Abstract
Background:
Conflicting results have been reported on the association of poststroke depression with recurrent stroke events. This meta-analysis of prospective studies aims to evaluate whether poststroke depression is an independent predictor of stroke recurrence among stroke patients.
Methods:
A systematic search of articles in PubMed and Embase databases from their inception to October 2018 was conducted. Prospective studies reporting risk estimates of stroke recurrence by depression status in stroke patients were included and pooled risk ratio (RR) with 95% confidence intervals (CIs) of stroke recurrence was calculated for patients with or without poststroke depression.
Results:
Six studies with 4648 stroke patients were finally included, and the prevalence of poststroke depression was found to from 15.9% to 40.5%. The pooled adjusted RR for stroke recurrence in patients suffering from poststroke depression was 1.48 (1.22–1.79) in a fixed-effect model. Subgroup analyses indicated that poststroke depression significantly increased stroke recurrence (RR 1.64; 95% CI, 1.28–2.10) among ischemic stroke patients but not in total stroke patients (RR 1.28; 95% CI, 0.96–1.73).
Conclusions:
This meta-analysis suggests that poststroke depression may be an independent predictor of stroke recurrence among ischemic stroke patients. Further studies are required to investigate whether treatment of poststroke depression can reduce the risk of stroke recurrence.
Keywords: depression, meta-analysis, poststroke depression, recurrent stroke
1. Introduction
Stroke remains the main cause of morbidity and mortality worldwide.[1] Survivors of stroke, particularly the ischemic type, are known to be at high-risk for stroke recurrence. Common conventional risk factors for recurrent stroke include hypertension, diabetes mellitus, hyperlipidemia, sleep apnea, obesity, cardiac disease, and stroke subtype.[2] The cumulative risk of recurrent stroke at 1 year was estimated to be 6.7% in northeastern Greece[3] and 9.3% in the Black Afro-Caribbean population.[4] Globally, the risk of recurrent stroke is estimated to be approximately 11% within the first year after stroke.[5] As recurrent stroke is associated with high mortality and poor functional recovery, identification of additional risk factors of stroke recurrence is of great importance.
Poststroke depression (PSD) is the most common psychiatric problem after stroke.[6,7] Approximately one-third of all stroke survivors suffer from depression either in the early or late period after stroke.[8] PSD is considered a serious public health concern due to its negative influence on stroke outcomes. Stroke survivors with PSD are at greater risk of, poor functional recovery, recurrent vascular events, deterioration of quality of life, and high mortality than those without depression.[9] However, inconsistent findings[10–15] regarding the association between PSD and recurrent stroke risk have been reported. Despite a recently published systematic review and meta-analysis[16] assessing the predictive value of PSD in stroke recurrence, this outcome has only been analyzed in 2 studies.[11,13]
To the best of our knowledge, no other meta-analysis has yet focused on PSD in predicting of recurrent stroke. Therefore, we conducted a meta-analysis of prospective studies to evaluate whether PSD is an independent predictor of stroke recurrence among stroke survivors.
2. Materials and methods
2.1. Data source and search strategy
The present study was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The Pubmed and Embase databases were comprehensively searched from their inception to October 30, 2018. The search strategy included combinations of the following terms: “depression” OR “depressive” AND “recurrent” OR “recurrence” OR “relapse” AND “stroke” OR “cerebrovascular disease” AND “follow-up” OR “longitudinal.” The articles were restricted to English and Chinese language publications. In addition, reference lists of all related articles were manually examined to identify any gray literature. Ethical approval was not required because this study reviewed the study-level data.
2.2. Study selection
The selection criteria of the eligible studies included a prospective design, focus on patients with previous stroke, depression after stroke as exposure, and reported multivariate-adjusted risk estimates of stroke recurrence events among patients with and without PSD. Articles were excluded if depression was diagnosed before stroke onset, the study design was a case–control study or retrospective in nature, outcome reported was not of interest, and reviews or conference abstracts.
2.3. Data extraction and quality assessment
The first and second authors independently extracted the following data: first author's surname, year of publication, origin of study, study design, population, sample size, proportion of men, age, assessment method of depression, prevalence of PSD, duration of follow-up, number of recurrent stroke events, multivariate-adjusted risk ratio (RR) or hazard ratio (HR) and 95% confidence intervals (CIs) of stroke recurrence, and covariates adjusted in statistical model. Methodological quality was assessed according to the Newcastle-Ottawa Scale (NOS) for the cohort studies[17] with the following aspects: selection bias, detection bias, and attrition bias. Studies awarding 7 stars or over were deemed as good quality. In case of discrepancy between the 2 authors in terms of data extraction and quality assessment, consensus was achieved through discussion.
2.4. Statistical analysis
Data analyses applied the most fully adjusted risk estimate. HRs were directly considered equivalent to RR. The association of PSD with recurrent stroke risk was pooled for the PSD versus without depressed patients. Cochran's Q test (significance level at P < .10) and I2 statistic (significance level at I2 > 50%) were used to assess the heterogeneity across studies. We selected a random-effect model in the presence of significant heterogeneity; otherwise, a fixed-effect model was chosen. Publication bias was evaluated using a funnel plot, Begg's rank correlation test,[18]and Egger linear regression test.[19] To test for the robustness of the pooling results, we performed the sensitivity analyses by sequentially removing one study at each time. Subgroup analyses were performed based on follow-up duration, sample size, type of stroke, study region, study quality, and whether excluded patients with prestroke depression. All statistical analyses were conducted using the STATA 12.0 (Stata Corp LP, College Station, TX).
3. Results
3.1. Search results and study characteristics
Our preliminary literature search yielded 753 potential records. After removing duplicate publication and irrelevant articles, 37 articles were retrieved for full-text assessment. After reviewing the full-text articles, 31 studies were further excluded because they did not specify the outcome of interest, included prestroke depression as exposure, included populations overlapping those in other studies, or were designed as reviews and conference abstracts. Finally, 6 studies[10–15] were included in the current meta-analysis. A flow chart of the literature search and selection process is shown in Figure 1.
Figure 1.
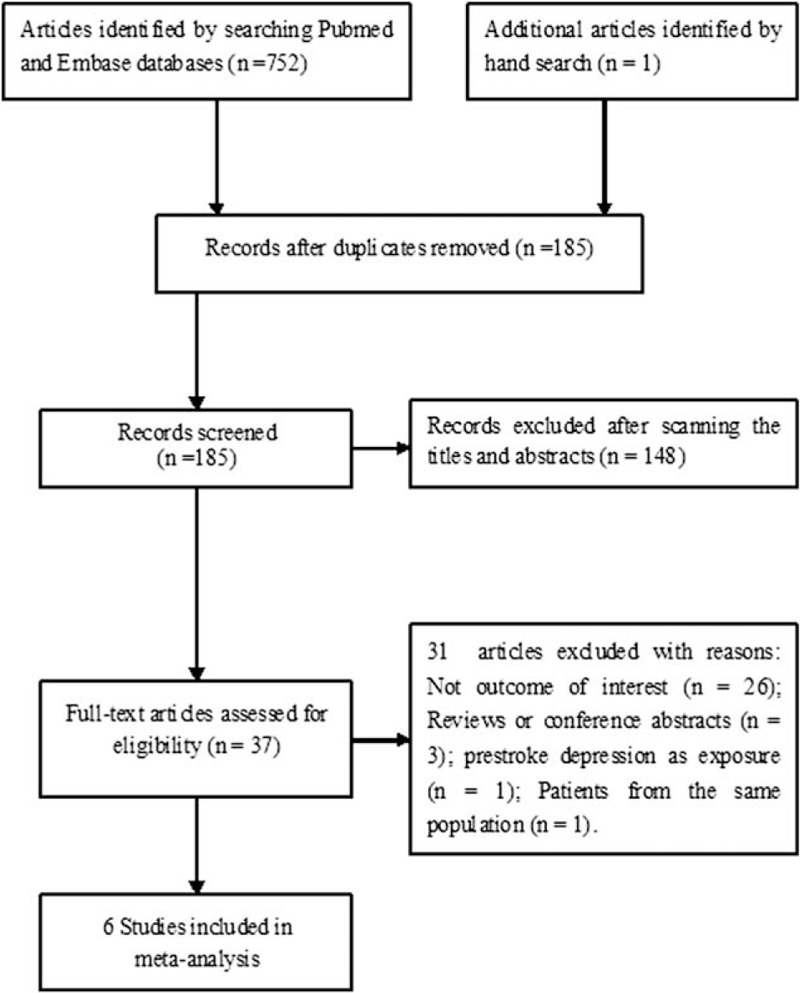
Flow chart of the study selection process.
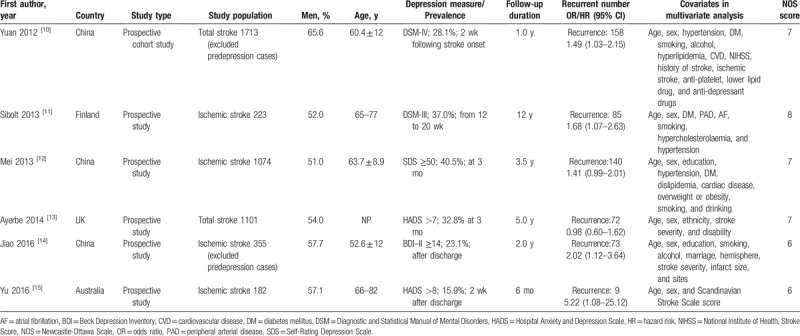
The main characteristics of the selected studies are shown in Table 1. All of the included studies were published between 2012 and 2016, and the sample size ranged from 182 to 1713. The overall number of patients included in the current meta-analysis was 4648, with 537 recurrent stroke events. The follow-up period was between 6 months and 12 years. Depression was determined by and Hospital Anxiety and Depression Scale,[13,15] Diagnostic and Statistical Manual of Mental Disorders,[10,11] Beck Depression Inventory,[14] and Self-Rating Depression Scale.[12] The methodological quality of the included studies was quantified using the NOS (6–8 stars) and considered to be moderate to high.
Table 1.
Main characteristics of studies included in the meta-analysis.
3.2. Association of PSD with recurrent stroke
All the included studies reported recurrent stroke outcome. As shown in Figure 2, PSD was associated with an increased risk of recurrent stroke (RR 1.48, 95% CI, 1.22–1.79) than those without depressed patients in a fixed-effect model. No significant heterogeneity across studies was found (I2 = 23.8%, P = .255). Begg's rank correlation test (P = .133) and Egger's linear regression test (P = .209) did not observe evidence of publication bias.
Figure 2.
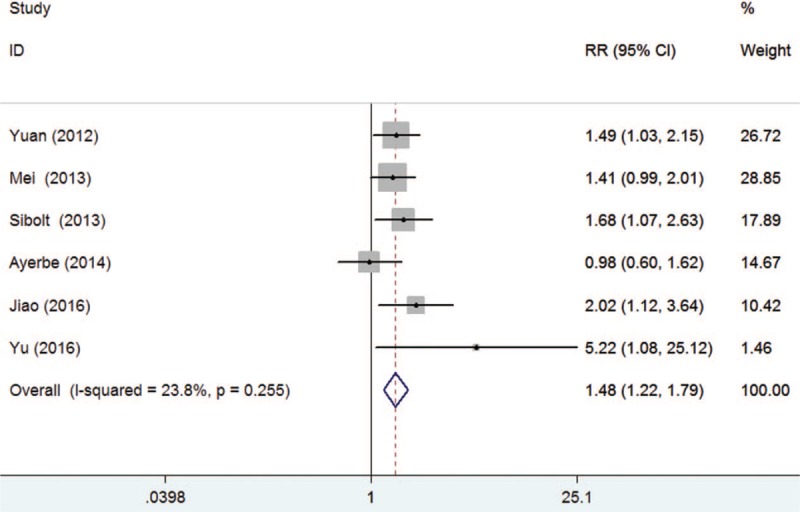
Forest plots showing risk ratio and 95% confidence interval of recurrent stroke event for poststroke depression versus without depressed stroke patients.
3.3. Subgroup analyses and sensitivity analyses
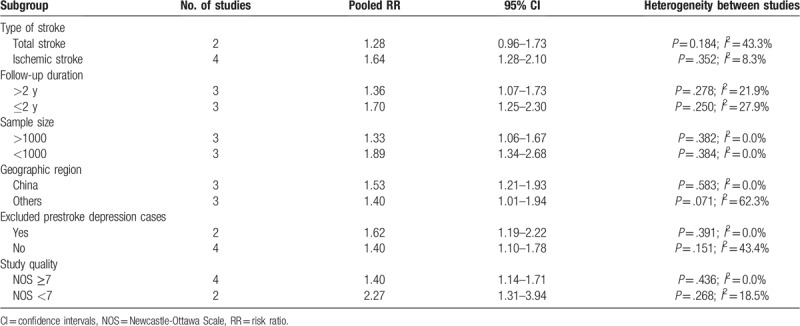
PSD significantly increased the risk of recurrent stroke in most subgroups except the total stroke subgroup. Thin increase in risk appeared to be strongest in ischemic stroke, follow-up ≤2 years, and exclusion of the prestroke depression case subgroup. Details of the subgroup analyses are shown in Table 2. Sensitivity analyses showed a minimal influence on the quantitative pooled risk estimate (RR ranged 1.43–1.59 and low 95% CI ranged from 1.18–1.29) when each of the studies was omitted one by one.
Table 2.
Subgroup analysis for recurrent stroke risk associated with poststroke depression.
4. Discussion
This meta-analysis evaluated the impact of PSD on recurrent stroke in stroke survivors. The result of this meta-analysis demonstrated that PSD is associated with an increased risk of recurrent stroke among stroke survivors. Stroke survivors with PSD had a 48% higher risk of recurrent stroke than those without. The prevalence of PSD varied from 15.9% to 40.5%. In addition, self-described prestroke depressive symptoms were associated with increased risk of stroke recurrence.[20] Early screening and treatment of depressive symptoms may thus present potential benefits in decreasing recurrent stroke rate.
Subgroup analyses showed that studies with the follow-up durations <2 years had a strong association between PSD and stroke recurrence risk. The risk of recurrent stroke weakened with increasing follow-up period, thus suggesting that this association is greatest after the initial stroke onset. Indeed, depressive symptoms most frequently developed within the first year after a stroke.[21] Nevertheless, PSD remained both a long- and short-term prognostic marker of stroke recurrence risk. The predictive significance observed persisted and remained statistically significant across several subgroups stratified by whether removal of prestroke depression cases at enrollment, geographic region, sample size, and study quality. However, subgroup analyses indicated that the pooled risk estimate (RR 1.28; 95% CI, 0.96–1.73) for studies with total stroke patients was not significant.
Avoiding stroke recurrence is the primary target of secondary stroke prevention in order to improve long-term patient prognosis.[22] Apart from recurrent stroke outcome, previous meta-analyses have suggested that besides recurrent stroke outcomes, patients suffering from PSD had a 22% higher risk of mortality than those who do not suffer from depression.[23] Stroke recurrence may further increase morbidity and mortality risk. Therefore, PSD deserves more clinical and research attention.
Given the negative impacts of PSD, whether antidepressant therapy could prevent recurrent stroke outcomes is an interesting subject. One-quarter of 15747 Swedish stroke survivors reported using antidepressant agent at 3 months.[24] Antidepressant use may reflect more severe depression. However, only one included study[10] considered the use of antidepressant drugs in the multivariable statistical models and this study showed a lack of statistical significance (OR 1.96, 95% CI, 0.95–4.04) in the association between PSD and recurrent stroke risk among patients receiving antidepressant drugs. As for individual studies have yielded conflicting evidence on the long-term survival of antidepressant therapy,[25,26] the impact of antidepressant agents in preventing stroke recurrence requires further investigation.
The association between particular lesion location after stroke and the development of PSD remains under debate.[27] A more recent published meta-analysis suggested that left-hemisphere lesions were more susceptible to PSD than right-hemisphere lesions during the subacute phase of stroke.[28] The underlying mechanisms linking PSD with stroke recurrence have not been fully elucidated. Possible explanations include depression is more common in patients with severe stroke than in those with mild stroke, stroke survivors suffering from PSD may be less compliant with treatment than those who do not suffer from depression, and PSD can affect stroke outcomes via the multitude of variables, including as those associated with cerebellar metabolism,[29] cerebral perfusion,[30] inflammatory response,[31] and immune reaction.[32]
Depressive symptoms are common among stroke survivors.[33] The pathogenesis of PSD involves biological and social psychological factors, such as hypothalamic–pituitary–adrenal axis abnormalities, monoamine neurotransmitter change, immunological changes, inflammation, and genetic variants.[34,35] Therefore, management of stroke patients will increasingly require a comprehensive assessment of both laboratory and clinical factors, which would help to improve clinical decision-making and outcome prognostication.
In interpreting the findings of this meta-analysis, several potential limitations should be mentioned. First, the included studies used self-reported scales to determine depression, which may lead to selection bias and underestimate the risk associated with recurrent stroke. Second, depression subtypes were not reported in the included studies and we failed to distinguish the impacts of minor or major depression. Third, information on antidepressant agent use was not reported in most of the original studies, and antidepressant drugs may affect the association between PSD and stroke recurrence. Fourth, the results of subgroup analysis may be unreliable due to the small number of studies analyzed. Fifth, despite the absence of significant heterogeneity in our pooled risk of stroke recurrence, determination of PSD based on different scales and time points presents obvious clinical heterogeneity, which may overestimate or underestimate the actual risk summary. Finally, the predictive significance of recurrent stroke associated with hemorrhagic type of recurrent stroke remains remained unclear. Future studies are warranted to investigate whether the impact of PSD on recurrent stroke is similar for patients with ischemic and hemorrhagic stroke.
5. Conclusions
This meta-analysis indicates that PSD may be an independent risk factor of recurrent stroke among stroke survivors. Thus, clinicians should routinely assess depressive symptoms after stroke. Future studies are warranted to investigate the impact of antidepressant agents on recurrent stroke among stroke survivors.
Author contributions
Conceptualization: Xin Shi.
Data curation: Quan-e Wu, Ai-min Zhou.
Formal analysis: Yun-peng Han, Yan-ming Liu.
Investigation: Quan-e Wu, Ai-min Zhou.
Methodology: Ai-min Zhou, Xin Shi.
Project administration: Xin Shi.
Resources: Quan-e Wu, Yang Yang.
Software: Yun-peng Han, Yan-ming Liu.
Supervision: Xin Shi.
Validation: Xin Shi.
Visualization: Quan-e Wu.
Writing – original draft: Yang Yang.
Writing – review and editing: Xiao-meng Wang.
Xin Shi orcid: 0000-0003-1888-955X.
Footnotes
Abbreviations: CI = confidence intervals, NOS = Newcastle-Ottawa Scale, PSD = poststroke depression, RR = risk ratio.
How to cite this article: Wu Qe, Zhou Am, Han Yp, Liu Ym, Yang Y, Wang Xm, Shi X. Poststroke depression and risk of recurrent stroke. Medicine. 2019;98:42(e17235).
This work was supported by Doctoral Foundation of Hebei College of Traditional Chinese Medicine (BSZ2017013).
The authors have no conflicts of interest to disclose.
References
- [1].Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res 2017;120:439–48.. [DOI] [PubMed] [Google Scholar]
- [2].Singh RJ, Chen S, Ganesh A, et al. Long-term neurological, vascular, and mortality outcomes after stroke. Int J Stroke 2018;13:787–96.. [DOI] [PubMed] [Google Scholar]
- [3].Tsivgoulis G, Katsanos AH, Patousi A, et al. Stroke recurrence and mortality in northeastern Greece: the Evros Stroke Registry. J Neurol 2018;265:2379–87.. [DOI] [PubMed] [Google Scholar]
- [4].Olindo S, Saint-Vil M, Jeannin S, et al. One-year disability, death and recurrence after first-ever stroke in a Black Afro-Caribbean population. Int J Stroke 2017;12:844–50.. [DOI] [PubMed] [Google Scholar]
- [5].Mohan KM, Wolfe CD, Rudd AG, et al. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 2011;42:1489–94.. [DOI] [PubMed] [Google Scholar]
- [6].Alajbegovic A, Djelilovic-Vranic J, Nakicevic A, et al. Post stroke depression. Med Arch 2014;68:47–50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Arwert HJ, Meesters JJL, Boiten J, et al. Poststroke Depression: A Long-Term Problem for Stroke Survivors. Am J Phys Med Rehabil 2018;97:565–71.. [DOI] [PubMed] [Google Scholar]
- [8].Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke 2014;9:1017–25.. [DOI] [PubMed] [Google Scholar]
- [9].Towfighi A, Ovbiagele B, El Husseini N, et al. Poststroke depression: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017;48:e30–43.. [DOI] [PubMed] [Google Scholar]
- [10].Yuan HW, Wang CX, Zhang N, et al. Poststroke depression and risk of recurrent stroke at 1 year in a Chinese cohort study. PLoS One 2012;7:e46906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sibolt G, Curtze S, Melkas S, et al. Post-stroke depression and depression-executive dysfunction syndrome are associated with recurrence of ischaemic stroke. Cerebrovasc Dis 2013;36:336–43.. [DOI] [PubMed] [Google Scholar]
- [12].Mei LP, Liu HJ, Fang XH, et al. Relationship between post-stroke depression and recurrent stroke and death in Beijing communities. Chin J Cerebrovasc Dis 2013;10:239–43.. [Google Scholar]
- [13].Ayerbe L, Ayis S, Crichton S, et al. The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J Neurol Neurosurg Psychiatry 2014;85:514–21.. [DOI] [PubMed] [Google Scholar]
- [14].Jiao JT, Cheng C, Ma YJ, et al. Association between inflammatory cytokines and the risk of post-stroke depression, and the effect of depression on outcomes of patients with ischemic stroke in a 2-year prospective study. Exp Ther Med 2016;12:1591–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yu S, Arima H, Bertmar C, et al. Depression but not anxiety predicts recurrent cerebrovascular events. Acta Neurol Scand 2016;134:29–34.. [DOI] [PubMed] [Google Scholar]
- [16].Cai W, Mueller C, Li YJ, et al. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev 2019;50:102–9.. [DOI] [PubMed] [Google Scholar]
- [17].Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed March 15, 2019. [Google Scholar]
- [18].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101.. [PubMed] [Google Scholar]
- [19].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Morgenstern LB, Sanchez BN, Skolarus LE, et al. Fatalism, optimism, spirituality, depressive symptoms, and stroke outcome: a population-based analysis. Stroke 2011;42:3518–23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang N, Wang CX, Wang AX, et al. Time course of depression and one-year prognosis of patients with stroke in mainland China. CNS Neurosci Ther 2012;18:475–81.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pandian JD, Gall SL, Kate MP, et al. Prevention of stroke: a global perspective. Lancet 2018;392:1269–78.. [DOI] [PubMed] [Google Scholar]
- [23].Bartoli F, Lillia N, Lax A, et al. Depression after stroke and risk of mortality: a systematic review and meta-analysis. Stroke Res Treat 2013;2013:862978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Eriksson M, Asplund K, Glader EL, et al. Self-reported depression and use of antidepressants after stroke: a national survey. Stroke 2004;35:936–41.. [DOI] [PubMed] [Google Scholar]
- [25].Robinson RG, Jorge RE, Long J. Prevention of poststroke mortality using problem-solving therapy or escitalopram. Am J Geriatr Psychiatry 2017;25:512–9.. [DOI] [PubMed] [Google Scholar]
- [26].Ried LD, Jia H, Feng H, et al. Selective serotonin reuptake inhibitor treatment and depression are associated with poststroke mortality. Ann Pharmacother 2011;45:888–97.. [DOI] [PubMed] [Google Scholar]
- [27].Das J, G KR. Post stroke depression: the sequelae of cerebral stroke. Neurosci Biobehav Rev 2018;90:104–14.. [DOI] [PubMed] [Google Scholar]
- [28].Zhang Y, Zhao H, Fang Y, et al. The association between lesion location, sex and poststroke depression: meta-analysis. Brain Behav 2017;7:e00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lattanzi S, Bartolini M, Provinciali L, et al. Glycosylated Hemoglobin and Functional Outcome after Acute Ischemic Stroke. J Stroke Cerebrovasc Dis 2016;25:1786–91.. [DOI] [PubMed] [Google Scholar]
- [30].Lattanzi S, Silvestrini M. Blood pressure in acute intra-cerebral hemorrhage. Ann Transl Med 2016;4:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lattanzi S, Cagnetti C, Rinaldi C, et al. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci 2018;387:98–102.. [DOI] [PubMed] [Google Scholar]
- [32].Zangari R, Zanier ER, Torgano G, et al. Early ficolin-1 is a sensitive prognostic marker for functional outcome in ischemic stroke. J Neuroinflamm 2016;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mitchell AJ, Sheth B, Gill J, et al. Prevalence and predictors of post-stroke mood disorders: a meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen Hosp Psychiatry 2017;47:48–60.. [DOI] [PubMed] [Google Scholar]
- [34].Villa RF, Ferrari F, Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Ther 2018;184:131–44.. [DOI] [PubMed] [Google Scholar]
- [35].Wang Z, Shi Y, Liu F, et al. Diversiform etiologies for post-stroke depression. Front Psychiatry 2018;9:761. [DOI] [PMC free article] [PubMed] [Google Scholar]


