Abstract
As research progressed, the recommended duration of endocrine therapy for breast cancer patients has been extended from 5 to 10 years. This study aimed to investigate how the duration of endocrine medication and therapy affect survival rate in the real world. By using the National Health Insurance Research Database (NHIRD), this study examined 1002 breast cancer patients newly diagnosed between 2000 and 2005 as research subjects, and conducted follow-up until 2013. Among these subjects, 51 used aromatase inhibitors (AIs), 561 used tamoxifen, and 390 alternated between the use of tamoxifen and AIs. The mean follow-up period in this study was 9.63 years, and the mean duration of taking endocrine medication was 4.04 years. The tamoxifen group had the longest follow-up period (9.87 years), shortest endocrine therapy duration (3.29 years), and best survival rate (86.1%). Patients were divided into 3 groups based on the duration of endocrine therapy: under 2 years, 2 to 5 years, and over 5 years. It was found that patients who received medication for less than 2 years showed the lowest survival rate with statistically significant differences (P < .001). Therefore, the extension of endocrine therapy duration is critical in improving breast cancer patients’ survival rate.
Keywords: breast cancer, duration of endocrine therapy, survival rate, tamoxifen
1. Introduction
In 1896, Beatson observed that using oophorectomy to inhibit estrogen was a feasible method of treatment for breast cancer patients who could not be surgically treated.[1] Tamoxifen is a selective estrogen receptor modulator that can antagonize the effects of estrogen. Its initial application as a contraceptive did not produce desirable outcomes. Subsequently, tamoxifen was applied to breast cancer therapy and became a gold standard for endocrine therapy. Other than tamoxifen, postmenopausal women can also choose aromatase inhibitors (AIs) as an adjuvant therapy for estrogen receptor positive breast cancer.
The 2001 NSABP B-14 study[2] randomly divided 1172 disease-free patients who completed 5 years of tamoxifen treatment into either the placebo group (n = 579) or the tamoxifen group (n = 593). The study found that patients with 5 years of tamoxifen treatment showed better disease free survival than patients with 10 years of treatment (82% vs 78%, P = .03), and no statistically significant differences in survival rate were found between these 2 groups (94% vs 91%, P = .07). The Scottish study[3] published in the same year also found similar results, hence the recommended duration of tamoxifen treatment does not need to exceed 5 years.
Published in 2013, the ATLAS study[4] randomly divided 6846 patients who completed 5 years of tamoxifen treatment into 2 groups: one group continued taking tamoxifen until 10 years (n = 3428) while the other group stopped (n = 3418, open control). The continued use of tamoxifen was found to reduce the risk of breast cancer recurrence (617/3428 vs 711/3418, P = .002), and lower breast cancer mortality (331 deaths vs 397 deaths, P = .01) as well as overall mortality (639 deaths vs 722 deaths, P = .01). In addition to these findings, other studies that applied 3 to 5 years of AIs treatment (letrozole,[5] anastrozole,[6] and exemestane[7]) after 5 years of tamoxifen treatment also found the extension of endocrine therapy beyond 5 years to be beneficial.
What is the recommended duration of endocrine therapy? The ASCO and ESMO guidelines[8,9] recommend 10 years unless patients have a very low risk disease. As new evidence in support of extended endocrine therapy emerged, this study aimed to examine the duration of endocrine therapy for breast cancer patients in the real world and the effects of therapy duration on survival rate.
2. Methods
2.1. Data source
This retrospective cohort study is based on data from the National Health Insurance Research Database (NHIRD) between 2000 and 2013. Launched in 1995, Taiwan's National Health Insurance (NHI) program contains health care data from over 99% of the 23 million population. The NHIRD records 1 million beneficiaries that are randomly sampled from NHI beneficiaries. This retrospective study was conducted after being granted ethics approval from the Institutional Review Board of Taipei City Hospital (TCHIRB-10604115-W).
2.2. Study subjects
The first batch of data was filtered by using the diagnostic code for breast cancer on major illness/injury certificates (The International Classification of Disease, Ninth Revision, Clinical Modification, ICD-9-CM code174) to find patients who were diagnosed with breast cancer for the first time between January 1, 2000 and December 31, 2005. The Longitudinal Health Insurance Database does not provide information about the stages of cancer. In order to exclude mortality due to severe illness instead of different medication, this study set new breast cancer patients who passed away within 180 days and patients with other cancer diagnosis records (ICD9 140-208) 2 years prior to breast cancer diagnosis as criteria for exclusion to mitigate the issue of bias.
After excluding the cases of mortality within 180 days from the new cases of breast cancer between 2000 and 2005, a total of 1002 patients underwent surgery and received endocrine therapy. Among these patients, 51 used AIs, 561 used tamoxifen, and 390 alternated between both tamoxifen and AIs. This study examined the effects of different durations of endocrine therapy on patient survival.
2.3. Variables and measures
The research subjects were tracked from the day of their breast cancer diagnosis to December 31, 2013, and information such as age, low-income status, comorbidity, type of endocrine therapy, duration of endocrine therapy, and survival status were collected. Charlson comorbidity index (CCI) was used as comorbidity indices. The application of endocrine therapy was classified into 3 groups based on their pharmacological effects: group I only used AIs including 3 medications: anastrozole, exemestane, and letrozole; group II only used tamoxifen; and group III alternated between both tamoxifen and AIs.
The quantitative variables in this study included the age of breast cancer diagnosis, CCI score, and duration of endocrine therapy. The age of breast cancer diagnosis was divided into 3 groups: less than 50 years old, 50 to 55 years old, and over 55 years old. Most patients above age 55 have undergone menopause while most patients under age 50 have not undergone menopause; it was harder to judge whether patients between ages 50 and 55 have undergone menopause or not. Disease comorbidity was recorded and scored by using the CCI. Patients were divided into 3 groups: normal (CCI score of 0); mild (CCI score of 1−2); and severe (CCI score ≥3). The duration of endocrine therapy was the length of time in using endocrine therapy, which was divided into 3 groups: less than 2 years, 2 to 5 years, and over 5 years.
2.4. Statistical analysis
Statistical analysis was performed by the SAS 9.4 package software. In terms of descriptive statistics, the distribution of continuous variables was displayed by mean and the distribution of categorical variables was displayed by percentage. Then, survivorship curves were constructed for patients by using the Kaplan−Meier method. In respective to inferential statistics, the mean values were analyzed by Fisher exact test, and percentages were analyzed by the chi-squared test. The log-rank test was applied to test patients’ survival rate, and the Cox proportional hazard model was applied to estimate the adjusted hazard ratio in breast cancer patient mortality and the 95% confidence interval. The Cox proportional hazards model was used to analyze the effects of the following 5 variables on survival: endocrine therapy, age, low-income family, CCI type, and medication period. The adjusted hazard ratio controlled for 4 of the variables to only demonstrate the effects of one of the variables on survival. The control for confounding was achieved in this way. The significance level for statistical analysis was set at P < .05.
3. Results
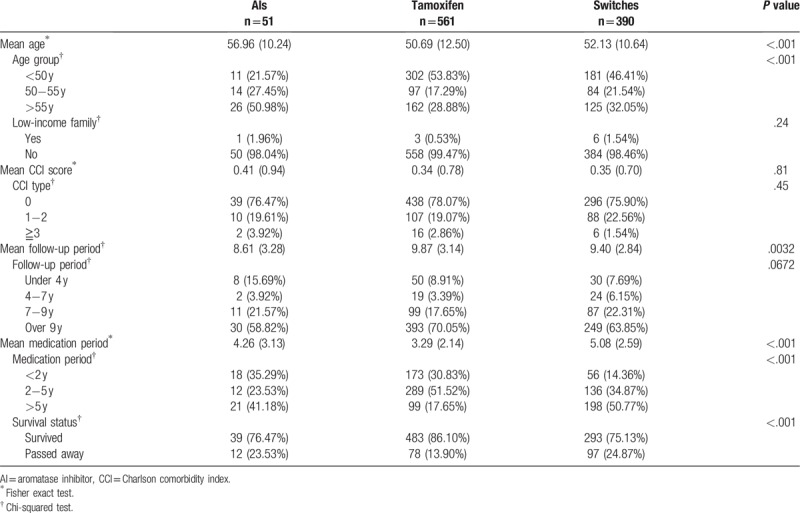
The first-time breast cancer patients had a mean age of 51.57 years. The tamoxifen group was the youngest group with 53.83% of breast cancer patients below 50 years old; the AIs group was the oldest group with 50.98% of patients above age 55. The mean follow-up period in this study was 9.63 years, and the mean duration of taking endocrine medications was 4.04 years. The tamoxifen group had the longest follow-up period (9.87 years), shortest endocrine therapy duration (3.29 years), and best survival rate (86.1%). Please refer to Table 1 for details.
Table 1.
A basic comparison between different types of endocrine therapy (n = 1002).
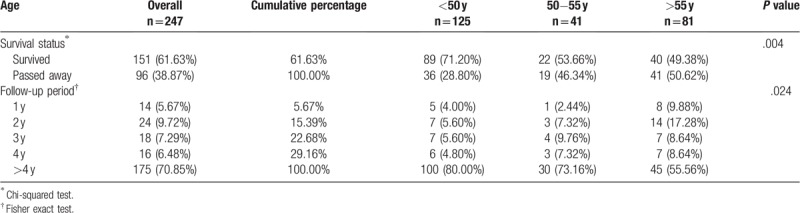
The Kaplan−Meier survivorship curve was applied to analyze the effects of different endocrine therapy durations on patient survival. Based on the duration of endocrine therapy, patients were divided into 3 groups: under 2 years, 2 to 5 years, and over 5 years. Patients who received medication for less than 2 years showed the lowest survival rate, and the differences between these 3 groups were statistically significant (P < .001). Please see Figure 1 for more details. A further analysis on patients with endocrine therapy for less than 2 years showed that 96 (38.87%) of the 247 patients passed away by 2013; 14 patients passed away within 1 year; and 24 patients passed away within 1 to 2 years. In other words, 209 of the patients (84.61%) who stopped receiving endocrine therapy remained alive beyond 2 years. Please see Table 2 for details.
Figure 1.
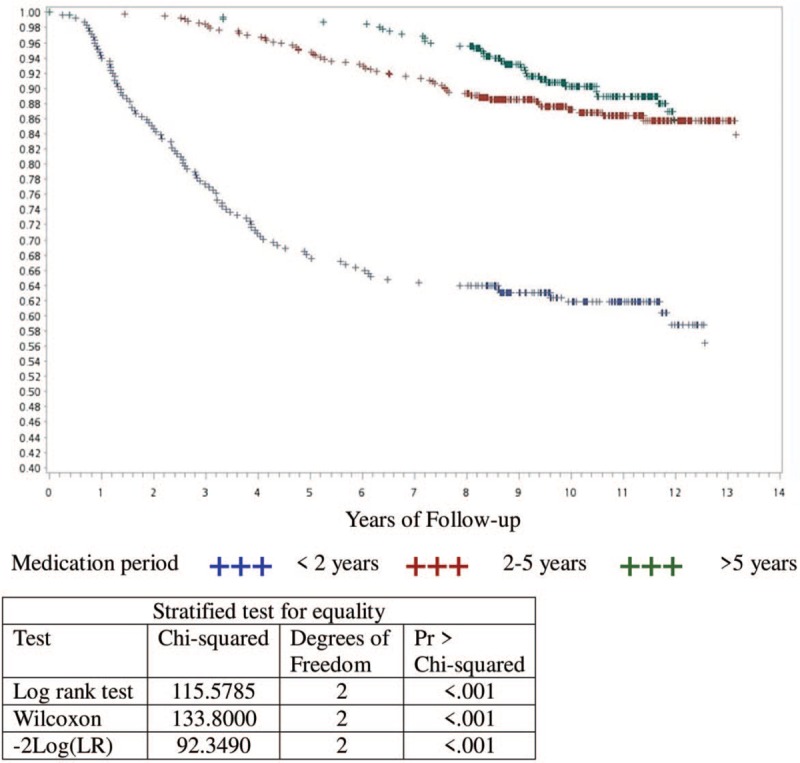
Kaplan–Meier survivorship curve - according to the time of endocrine therapy.
Table 2.
Basic information on breast cancer patients who received endocrine therapy for less than 2 y (n = 247).
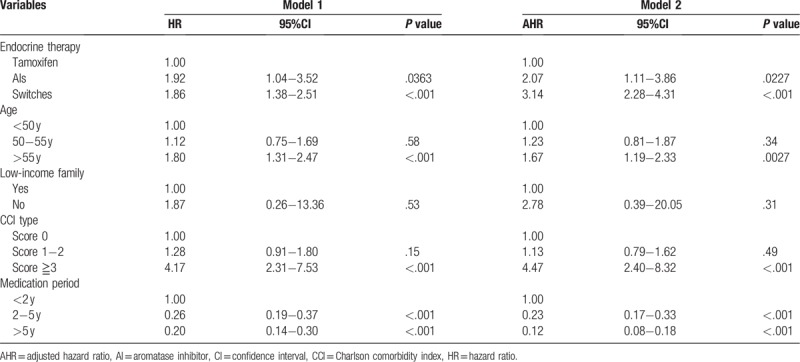
Cox regression was applied to analyze the factors that affected breast cancer patient survival. Compared to using tamoxifen, the mortality in taking AIs and both medications was 2.07 and 3.14 times, respectively. These differences are statistically significant. In respective to age groups, the mortality in patients over age 55 was 1.67 times that of patients under age 50, and this difference was statistically significant. In regards to the duration of medication therapy, the mortality in patients who took medications for 2 to 5 years and over 5 years was 0.23 and 0.12 times that of patients who took medications for less than 2 years with statistical significance. Please see Table 3 for details.
Table 3.
Cox regression analysis on factors that affected breast cancer patients survival (n = 1002).
4. Discussion
According to the 2014 study published by Hsieh et al,[10] survival was affected by interruption and nonadherence when breast cancer patients received endocrine therapy. Their data were collected between 2003 and 2010 with follow-up until 2011, while this study data collected between 2000 and 2005 with follow-up until 2013. Although both studies were conducted by using the NHIRD, the data collection at different times resulted in different distributions of endocrine therapy. In the study by Hsieh et al, the percentage of patients in the Tamoxifen-only, AIs-only, and Switches groups was 65.9%, 10.7%, and 23.4%, respectively. In contrast, the percentages in this study were 56%, 5.1%, and 38.9%. The data in this study were collected at an earlier time point when AIs remained relatively new and had to meet the NHI Payment Guidelines to be prescribed. As a result, only 5.1% of the patients used AIs in this study. In addition, the follow-up period was longer in this study, hence there was a relatively higher proportion of patients in the Switches group.
This study collected data between 2000 and 2005, and conducted follow-up until 2013. At that time, the application of 5-year endocrine therapy was already established and some large-scale studies[4–7] gradually published findings in support of extended therapy. In 2014 to 2015, the ASCO and ESMO guidelines[8,9] finally confirmed the recommended use of 10-year endocrine therapy. In reality, endocrine medications had the issue of having a shorter course of treatment. The median duration of using tamoxifen was 2.42 years in a retrospective cohort study[11] in the Tayside region of Scotland between 1993 and 2002. In comparison, the mean duration of using endocrine therapy was 3.6 years in the study by Hsieh et al.[10] In this study, the mean duration of using endocrine therapy was 4.04 years, and that of using tamoxifen had the shortest duration of only 3.29 years. Although the duration was longer than what was reported in the 2 earlier studies,[10,11] it was still shorter than the recommended 5 to 10 year duration. Moreover, the proportion of patients receiving treatment over 5 years was lowest (17.6%) in the tamoxifen group, while accounting for 40% to 50% in the AIs or Switches group.
In 1996, Saphner et al analyzed the time of recurrence in 3585 postsurgery breast cancer patients and discovered that the peak hazard of recurrence occurred in the interval of 1 to 2 years. The hazard decreased consistently in the interval of 2 to 5 years.[12] Postmenopausal breast cancer patients were randomly assigned to receive tamoxifen treatment for 2 or 5 years, and the patients who continued therapy for 5 years performed better in event-free survival and overall survival.[13] In their study, Hsieh et al found that during the period of endocrine therapy, the first occurrence of interruption in the 2nd and 3rd year of endocrine therapy was significantly associated with increased mortality comparing with the endocrine therapy persistence group.[10] This study analyzed the survival rate based on different durations of endocrine therapy and found that patients with less than 2 years of therapy showed the lowest survival rate.
For patients who took medications for less than 2 years, this study further analyzed their survival after stopping the medications to determine whether the interruption was caused by mortality or other reasons. A total of 209 patients (84.61%) stopped taking endocrine medications but remained alive for more than 2 years in our follow-up. Therefore, it is critical to find these patients and deal with the reasons that interrupted the therapy, in order to improve the survival rate in endocrine therapy. Hsieh et al[14] studied the predictors for endocrine therapy interruption and found younger breast cancer patients (under age 50) and patients who either switched medications or experienced adverse reactions during the hormone treatment process were more likely to interrupt their therapy. In another study, 32.4% discontinued initial AIs therapy within 2 years because of adverse effects; 24.3% discontinued specifically because of musculoskeletal symptoms. Younger age and taxane-based chemotherapy were associated with higher likelihood of treatment discontinuation.[15]
The limitations in this study are outlined below: first, the severity of illness can affect survival rate. This effect cannot be ruled out since the NHIRD does not specify the stages of cancer. This study removed terminal breast cancer patients by excluding patients who passed away within 180 days after confirmed diagnosis. This reduced the effects of different stages of cancer on patient survival. Next, this study collected data from 2000 to 2005 and performed follow-up until 2013 in order to track the long-term outcomes of taking the medications. This resulted in relatively fewer patients in the AIs group. Third, this study did not have information on self-paid medications and it had to use prescribed medications according to the NHI Payment Guidelines. The payment guidelines were stricter for AIs than tamoxifen, hence the patients in the AIs group had more severe illness than the tamoxifen group.
Author contributions
Conceptualization: Chuan-Hsun Chang, Chun-Wen Huang, Chien-Ming Huang, You-Min Lu.
Data curation: Chun-Wen Huang, Tzu-Chi Ou, Chu-Chieh Chen.
Formal analysis: Tzu-Chi Ou, Chu-Chieh Chen.
Investigation: Chun-Wen Huang, You-Min Lu.
Methodology: Chuan-Hsun Chang, Chun-Wen Huang, Chien-Ming Huang, Tzu-Chi Ou, Chu-Chieh Chen.
Project administration: Chuan-Hsun Chang, You-Min Lu.
Software: Tzu-Chi Ou, Chu-Chieh Chen.
Supervision: Chuan-Hsun Chang, Chien-Ming Huang.
Writing – original draft: You-Min Lu.
Writing – review & editing: Chuan-Hsun Chang, Chun-Wen Huang, Chien-Ming Huang.
You-Min Lu orcid: 0000-0001-7739-4050.
Footnotes
Abbreviations: AI = aromatase inhibitor, CCI = Charlson comorbidity index, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database.
How to cite this article: Chang CH, Huang CW, Huang CM, Ou TC, Chen CC, Lu YM. The Duration of Endocrine Therapy and Breast Cancer Patients’ Survival—A nationwide population-based cohort study. Medicine. 2019;98:43(e17746).
This retrospective study was conducted after being granted ethics approval from the Institutional Review Board of Taipei City Hospital (TCHIRB-10604115-W).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Beatson G. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet 1896;2:104–7.. [PMC free article] [PubMed] [Google Scholar]
- [2].Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst 2001;93:684–90.. [DOI] [PubMed] [Google Scholar]
- [3].Stewart HJ, Prescott RJ, Forrest APM. Scottish adjuvant tamoxifen trial: a randomized study updated to 15 years. J Natl Cancer Inst 2001;93:456–62.. [DOI] [PubMed] [Google Scholar]
- [4].Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805–16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003;349:1793–802.. [DOI] [PubMed] [Google Scholar]
- [6].Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst 2007;99:1845–53.. [DOI] [PubMed] [Google Scholar]
- [7].Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the national surgical adjuvant breast and bowel project B-33 trial. J Clin Oncol 2008;26:1965–71.. [DOI] [PubMed] [Google Scholar]
- [8].Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. J Clin Oncol 2014;32:2255–69.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol 2015;26Suppl 5:v8–30.. [DOI] [PubMed] [Google Scholar]
- [10].Hsieh KP, Chen LC, Cheung KL, et al. interruption and non-adherence to long-term adjuvant hormone therapy is associated with adverse survival outcome of breast cancer women − an Asian population-based study. PLoS ONE 2014;9:e87027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 2008;99:1763–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996;14:2738–46.. [DOI] [PubMed] [Google Scholar]
- [13].Swedish Breast Cancer Cooperative Group Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. J Natl Cancer Inst 1996;88:1543–9.. [DOI] [PubMed] [Google Scholar]
- [14].Hsieh KP, Chen LC, Cheung KL, et al. A competing risk analysis of hormone therapy interruption in Asian women with breast cancer. Pharmacoepidemiol Drug Saf 2015;24:301–9.. [DOI] [PubMed] [Google Scholar]
- [15].Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 2012;30:936–42.. [DOI] [PMC free article] [PubMed] [Google Scholar]


