Abstract
Background:
To evaluate if splenectomy results in severely impaired immune responses against primary cytomegalovirus (CMV) infection compared to the general immunocompetent population.
Methods:
We performed a systemic literature review to study CMV infections in splenectomized individuals, a special population group presently considered immunocompetent to viral infections. We retrieved 30 cases with established CMV infection post-splenectomy and we recorded their disease manifestations, laboratory findings, immunological studies, and histopathology reports. In addition, we retrieved numerous multidisciplinary articles in view of post-splenectomy immunology defects, as well as of immune responses to primary invading CMV in the absence of the spleen. Two clinical studies directly comparing splenectomized with nonsplenectomized individuals under severe iatrogenic immunosuppression as well as the numerically largest review articles of CMV infections in immunocompetent were retained.
Results:
Splenectomy results in the loss of spleen's ability to fend-off blood-borne pathogens and impairs the link between innate and adaptive immunity. The major post-splenectomy immune-defects against CMV are: weakened, delayed or absent anti-CMV IgM, and compensatory marked IgG response; severely impaired B-cell and CD4+, CD8+ T-cells function responses; and post-splenectomy, bone marrow compensates for the absence of spleen's immune responses against CMV, mimicking a monoclonal T-cell lymphoproliferative process.
Conclusion:
The puzzled diagnosis of the CMV syndrome post-splenectomy is of the most challenging and misleading, resulting in risky and costly interventions and a subsequent prolonged hospitalization (2 months). The mounting multi-disciplinary literature evidence renders us to suggest that splenectomized individuals are not only prone to encapsulated bacteria but also behave as immunocompromised to CMV.
Keywords: cytomegalovirus, ganciclovir, immunity, immunocompromise, splenectomized, splenectomy
1. Introduction
Cytomegalovirus (CMV) is a ubiquitous herpesvirus (HHV-5, β-herpesvirus) highly prevalent worldwide (50%–100% seropositive adult individuals).[1] It is usually acquired early in life and is well-controlled by an intact host immune system. Infection is generally asymptomatic/subclinical or may present as a self-limiting mononucleosis-like syndrome.[2] However, when the immune system has not fully developed yet (fetus and neonates), or when suppressed by a disease (acquired immunodeficiency syndrome [AIDS]) or treatment (transplant recipients, chemotherapy in oncologic/hematologic patients), the infection may be severe or fatal. After primary infection, CMV establishes latency/persistence.[3] Viral DNA has been detected in monocytes, dendritic cells (DCs), megakaryocytes, and myeloid progenitor cells in the bone marrow (BM).[4]
Besides primary infection and reactivation, reinfection with a different CMV strain can cause recurrent CMV infection of mothers who were CMV seropositive during pregnancy with subsequent intrauterine viral transmission to infants.[5] The latter results in symptomatic congenital infection, which is a major public health problem causing brain damage, sensorineural hearing loss, and visual impairment. Such reinfection can also occur during organ transplantation from a donor with preexisting immunity against 1 strain of CMV to a recipient with antibodies against another strain, resulting in CMV transmission.
It is well-known that splenectomized individuals are prone to life-threatening infections by encapsulated bacteria. Severe blood protozoan infections (malaria, babesiosis) and cases of Capnocytophaga canimorsus sepsis and meningitis have also been reported.[6] However, the importance of viral infections post-splenectomy is poorly studied, or even ignored.
2. Methods
2.1. Ethical review
The meta-analysis data was from published research studies. Therefore, ethical review is not applicable.
2.2. Literature search
We performed a systemic literature review of CMV infections in splenectomized individuals who had no medical history of immunosuppression. PubMed and Scopus were searched between 1960 and April 2019. Search terms applied were “Cytomegalovirus,” “infection,” “immunocompetent,” “splenectomized,” or “splenectomy” in various combinations. English-, non-English-language literature and citations within the retrieved papers were carefully reviewed.
2.3. Study selection criteria
We included each established case of CMV infection following splenectomy, with the requisite condition that the patient was apparently immunocompetent, as defined by the absence of immunodeficiency syndromes, AIDS, hematological/oncological malignancies, and immunosuppressive therapy administered for any cause. Laboratory CMV diagnosis was established by at least one of the following methods: serology (immunoglobulin M [IgM] and IgG antibodies) in paired specimens obtained at least 2 to 4 weeks apart; detection of CMV-DNA in biological samples or of CMV protein pp65 antigenemia; characteristic viral inclusion bodies in tissue samples; and positive CMV cultures of any specimen. A laboratory CMV diagnosis should necessarily accompany clinical manifestations and laboratory features consistent with CMV mononucleosis with or without end-organ involvement to be finally eligible for inclusion. Other causes of infectious mononucleosis should have been excluded in each eligible case-study.
2.4. Study collection process
Data were collected independently from every eligible study and were extracted on a piloted form, comprising: demographics, medical history, time and etiology of splenectomy, presenting symptoms, laboratory findings, diagnostics, disease duration, treatment, and outcome. No assumptions or simplifications were made. Means and median values of numeric data were calculated.
3. Results
The literature search yielded 125 articles with potential relevance to our study. Most of them were excluded because they referred to CMV infections in nonsplenectomized, or to CMV-related spontaneous splenic rupture, immune thrombocytopenia, and hemolytic anemia. Totally, 20 studies reporting on 30 different patients were considered eligible for inclusion.[7–26]
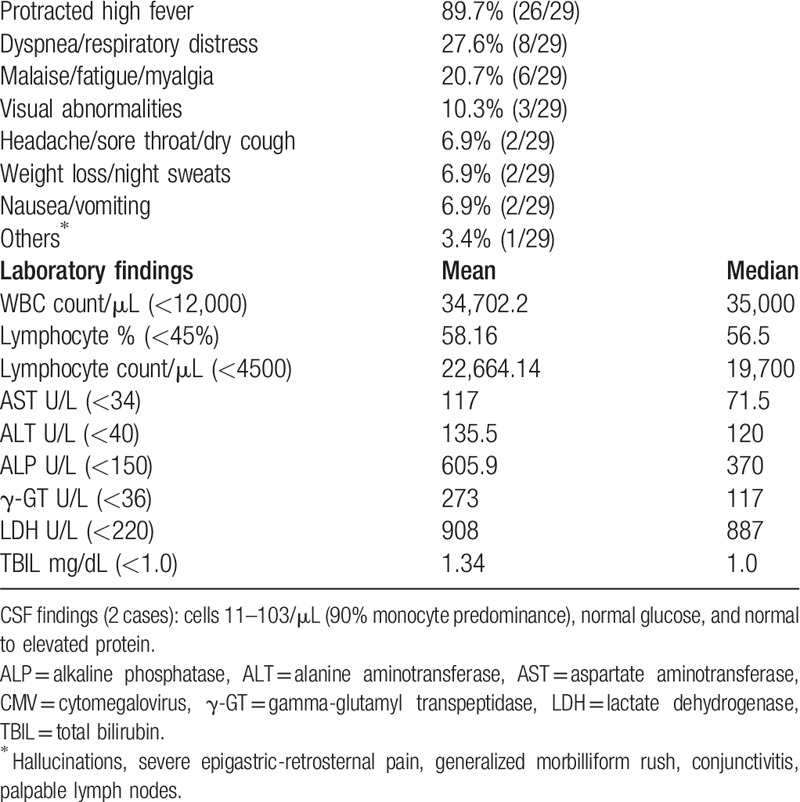
Patients’ mean age was 36-year-old with male predominance. The most common etiology of splenectomy was injury (Table 1). Typically, CMV presented with protracted daily spiking (peak 39.7oC) fever pattern. On auscultation, chest rales and bilateral diffuse crackles were found in the one-third. Clinical-laboratory features are shown in Table 2. The radiological features in cases with pneumonitis were bilateral interstitial infiltrates with a micronodular interstitial pattern of both lungs toward the lower lobes, with/without pleural effusions.
Table 1.
Clinical and demographic data of the retrieved splenectomized cases (n = 30).
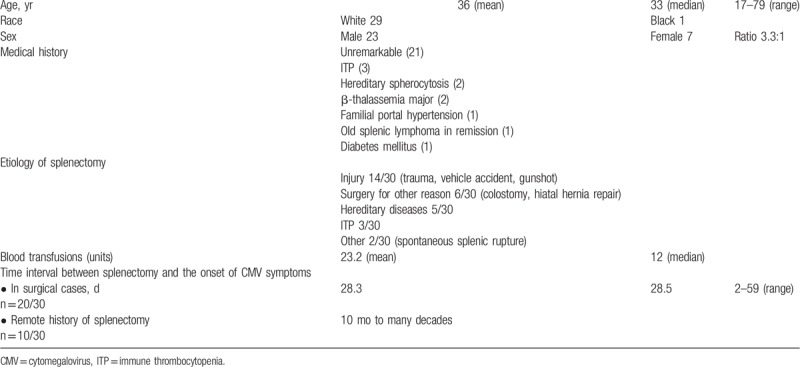
Table 2.
Clinical manifestations (data available for 29 patients) and laboratory findings in splenectomized with severe, primary CMV infection.
The CMV diagnosis was based upon serology alone (10/30 cases) or in combination with other methods (20/30). Before 1984, total CMV antibody titers were determined by complement fixation techniques, and thereafter by immunofluorescence and/or enzyme-linked immunosorbent assay. Weakly positive or negative IgM (8/16) and strong IgG (6/16) responses were detected. CMV cultures were positive in 9 cases (urine 5, throat 3, blood and saliva each 1 of 2, and an autopsy liver culture). Molecular techniques were applied in 10 cases including reverse transcription-polymerase chain reaction (rt-PCR) for the detection of CMV viremia, and immunofluorescent assays for the detection of CMV proteins (pp65) in peripheral leukocytes (range 2–85 cells/100,000 leukocytes). Positive PCR was also reported in ocular samples (vitreous and anterior chamber), and in a bronchoalveolar lavage (BAL) specimen. Histopathology reports showed: disseminated interstitial pneumonitis with generalized cytomegalic inclusions in 4, and acute esophagitis with viral inclusions in 1. Liver biopsies revealed: centrilobular necrosis with some viral inclusions in 1; in 2 cases with cholestasis, portal and periportal inflammation, and massive sinusoidal lymphocytic infiltration staining positive for CD3+CD45RO+ (CD4+) and for leukocyte common antigen markers.
Concerning the 10 cases[9,11,12,15,16,20,24] in which laboratory CMV diagnosis was based upon serology alone, 9 of them demonstrated a fourfold or greater increase in CMV-specific IgG titers in paired specimens obtained at least 2 to 8 weeks apart. Necessary and sufficient condition for inclusion in our cohort was for the serological diagnosis to accompany clinical/laboratory features consistent with CMV mononucleosis, a prerequisite fulfilled in all: protracted (mean 33 days) spiking fever, very increased white blood cell (WBC) (mean 47,200/μL) and lymphocytes (mean 27,523/μL) count with atypical forms, and impaired hepatic function. In addition, other common causes of infectious mononucleosis (mostly Epstein-Barr virus, toxoplasma) were excluded. In only 1 case[24] out of the 10, the initial positive serology was not followed by convalescent-phase antibody detection. Authors, however, concluded that their patient suffered an acute CMV infection because of his immediate and impressive response to ganciclovir when commenced (therapeutic criterion).
Blood flow cytometry analysis was applied in 7 cases. The most important findings were mature B-cell lymphopenia, low T-helper (CD3+CD4+), increased T-cytotoxic (CD3+CD8+) cells, with inversion of the CD4+/CD8+ ratio. T-cells expressing CD3+, CD8+, and CD57+ markers (large granular lymphocytes [LGLs]) were increased in the blood and BM. Abnormalities of the natural killer (NK) cells were also reported such as increased number with the expression of CD56+ and CD16+. BAL cytometry results (2 cases) were in accordance with blood cytometry: decreased T-helper, elevated T-suppressor/cytotoxic cells with inversion of the CD4+/CD8+ ratio. BM examination demonstrated hypercellular marrow in 2 cases; in another, increased LGLs with T-cell receptor (TCRγ) gene rearrangements were detected; in the fourth case, increased cytotoxic T-cells/LGLs accounted for 68% of the lymphocytes, while 50% of the CD3+/CD8+ cells showed expression of TCR V-β. TCR β gene rearrangements were also demonstrated by PCR.
In terms of the CMV disease treatment data were available for 28 cases. In 12, treatment was supportive. In 10, a remarkably large number of advanced, broad-spectrum antibiotics (third-generation cephalosporins, piperacillin-tazobactam, quinolones, carbapenems, vancomycin, daptomycin, and teicoplanin) were administered revealing how puzzled the diagnosis of post-splenectomy CMV disease is, especially in the settings of the protracted fever and of the marked leukocytosis/lymphocytosis. Ganciclovir iv (5 mg/kg body weight) was commenced in 8 cases for a duration of 1 to 3 weeks; oral ganciclovir was given in 2 for treatment completion (2 weeks and 6 months for recurrent retinitis); ganciclovir ocular implants and intravitreal injections were also administered. Valganciclovir per os (3 weeks) was administered in 3 cases; foscarnet (2 weeks), oseltamivir, and prednisone in 1 case each. Hospitalization was very prolonged (mean 56.3 days), another remarkable finding reflecting the severity and the difficulty in the timely diagnosis of the CMV syndrome post-splenectomy. As for outcome results, 4 out of the 29 patients succumbed (mortality rate 13.8%). Three died from bilateral interstitial pneumonitis, and another from “generalized cytomegalic inclusion disease.” Among those who were administered ganciclovir, only 1 succumbed, but he was commenced on ganciclovir too late in the disease course.[17] In addition, ganciclovir was approved for medical use only after 1989, when already 19/30 cases had occurred; therefore, no safe conclusions about its efficacy can be drawn. We could speculate that the mortality rate in our cohort could have underestimated the real magnitude of the CMV disease mortality post-splenectomy, considering that almost all of our study-cases were young and they had no other comorbidities affecting immune responses (diabetes, renal failure, heart failure, etc).
An interesting issue arising from this analysis is whether our cohort patients suffered primary CMV infection or reactivation and if a splenectomy is associated with reactivation in CMV-positive patients. CMV reactivation occurs commonly in critically ill patients and is linked to increased length of hospital/intensive care unit stay, duration of mechanical ventilation, severe sepsis, and mortality. Our entire study-cases were considered by their authors as primary infections and in most of them, CMV was acquired from blood transfusions. In 1 case[21], however, when the fever started just 2 days post-surgery, authors quested about the CMV acquisition. Resolving this issue they noted that CMV antigenemia is usually positive within 2 weeks of primary infection (not reactivation),[3,27] as in their case. Reactivated CMV viremia in AIDS, BM transplants or hematology cancers is caused by profound lymphopenia and/or dysfunction of lymphocytes, and it is lymphocytes that keep CMV in check in normal individuals.[27] On the contrary, their patient had prominent lymphocytosis as a vigorous response to the sudden CMV insult. The lack of IgM, on the other hand, should not be taken as evidence of reactivation in the setting of splenectomy because the capability of IgM response by the IgM memory B-cells is gone with the spleen.[28] The short post-splenectomy incubation could be explained by the coincidence that the patient had already been in the incubation period (before the release of anti-CMV IgM from the spleen) and the inflamed spleen was prone to trauma injury resulting in splenectomy that further brought the infection to nearly fulminant scale.
4. Discussion
4.1. Overview of impaired immunology post-splenectomy
In post-splenectomy patient populations, a variety of abnormalities in host defense have been described contributing to an undue susceptibility to infectious agents and their complications.[29] The spleen is the greatest single secondary lymph organ. It fends off blood-borne pathogens (encapsulated bacteria and CMV) and it is crucial in regulating immune homeostasis through its ability to link innate (filtration and phagocytocis) and adaptive (antibody production) immunity. The clearance of antibody- and complement-coated microorganisms by phagocytic cells of the spleen is very rapid and prevents the dissemination of infectious organisms to significant organs (CNS, kidneys, and lungs). Other innate defects post-splenectomy include decreased levels of opsonins (properdin, tuftsin) and a resulting defective alternative pathway complement activation.[6]
Early reports described post-splenectomy impairment in antibody production to intravenously injected antigens,[30] decreased levels of serum IgM[31] and impaired B-cell function in vitro.[32,33] Splenectomized mount a poor antibody response to vaccines and studies suggest that it is the IgM response that is impaired not IgG.[34] The common denominator in splenectomized with increased susceptibility to Streptococcus pneumoniae infections is the lack of IgM memory B-cells which are circulating splenic marginal zone (SMZ) B-cells,[35] and are identified by the expression of CD27+, IgM, and IgD that account for the one-third of peripheral B-cells.[28,36] Memory B-cells are the most sophisticated actor in adaptive immune response: in the germinal centers (GCs) of secondary lymph organs, mature B-cells proliferate, differentiate, mutate their antibody genes, and switch their antibody class (for example from IgM to IgG), during the normal immune response to infection. The final products of the GCs reaction are high-affinity memory B-cells. Several lines of evidence, however, indicate that the IgM memory B-cells can be generated in the absence of GCs.[37] Human IgM memory B-cells cannot be detected in splenectomized patients thus making spleen indispensable for their generation and survival and for protection against infections.[36] A fundamental function of this B-cell population is to generate fast protective responses, as natural antibodies, to T-independent antigens carried by infectious agents such as bacterial polysaccharidic and viral repetitive surface determinants.[28,37]
The effect of splenectomy on cell-mediated immunity is not well-studied. In otherwise healthy adults studied years after traumatic splenectomy, a significant and long-term reduction of the levels of CD4+ T-cells co-expressing the high molecular weight isoform of CD45+, CD45RA+, which characterizes the T-cell subset critically involved in responsiveness to a novel antigen (naïve), has been determined. CD45RA+ show a higher proliferative capacity in response to mitogenic stimulation and respond to an in vitro primary T-cell stimulation[38]; in contrast, CD45RO+ CD4+ T-cells produce a wider variety and increased amounts of T-cell cytokines (interferon [IFN]-g, interleukin [IL]-4, IL-5), they are the predominant T-cell subset responding to recall antigens[39] and remain unchanged post-splenectomy.[29] Splenectomized present impaired primary antibody responses to the inactivated hepatitis A virus vaccine, a clinically relevant T-cell dependent antigen.[29] A statistically significant correlation between decreased relative levels of CD4+ CD45RA+ T-cells with reduced anti-HAV antibody responses was found when splenectomized and control individuals were analyzed together. The later could reflect a disturbance in the relation between memory and naïve CD4+ T-cell subsets as present in the secondary lymphoid tissues, the principal site of induction of an immune response.[29]
Although BM is known as a primary lymphoid organ, it can potentially serve as a secondary one.[40] BM is capable of antigen presentation and initiation of primary T-cell responses, and contains follicle-like structures similar to lymph nodes or spleen. It harbors DCs that capture, process and present blood-borne antigen to naïve CD4+ and CD8+ T-cells resulting in primary immune responses. Systemic antigen application into splenectomized mutant mice lacking secondary lymph organs leads to the generation of primary T-cell responses in the BM, as revealed by the formation of multicellular clusters with DCs and clonal expansion with subsequent cytotoxic T-lymphocyte activity.[40] The frequency of lymphoid follicles/aggregates in the BM can increase during infection, inflammation, and autoimmunity. Benign lymphoid nodules in trephine BM biopsies have been associated with several viral infections (human immunodeficiency virus-myelopathy, hepatitis B virus and hepatitis C virus),[41] mycobacteriosis, fungal, and bacterial infections.[42] GCs, however, are rarely seen in lymphoid aggregates of BM aspirates and when present they are mostly associated with malignancy. There is no evidence of impaired GCs responses in splenectomized individuals. GCs in the BM have never been reported in CMV infection, nor in post-splenectomy CMV cases.
4.2. Post-splenectomy major defective immune responses against CMV
During CMV infection splenomegaly is a common clinical sign. Several cases of CMV-related spontaneous splenic rupture have been reported with the identification of viral inclusions in it.[20] On the other hand, the spleen's role in early innate control of murine CMV replication/spread is well-established initially through the production of IFαβ by the SMZ cells with a subsequent promotion of NK-cytotoxicity; splenic plasmacytoid DCs eventually are the major producers of IFNαβ, TNF, and IL-12 against murine CMV infection.[43,44] In short, the above data complement each other in intimating that spleen is a replication site of and thus, early immunity against CMV.[20]
4.2.1. Importance of IgM antibodies in primary encounter with viruses. Weakened, delayed, or even absent anti-CMV IgM response post-splenectomy and compensatory marked IgG response
IgM antibodies inhibit infection by directly enhancing phagocytosis in lymphoid tissue by activating complement and by priming the adaptive immune responses. Primary immunodeficiency patients with very low serum IgM levels <0.2 g/L show increased susceptibility to several respiratory viruses.[45] The low initial serum anti-Japanese encephalitis virus IgM at admission in pediatric patients is an independent risk factor for death or severe neurologic deficits.[46] Low levels of serum anti-West Nile virus IgM antibodies are an independent risk factor for mortality because the early neutralizing IgM response limits viremia and dissemination into the CNS.[47] The natural IgM antibodies, secreted by the IgM IgD CD27+ subset of B-cells that is lost post-splenectomy, after infection with vesicular stomatitis virus, vaccinia virus, or lymphocytic choriomeningitis virus restrict virus distribution and early dissemination and also direct virus to secondary lymphatic organs to accelerate immune responses.[48] In mice, humoral responses to influenza virus infection are provided by both B-1 and B-2 cells. Natural IgM, secreted mainly by CD5+B(B-1) cells which are the murine counterpart of human IgM memory B-cells,[36] constitute most of the circulating IgM and tend to be poly-reactive to both foreign and self-components.[49] The spleen is indispensable for the generation and survival of B(B-1) cells.[50]
Many authors have underlined the problematic IgM response observed in primary CMV infection post-splenectomy.[16,20,21,23,25] A remarkable finding in half of our splenectomized cohort also was the low or totally absent anti-CMV IgM antibody titers, anytime tested during the disease course. Considering the numerous literature data mentioned above we could suggest 1 cardinal reason why splenectomized individuals are prone to primary CMV infection: the insufficient production of IgM antibodies in the absence of IgM IgD CD27+ subset of memory B-cells. In contrast, a marked IgG response was present in all the cases with inadequate IgM response. This strong IgG component could be attributed to the host's attempt to compensate for the prolonged disease without adequate IgM while BM seems to play a crucial role in this response (Section 4.2.3.). The detection of IgM in some cases than no IgM response at all may be attributed to the presence of accessory or residual spleen.[23]
4.2.2. Impaired B- and CD4+/CD8+ T-cells responses in primary CMV disease
Another cardinal observation in our cohort that advocates the immunity defects of splenectomized against CMV is the mature B-cell lymphopenia,[17,20,24] the lowered T-helper (CD3+CD4+),[17,19–25] and the increased T-cytotoxic (CD3+CD8+)[17,19–21,23–25] cells, with inversion of the CD4+/CD8+ ratio.[19–21,23–25] The post-traumatic splenectomy results in a significant and long-term reduction of the levels of CD45RA+CD4+ T-cells which characterize the subset that has been released from the thymus into the periphery but has not yet encountered an antigen (naïve).[29] Marker analysis with monoclonal antibodies of the peripheral blood of splenectomized individuals revealed normal proportions except for CD4+ and B1 (peripheral B-cells) positive cells.[51] The proliferative responses of lymphocytes to phytohemagglutinin were weakened, delayed, or diminished.
4.2.3. BM compensates for the absence of spleen's immune responses against CMV
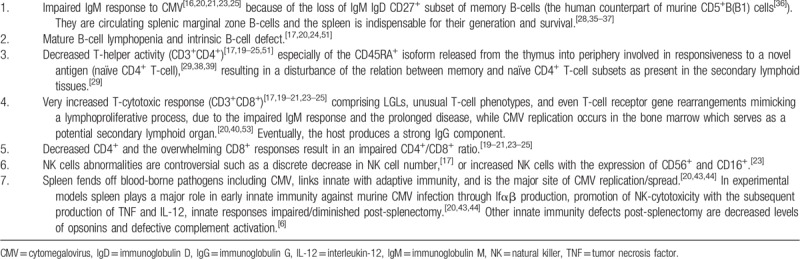
In our cohort, the mean WBC count was markedly elevated and the absolute lymphocyte count was 3-fold increased (22,664/μL) compared to the counts reported for primary CMV infection in the general immunocompetent population (<7000–7500/μL).[16,52] Atypical lymphocytes were seen in 62% of our cases. The lack of splenic storage and sequestration could partially explain the marked lymphocytosis. However, post-splenectomy, CMV replication occurs in the BM, in view of its marked lymphoproliferation without significant lymphadenopathy elsewhere.[20] Cells of the myeloid lineage are one site of CMV carriage in vivo while in myeloid CD34+ DCs progenitors the viral genome is carried latently in the absence of viral lytic gene expression.[53] BM can potentially serve as a secondary lymphoid organ. Naïve, antigen-specific T-cells home to BM, where they can be primed.[40] In our study, blood flow cytometric analysis when performed, showed increased T-cells expressing LGL markers, in some cases with an unusual T-cell phenotype because of the loss of CD5+ and CD7+ markers. It seems that without adequate IgM, CMV causes prolonged illness and the host attempts to compensate by an overwhelming cytotoxic T-cell response and eventually a strong IgG component. BM cytometry showed hypercellular marrow with trilineage hematopoiesis, increased cytotoxic T-cells/LGLs (>50% of lymphocytes), while TCRγ, TCRVβ 5.1, and TCRβ gene rearrangements were demonstrated by PCR indicating a possible monoclonal or oligoclonal T-cell lymphoproliferative process. Table 3 summarizes the major immunology defects against CMV post-splenectomy.
Table 3.
Synopsis of the major multi-factorial defects of host immunity against CMV post-splenectomy that render splenectomized individuals immunocompromised to CMV infection.
4.3. CMV disease in splenectomized versus nonsplenectomized
We acknowledge that we do not provide indisputable proofs that splenectomized behave as immunocompromised against CMV infection. Uncontested evidence would only be drawn from prospective controlled-trials directly comparing them to the immunocompetent general population. There are only two published clinical studies involving this direct comparison: in 700 orthotopic liver transplant recipients under immunosuppressive induction regimen, simultaneous splenectomy increases significantly (P < .05) the risk for the occurrence of opportunistic pneumonia[54] with CMV being the most common pathogen (40%). Authors concluded that even in iatrogenic, severe immunosuppression, splenectomy retains an exceptional and additional immunosuppressive effect. Furthermore, among ABO-incompatible pediatric kidney transplant recipients, 21 were pretreated with rituximab and 14 with splenectomy.[55] In the rituximab group, 6/21 had CMV viremia but no CMV disease; 5/14 of the splenectomy group, however, developed CMV disease with viremia. Authors also concluded that CMV disease was much higher post-splenectomy.
4.4. Limitations and conclusions
Our study has many limitations. It is a retrospective analysis based on a very small number of cases, thus no statistical analysis could be applied. The risk of publication bias cannot be excluded. Nevertheless, the numerous, multi-disciplinary evidence collected in this study, converge to the conclusion that splenectomized behave rather as immunosuppressed against primary CMV infection.
The role of viral infections post-splenectomy has been dramatically underestimated. Characteristically in a very large study of infection in splenectomized, viral infections were even excluded from the inclusion criteria.[56] Despite Stone et al reported in 1967 10 instances of hepatitis developing within a 6-month period following splenectomy with an impressive case-mortality rate of 80%,[57] it seems that the scientific community still considers viral infections post-splenectomy of low or even no importance.
Manifestations of this puzzling syndrome comprise a seriously ill patient with protracted fever, severe lymphocytosis with atypical lymphocytes, mild hepatitis and dyspnea/hypoxia, or mental status disturbances. If timely recognized, unneeded risky interventions (such as biopsies), and time-consuming expensive investigations can be avoided. But most importantly, when postsplenectomy CMV syndrome involves end-organs (lungs, brains, retina, and esophagus), prompt recognition is substantial for the early introduction to antiviral treatment that may be life-saving.
Acknowledgments
We thank Eirini Delikoura, librarian of the “Hippokration” General Hospital's library and the Greek National Documentation Centre which supplied us with several of the searched articles.
Author contributions
Conceptualization: George Dimitrios Liatsos.
Data curation: George Dimitrios Liatsos.
Formal analysis: George Dimitrios Liatsos.
Funding acquisition: George Dimitrios Liatsos.
Investigation: George Dimitrios Liatsos.
Methodology: George Dimitrios Liatsos.
Project administration: George Dimitrios Liatsos.
Resources: George Dimitrios Liatsos.
Software: George Dimitrios Liatsos.
Supervision: George Dimitrios Liatsos.
Validation: George Dimitrios Liatsos.
Visualization: George Dimitrios Liatsos.
Writing – original draft: George Dimitrios Liatsos.
Writing – review & editing: George Dimitrios Liatsos.
George Dimitrios Liatsos orcid: 0000-0002-8203-2748.
Footnotes
Abbreviations: BAL = bronchoalveolar lavage, BM = bone marrow, CMV = cytomegalovirus, CNS = central nervous system, GC = germinal center, IFN = interferon, IL = interleukin, LGLs = large granular lymphocytes, MCMV = murine CMV, NK = natural killer, PCR = polymerase chain reaction, SMZ = splenic marginal zone, TCR = T-cell receptor, WBC = white blood cell.
How to cite this article: Liatsos GD. The immunity features and defects against primary cytomegalovirus infection post-splenectomy indicate an immunocompromised status. Medicine. 2019;98:43(e17698).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Staras SA, Dollard SC, Radford KW, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis 2006;43:1143–51.. [DOI] [PubMed] [Google Scholar]
- [2].Eddleston M, Peacock S, Juniper M, et al. Severe cytomegalovirus infection in immunocompetent patients. Clin Infect Dis 1997;24:52–6.. [DOI] [PubMed] [Google Scholar]
- [3].Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis 2004;4:725–38.. [DOI] [PubMed] [Google Scholar]
- [4].Söderberg-Nauclér C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 1997;91:119–26.. [DOI] [PubMed] [Google Scholar]
- [5].Ishibashi K, Tokumoto T, Shirakawa H, et al. Strain-specific seroepidemiology and reinfection of cytomegalovirus. Microbes Infect 2008;10:1363–9.. [DOI] [PubMed] [Google Scholar]
- [6].Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev 2010;23:740–80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Henson D. Cytomegalic inclusion disease following multiple blood transfusions. JAMA 1967;199:278–80.. [PubMed] [Google Scholar]
- [8].Drew WL, Miner RC. Transfusion-related cytomegalovirus infection following noncardiac surgery. JAMA 1982;247:2389–91.. [PubMed] [Google Scholar]
- [9].Baumgartner JD, Glauser MP, Burgo-Black AL, et al. Severe cytomegalovirus infection in multiply transfused, splenectomized, trauma patients. Lancet 1982;2:63–6.. [DOI] [PubMed] [Google Scholar]
- [10].Kapsenberg JG, Langenhuysen AC, Nieweg HO, et al. Post transfusion mononucleosis with heterophil antibodies. Simultaneous infection with cytomegalovirus and EB virus. Acta Med Scand 1970;187:79–82.. [PubMed] [Google Scholar]
- [11].Lerner PI, Sampliner JE. Transfusion-associated cytomegalovirus mononucleosis. Ann Surg 1977;185:406–10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Okun DB, Tanaka KR. Profound leukemoid reaction in cytomegalovirus mononucleosis. JAMA 1978;240:1888–9.. [PubMed] [Google Scholar]
- [13].Lanng C, Bentsen KD. Cytomegalovirus infection after splenectomy. Ugeskr Laeger 1984;146:1129–31.. [PubMed] [Google Scholar]
- [14].Villar LA, Massanari RM, Mitros FA. Cytomegalovirus infection with acute erosive esophagitis. Am J Med 1984;76:924–8.. [DOI] [PubMed] [Google Scholar]
- [15].Rader DL, Mucha P, Jr., Moore SB, et al. Cytomegalovirus infection in patients undergoing noncardiac surgical procedures. Surg Gynecol Obstet 1985;160:13–6.. [PubMed] [Google Scholar]
- [16].Horwitz CA, Henle W, Henle G, et al. Clinical and laboratory evaluation of cytomegalovirus-induced mononucleosis in previously healthy individuals. Report of 82 cases. Medicine (Baltimore) 1986;65:124–34.. [DOI] [PubMed] [Google Scholar]
- [17].deGórgolas Hernández-Mora M, Jiménez Moreno A, Fernández Guerrero ML, et al. Fatal infection by cytomegalovirus in a patient after splenectomy and transfusion following trauma. Med Clin (Barc) 2001;116:439. [PubMed] [Google Scholar]
- [18].Watanabe S, Hanatsuka K, Yoshizawa M, et al. Cytomegalovirus infection in a patient after splenectomy. Nihon Naika Gakkai Zasshi 2003;92:2253–4.. [DOI] [PubMed] [Google Scholar]
- [19].Vote B, Russell M, Polkinghorne P. Recurrent cytomegalovirus retinitis in a patient with a normal lymphocyte count who had undergone splenectomy for lymphoma. Retina 2005;25:220–1.. [DOI] [PubMed] [Google Scholar]
- [20].Han XY, Lin P, Amin HM, et al. Post splenectomy cytomegaloviral mononucleosis: marked lymphocytosis, TCR gamma gene rearrangements, and impaired IgM response. Am J Clin Pathol 2005;123:612–7.. [DOI] [PubMed] [Google Scholar]
- [21].Assy N, Gefen H, Schlesinger S, et al. Reactivation versus primary CMV infection after splenectomy in immunocompetent patients. Dig Dis Sci 2007;52:3477–9.. [DOI] [PubMed] [Google Scholar]
- [22].Hoang QV, Simon DM, Kumar GN, et al. Recurrent CMV retinitis in a non-HIV patient with drug-resistant CMV. Graefes Arch Clin Exp Ophthalmol 2010;248:737–40.. [DOI] [PubMed] [Google Scholar]
- [23].Han XY, Hellerstedt BA, Koller CA. Postsplenectomy cytomegalovirus mononucleosis is a distinct clinicopathologic syndrome. Am J Med Sci 2010;339:395–9.. [DOI] [PubMed] [Google Scholar]
- [24].Torres D, Parrinello G, Bellanca M, et al. Salvage treatment with ganciclovir in a splenectomized, polytransfused patient affected by systemic inflammatory response syndrome. Int J Immunopathol Pharmacol 2014;27:267–72.. [DOI] [PubMed] [Google Scholar]
- [25].Liatsos GD, Pirounaki M, Lazareva A, et al. Cytomegalovirus infection in a splenectomized with β-thalassemia major: immunocompetent or immunosuppressed? Clin Case Rep 2017;5:1063–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fragkiadakis K, Ioannou P, Papadakis JA, et al. Cytomegalovirus pneumonitis in a patient with homozygous β-thalassemia and splenectomy. Jpn J Infect Dis 2018;71:370–2.. [DOI] [PubMed] [Google Scholar]
- [27].Slifkin M, Tempesti P, Poutsiaka DD, et al. Late and atypical cytomegalovirus disease in solid-organ transplant recipients. Clin Infect Dis 2001;33:E62–8.. [DOI] [PubMed] [Google Scholar]
- [28].Kruetzmann S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells controlling streptococcus pneumoniae infections are generated in the spleen. J Exp Med 2003;197:939–45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wolf HM, Eibl MM, Georgi E, et al. Long-term decrease of CD4 + CD45RA+ T cells and impaired primary immune response after post-traumatic splenectomy. Br J Haematol 1999;107:55–68.. [DOI] [PubMed] [Google Scholar]
- [30].Sullivan JL, Ochs HD, Schiffman G, et al. Immune response after splenectomy. Lancet 1978;1:178–81.. [DOI] [PubMed] [Google Scholar]
- [31].Eibl M. Immunological consequences of splenectomy. Prog Pediatr Surg 1985;18:139–45.. [DOI] [PubMed] [Google Scholar]
- [32].Drew PA, Kiroff GK, Ferrante A, et al. Alterations in immunoglobulin synthesis by peripheral blood mononuclear cells from splenectomized patients with and without splenic regrowth. J Immunol 1984;132:191–206.. [PubMed] [Google Scholar]
- [33].Muller C, Mannhalter JW, Ahmad R, et al. Peripheral blood mononuclear cells of splenectomized patients are unable to differentiate into immuno-globulin-secreting cells after pokeweed mitogen stimulation. Clin Immunol Immunopathol 1984;31:118–23.. [DOI] [PubMed] [Google Scholar]
- [34].Molrine DC, Siber GR, Samra Y, et al. Normal IgG and impaired IgM responses to polysaccharide vaccines in asplenic patients. J Infect Dis 1999;179:513–7.. [DOI] [PubMed] [Google Scholar]
- [35].Weller S, Braun MC, Tan BK, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 2004;104:3647–54.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Carsetti R, Pantosti A, Quinti I. Impairment of the antipolysaccharide response in splenectomized patients is due to the lack of immunoglobulin M memory B cells. J Infect Dis 2006;193:1189–90.. [DOI] [PubMed] [Google Scholar]
- [37].Weller S, Faili A, Garcia C, et al. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci U S A 2001;98:1166–70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Young JL, Ramage JM, Gaston JS, et al. In vitro responses of human CD45RObrightRA- and CD45RO-RAbright T cell subsets and their relationship to memory and naive T cells. Eur J Immunol 1997;27:2383–90.. [DOI] [PubMed] [Google Scholar]
- [39].Sanders ME, Makgobam MW, Shaw S. Human naïve and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today 1998;9:195–9.. [DOI] [PubMed] [Google Scholar]
- [40].Feuerer M, Beckhove P, Garbi N, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med 2003;9:1151–7.. [DOI] [PubMed] [Google Scholar]
- [41].Thiele J, Zirbes TK, Kvasnicka HM, et al. Focal lymphoid aggregates (nodules) in bone marrow biopsies: differentiation between benign hyperplasia and malignant lymphoma – a practical guideline. J Clin Pathol 1999;52:294–300.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Naemi K, Brynes RK, Reisian N, et al. Benign lymphoid aggregates in the bone marrow: distribution patterns of B and T lymphocytes. Hum Pathol 2013;44:512–20.. [DOI] [PubMed] [Google Scholar]
- [43].Hsu KM, Pratt JR, Akers WJ, et al. Murine cytomegalovirus displays selective infection of cells within hours after systemic administration. J Gen Virol 2009;90:33–43.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Loewendorf A, Benedict CA. Modulation of host innate and adaptive immune defenses by cytomegalovirus: timing is everything. J Intern Med 2010;267:483–501.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kainulainen L, Vuorinen T, Rantakokko-Jalava K, et al. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol 2010;126:120–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Libraty DH, Nisalak A, Endy TP, et al. Clinical and immunological risk factors for severe disease in Japanese encephalitis. Trans R Soc Trop Med Hyg 2002;96:173–8.. [DOI] [PubMed] [Google Scholar]
- [47].Diamond MS, Sitati EM, Friend LD, et al. A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med 2003;198:1853–62.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ochsenbein AF, Fehr T, Lutz C, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science 1999;286:2156–9.. [DOI] [PubMed] [Google Scholar]
- [49].Baumgarth N, Herman OC, Jager GC, et al. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med 2000;192:271–80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wardemann H, Boehm T, Dear N, et al. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med 2002;195:771–80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Downey EC, Shackford SR, Fridlund PH, et al. Long-term depressed immune function in patients splenectomized for trauma. J Trauma 1987;27:661–3.. [DOI] [PubMed] [Google Scholar]
- [52].Just-Nübling G, Korn S, Ludwig B, et al. Primary cytomegalovirus infection in an outpatient setting – laboratory markers and clinical aspects. Infection 2003;31:318–23.. [DOI] [PubMed] [Google Scholar]
- [53].Sinclair J. Human cytomegalovirus: latency and reactivation in the myeloid lineage. J Clin Virol 2008;41:180–5.. [DOI] [PubMed] [Google Scholar]
- [54].Neumann UP, Langehr JM, Kaisers U, et al. Simultaneous splenectomy increases risk for opportunistic pneumonia in patients after liver transplantation. Transpl Int 2002;15:226–32.. [DOI] [PubMed] [Google Scholar]
- [55].Hamasaki Y, Aikawa A, Itabashi Y, et al. Efficacy of 2 doses of rituximab on B-cell and antidonor antibody and outcomes of ABO-incompatible living-donor pediatric kidney transplant. Exp Clin Transplant 2019;17:105–9.. [DOI] [PubMed] [Google Scholar]
- [56].Bisharat N, Omari H, Lavi I, et al. Risk of infection and death among post-splenectomy patients. J Infect 2001;43:182–6.. [DOI] [PubMed] [Google Scholar]
- [57].Stone HH, Stanley DG, DeJarnette RH. Post splenectomy viral hepatitis. JAMA 1967;199:851–3.. [PubMed] [Google Scholar]


