Abstract
Background:
Previous studies have shown that microRNA-32 (miRNA-32) is an exosome microRNA that affects the proliferation and metastasis of non-small cell lung cancer (NSCLC) cells. In this study, our goal was to assess the expression of plasma microRNA-32 and its potential as a biomarker to predict the tumor response and survival of patients with NSCLC undergoing platinum-based chemotherapy.
Methods:
Plasma microRNA-32 levels before and after 1 cycle of platinum-based chemotherapy in 43 patients with NSCLC were measured using a quantitative real-time polymerase chain reaction assay (qPCR). In addition, the demographic and survival data of the patients were collected for analysis.
Results:
A significant correlation was observed between the changes in microRNA-32 levels before and after 1 chemotherapy cycle and the treatment response (P = .035). In addition, Kaplan–Meier analysis showed that the level of microRNA-32 after 1 chemotherapy cycle was significantly correlated with the prognosis of patients. The median progression-free survival (P = .025) and overall survival (P = .015) of patients with high microRNA-32 levels (≥7.73) after 1 chemotherapy cycle was 9 and 21 months, respectively. In contrast, the median survival of patients with low microRNA-32 levels (<7.73) was 5 and 10 months, respectively.
Conclusions:
The plasma levels of microRNA-32 correlated with the efficacy of platinum-based chemotherapy and survival, indicating that microRNA-32 may be useful for predicting the effectiveness of platinum-based chemotherapy and prognosis in NSCLC.
Keywords: chemotherapy, microRNA-32, non-small cell lung cancer, prognosis
1. Introduction
Lung cancer is currently one of the most common malignant tumors worldwide, ranking first in incidence and mortality among malignant tumors.[1] Among lung cancers, non-small cell lung cancer (NSCLC) accounts for 80% to 85% of all lung cancer.[2] Since most NSCLC patients are diagnosed at the advanced stage, chemotherapy and radiotherapy have become the primary treatments for such patients, with the platinum-based chemotherapy regimen being the standard first-line chemotherapy regimen for NSCLC. Patients undergoing chemotherapy need to be monitored to assess tumor progression. However, there are very few biomarkers that can predict the response of patients to conventional chemotherapy drugs, and patients often need to go through 2 to 3 relatively toxic treatment cycles before radiological evaluation of the response can be conducted. Early treatment of NSCLC involves multiple predictive response methods, including imaging examinations that are performed alone and in combination with specific biomarkers.[3–5] However, due to the lack of sufficient specificity and sensitivity,[6] existing biomarkers and predictors of NSCLC are unsatisfactory. Thus, new markers of NSCLC progression in chemotherapy patients need to be identified.
Studies have confirmed that some tumor chemotherapy drugs can cause changes in microRNAs (miRNAs) expression profiles in various malignant tumor cells,[7] indicating that the mechanism of action of tumor chemotherapy drugs is associated with miRNAs to some extent. This possibility may lead to new approaches for the treatment of lung cancer with miRNA and chemotherapy drugs. MicroRNA-32 (miR-32) is associated with a variety of tumors[8–11] and is involved in a wide range of cellular regulatory processes.
In this study, we explored the relationship between miR-32 expression and the efficacy and prognosis of NSCLC chemotherapy by assessing the expression of plasma miR-32 in 43 patients with NSCLC before and after platinum-based chemotherapy.
2. Methods
2.1. Study subjects
NSCLC patients confirmed by histological or cytological diagnosis were enrolled in the Affiliated Hospital of Shandong Academy of Medical Sciences from February 2013 to June 2016. All cases were based on an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2, and their expected survival was at least 3 months. All patients had no prior history of therapy, including surgery, chemotherapy, radiotherapy, or antibiotic therapy, and received platinum-based chemotherapy every 3 weeks.
The first-line chemotherapy used in patients included cisplatin in 28 cases (docetaxel + cisplatin in 3 cases, his + cisplatin dorsey in 6 cases, gemcitabine plus cisplatin in 15 cases, and pemetrexed plus cisplatin in 4 cases), 12 cases with carboplatin solution (docetaxel plus carboplatin in 5 cases, gemcitabine plus carboplatin in 5 cases, and pemetrexed plus carboplatin in 2 cases), and 3 cases with nida containing platinum (nida, gemcitabine plus cisplatin). The proportion of chemotherapy regiments containing cisplatin, carboplatin, and nedaplatin was 65.1%, 27.9%, and 7.0%, respectively. After 2 cycles of treatment, objective tumor responses to treatment based on the RECIST criteria evaluation showed complete remission (CR), partial response (PR), stable disease (SD), or progressive disease (PD).
The patients included 37 men and 6 women, aged from 45 to 78 years old (mean age, 64.00 years old). Of the 43 cases, 31 were squamous cell carcinomas and 12 were adenocarcinomas. Analysis of tumor-node metastasis (TNM) staging indicated that 6 tumors were stage II, 14 tumors were stage III, and 23 tumors were stage IV. Tumors were staged according to the TNM staging system of the American Joint Committee on Cancer. All subjects in this study provided written informed consent. The study protocol was approved by the Medical Ethics Committee of the Affiliated Hospital of Shandong Academy of Medical Sciences. Depending upon the histological type and tumor grade, tumors staging was performed according to the guidelines of the American Joint Committee on Cancer (AJCC)[3,4]. Twenty peripheral blood samples from 20 healthy volunteers without previous history of cancer were used as a control group.
2.2. Isolation of human plasma
Blood specimens were collected at 2 time points, 1 to 3 days before the first cycle of chemotherapy and 1 to 3 days after the first cycle of chemotherapy. All participants were asked to fast overnight before the collection of blood samples. Five milliliters of peripheral venous blood was collected in an anticoagulant tube with ethylenediaminetetraacetic acid (EDTA). Subsequently, the blood samples were centrifuged in primary blood collection tubes for 10 minutes at 1900 × g (3000 rpm) and 4 °C using a swinging bucket rotor. The supernatant was then transferred to a clean 1.5-mL centrifuge tube, centrifuged at 16,000 × g and 4 °C for 10 minutes and stored at –80 °C until analysis.
2.3. Plasma RNA extraction
Total RNA was extracted and purified from 200 μL of plasma using a QIAGEN miRNeasy Serum/Plasma kit (catalog no. 217184; QIAGEN, Germany). The primer sequence (5′-CCCCTATTGCACATTACTAAGT-3′) was obtained from the Sanger miR-32 sequence database. To eliminate differences among the samples, a C. elegans miR-39 miRNA mimic (catalogue no. 219610; QIAGEN, Germany) was utilized for normalization. The primer sequence, obtained from the Sanger miR sequence database, was 5′-TCACCGGGTGTAAATCAGCTTG -3′. MiR-39 was stably expressed in plasma samples, and its mean value was the same across all cohorts. RNA quantification and quality determination was performed using an MD2000 Series spectrophotometer (Biofuture, Britain, UK).
2.4. Reverse transcription and real-time PCR analysis
The manufacturer's instructions were followed for under kits utilized in this study. Mir-X miRNA First-Strand Synthesis and SYBR Premix Ex Taq kits (Tli RNaseH Plus) (Takara Bio, China) were used separately for microRNA reverse transcription and real-time polymerase chain reaction (RT-PCR), respectively. Specific and universal primer pairs (Takara Bio, China) were used to amplify microRNA. The qPCR reactions were incubated in 96-well plates at 95 °C for 30 seconds, followed by 45 cycles of amplification (95 °C for 5 seconds and 63 °C for 30 seconds). MicroRNA expression was calculated using the 2–ΔΔCt method and was subjected to statistical analysis. DdH2O was used as a negative control, and each sample was assayed in triplicate. The data are reported with standard deviations.
2.5. Statistical analysis
The data were analyzed using SPSS20.0 (SPSS Inc., Chicago, IL). Due to a non-normal distribution, the miR-32 expression results are presented as the median and range. The median of the baseline plasma miR-32 expression before and after treatment was used as the cut-off point and was divided into 2 groups, high and low, respectively. The Mann–Whitney U test and χ2 test were used to compare the differences in continuous and categorical variables between the groups, respectively. Progression free survival (PFS) is the time between when a patient with a tumor disease begins receiving treatment and when the disease is observed to have progressed or the individual dies of any cause. Overall survival (OS) was defined as the interval between the start of treatment to death. Survival curves were generated using the Kaplan–Meier method, and differences were compared using the log-rank test. The COX proportional hazard model was used to analyze the possible factors affecting the survival of NSCLC patients. A P value of <.05 was considered to be significant.
3. Results
3.1. Patient characteristics
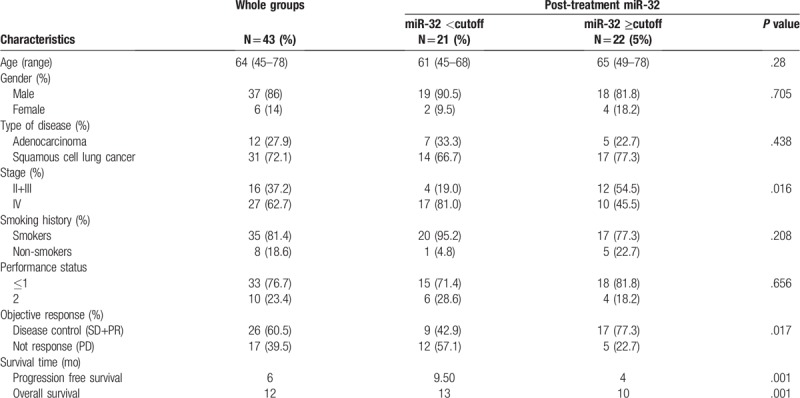
The characteristics of the 43 patients included in this study are presented in Table 1, of whom 12 had adenocarcinoma and 31 had squamous-cell carcinoma. Of note, 6 patients had stage II disease, 14 patients had stage III disease, and 23 patients had stage IV disease (Table 1).
Table 1.
Patient characteristics.

3.2. Plasma levels of miR-32 and association with tumor response to chemotherapy
The miR-32 levels of 43 NSCLC patients before and after treatment and those of individuals in the control group are shown in Fig. 1. Twenty-six patients (PR+SD) showed disease control (disease control rate, DCR 60.5%) and 17 patients (39.5%) progressed during the platinum-based chemotherapy. No chemotherapeutic reaction was observed in any of the patients. The plasma levels of miR-32 in the 43 patients with NSCLC pre- and post-treatment and in the control group individuals are shown in Fig. 1. The levels of plasma miR-32 in the NSCLC patients pre- and post-treatment were not significantly different than those observed in the control group (P = .291, P = .343, respectively). However, plasma miR-32 levels after chemotherapy were significantly higher than those observed before chemotherapy (P = .035), indicating that plasma miR-32 levels were significantly increased after 1 chemotherapy cycle. Subsequently, we evaluated the relationship between changes in miR-32 levels before and after 2 cycles of chemotherapy and the imaging evaluation of the treatment response.
Figure 1.
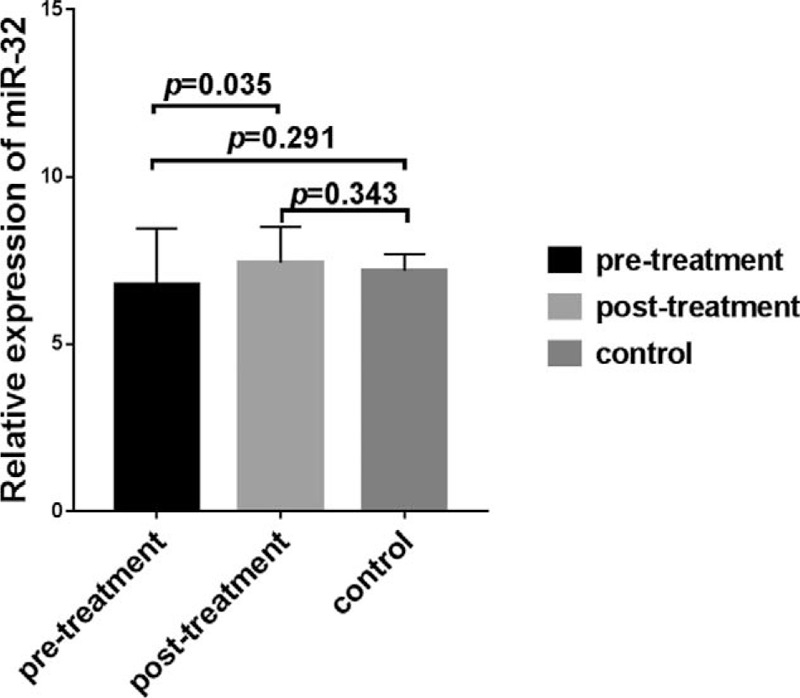
Plasma expression of miR-32 before and after treatment of non-small cell lung cancer (NSCLC) patients and in control group individuals. Plasma miR-32 levels at after chemotherapy was significantly higher that was observed before chemotherapy (P = .0351). The pre- and post-treatment plasma levels of miR-32 in NSCLC patients were not significantly different than in the control group (P = .291 and .343, respectively).
The median plasma miR-32 level at baseline (7.73 after chemotherapy) was used as positive cut-off values for the 43 patients (Table 2). The 2 groups of patients were comparable with respect to all major clinical characteristics, such as age, sex, type of disease, clinical stage, smoking history, performance status, objective response, and survival time. No significant association in miR-32 expression levels was observed between the 2 groups with respect to other clinical features, including age (P = .28), sex (P = .705), type of disease (P = .438), smoking history (P = .208), performance status (P = .656). However, there were significant differences in the expression levels of miR-32 between the 2 groups with respect to clinical staging (P = .016), objective chemotherapy response (P = .017), PFS (P = .001), and OS (P = .001).
Table 2.
Main clinical characteristics and results for the whole patients population and according to miR-32 post-treatment values (above or below the respective cut-off).
As shown in Fig. 2, the miR-32 levels in the 43 patients after 1 chemotherapy cycle were significantly higher than those observed before chemotherapy (P = .035). In addition, the miR-32 levels in patients with PR+SD significantly increased after 1 chemotherapy cycle (P < .001). However, no significant differences in miR-32 levels before and after 1 chemotherapy cycle were observed in patients with PD (P = .619).
Figure 2.
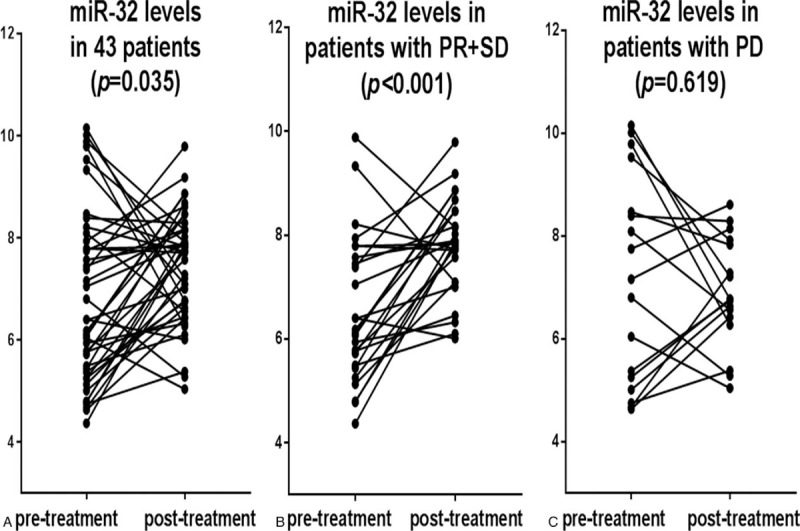
Plasma miR-32 levels in patients with non-small cell lung cancer (NSCLC) before and after 1 cycle chemotherapy (P = .035). The changes in plasma miR-32 levels of 43 NSCLC patients between pre- and post-treatment with partial response + stable disease (PR+SD) (P < .001) and progressive disease (PD) (P = .619), respectively.
3.3. High miR-32 levels are associated with better chemotherapy efficacy
The follow-up data from the 43 cases of NSCLC were analyzed, which dated up to June 2018. The results of Kaplan–Meier survival curves further demonstrated that NSCLC patients with high miR-32 levels have substantially longer PFS (P = .025) and OS (P < .015) (Fig. 3) rates compared with those observed in patients with low miR-32 expression. The median PFS time of patients post-treatment with high miR-32 expression (≥7.73) was 9.0 months, while the median PFS time of patients with low miR-32 expression (<7.73) was 5.0 months (Fig. 3A). However, the median OS time post-treatment was 21.0 months in patients with high miR-32 levels. The median OS time was 10.0 months in patients with a low chemotherapy ratio (Fig. 3B). These results suggest that the level of plasma miR-32 expression post-chemotherapy may be related to the prognosis of patients with NSCLC.
Figure 3.

Kaplan–Meier curve analysis of the effect of miR-32 expression after chemotherapy on the survival time of patients with non-small cell lung cancer. (A) Progress-free survival (PFS) (P = .025); (B) overall survival (months) (P = .015).
4. Discussion
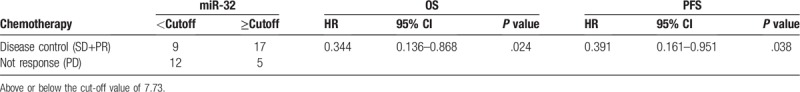
In first-line chemotherapy, the lack of clinical and biological factors that can predict the prognosis of patients is one of the most critical factors hindering NSCLC treatment. In this study, we investigated the role of plasma miR-32 levels in the chemosensitivity and prognosis of NSCLC patients receiving platinum-based chemotherapy. Plasma miR-32 levels were significantly increased after 1 chemotherapy cycle. Our data showed that plasma miR-32 levels can discriminate between patients with a partial response and stable disease (PR+SD) from those who have progressive disease. For patients with NSCLC who are sensitive to chemotherapeutic drugs, the higher plasma miR-32 levels observed after chemotherapy were more pronounced than in the chemotherapy-resistant patients. Patients with low miR-32 expression after chemotherapy often suggest poor prognosis in patients with NSCLC. Meanwhile, we also assessed the relationship between changes in plasma miR-32 levels and prognosis via Kaplan–Meier survival analysis. The results showed that the survival of NSCLC patients with significantly higher miR-32 levels was significantly longer than that of patients with no obvious changes in miR-32 expression. A lack of an obvious change in miR-32 expression suggests a poor clinical outcome compared with significantly increased miR-32 expression (Table 3).
Table 3.
Univariate analysis of miR-32 expression and survival time in patients with non-small cell lung cancer.
MiRNAs are small non-coding RNA molecules with lengths of approximately 22 nucleotides[12,13] and are believed to play vital roles in the diagnosis, prognosis, and recurrence of lung cancer.[14–16] A number of studies have reported the potential clinical applications of miRNAs in lung cancer chemotherapy. Some miRNAs enhance anticancer therapy by regulating the cell cycle, cell proliferation and apoptosis, DNA damage repair, and tumor angiogenesis.[17–19] For example, miR-1 overexpression increases the chemosensitivity of non-small cell lung cancer cells by inhibiting autophagy-related 3-mediated autophagy and increasing apoptosis.[20] Furthermore, the overexpression of miR-202 has been shown to promote chemosensitivity by inhibiting the Kras gene in NSCLC.[21] Increasing evidence has shown that circulating miRNAs can be potential biomarkers for the early diagnosis and prognosis of lung cancer.[22,23] In addition, decreased miR-215 expression has been shown to be a predictor of poor prognosis in patients with acute myeloid leukemia.[24] In addition, the upregulation of miR-200c has been observed to inhibit cell migration and enhance chemosensitivity in breast cancer.[25] However, the role of plasma miRNA in predicting chemotherapy sensitivity in patients with NSCLC is rarely reported.
In this study, we investigated the correlation between plasma miR-32 levels and chemotherapy sensitivity in patients with NSCLC. MiR-32 has been shown to be a sensitive indicator of chemotherapy with respect to tumor response. It is well known that miRNAs typically downregulate target genes by inhibiting translation.[26–28] Interestingly, Li and Wu[8] observed a negative correlation between the level of miR-32 expression and NSCLC cell proliferation, epithelial-mesenchymal transition (EMT), and metastasis, which were inhibited by upregulated miR-32 expression. They also confirmed that TWIST1 is a direct target of miR-32 and that miR-32 regulates NSCLC cell proliferation, EMT, and metastasis at least in part by regulating TWIST1. MiRNA expression is often altered during disease progression and anti-tumor therapy. After chemotherapy, chemotherapy-resistant miRNAs were observed to be downregulated and chemotherapy-sensitive miRNAs were was upregulated in the treatment group. However, it is a timely and effective choice for patients with NSCLC to adjust treatment plans by evaluating the dynamic levels of miR-32 during short-term chemotherapy treatment (1 chemotherapy cycle) to predict its effect. Alexios et al[6] have performed similar studies, and Tetsuro et al[29] reported that changes in the torque teno virus DNA titer in patients with primary lung cancer after chemotherapy was related to tumor growth. Thus, it is important to study the relationship between gene expression and tumor growth after chemotherapy.
The reduction in miR-32 expression in NSCLC tissues was associated with tumor staging and lymph node metastasis, and the Kaplan–Meier analysis results showed that the overall survival time of patients with low miR-32 expression was shorter than individuals with high miR-32 expression.[12] Circulating miRNA has been reported to be an important biomarker for cancer diagnosis due to its abundance, stability and convenient blood circulation.[30] In our study, the survival time of NSCLC patients with significantly increased miR-32 expression was significantly longer than those without significant changes in miR-32 expression. A lack of obvious changes in miR-32 expression suggests a poor clinical outcome compared with significantly increased miR-32 expression. In addition, by analyzing published miRNA microarray studies, miR-32 was observed to be significantly reduced in NSCLC tissues compared with non-tumor tissues.[31,32] Interestingly, the upregulation of miR-32 expression inhibits the proliferation, epithelial mesenchymal transformation, and metastasis of NSCLC cells,[9] which is consistent with the results of our study.
In summary, our data showed that the elevation of plasma miR-32 levels in NSCLC patients receiving platinum-based chemotherapy can predict the sensitivity of chemotherapy regimens and predict the prognosis of patients. Thus, changes of plasma miR-32 levels in platinum-based chemotherapy patients with lung cancer are a prognostic indicator.
Acknowledgments
The authors thank all of the individuals involved in the present study. The authors are also grateful to Miss Yanxin Wei at the School of Public Health, Shandong University, for statistical advice.
Author contributions
Data curation: Shuyuan Xu, Jing Li.
Formal analysis: Shuyuan Xu, Jing Li, Ling Chen.
Funding acquisition: Shuyuan Xu.
Investigation: Shuyuan Xu, Jing Li, Ling Chen, Li Guo.
Methodology: Li Guo, Mei Ye, Yuanyuan Wu.
Project administration: Quanjiang Ji.
Supervision: Quanjiang Ji.
Writing – original draft: Shuyuan Xu, Quanjiang Ji, Jing Li.
Writing – review & editing: Mei Ye, Yuanyuan Wu, Quanjiang Ji.
Footnotes
Abbreviations: miRNA-32 = microRNA-32, NSCLC = non-small cell lung cancer, qPCR = quantitative real-time PCR, ROC = receiver operating characteristic.
How to cite this article: Xu S, Li J, Chen L, Guo L, Ye M, Wu Y, Ji Q. Plasma miR-32 levels in non-small cell lung cancer patients receiving platinum-based chemotherapy can predict the effectiveness and prognosis of chemotherapy. Medicine. 2019;98:42(e17335).
Mini Abstract: Plasma miR-32 levels can predict chemotherapy sensitivity and prognosis in NSCLC patients.
This work was supported by the youth fund of the Shandong Academy of Medical Sciences of China (No.2015-70) and the Innovation Project of Shandong Academy of Medical Sciences.
The authors have no conflicts of interest to disclose.
References
- [1].Junichi S, Norihito O, Masao N, et al. Randomized feasibility study of S-1 for adjuvant chemotherapy in completely resected Stage IA non–small-cell lung cancer: results of the Setouchi Lung Cancer Group Study 0701. Jpn J Clin Oncol 2016;46:741–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang P. Epidemiology of lung cancer prognosis: quantity and quality of life. Methods Mol Biol 2009;471:469–86.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jarrod TB, Jan ME, Amanda KA, et al. Timeliness of treatment initiation and associated survival following diagnosis of non-small-cell lung cancer in South Carolina. South Med J 2017;110:107–13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xu CH, Yang Y, Wang YC, et al. Prognostic significance of serum chemerin levels in patients with non-small cell lung cancer. Oncotarget 2017;8:22483–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shigehiro Y, Hidehito H, Kuniko SS, et al. Impact of KRAS mutation on response and outcome of patients with stage III non-squamous non-small cell lung cancer. Cancer Sci 2015;106:1402–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Alexios SS, Udai B, Parames T, et al. Percentage change in plasma cytokeratin 18 is associated with clinical outcomes in patients receiving pemetrexed and carboplatin for the adenocarcinoma subtype of NSCLC. Oncology 2015;89:53–9.. [DOI] [PubMed] [Google Scholar]
- [7].Sun D, Li X, Ma M, et al. The predictive value and potential mechanisms of miRNA-328 and miRNA-378 for brain metastases in operable and advanced non-small-cell lung cancer. Jpn J Clin Oncol 2015;45:464–73.. [DOI] [PubMed] [Google Scholar]
- [8].Li L, Wu D. MiR-32 inhibits proliferation, epithelial-mesenchymal transition, and metastasis by targeting TWIST1 in non-small-cell lung cancer cells. Onco Targets Ther 2016;9:1489–98.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fu X, Liu M, Qu S, et al. Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res 2018;37:52. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [10].Wang N, Guo H, Dong Z, et al. Establishment and validation of a 7-microRNA prognostic signature for non-small cell lung cancer. Cancer Manage Res 2018;10:3463–71.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bai Y, Wang Y, Yao W, et al. Expression of miR-32 in human non-small cell lung cancer and its correlation with tumor progression and patient survival. Int J Clin Exp Pathol 2015;8:824–9.. [PMC free article] [PubMed] [Google Scholar]
- [12].Bartel DP. MicroRNAs target recognition and regulatory functions. Cell 2009;136:215–33.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Josie H, Pier PP, Sean L. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 2014;20:8. [DOI] [PubMed] [Google Scholar]
- [14].Skrzypski M, Czapiewski P, Goryca K, et al. Prognostic value of microRNA expression in operable non-small cell lung cancer patients. Br J Cancer 2014;110:991–1000.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yang J, Liu H, Wang H, et al. Down-regulation of microRNA-181b is a potential prognostic marker of non-small cell lung cancer. Pathol Res Pract 2013;209:490–4.. [DOI] [PubMed] [Google Scholar]
- [16].Zhu W, He J, Chen D, et al. Expression of miR-29c, miR-93, and miR-429 as potential biomarkers for detection of early stage non-small lung cancer. PLoS One 2014;9:e87780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Giulia B, Barbara P, George AC, et al. Targeting the microRNA-regulating DNA damage/repair pathways in cancer. Expert Opin Biol Ther 2014;14:1667–83.. [DOI] [PubMed] [Google Scholar]
- [18].Bonanno L, Favaretto A, Rosell R. Platinum drugs and DNA repair mechanisms in lung cancer. Anticancer Res 2014;34:493–501.. [PubMed] [Google Scholar]
- [19].Ivana S, Juliana S, Margarita M, et al. Pharmacogenetics of the DNA repair pathways in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Cancer Lett 2014;353:160–6.. [DOI] [PubMed] [Google Scholar]
- [20].Li H, Guirong Z, Jianguo W. MicroRNA-1 overexpression increases chemosensitivity of non-small cell lung cancer cells by inhibiting autophagy related 3-mediated autophagy. Cell Biol Int 2018;42:1240–9.. [DOI] [PubMed] [Google Scholar]
- [21].Sun W, Ping W, Tian Y, et al. MiR-202 enhances the anti-tumor effect of cisplatin on non-small cell lung cancer by targeting the Ras/MAPK pathway. Cell Physiol Biochem 2018;51:2160–71.. [DOI] [PubMed] [Google Scholar]
- [22].Hu Z, Chen X, Zhao Y, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol 2010;28:1721–6.. [DOI] [PubMed] [Google Scholar]
- [23].Shen J, Todd NW, Zhang H, et al. Plasma microRNAs as potential biomarkers for non-small-cell lung cancer. Lab Invest 2011;91:579–87.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang Y, Zhang T, Yang D, et al. Reduced miR-215 expression predicts poor prognosis in patients with acute myeloid leukemia. Jpn J Clin Oncol 2016;46:350–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Q, Cheng Y, Wang Y, et al. Tamoxifen reverses epithelial–mesenchymal transition by demethylating miR-200c in triple-negative breast cancer cells. BMC Cancer 2017;17:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang W, Zhang E, Lin C. MicroRNAs in tumor angiogenesis. Life Sci 2015;136:28–35.. [DOI] [PubMed] [Google Scholar]
- [27].Marilena VI, Carlo MC. MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol 2009;27:5848–56.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang X, Hu S, Zhang X, et al. MicroRNA-7 arrests cell cycle in G1 phase by directly targeting CCNE1 in human hepatocellular carcinoma cells. Biochem Biophys Res Commun 2014;443:1078–84.. [DOI] [PubMed] [Google Scholar]
- [29].Tetsuro S, Masashi B, Masayuki N, et al. Clinical significance of changes in Torque teno virus DNA titer after chemotherapy in patients with primary lung cancer. Respir Investig 2018;56:173–8.. [DOI] [PubMed] [Google Scholar]
- [30].He WJ, Li WH, Jiang B, et al. MicroRNAs level as an initial screening method for early-stage lung cancer: a bivariate diagnostic random-effects meta-analysis. Int J Clin Exp Med 2015;8:12317–26.. [PMC free article] [PubMed] [Google Scholar]
- [31].Nozomu Y, Natasha C, Elise B, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 2006;9:189–98.. [DOI] [PubMed] [Google Scholar]
- [32].Sanja D, Lindsey K, Yongli S, et al. MiRNA expression profiling of lung adenocarcinomas: correlation with mutational status. Mod Pathol 2010;23:1577–82.. [DOI] [PubMed] [Google Scholar]


