Abstract
Background:
Extra virgin olive oil (EVOO) has shown beneficial effects on the lipid profile and inflammatory parameters in general population. Our goal is to analyze these changes together with those of intestinal microbiota in human immunodeficiency virus (HIV)-infected patients over 50 years of age.
Methods:
Experimental single arm open study. HIV patients over the age of 50 with undetectable viral load were selected. EVOO was distributed among the patients so that each one consumed 50 g daily for 12 weeks. Lipid profile, C-reactive protein (CRP), and intestinal microbiota composition were analyzed at the beginning and at the end of the intervention.
Results:
Total cholesterol decreased significantly (5 mg/dL), and a nonsignificant decrease in low-density lipoprotein cholesterol (12 mg/dL), triglycerides (21 mg/dL), and CRP (1.25 mg/dL) was observed. There was a significant increase in alpha diversity after the intervention in men and a decrease in proinflammatory genera such as Dethiosulfovibrionaceae was observed. Differences were also observed in the microbiota of men and women and according to the type of antiretroviral treatment.
Conclusion:
Sustained consumption of 50 g of EVOO in elderly HIV-infected patients might be associated with an improvement in lipid profile and alfa diversity of intestinal microbiota.
Keywords: aging, cholesterol, extra virgin olive oil, HIV, microbiota
1. Introduction
Human immunodeficiency virus (HIV) infection conditions a chronic inflammatory state that is not completely reversed with an effective antiretroviral treatment (ART).[1] This inflammatory state is associated with an increase in the incidence of non-AIDS events, such as cancer or cardiovascular events, among others.[2–4] A key point in maintaining this condition is the intestinal microbiota,[5–7] which is modified by HIV itself, increasing proinflammatory bacterial species, such as Firmicutes and Prevotella and decreasing Bacteroides.[8–10] The use of probiotics has been tested in patients with HIV infection,[11,12] with decreases in inflammatory markers such as IL-6 or D-dimers, as well as prebiotics[13] and symbiotics.[14]
Olive oil is a fundamental component of the Mediterranean diet. Virgin olive oil is obtained by mechanical extraction from olives. Extra virgin olive oil (EVOO) is extracted from the highest quality olives, with an acidity of less than 1%. In patients not infected by HIV, with high vascular risk, daily consumption of 50 g of EVOO has been associated with a decrease in mortality and cardiovascular events (HR 0.69) compared to a low-fat diet.[15] This reduction was dependent on the dose of polyphenols received.[16] These beneficial clinical effects seem to be a result of different mechanisms. A crucial milestone in the formation of atheromatous plaque is the oxidation of low-density lipoprotein (LDL) cholesterol and high-density lipoprotein cholesterol, since these oxidized particles will deposit their content in macrophages. It has been proven how the percentage of oxidized LDL decreases as the intake of polyphenolic compounds in olive oil increases along with an increase in high-density lipoprotein cholesterol levels in a dose-dependent manner.[17] The administration of olive oil rich in polyphenols has also demonstrated the improvement of endothelial function[18] and reduction of levels of inflammatory markers.[19] In addition, nonabsorbable EVOO polyphenols are metabolized by the gut microbiota through decarboxylation and demethylation processes, increasing Lactobacillus[20] and Bacteroidaceae.[21] The increase in Lactobacillus, which codifies a hydroxylase of the bile salts, conditions the division of bile acids, which prevents their reabsorption and they are excreted along with cholesterol in the feces. Moreover, the polyphenols in olive oil have shown to inhibit the growth of intestinal pathogens such as Escherichia coli[22] or Helicobacter pylori[23] in vitro. Different studies have also demonstrated the antiproliferative effect of EVOO in neoplastic cell lines, as well as showing an antioxidant and antiinflammatory effect.[24,25]
The published experience on the effects of the use of EVOO in patients with HIV infection is very scarce,[26] suggesting a positive effect on inflammatory markers such as ultrasensitive C-reactive protein (CRP), so we aimed to observe the effects of daily EVOO intake in a group of patients with chronic HIV infection and long-term ART use, analyzing its effects on the lipid profile, inflammatory parameters, and intestinal microbiota.
2. Methods
An experimental open study of a single arm was performed at the Costa del Sol Hospital (Marbella, Spain) during the first semester of 2018. Patients with chronic HIV infection, on ART for at least 1 year before inclusion in the study or elite controllers, aged between 50 and 75 years at the time of inclusion in the study were selected. They needed to have presented undetectable viral load (VL) for at least 6 months prior to inclusion and a CD4 lymphocyte count in peripheral blood less or equal to 200 cells/μL in the previous 12 months. Previously, a questionnaire on dietary habits was performed on all patients in the cohort over 50 years old, entering the study those who presented figures of daily EVOO consumption of less than 10 g per day. Exclusion criterion was the presentation of diarrhea or antibiotics in the 8 weeks prior to inclusion, consumption of illicit drugs, HBV or HCV coinfection, any AIDS event in the previous year, daily alcohol consumption of at least 20 g, chronic liver disease, inflammatory bowel disease (IBD), previous cardiovascular events and cancer of any type in the previous 5 years (excluding nonmelanoma skin cancer and anal cancer as long as it was in situ carcinoma), and use of nonsteroidal antiinflammatory drugs, corticosteroids, immunosuppressants, or probiotics in the last 12 weeks. After signing the informed consent, blood samples were taken, fecal samples were given by patients for microbiota investigation, and a physical activity questionnaire was completed (International Physical Activity Questionnaire). The study was approved by the local Ethics Committee. Once the samples had been collected, the patients received 750 mL bottles of EVOO donated by Bravoliva Ltd, this EVOO was a mixture of Hojiblanca olive varieties (80%), chamomile (10%), and picuda (10%) with an acidity of 0.25%. Sufficient bottles were given fortnightly to guarantee a daily consumption of 50 mL. In addition, a weekly call was made to patients to reinforce adherence to the intervention and to identify the presence of side effects and to record if diarrhea or antibiotics use had occurred. The intervention was maintained for 12 weeks.
At the end of the intervention, glucose levels, CRP, lipid profile, homeostatic model assessment index, glomerular filtration rate estimated by the Chronic Kidney Disease-Epidemiology Collaboration formula, and N-terminal pro B-type natriuretic peptide levels were determined. HIV-1 VL along with CD4 and CD8 lymphocytes counts was measured. Results are shown as median and interquartile range (IQR) for quantitative variables, and as percentage for qualitative variables. Quantitative values were compared through nonparametric tests (Mann–Whitney U) and Chi square for categorical variables.
Fecal samples were collected in plastic sterile containers from each volunteer and then transferred to −80 °C conservation conditions until analysis. Fecal samples were homogenized in a Stomacher-400 blender. Deoxyribonucleic acid (DNA) was extracted using a QIAamp DNA Stool Mini Kit (QIAGEN, Barcelona, Spain) as directed by the manufacturer, with the exception that samples were mixed with the lysis buffer and incubated at a temperature of 95 °C instead of 70 °C to ensure lysis of both gram-positive and gram-negative bacteria. Quantification was conducted with a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, DE). The DNA yield was determined by measuring absorbance ratios spectrophotometrically, and the measurement includes A260/280 nm for protein contamination and A260/230 nm for salt and phenol contamination.
Microbiome was analyzed using the V3 chemistry in a MySeq Illumina platform. The extracted DNA was polymerase chain reaction (PCR) amplified using the primer pairs, with a 16S Amplicon PCR Forward Primer: 5′TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG, and 16S Amplicon PCR Reverse Primer: 5′GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC targeting the V3 and V4 hypervariable regions of the bacterial 16S rRNA gene. All PCRs were performed in 25 μL reaction volumes containing 12.5 μL 2X KAPA HiFi Hotstart ready mix (KAPA Biosystems, Woburn, MA), 5 μL of each forward and reverse primers (1 μM) and 2.5 μL of extracted DNA (10 ng) under the following cycling conditions: initial denaturation at 95 °C for 3 minutes, followed by cycles of denaturation at 95 °C for 30 seconds, annealing at 55 °C for 30 seconds, and elongation at 72 °C for 30 seconds, with a final extension at 72 °C for 5 minutes. PCR clean-up was performed using AMPure XP beads (Beckman Coulter, Indianapolis, IN) to purify the 16S V3 and V4 amplicon from free primers and primer dimer species. Then the next step was the PCR index, in this step attaches dual indices and Illumina sequencing adapters using the Nextera XT Index Kit (Illumina, San Diego, CA), PCR conditions were: 95 °C for 3 minutes; 8 cycles of 95 °C for 30 seconds, 55 °C for 30 seconds, 72 °C for 30 seconds; 72 °C for 5 minutes, and hold at 4 °C. The pooled PCR products were purified using AMPure XP beads (Beckman Coulter, Indianapolis, IN) before quantification. The resultant amplicons were sequenced at MiSeq (Illumina, San Diego, CA), using paired-end (2x300nt) Illumina MiSeq sequencing system (Illumina).
2.1. Microbiota analyses
We used Usearch (v11, https://drive5.com/usearch/) suite to make the operational taxonomic unit (OTU) analysis. First, we checked the quality and metrics of the samples using FastQC. Trimming and merging of the reads were done by Usearch command fasq_mergepairs. The fastq_filter command was used to filter the merged sequences, only retaining those that had at least 335 pb and a maximum of 1 error (maxee 1.0). We followed the uparse pipeline from Usearch (http://www.drive5.com/usearch/manual/uparse_pipeline.html) for the OTU analysis. First of all, we dereplicated the sequences, identifying unique sequences. Next we used the clusterotus command to discard single unique reads (with the option, minsize 2), eliminate the chimeras, and cluster the OTUs, using the UPARSE algorithm (http://www.drive5.com/usearch/manual/uparseotu_algo.html). This clusterization was done at 97% identity. The original input sequences were mapped onto the OTUs with 97% similarity using the otutab command. The taxonomy assignation of the OTUs was made using the SINTAX algorithm with the command sintax at 99% of identity, and the GreenGenes database v13.5.
Six different α-diversity indices were estimated: richness/observed OTU, Chao1 Richness, Abundance-based Coverage Estimator, Shannon–Wiener index, Equitability, Simpson, Inverse Simpson, and Dominance for diet, gender, and ART. Differences in α-diversity were estimated using a paired Wilcoxon rank test for diet for univariate analysis and Nonlinear Mixed-Effects Models for multivariate analysis.
For β-diversity analyses, Bray–Curtis (BC), unweighted, and weighted UniFrac distances (wUniFrac) were calculated with permutational multivariate analysis of variance (PERMANOVA) which was performed on the OTU abundance profile of all samples to assess the effect of diet on β-diversity, 999 permutations were used to obtain the permuted P-value in R.
The relative abundance of each OTU was calculated for each sample, and the OTUs were combined at phylum, genus, and species levels. For each sample type, the overall abundance of each level was calculated by taking the sum of relative abundances by levels in all subjects. Taxa with a lower abundance of 1% were joined into the same category.
LefSe analysis (linear discriminant analysis [LDA] effect size) was used for discovery of diet, gender, and ART-related bacterial genus and species taxa. The LefSe algorithm uses a nonparametric factorial Kruskal–Wallis sum rank test, unpaired Wilcoxon rank-sum test, and LDA to estimate the effect size of each differentially abundant OTU/taxon. The threshold on the logarithmic score of LDA analysis was set to 1.0 for diet and ART and set to 2.0 for gender.
All analyses were adjusted for multiple testing using the Benjamini and Hochberg procedure (FDR < 0.05). Statistical analyses were performed using R 3.5.1.
3. Results
A total of 20 patients were recruited, of which 18 completed the intervention. The remaining 2 did not attend the scheduled appointments. Adherence to the intervention was 95%, there were no cases of diarrhea or antibiotic consumption during the study and no interruptions of the intervention.
Median age of the patients was 54.5 years (IQR: 7), 7 (38.9%) were women and 11 (61.1%) were men. Median number of years since diagnosis was 9.5 (IQR: 17) and median duration of ART was 9 years (IQR: 6.5). Regarding the route of infection, 10 (55.6%) reported unprotected heterosexual practices, 4 (22.2%) were men who have sex with men (MSM), and 4 (22.2%) were former injecting drug addicts.
Regarding ART, 8 patients were receiving the combination of tenofovir alafenamide (TAF), emtricitabine (FTC), and rilpivirine (RPV); 3 received TAF, FTC, and elvitegravir-cobicistat; 3 the combination of abacavir (ABC), lamivudine (3TC), and dolutegravir. Three of the remaining 4 cases received a different ART: dual therapy with darunavir-cobicistat (DRV-cb) + RPV, TAF, FTC and DRV-cb and ABC + 3TC + etravirine. One patient was an elite controller and did not receive any ART. The CD4 lymphocyte nadir was 263 cells/μL (IQR: 277).
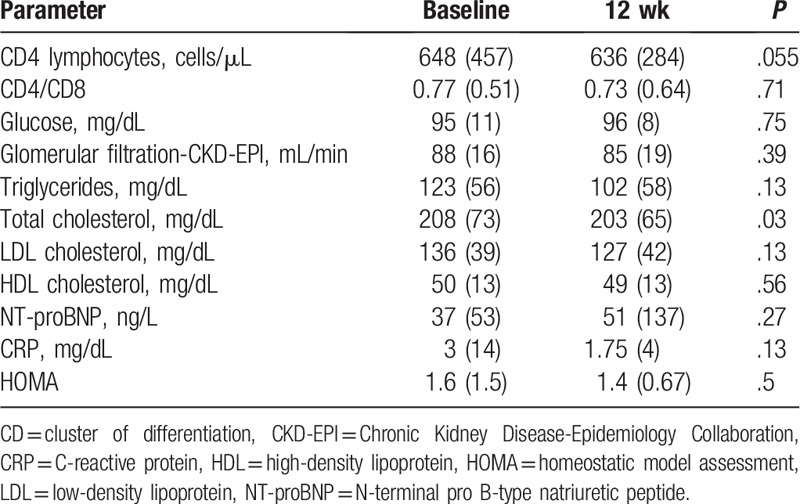
A total of 10 patients (55.6%) did not take nonantiretroviral medication. The rest of the patients took benzodiazepines (3 cases), calcium, vitamin D, and alendronate (3 cases), antagonists of angiostensin II receptors, proton pump inhibitors, statins, and tamsulosin (2 cases for each medication). Seven patients (38.9%) were active smokers, and 5 were ex-smokers (27.8%). Median BMI was 25 kg/m2 (IQR: 4.68). At the time of inclusion in the study, all patients had VL < 50 copies/mL, except one who had VL of 57 copies/mL. At the end of the intervention, all patients had VL < 50 copies/mL. Main results of the different parameters studied at baseline and at 12 weeks of the intervention are shown in Table 1. There were only significant changes in total cholesterol levels, with a substantial decrease in the levels of CRP, LDL cholesterol, and triglycerides.
Table 1.
Results of the main analytical parameters: median and interquartile range.
3.1. Analysis of the microbiota
The samples from 18 patients could be analyzed, but in 2 of them only baseline samples were taken, which makes a total of 32 samples from 16 patients.
When analyzing the alpha diversity indices (Richness, Shannon–Wiener, Chao1, Abundance-based Coverage Estimator, Equitability, Simpson, Dominance, Inverse Simpson), no statistically significant differences were observed before and after the intervention.
However, when adjusting the effect of diet by gender, as can be seen in Figure 1, statistically significant differences were observed for men in the Shannon–Wiener index (P = .048) and Equitability index (P = .036).
Figure 1.
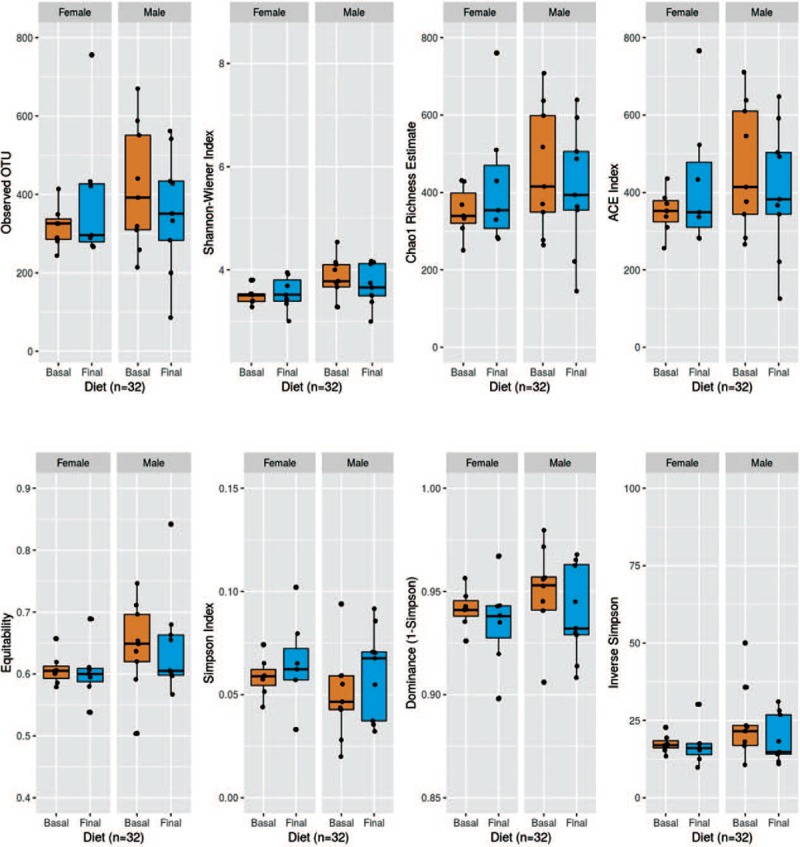
Alpha diversity indexes for intervention, controlling for gender.
Regarding the beta-diversity, no significant differences in the interindividual composition of the microbiota before and after the intervention (BC P = .834, wUniFrac P = .997, UniFrac P = 1000) were observed, even when adjusting by gender and ART of patients (BC P = .994, wUniFrac P = .993, UniFrac P = .997).
When studying significant gain of genus with the intervention, an increase in Gardnerella was observed, and a decrease of Mogibacterium, Dethiosulfovibrionaceae, and Coprococcus (Fig. 2). As for species, a gain of Bulleidia moorei and a decrease of the Bacilli species were observed.
Figure 2.
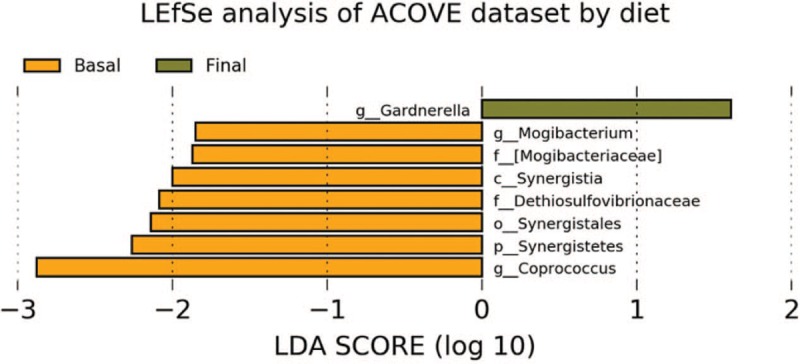
LEfSe analysis of ACOVE dataset by diet.
Significant differences were observed depending on gender of the patients, as shown in Figure 3, with an increase of Prevotella, Bacteroidetes, Bifidobacterium, Erysipelotrichaceae, and Eubacterium in males, while in women Eggerthella, Ruminococcus, Lachnospiraceae, Parabacteroides, and Akkermansia were more abundant.
Figure 3.
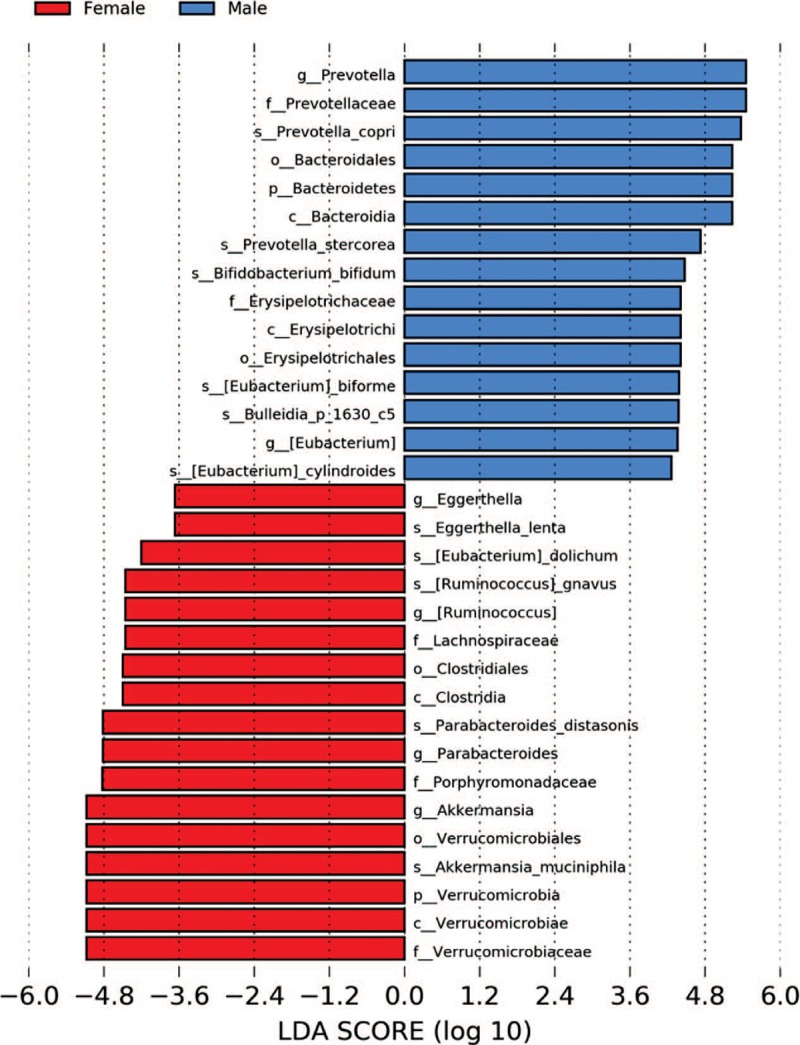
LDA score by gender. LDA = linear discriminant analysis.
Interestingly, significant differences were observed depending on whether patients were on an integrase inhibitor (II) or a nonnucleoside reverse transcriptase inhibitors (NNRTI) based ART. In those who used an II, Bifidobacterium and Actinobacterium were more abundant, while those using NNRTI had a greater abundance of Bacteroidales and Bulleidia (Fig. 4). Receiving one or the other ART did not influence the effect of EVOO on microbiota.
Figure 4.
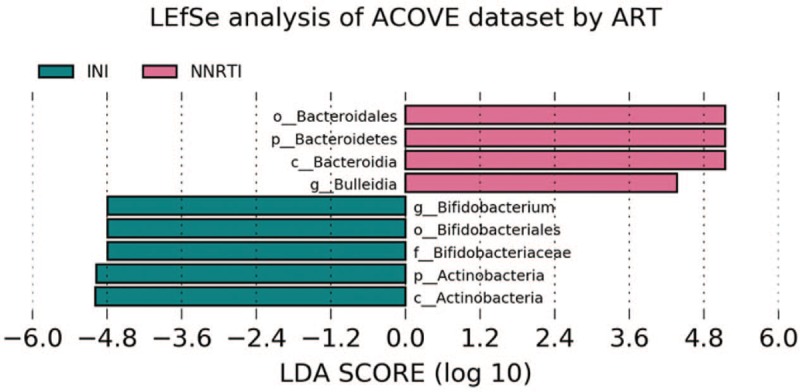
LDA score by ART. ART = antiretroviral treatment, LDA = linear discriminant analysis.
4. Discussion
In this study, the use of EVOO in patients of over 50 years of age with HIV infection showed a significant decrease in total cholesterol, as well as an increase in the alpha diversity of the microbiota in males. In addition, the use of EVOO showed a decrease in proinflammatory genera such as Dethiosulfovibrionaceae. Finally, a different profile in the composition of the microbiota according to the type of ART received was found. These findings suggest a beneficial effect of sustained consumption of 50 g of EVOO in elderly HIV-infected patients.
Previous studies in non-HIV population have shown that EVOO rich in nonabsorbable polyphenols is associated with significant decrease in CRP levels.[18] Experience in HIV-infected patients is limited, although there has also been evidence of a decrease in CRP levels in patients who used older ART regimens that are no longer in use.[26]
In addition, the use of EVOO showed an increase in Gardnerella and a decrease in proinflammatory genera such as Dethiosulfovibrionaceae. In this study, a significant increase in alpha diversity was observed in males. The diversity of the intestinal microbiota is reduced in HIV-infected patients compared to uninfected individuals,[27–29] as in patients with IBD,[30] there is a relationship between a greater alpha diversity and a higher level of CD4 lymphocytes in peripheral blood of naive patients, as well as with lower levels of markers of activation of peripheral blood monocytes and bacterial translocation.[31] In this study, it has been found that a simple dietary intervention can increase alpha diversity in elderly patients with effective ART. This effect of increased alpha diversity is the same as the effect observed in patients who initiate ART with a degree of severe immunosuppression,[32] although the same has not been demonstrated in patients with better immunological status.[5] In fact, some studies have associated the use of ART to a reduction in alpha diversity.[31] In these patients, part of the beneficial effect on alpha diversity can be explained by the immune reconstitution after an effective ART; however, this would not be the case of the patients included in this study, which had a long period of ART with a very good viroimmunological situation, so we believe that the effect on alpha diversity can only be attributed to the EVOO intake. Changes in diet rapidly induce changes in the diversity and composition of the microbiota,[33] at least in individuals not infected with HIV. In this study, the intervention was maintained for 12 weeks, trying to mimic the effect of introducing EVOO into diet in a sustained manner. Other authors have also reported a differential effect of diverse types of ART on the modification of alpha diversity in the intestinal microbiota, increasing in patients that receive triple therapy based on II or NNRTI.[27] In this study, EVOO increased alpha diversity in men regardless of ART received. The effect of EVOO on the microbiota seems to be mediated by nonabsorbable polyphenols,[34] which reach the intestinal lumen, being metabolized by the microbiota.[35] Previous studies in non-HIV hypercholesterolemic patients have shown an increase in the proportion of Bifidobacterium associated with the consumption of virgin olive oil enriched in polyphenols.[36]
On the other hand, Dethiosulfovibrionaceae increases its frequency in the microbiota of other chronic inflammatory conditions such as IBD, and can produce compounds that are toxic to human cells,[37,38] so the effect of EVOO would be clearly positive in this case.
Previous studies with probiotics (a mixture of fructo-oligosaccharides, galacto-oligosaccharides, and glutamine) have not shown to increase alpha diversity, but have in beta diversity, thus inducing changes in microbiota structure without inducing an increase in the diversity of the species.[39] Specifically, an increase in Bifidobacteriaceae was observed in viremic patients without ART, without observing any significant change in patients following ART.
In this study, differences were observed between the composition of the microbiota in men and women. Although it is known that the composition of the microbiota may be modified partially by sexual habits in MSM,[29,40] this should not be the case for this study, as only 4 of the male participants were MSM. An enrichment was observed in species such as Prevotella copri was observed in males, which has already been evidenced in other studies[5,6,28,39] and also in Erysipelotrichaceae. In the case of the latter, its in vitro capacity to induce the increase in the production of tumor necrosis alpha and interleukin-10 of non-HIV individuals[6] has been shown. Prevotella has been associated with an increase in activated CD4 lymphocytes[8] and in proatherogenic metabolites.[41] On the other hand, genera linked to the production of butyrate such as Lachnospira[42] or Ruminococcus,[43] or linked to the antiinflammatory response, such as Akkermansia,[44] showed greater abundance in the group of women.
Significant differences were also observed in the composition of the microbiota according to the ART received by the patients, with an increase in Bacteroidales in patients who continued treatment with NNRTI and an increase in Bifidobacterium and Actinobacteria in those receiving II. The increase in the proportion of Bacteroidales has been associated in other studies to the activation of CD8 + T cells[45]; on the contrary, depletion of Bifidobacterium is one of the changes of the microbiota associated with aging,[46] its abundance is linked to the antiinflammatory response.[44] There was no influence of ART type on alpha or beta diversity, as has already been observed in individuals who initiate ART with a good immunological situation.[32] In NNRTI's case, at least efavirenz has been shown to inhibit the growth of E coli and Bacillus subtilis,[47] thus influencing the composition of the intestinal microbiota. In this study, the NNRTI used was RPV, of which no data on influence on the composition of microbiota is available so far.
Generally, studies on the microbiota of HIV patients have been carried out in patients of a younger age,[10,27,29,32,39] but only elderly patients were recruited in this study, because they are the ones with the highest incidence of non-AIDS events. The patients had a CD4 lymphocyte count of more than 600 cells/μL, so it remains to be seen if in patients with a worse immune status, EVOO would produce the same effects.
This study has several limitations. First, the small sample size has possibly limited the fact that we have seen significant differences in the improvement of the lipid profile or the CRP. In second place, all patients were elderly with ART based on NNRTI or II along with 2 nucleos(t)ide analogues, so the effect observed in these patients cannot be extrapolated to other scenarios such as dual therapy or triple therapy based on protease inhibitors. Third, this study was a single-arm study, with no control arm. Fourth, an objective measure of adherence to the intervention, as the determination of hydroxytyrosol in urine, was not performed, but a weekly telephone follow-up was made.
In conclusion, the findings of the present study, for the first time ever, showed that a beneficial effect of the sustained consumption of EVOO (12 weeks) was associated with a decrease in total cholesterol and an increase in the alpha-diversity of the microbiota in males. As these findings suggest a beneficial effect of the sustained consumption of 50 g of EVOO in elderly HIV-infected patients, we believe that this pilot study merits confirmation of its conclusions in a study with larger population and with a longer follow-up.
Acknowledgments
The authors thank the patients who collaborated in the development of this project and to the olive company Bravoliva S.L., who selflessly donated the extra virgin olive oil necessary for the project. The authors thank the Grants from Plan Nacional de I+D+I and Fondo Europeo de Desarrollo Regional-FEDER (RD16/0025/0040; http://www.isciii.es/isciii/es/contenidos/fd-investigacion/fd-ejecucion/fd-centros-participados/centros-participados-redesretics.shtml) and Fondo de Investigación Sanitaria (PI 18/00819) for the financial support. And the authors also thank Josefa López-Bueno for assistance in the analysis of the microbiota by next generation sequencing.
Author contributions
Conceptualization: Julián Olalla, Federico García.
Data curation: Julián Olalla, José M García de Lomas, Natalia Chueca, Xavier Pérez-Stachowski, Adolfo De Salazar, Fernando Fernández-Sánchez.
Formal analysis: Julián Olalla, Natalia Chueca, Adolfo De Salazar, Alfonso Del Arco, Julio Plaza-Díaz, Federico García.
Investigation: Julián Olalla, José M García de Lomas, Natalia Chueca, Xavier Pérez-Stachowski, Adolfo De Salazar, Alfonso Del Arco, Julio Plaza-Díaz, Javier De la Torre, José Luis Prada, Javier García-Alegría, Federico García, Fernando Fernández-Sánchez.
Methodology: Julián Olalla, Federico García.
Project administration: Julián Olalla, Federico García.
Resources: Julián Olalla, José M García de Lomas, Natalia Chueca, Javier De la Torre, Javier García-Alegría, Federico García, Fernando Fernández-Sánchez.
Supervision: Julián Olalla, Federico García.
Writing – original draft: Julián Olalla.
Writing – review & editing: Julián Olalla, José M García de Lomas, Natalia Chueca, Xavier Pérez-Stachowski, Adolfo De Salazar, Alfonso Del Arco, Julio Plaza-Díaz, Javier De la Torre, José Luis Prada, Javier García-Alegría, Federico García, Fernando Fernández-Sánchez.
Footnotes
Abbreviations: ART = antiretroviral treatment, BC = Bray–Curtis, CRP = C-reactive protein, DNA = deoxyribonucleic acid, EVOO = extra virgin olive oil, FTC = emtricitabine, HIV = human immunodeficiency virus, IBD = inflammatory bowel disease, II = integrase inhibitor, IQR = interquartile range, LDA = linear discriminant analysis, LDL = low-density lipoprotein, MSM = men who have sex with men, NNRTI = nonnucleoside reverse transcriptase inhibitors, OTU = operational taxonomic unit, PCR = polymerase chain reaction, RPV = rilpivirine, TAF = tenofovir alafenamide, VL = viral load.
How to cite this article: Olalla J, de Lomas JMG, Chueca N, Pérez-Stachowski X, De Salazar A, Del Arco A, Plaza-Díaz J, De la Torre J, Prada JL, García-Alegría J, Fernández-Sánchez F, García F. Effect of daily consumption of extra virgin olive oil on the lipid profile and microbiota of HIV-infected patients over 50 years of age. Medicine. 2019;98:42(e17528).
Funding: This work was financed in part by Grants from Plan Nacional de I+D+I and Fondo Europeo de Desarrollo Regional-FEDER (RD16/0025/0040; http://www.isciii.es/isciii/es/contenidos/fd-investigacion/fd-ejecucion/fd-centros-participados/centros-participados-redesretics.shtml) and Fondo de Investigación Sanitaria (PI 18/00819).
The authors have no conflicts of interest to disclose.
References
- [1].Chen J, Shao J, Cai R, et al. Anti-retroviral therapy decreases but does not normalize indoleamine 2,3-dioxygenase activity in HIV-infected patients. PLoS One 2014;9:e100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hsu DC, Sereti I, Ananworanich J. Serious non-AIDS events: immunopathogenesis and interventional strategies. AIDS Res Ther 2013;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nix LM, Tien PC. Metabolic syndrome, diabetes, and cardiovascular risk in HIV. Curr HIV/AIDS Rep 2014;11:271–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Paiardini M, Muller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev 2013;254:78–101.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ling Z, Jin C, Xie T, et al. Alterations in the fecal microbiota of patients with HIV-1 infection: an observational study in a Chinese population. Sci Rep 2016;6:30673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lozupone CA, Li M, Campbell TB, et al. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe 2013;14:329–39.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013;5:193ra191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dillon SM, Lee EJ, Kotter CV, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol 2014;7:983–94.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sun Y, Ma Y, Lin P, et al. Fecal bacterial microbiome diversity in chronic HIV-infected patients in China. Emerg Microbes Infect 2016;5:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee SC, Chua LL, Yap SH, et al. Enrichment of gut-derived Fusobacterium is associated with suboptimal immune recovery in HIV-infected individuals. Sci Rep 2018;8:14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stiksrud B, Nowak P, Nwosu FC, et al. Reduced levels of D-dimer and changes in gut microbiota composition after probiotic intervention in HIV-infected individuals on stable ART. J Acquir Immune Defic Syndr 2015;70:329–37.. [DOI] [PubMed] [Google Scholar]
- [12].Villar-Garcia J, Hernandez JJ, Guerri-Fernandez R, et al. Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: a double-blind, randomized, placebo-controlled trial. J Acquir Immune Defic Syndr 2015;68:256–63.. [DOI] [PubMed] [Google Scholar]
- [13].Gori A, Rizzardini G, Van’t Land B, et al. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the “COPA” pilot randomized trial. Mucosal Immunol 2011;4:554–63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schunter M, Chu H, Hayes TL, et al. Randomized pilot trial of a synbiotic dietary supplement in chronic HIV-1 infection. BMC Complement Altern Med 2012;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90.. [DOI] [PubMed] [Google Scholar]
- [16].Tresserra-Rimbau A, Rimm EB, Medina-Remon A, et al. Polyphenol intake and mortality risk: a re-analysis of the PREDIMED trial. BMC Med 2014;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Castaner O, Fito M, Lopez-Sabater MC, et al. The effect of olive oil polyphenols on antibodies against oxidized LDL. A randomized clinical trial. Clin Nutr 2011;30:490–3.. [DOI] [PubMed] [Google Scholar]
- [18].Moreno-Luna R, Munoz-Hernandez R, Miranda ML, et al. Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. Am J Hypertens 2012;25:1299–304.. [DOI] [PubMed] [Google Scholar]
- [19].Fito M, Cladellas M, de la Torre R, et al. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: a randomized, crossover, controlled trial. Eur J Clin Nutr 2008;62:570–4.. [DOI] [PubMed] [Google Scholar]
- [20].Corona G, Tzounis X, Assunta Dessi M, et al. The fate of olive oil polyphenols in the gastrointestinal tract: implications of gastric and colonic microflora-dependent biotransformation. Free Radic Res 2006;40:647–58.. [DOI] [PubMed] [Google Scholar]
- [21].Patterson E, O’Doherty RM, Murphy EF, et al. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br J Nutr 2014;111:1905–17.. [DOI] [PubMed] [Google Scholar]
- [22].Medina E, Brenes M, Romero C, et al. Main antimicrobial compounds in table olives. J Agric Food Chem 2007;55:9817–23.. [DOI] [PubMed] [Google Scholar]
- [23].Romero C, Medina E, Vargas J, et al. In vitro activity of olive oil polyphenols against Helicobacter pylori. J Agric Food Chem 2007;55:680–6.. [DOI] [PubMed] [Google Scholar]
- [24].Martin-Pelaez S, Covas MI, Fito M, et al. Health effects of olive oil polyphenols: recent advances and possibilities for the use of health claims. Mol Nutr Food Res 2013;57:760–71.. [DOI] [PubMed] [Google Scholar]
- [25].Gavahian M, Khaneghah AM, Lorenzo JM, et al. Health benefits of olive oil and its components: impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci Technol 2019;88:220–7.. [Google Scholar]
- [26].Kozic Dokmanovic S, Kolovrat K, Laskaj R, et al. Effect of extra virgin olive oil on biomarkers of inflammation in HIV-infected patients: a randomized, crossover, controlled clinical trial. Med Sci Monit 2015;21:2406–13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Villanueva-Millan MJ, Perez-Matute P, Recio-Fernandez E, et al. Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients. J Int AIDS Soc 2017;20:21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mutlu EA, Keshavarzian A, Losurdo J, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog 2014;10:e1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Noguera-Julian M, Rocafort M, Guillen Y, et al. Gut microbiota linked to sexual preference and HIV infection. EBioMedicine 2016;5:135–46.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Manichanh C, Borruel N, Casellas F, et al. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol 2012;9:599–608.. [DOI] [PubMed] [Google Scholar]
- [31].Nowak P, Troseid M, Avershina E, et al. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS 2015;29:2409–18.. [DOI] [PubMed] [Google Scholar]
- [32].Ji Y, Zhang F, Zhang R, et al. Changes in intestinal microbiota in HIV-1-infected subjects following cART initiation: influence of CD4+ T cell count. Emerg Microbes Infect 2018;7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cardona F, Andres-Lacueva C, Tulipani S, et al. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem 2013;24:1415–22.. [DOI] [PubMed] [Google Scholar]
- [35].Mosele JI, Martin-Pelaez S, Macia A, et al. Faecal microbial metabolism of olive oil phenolic compounds: in vitro and in vivo approaches. Mol Nutr Food Res 2014;58:1809–19.. [DOI] [PubMed] [Google Scholar]
- [36].Martin-Pelaez S, Mosele JI, Pizarro N, et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: implications of human gut microbiota. Eur J Nutr 2017;56:119–31.. [DOI] [PubMed] [Google Scholar]
- [37].Christophersen CT, Morrison M, Conlon MA. Overestimation of the abundance of sulfate-reducing bacteria in human feces by quantitative PCR targeting the Desulfovibrio 16S rRNA gene. Appl Environ Microbiol 2011;77:3544–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Verma R, Verma AK, Ahuja V, et al. Real-time analysis of mucosal flora in patients with inflammatory bowel disease in India. J Clin Microbiol 2010;48:4279–82.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Serrano-Villar S, Vazquez-Castellanos JF, Vallejo A, et al. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol 2017;10:1279–93.. [DOI] [PubMed] [Google Scholar]
- [40].Yu G, Fadrosh D, Ma B, et al. Anal microbiota profiles in HIV-positive and HIV-negative MSM. AIDS 2014;28:753–60.. [DOI] [PubMed] [Google Scholar]
- [41].Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–85.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Meehan CJ, Beiko RG. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol Evol 2014;6:703–13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 2009;294:1–8.. [DOI] [PubMed] [Google Scholar]
- [44].Biagi E, Franceschi C, Rampelli S, et al. Gut microbiota and extreme longevity. Curr Biol 2016;26:1480–5.. [DOI] [PubMed] [Google Scholar]
- [45].Ellis CL, Ma ZM, Mann SK, et al. Molecular characterization of stool microbiota in HIV-infected subjects by panbacterial and order-level 16S ribosomal DNA (rDNA) quantification and correlations with immune activation. J Acquir Immune Defic Syndr 2011;57:363–70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Drago L, Toscano M, Rodighiero V, et al. Cultivable and pyrosequenced fecal microflora in centenarians and young subjects. J Clin Gastroenterol 2012;46Suppl:S81–4.. [DOI] [PubMed] [Google Scholar]
- [47].Shilaih M, Angst DC, Marzel A, et al. Antibacterial effects of antiretrovirals, potential implications for microbiome studies in HIV. Antivir Ther 2018;23:91–4.. [DOI] [PubMed] [Google Scholar]


