS compared with patients without anxiety, and patients with depression displayed worse EFS, and also OS compared with patients without depression, which might indicate that anxiety and depression may have the potentiality to predict survival in AML patients after treatment. Concerning the possible reasons for these results, there might include the following aspects: anxiety and depression might directly harm the survival of patients by influencing the treatment efficacy, medication compliance, and disease-related stress[11,25]; anxiety and depression may also indirectly lead to worse survival profiles of patients through their interaction of worse clinical properties and symptoms.
Here were several limitations in this study: first, our study was conducted in a single center, which might lead to selection bias; second, anxiety and depression were assessed only by HADS in our study, which might need to be validated by other anxiety and depression related scales as well; third, the follow-up duration was relatively short in our study, and thus, the results needed to be validated in long-duration study in the future; fourth, only de novo AML patients were included in our study, thus prevalence and prognostic value of anxiety and depression in refractory/relapsed AML patients needed to be further explored.
5. Conclusions
In conclusion, anxiety and depression are much more frequent and severe in AML patients compared with HCs, and both of them predict unfavorable survival profiles in AML patients.
Author contributions
Conceptualization: Liwen Xu, Jing Lin.
Data curation: Ting Ding, Xin Wang, Adan Fu.
Formal analysis: Ting Ding, Xin Wang, Adan Fu.
Funding acquisition: Liwen Xu, Jing Lin.
Investigation: Ting Ding, Xin Wang, Adan Fu.
Methodology: Ting Ding, Xin Wang, Adan Fu.
Resources: Liwen Xu, Jing Lin.
Writing – original draft: Ting Ding, Xin Wang, Adan Fu.
Writing – review & editing: Liwen Xu, Jing Lin.
Footnotes
How to cite this article: Ding T, Wang X, Fu A, Xu L, Lin J. Anxiety and depression predict unfavorable survival in acute myeloid leukemia patients. Medicine. 2019;98:43(e17314).
Abbreviations: AML = acute myeloid leukemia, CEBPA = CCAAT/enhancer-binding protein α, CR = complete remission, EFS = event-free survival, FAB = French-American-British, FLT3-ITD = FMS-like tyrosine kinase 3, HADS = Hospital Anxiety and Depression Scale, HADS-A = HADS—anxiety, HADS-D = HADS—depression, HCs = healthy controls, HSCT = hematopoietic stem cell transplantation, MK = monosomal karyotype, NPM1 = nucleophosmin, OS = overall survival, SD = standard deviation, WBC = white blood cell.
TD, XW, and AF contributed equally to this work and are the co-first authors.
Funding: This study was supported by Wuhan Municipal Health Commission in 2014 (No. WG14B11) and Health Commission of Hubei Province in 2015 (No. WJ2015HB025).
The authors have no conflicts of interest to disclose.
References
- [1].Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer 2006;107:2099–107.. [DOI] [PubMed] [Google Scholar]
- [2].Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015;373:1136–52.. [DOI] [PubMed] [Google Scholar]
- [3].Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood 2009;114:7–19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood 2016;127:53–61.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 2013;369:111–21.. [DOI] [PubMed] [Google Scholar]
- [6].Loh KP, Tooze JA, Nicklas BJ, et al. Inflammatory biomarkers, geriatric assessment, and treatment outcomes in acute myeloid leukemia. J Geriatr Oncol 2019;doi: 10.1016/j.jgo.2019.03.014. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].El-Jawahri A, Abel GA, Traeger L, et al. Quality of life and mood of older patients with acute myeloid leukemia (AML) receiving intensive and non-intensive chemotherapy. Leukemia 2019;33:2393–402.. [DOI] [PubMed] [Google Scholar]
- [8].Derry HM, Maciejewski PK, Epstein AS, et al. Associations between anxiety, poor prognosis, and accurate understanding of scan results among advanced cancer patients. J Palliat Med 2019;22:961–5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Arrieta O, Angulo LP, Nunez-Valencia C, et al. Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non-small cell lung cancer. Ann Surg Oncol 2013;20:1941–8.. [DOI] [PubMed] [Google Scholar]
- [10].Kim SA, Roh JL, Lee SA, et al. Pretreatment depression as a prognostic indicator of survival and nutritional status in patients with head and neck cancer. Cancer 2016;122:131–40.. [DOI] [PubMed] [Google Scholar]
- [11].Sogawa R, Kimura S, Yakabe R, et al. Anxiety and depression associated with tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia. Int J Clin Oncol 2018;23:974–9.. [DOI] [PubMed] [Google Scholar]
- [12].Jaffe ES, Harris NL, Stein H. Introduction and overview of the classification of the lymphoid neoplasms. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. 2008;Lyon: IARC, 158–166. [Google Scholar]
- [13].Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med 1985;103:620–5.. [DOI] [PubMed] [Google Scholar]
- [14].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70.. [DOI] [PubMed] [Google Scholar]
- [15].Meeske KA, Siegel SE, Globe DR, et al. Prevalence and correlates of fatigue in long-term survivors of childhood leukemia. J Clin Oncol 2005;23:5501–10.. [DOI] [PubMed] [Google Scholar]
- [16].Tomaszewski EL, Fickley CE, Maddux L, et al. The patient perspective on living with acute myeloid leukemia. Oncol Ther 2016;4:225–38.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cheng MJ, Smith BD, Hourigan CS, et al. A single center survey of health-related quality of life among acute myeloid leukemia survivors in first complete remission. J Palliat Med 2017;20:1267–73.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Klepin HD, Geiger AM, Tooze JA, et al. The feasibility of inpatient geriatric assessment for older adults receiving induction chemotherapy for acute myelogenous leukemia. J Am Geriatr Soc 2011;59:1837–46.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol 2011;29:3636–42.. [DOI] [PubMed] [Google Scholar]
- [20].Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012;118:3377–86.. [DOI] [PubMed] [Google Scholar]
- [21].Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457–65.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Klepin HD, Tooze JA, Pardee TS, et al. Effect of intensive chemotherapy on physical, cognitive, and emotional health of older adults with acute myeloid leukemia. J Am Geriatr Soc 2016;64:1988–95.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Amler S, Sauerland MC, Deiters C, et al. Factors influencing life satisfaction in acute myeloid leukemia survivors following allogeneic stem cell transplantation: a cross-sectional study. Health Qual Life Outcomes 2015;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 2013;121:4287–94.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gheihman G, Zimmermann C, Deckert A, et al. Depression and hopelessness in patients with acute leukemia: the psychological impact of an acute and life-threatening disorder. Psychooncology 2016;25:979–89.. [DOI] [PubMed] [Google Scholar]


