Abstract
In 2014/2015, tyrosine kinase inhibitors (TKIs) were introduced as a secondary treatment for refractory differentiated thyroid cancer (DTC) in Japan. While renal dysfunction is an adverse event of TKI, data on this adverse event in TKI-treated DTC remains insufficient. Here, we investigated renal function in patients undergoing TKI treatment for DTC and evaluated the efficacy of dose reduction/withdrawal for cases of renal dysfunction.
A total of 73 cases of radioactive iodine-refractory DTC treated with sorafenib (n = 22) or lenvatinib (n = 51) were included. Patient data evaluated were TKI treatment period, estimated glomerular filtration rate (eGFR) before and after TKI therapy, incidence and degree (maximum value at time of TKI treatment) of proteinuria, and albumin levels before and after TKI therapy were compared.
The mean ΔeGFR was −6.75% with lenvatinib and +5.90% with sorafenib. It was not significant (P = .15). The mean Δalbumin was −8.90% and −5.85% with lenvatinib and sorafenib, respectively; there was no significant difference between the lenvatinib and sorafenib groups (P = .77). According to our program of TKI dose reduction and withdrawal, all patients except 2 with diabetes were successfully continuing treatment.
Overall, the present results demonstrated that renal function is negatively affected by long-term TKI treatment for RAI-refractory DTC. However, heightened proteinuria, decreased eGFR and albumin levels, and significant but apparently reversible renal dysfunction were more frequent with lenvatinib than sorafenib.
Keywords: differentiated thyroid cancer, lenvatinib, renal dysfunction, tyrosine kinase inhibitor
1. Introduction
Differentiated thyroid carcinoma (DTC), consisting of papillary and follicular carcinoma, generally has a slow progression and good prognosis. However, some aggressive cases can develop progressive metastases and become life-threatening. While surgical resection is the first line of therapy in all DTC cases, radioactive iodine (RAI) and other various therapies specific to affected organs are indicated for recurrent lesions and/or distant metastases. In 2014/2015, therapies using anti-angiogenic tyrosine kinase inhibitors (TKIs), such as sorafenib[1] and lenvatinib,[2] for RAI-refractory metastatic or recurrent DTC became available in Japan as a second line of therapy.
TKIs are clinically introduced with various carcinomas and inhibit tumor angiogenesis mainly by suppressing the vascular endothelial growth factor pathway.[3] In these anti-VEGF agents, hypertension and asymptomatic proteinuria are common dose-related side-effects that frequently occur together.[4,5] The mechanism is also reported.[6] Regarding sorafenib, as multikinase inhibitor, the incidence of drug-induced proteinuria was reported as 11.6% for all grade and 0.9% for high grade.[7] Phase 3 study of lenvatinib showed proteinuria in 63% (grade 3 or higher in 20%).[8] Lenvatinib-induced renal failure has already been reported.[9] Unlike other carcinomas, RAI-refractory metastatic and recurrent DTCs typically require long-term TKI treatment. Therefore, management of potentially life-threatening adverse events associated with TKI therapy, such as renal dysfunction, is extremely important. However, data on renal dysfunction in TKI-treated DTC remains insufficient. The aim of this study was to evaluate the changes in renal function during sorafenib or lenvatinib therapy for patients with RAI-refractory DTC. In addition, the efficacy of our program of TKI dose reduction and/or withdrawal in cases of renal dysfunction was also examined.
2. Patients and methods
This retrospective study included a total of 73 patients (30 men, 43 women) diagnosed with RAI-refractory DTC and treated at Kanagawa Cancer Center (Yokohama City, Japan) between April 2015 and August 2018. The measured values obtained from patient records. Patients were split into 2 groups based on the TKI drug being administered, sorafenib (n = 22) or lenvatinib (n = 51); 15 cases were administered both TKIs. To eliminate the influence of the previous treatment, only data of medicine used in 1st line were adopted. The cancer board of Kanagawa Cancer Center (Yokohama, Kanagawa, Japan) approved sorafenib and lenvatinib treatment, including surgery, for patients with DTC. The study was approved by the Institutional Review Board of Kanagawa Cancer Center.
Only patients with normal renal function before TKI therapy were included, and those presenting with renal dysfunction prior to TKI therapy were excluded. Patient data evaluated included the total TKI dose and treatment period as well as the change in estimated glomerular filtration rate (eGFR), change in albumin levels before and after TKI treatment, and incidence and degree of proteinuria. These parameters were used as indicators of renal function [https://cdn.jsn.or.jp/guideline/pdf/2016-cancer-guideline-170706.pdf].[6,7,10] The formula used to calculate eGFR was specific for Japanese populations[11]:
eGFR creatinin (male) = 194 × (serum creatinine value)−1.094 × (age)−0.287
eGFR creatinin (female) = eGFR creatinin (male) × 0.739
Percent changes in eGFR (ΔeGFR) were determined by subtracting the eGFR determined before TKI administration (baseline) from the value obtained after TKI treatment or the latest eGFR determined during continued administration and then dividing by the baseline eGFR to obtain a percentage.[7] Percent changes in serum albumin levels (Δalbumin) were calculated in the same way.
The degree of proteinuria was determined on a scale from 0 (no proteinuria) to + 4 (Grade 4), with the maximum value being recorded. The 2016 Japanese Cancer Guidelines recommend TKI dosage reduction in cases of Grade 2 proteinuria (1.0–3.5 g/d) and discontinuation of treatment with Grade 3 proteinuria (>3.5 g/d) (https://cdn.jsn.or.jp/guideline/pdf/2016-cancer-guideline-170706.pdf). This is based on the definition of AKI of 2012 KDIGO Guidelines (http://www.kdigo.org). Our program of TKI dose reduction and withdrawal is shown in Table 1. In this scheme, the initial dose of lenvatinib was 24 mg, and was reduced to 20, 14, 10, and 8 mg. Sorafenib dosing began at 800 mg and was reduced to 600, 400, and 200 mg. Owing to the fact that the 2 drugs are administered in different doses, they were administered in separate dose reduction programs, and no one administered them at the same time. In withdrawal cases, the dosage was eventually reduced to 0 mg in both cases.
Table 1.
Proteinuria grade and correspondence program.

Categorical variables (TKI, sex, histopathology of thyroid cancer, and proteinuria) were compared using a Chi-square test, whereas continuous variables (age, treatment period, baseline eGFR, post-TKI eGFR, ΔeGFR, and Δalbumin) were compared by Student t test. Actual P values were calculated and P < .05 was considered significantly difference using EZR statistical software.[12] Categorical variables are presented as plain numbers and proportions. Distributed variables are presented as means ± standard deviations (interquartile range, 25th percentile–75th percentile). The total TKI doses are presented as medians but could not be statistically compared because TKI doses and dose units varied (Table 2). Pearson product-moment correlation test was used to calculate correlations and 95% confidence intervals. The correlation coefficients of each variable (ΔeGFR, Δalbumin, and proteinuria value and treatment period) were calculated by EZR software,[12] and a P < .05 was considered significantly different. Additionally, multiple regression analysis was performed using ΔeGFR as an objective variable. Explanatory variables included baseline eGFR, age, sex, pathology, proteinuria, TKI, treatment period, and Δalbumin.
Table 2.
Patients’ characteristics and renal functions after TKI treatment.
In this study, the drugs used in combination with TKI include therapeutic agents for hypertension, diabetes, hyperlipidemia, hyperuricemia, analgesics including narcotics, steroids, and digestive agents. The drugs added after the TKI treatment are mainly antihypertensive and digestive. Since non-steroidal anti-inflammatory drugs and antibiotics cause renal dysfunction, they were not used in combination with TKI.
3. Results
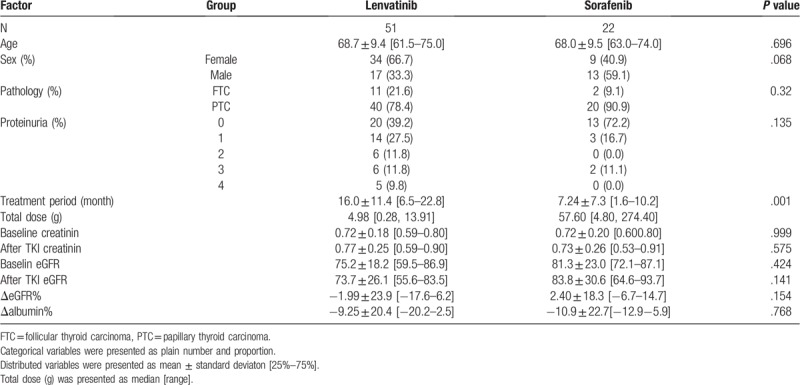
The patients’ background and parameters are shown in Table 2. Their mean age was 68.5 ± 14.6 years, with an interquartile range, 25th percentile for 62-year-olds and a 75th percentile for 75-year-olds. There were no significant differences in age, sex, or histopathology of thyroid cancer in the 51 patients given lenvatinib and 22 given sorafenib. The incidence of proteinuria (all grades) was 60.8% in the lenvatinib group and 27.8% in the sorafenib group, but there were no significant differences (Table 2). The sample size calculation to compare the incidences of proteinuria between the lenvatinib and sorafenib groups was 41. Because the number of patients in the lenvatinib group was 51, it was statistically more reliable than the sample size of 41; however, the sorafenib group, with 22 patients, did not achieve the required sample size. This was unavoidable because sorafenib has not been used as a 1st line medication in our institution since June 2015.[13] In the sorafenib group, there were no cases in which dose reduction was performed due to renal dysfunction. In the lenvatinib group, 11 patients (21.6%) had a semiquantitative proteinuria value of +3 (Fig. 1), indicating renal dysfunction, and they underwent dose reduction/withdrawal for at least 1 week. However, there were no negative proteinuria cases in either treatment group by the end of the treatment period. Overall, the median treatment period was significantly longer with lenvatinib (14.9 months) than with sorafenib (4.65 months; P = .001).
Figure 1.
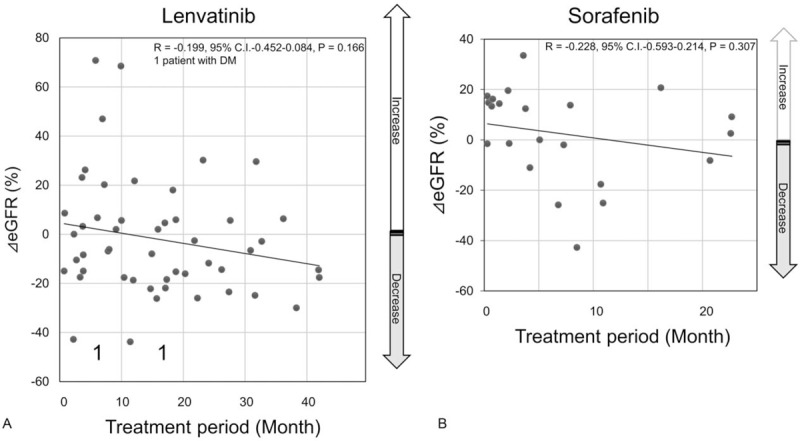
Scatter plots of ΔeGFR values for the TKI treatment period. The horizontal axis represents the treatment period (month), and the vertical axis represents the ΔeGFR. R, correlation coefficient. Graph A demonstrates lenvatinib group, and graph B demonstrates sorafenib group.
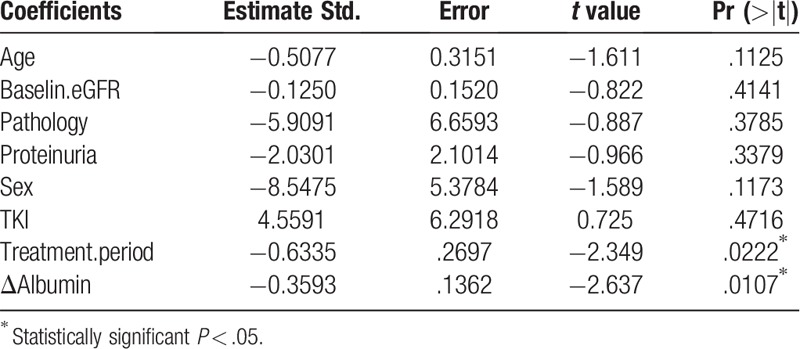
All the eGFRs after TKI treatment were distributed within stages 1 to 3 according to the Kidney Disease Improving Global Outcomes definition of chronic kidney disease (http://www.kdigo.org). The mean ΔeGFR was −6.75% with lenvatinib and +5.90% with sorafenib (Fig. 2). Although there was an obvious decrease in the lenvatinib group after treatment, it was not significant (P = .15). Conversely, in the sorafenib group, eGFR after treatment increased. The mean Δalbumin was −8.90% with lenvatinib and −5.85% with sorafenib (Fig. 3); there was no significant difference between the lenvatinib and sorafenib groups (P = .77). Similar results were obtained with the total dose; both ΔeGFR and Δalbumin tended to decrease over the treatment period for both drugs, but no correlation was observed. Furthermore, multiple regression analysis using ΔeGFRs as objective variables revealed that the treatment period and Δalbumin were significant factors (P < .05, Table 3).
Figure 2.
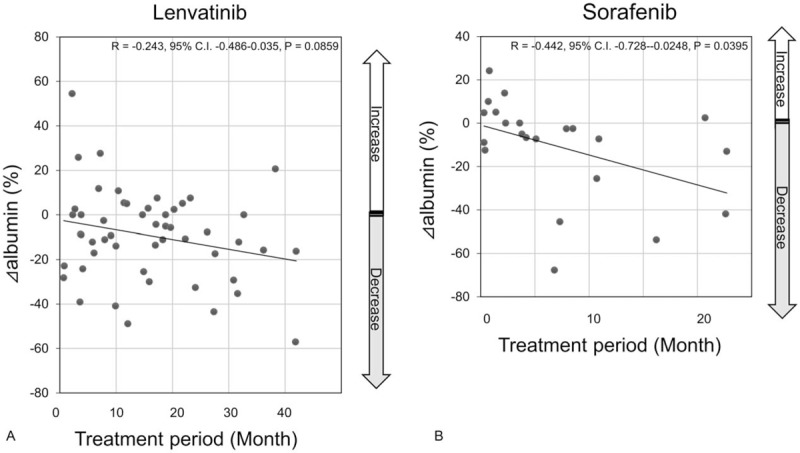
Scatter plots of Δalbumin values for the TKI treatment period. The horizontal axis represents the treatment period (month), and the vertical axis represents the Δalbumin. R, correlation coefficient.
Figure 3.
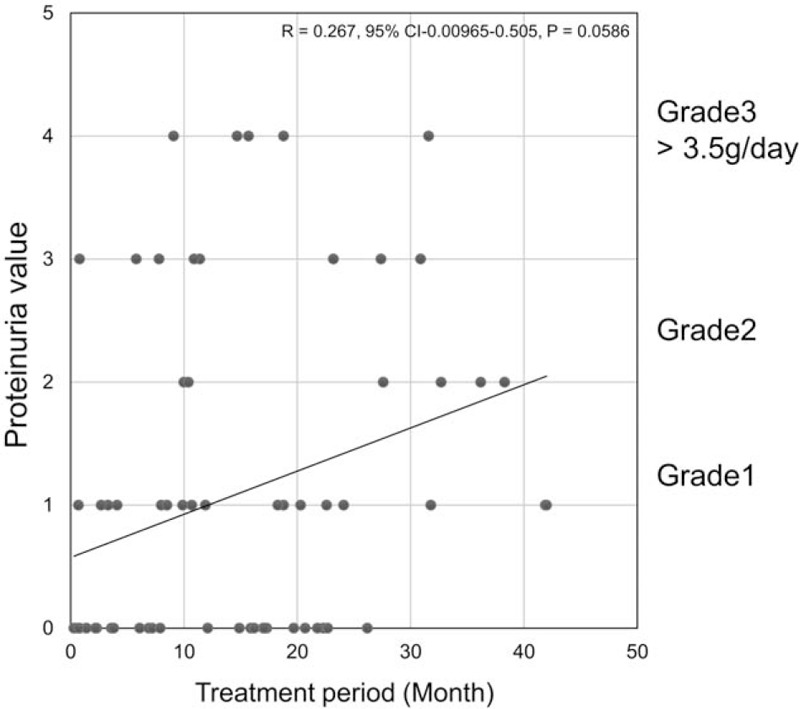
Scatter plots of maximum proteinuria values for the TKI treatment period. The horizontal axis represents the treatment period (month), and the vertical axis represents the maximum proteinuria value (from 0 to +4). R, correlation coefficient. Graph A demonstrates lenvatinib group, and graph B demonstrates sorafenib group.
Table 3.
Multiple regression analysis performed using ΔeGFR% as objective variables.
Two patients (3.9%) with diabetes receiving lenvatinib had to discontinue therapy due to renal dysfunction (Fig. 1). However, TKI discontinuation resulted in progressive disease, and both the patients resumed lenvatinib therapy at a reduced dose. All other cases are continuing treatment, and there are no other cases where TKI treatment was discontinued due to renal dysfunction.
4. Discussion
Although the precise mechanism of proteinuria onset during TKI treatment has not yet been elucidated,[14] it is speculated that the glomerular structure and filtration failure are caused by the inhibition of vascular endothelial growth factor production, which is important for glomerular epithelial cells.[15] Blood pressure control is also important as it reduces glomerular internal pressure and decreases proteinuria.[6] Proteinuria reportedly occurs in a dose-dependent manner, although its incidence differs with each anti-angiogenic TKI. For example, higher dosages of bevacizumab have been associated with an increased risk of proteinuria.[16] In one study, 80% (n = 28), 64% (n = 16), and 80% (n = 35) of patients on pazopanib, bevacizumab, and everolimus, respectively, were managed at the same dose at peak proteinuria with continued monitoring.[17] In cases where Grade 2 or higher proteinuria develops during treatment, dosage reduction or withdrawal, followed by the readministration of a lower dose, is often the course of action.[18] Although the continuous monitoring of renal function and the implementation of proteinuria coping strategies are helpful, patients who develop nephrotic syndrome during the administration of various anti-angiogenic TKIs have been reported.[19–21] Two cases of renal failure have also been reported for the first time with lenvatinib.[9] In contrast, another study reported that renal function does not fail even if it declines after TKI drug treatment.[22]
The incidence of proteinuria (all grades) in the phase 3 study of (E7080) Lenvatinib in Differentiated Cancer of the Thyroid (SELECT)[2] was 31%, which was not reported in the Decision test.[23] The incidence of proteinuria during sorafenib administration to 3335 patients with advanced renal cell carcinoma was purportedly only 0.71%, and no serious cases were reported (https://pharma-navi.bayer.jp/nexavar/static/pdf/usage-safty/rcc201504.pdf). These data are obtained from Bayer Yakuhin, Ltd. However, our results showed a much higher incidence of proteinuria for both lenvatinib (60.8%) and sorafenib (27.8%), together with decreased eGFR and serum albumin levels. This heightened incidence of proteinuria occurred probably because patients included in our study were taking TKIs long-term. Nonetheless, renal dysfunction did not differ significantly with either drug, although this adverse event was obviously more prevalent with lenvatinib as 11 patients had to reduce the dose or discontinue treatment. It has been suggested that sorafenib does not exacerbate proteinuria or renal impairment induced by lenvatinib and may be an effective treatment option for patients with RAI-refractory DTC who are unable to tolerate lenvatinib. Thus, switching from lenvatinib to sorafenib before renal function deteriorates may be an effective option.[24]
Of those patients on lenvatinib who underwent dose reduction or withdrawal, then readministration of a lower dose, all are continuing treatment, and there were no other patients in whom treatment was discontinued due to renal dysfunction. This not only implies a state of reversible damage[25] but also indicates that our program of dose reduction and/or withdrawal, followed by readministration is reasonable. Although it may be reasonable to continue therapy at the same initial dose in cases of Grade 1 or 2 proteinuria, treatment modification or discontinuation may be warranted with Grade 3 or 4.[17,18]
This retrospective study has some limitations. First, it is a retrospective study with a small sample size. Second, while semiquantitative determination of proteinuria is fast, readily available, and inexpensive, there are major discrepancies between dipstick and 24-hour proteinuria testing, especially because it does not enable the exact quantification of urine protein concentration. Furthermore, our results cannot be extrapolated or generalized to other populations.
Overall, the present results demonstrated that renal function is negatively affected by long-term TKI treatment for RAI-refractory DTC. However, heightened proteinuria, decreased eGFR and albumin levels, and significant but apparently reversible renal dysfunction were more frequent with lenvatinib than sorafenib. For those cases of diagnosed renal dysfunction (lenvatinib only), the implementation of our program of dosage reduction or withdrawal, followed by the readministration of a lower dose successfully reversed severe symptoms and was judged to be reasonable. Since TKI treatment of DTC is typically long-term, renal function should be continually monitored until the treatment is discontinued permanently.
Acknowledgments
The authors thank Akira Yoshida and Yoshio Kure for referring several patients to our hospital.
ORCID: 0000-0002-9724-8525
Author contributions
Conceptualization: Hiroyuki Iwasaki, Hirotaka Takasaki, Rika Sakai.
Data curation: Haruhiko Yamazaki, Hirotaka Takasaki, Nobuyasu Suganuma, Soji Toda.
Formal analysis: Rika Sakai, Hirotaka Nakayama, Soji Toda, Katsuhiko Masudo.
Investigation: Hiroyuki Iwasaki, Haruhiko Yamazaki.
Methodology: Hirotaka Takasaki, Hirotaka Nakayama.
Project administration: Hiroyuki Iwasaki.
Supervision: Hiroyuki Iwasaki, Katsuhiko Masudo.
Validation: Nobuyasu Suganuma.
Writing – original draft: Hiroyuki Iwasaki.
Hiroyuki Iwasaki orcid: 0000-0002-9724-8525.
Footnotes
Abbreviations: DTC = differentiated thyroid cancer, eGFR = estimated glomerular filtration rate, RAI = radioactive iodine, TKI = tyrosine kinase inhibitor.
How to cite this article: Iwasaki H, Yamazaki H, Takasaki H, Suganuma N, Sakai R, Nakayama H, Toda S, Masudo K. Renal dysfunction in patients with radioactive iodine-refractory thyroid cancer treated with tyrosine kinase inhibitors. Medicine. 2019;98:42(e17588).
The authors report no conflicts of interest.
References
- [1].Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014;384:319–28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621–30.. [DOI] [PubMed] [Google Scholar]
- [3].Siemann DW, Brazelle WD, Jurgensmeier JM. The vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor cediranib (Recentin; AZD2171) inhibits endothelial cell function and growth of human renal tumor xenografts. Int J Radiat Oncol Biol Phys 2009;73:897–903.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhu X, Wu S, Dahut WL, et al. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 2007;49:186–93.. [DOI] [PubMed] [Google Scholar]
- [5].Izzedine H, Rixe O, Billemont B, et al. Angiogenesis inhibitor therapies: focus on kidney toxicity and hypertension. Am J Kidney Dis 2007;50:203–18.. [DOI] [PubMed] [Google Scholar]
- [6].Izzedine H, Massard C, Spano JP, et al. VEGF signalling inhibition-induced proteinuria: mechanisms, significance and management. Eur J Cancer 2010;46:439–48.. [DOI] [PubMed] [Google Scholar]
- [7].Zhang ZF, Wang T, Liu LH, et al. Risks of proteinuria associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a systematic review and meta-analysis. PLoS One 2014;9:e90135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kiyota N, Schlumberger M, Muro K, et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci 2015;106:1714–21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cavalieri S, Cosmai L, Genderini A, et al. Lenvatinib-induced renal failure: two first-time case reports and review of literature. Expert Opin Drug Metab Toxicol 2018;14:379–85.. [DOI] [PubMed] [Google Scholar]
- [10].Kandula P, Agarwal R. Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int 2011;80:1271–7.. [DOI] [PubMed] [Google Scholar]
- [11].Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–92.. [DOI] [PubMed] [Google Scholar]
- [12].Kanda Y. Investigation of the freely available easy-to-use software ’EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Iwasaki H, Yamazaki H, Takasaki H, et al. Treatment outcomes of differentiated thyroid cancer with distant metastasis improve by tyrosin kinase inhibitors. Oncol Lett 2019;17:5292–300.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Advani A, Kelly DJ, Advani SL, et al. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc Natl Acad Sci U S A 2007;104:14448–53.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu S, Keresztes RS. Antiangiogenic agents for the treatment of nonsmall cell lung cancer: characterizing the molecular basis for serious adverse events. Cancer Invest 2011;29:460–71.. [DOI] [PubMed] [Google Scholar]
- [16].Wu S, Kim C, Baer L, et al. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol 2010;21:1381–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Land JD, Chen AH, Atkinson BJ, et al. Proteinuria with first-line therapy of metastatic renal cell cancer. J Oncol Pharm Pract 2016;22:235–41.. [DOI] [PubMed] [Google Scholar]
- [18].Hainsworth JD, Spigel DR, Burris HA, 3rd, et al. Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol 2010;28:2131–6.. [DOI] [PubMed] [Google Scholar]
- [19].Overkleeft EN, Goldschmeding R, van Reekum F, et al. Nephrotic syndrome caused by the angiogenesis inhibitor sorafenib. Ann Oncol 2010;21:184–5.. [DOI] [PubMed] [Google Scholar]
- [20].Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 2008;358:1129–36.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Costero O, Picazo ML, Zamora P, et al. Inhibition of tyrosine kinases by sunitinib associated with focal segmental glomerulosclerosis lesion in addition to thrombotic microangiopathy. Nephrol Dial Transplant 2010;25:1001–3.. [DOI] [PubMed] [Google Scholar]
- [22].Boursiquot BC, Zabor EC, Glezerman IG, et al. Hypertension and VEGF (vascular endothelial growth factor) receptor tyrosine kinase inhibition: effects on renal function. Hypertension 2017;doi: 10.1161/HYPERTENSIONAHA.117.09275. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brose MS, Nutting CM, Sherman SI, et al. Rationale and design of decision: a double-blind, randomized, placebo-controlled phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer 2011;11:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Goto H, Kiyota N, Otsuki N, et al. Successful treatment switch from lenvatinib to sorafenib in a patient with radioactive iodine-refractory differentiated thyroid cancer intolerant to lenvatinib due to severe proteinuria. Auris Nasus Larynx 2018;45:1249–52.. [DOI] [PubMed] [Google Scholar]
- [25].Patel TV, Morgan JA, Demetri GD, et al. A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. J Natl Cancer Inst 2008;100:282–4.. [DOI] [PubMed] [Google Scholar]


