Abstract
This study sought to assess the hypothesis that anemia is associated with diabetic retinopathy in type 2 diabetes mellitus (DM) and investigate the factors mediating the relationship between anemia and diabetic retinopathy.
In total, 1637 individuals with type 2 DM were examined in a cross-sectional study. Anemia was defined as hemoglobin level <120 g/L in women and <130 g/L in men. A logistic regression model was used to determine the association between anemia and diabetic retinopathy.
Anemia was more prevalent in individuals with diabetic retinopathy. Logistic regression analysis found a statistically significant association between anemia and diabetic retinopathy after adjustment for traditional risk factors (odds ratio, 1.44; 95% confidence interval, 1.10–1.89, P = .009). Further adjustment for serum bilirubin levels removed the statistically significant association.
In individuals with type 2 DM, anemia is related to diabetic retinopathy, and its association may be mediated by a correlated change in serum bilirubin levels.
Keywords: anemia, bilirubin, diabetic retinopathy
1. Introduction
Diabetic retinopathy is a common complication in individuals with diabetes mellitus (DM), leading to visual impairment and blindness.[1] Aside from its detrimental impact on vision, diabetic retinopathy might be associated with increased systemic vascular complications in individuals with type 2 DM.[1,2] Even though chronic hyperglycemia, diabetes duration, and hypertension have been well recognized as crucial risk factors for diabetes retinopathy, the risk of diabetic retinopathy could not be completely eliminated by controlling for blood glucose and blood pressure.[1] Hence, other factors may also be involved in the pathogenesis of diabetic retinopathy.
Anemia is frequently found in individuals with type 2 DM.[3] Many previous studies have shown a close link between anemia and hypoxia-induced organ damage, including heart failure, angina, and pedal ulceration, as well as increased mortality.[4,5] An increasing body of evidence also implicates anemia in diabetes-related organ damage.[6,7] Retinal hypoxia is also suggested to be associated with diabetic retinopathy.[5]
Although bilirubin, a metabolite of heme, was once considered to be a toxic waste product, it has recently emerged as an antioxidant and cytoprotectant.[8] Previous clinical studies have shown a protective role of bilirubin in diabetic microangiopathy.[9–11] Low serum bilirubin levels are reported to be associated with an increased risk of diabetic retinopathy.[12,13] As serum bilirubin is generated from the degradation of hemoglobin,[14] the effect of anemia on the retina might be related to a reduction in serum bilirubin levels with antioxidant activities. However, the relationship among anemia, bilirubin, and diabetic retinopathy has not yet been clarified.
The purpose of the current study was, therefore, to explore the associations between anemia, serum bilirubin concentrations, and diabetic retinopathy in individuals with type 2 DM.
2. Patients and methods
2.1. Participants
We conducted a cross-sectional study in 1637 randomly chosen individuals with type 2 DM who visited the diabetes clinic of our hospital from January 2017 to December 2018. Based on the “Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus”,[15] we made the diagnosis of type 2 DM. We defined the presence of anemia as a hemoglobin level <120 g/L in women and <130 g/L in men.[16] Anemia was categorized into two groups: grade I anemia with hemoglobin levels ≥110 g/L and grade II anemia with hemoglobin levels <110 g/L. Hypertension was defined as a blood pressure ≥140/90 mmHg or taking anti-hypertensive drugs. An individual was considered to have hyperlipidemia if total cholesterol levels were ≥6.5 mmol/L and/or triglycerides levels ≥2.3 mmol/L or if anti-hyperlipidemic agents were prescribed. We gathered information regarding smoking status, diabetes duration, and other health-associated parameters through a standardized questionnaire. Individuals with a history of pancreatitis, chronic liver disease (including hepatitis B or C), liver cirrhosis, glucocorticoid use, advanced renal dysfunction (serum creatinine more than 177 mmol/L), alcoholism, malignancy, infection, hemolysis, acute or chronic blood loss, hemoglobinopathies, or red blood cell transfusion were excluded from this study. In addition, individuals with serum bilirubin concentrations greater than the upper limit of normal (>22.2 mmol/L) and those with serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels more than twice the upper limit of normal (>74 U/L) were excluded. The study was approved by the ethics committee of Chonnam National University Hospital, with informed consent provided by all participants.
2.2. Measurement
After overnight fasting, venous blood samples were collected. We measured glycated hemoglobin (A1C) level using ion exchange liquid chromatography with a model HLC-723-GHbV apparatus (Tosoh, Tokyo, Japan). We determined hemoglobin levels using cyanmethemoglobin spectrophotometry (Beckman-Coulter Inc., Miami, FL). We quantified serum levels of AST and ALT using the AU5407 analyzer (Olympus, Tokyo, Japan). We measured serum bilirubin levels with an enzymatic method using bilirubin oxidase on a model AU5407 analyzer (Olympus, Tokyo, Japan) and assessed urinary albumin excretion using the urinary albumin: creatinine ratio (ACR) in random urine samples. We determined estimated glomerular filtration rate (eGFR) by the Chronic Kidney Disease Epidemiology Collaboration equation.[17] Nephropathy was categorized as a urinary ACR ≥ 300 mg/gCr or eGFR <60 mL/min/1.73 m2. To diagnose diabetic retinopathy, fundoscopy was conducted by ophthalmologists after pupil dilation. Study participants were categorized into three groups: no diabetic retinopathy, non-proliferative diabetic retinopathy (NPDR), and proliferative diabetic retinopathy (PDR). In this study, diabetic retinopathy included NPDR and PDR.
2.3. Statistical analyses
Data are presented as mean ± standard deviation or median (interquartile range). The Mann–Whitney U test or Student's t test was used for continuous variables, and the Chi-squared test was used for categorical variables. To explore the relationship between anemia and diabetic retinopathy, multivariable analysis was conducted using logistic regression models with identified factors and previously recognized risk factors. Logarithmic transformation was conducted prior to analysis for parameters with skewed distribution. Model 1 included sex and age as covariates. Model 2 was adjusted by Model 1 variables plus smoking habits, body mass index, ALT levels, hyperlipidemia, hypertension, diabetes duration, A1C levels, and nephropathy. Further, bilirubin level was entered as a covariate (Model 2a). SPSS version 20.0 (SPSS, Chicago, IL) was used for statistical analyses. A P value <.05 indicated statistical significance.
3. Results
Characteristics of individuals with type 2 DM are presented in Table 1. Individuals with diabetic retinopathy were older and had longer diabetes duration, lower body mass index, lower eGFR, higher urinary ACR, and lower serum bilirubin, ALT, and hemoglobin levels than those without diabetic retinopathy. Individuals with diabetic retinopathy had higher systolic blood pressures and higher A1C levels than those without diabetic retinopathy. Individuals with diabetic retinopathy were associated with a higher prevalence of hypertension and nephropathy than those without diabetic retinopathy. In addition, anemia was significantly more common in individuals with diabetic retinopathy than in those without diabetic retinopathy. Furthermore, the severity of diabetic retinopathy increased with the grade of anemia (P < .001, Fig. 1).
Table 1.
Characteristics of subjects with Type 2 diabetes mellitus.
Figure 1.
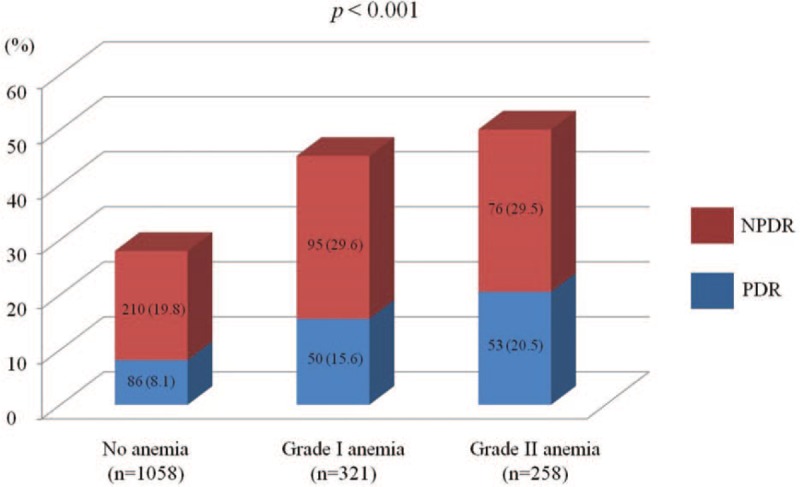
Diabetic retinopathy prevalence according to anemia grade. Diabetic retinopathy severity increased with anemia grade (P < .001). Data are represented as frequencies (percentages). NPDR = non-proliferative diabetic retinopathy, PDR = proliferative diabetic retinopathy
To explore the effects of anemia on diabetic retinopathy, we performed multivariable analyses using logistic regression models (Table 2, Fig. 2). The relationship between anemia and diabetic retinopathy was statistically significant after adjusting for sex, age, smoking habits, body mass index, ALT level, hypertension, hyperlipidemia, diabetes duration, A1C level, and nephropathy (odds ratio [OR]: 1.44; 95% confidence interval [CI]: 1.10–1.89, P = .009; Model 2). The addition of serum bilirubin concentrations to this model attenuated this association (OR: 1.30, 95% CI: 0.98–1.72, P = .074; Model 2a).
Table 2.
Odds ratio for diabetic retinopathy in individuals with Type 2 diabetes mellitus.
Figure 2.
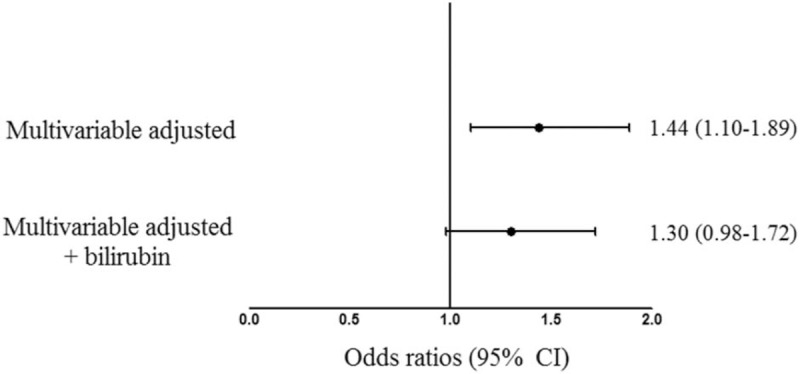
Odds ratios for diabetic retinopathy with anemia as an independent variable after adjusting for confounding factors. Odd ratios were adjusted for age, sex, smoking, body mass index, ALT, hypertension, hyperlipidemia, DM duration, A1C level, and nephropathy.
4. Discussion
We found a positive relationship between anemia and diabetic retinopathy in individuals with type 2 DM after adjusting for conventional risk factors including diabetes duration, A1C, and hypertension. Moreover, our findings suggest that the association between anemia and diabetic retinopathy might be partly mediated by a correlated change in serum bilirubin concentrations.
Individuals with type 2 DM commonly have anemia.[3] Regardless of the presence of nephropathy, hemoglobin levels continue to decline over time in individuals with type 2 DM.[3,18] Evidence points to anemia as a risk factor for adverse cardiovascular events including coronary heart disease, heart failure, and cardiovascular mortality.[4,19] In addition, anemia might be implicated in diabetes-related organ damage.[5] Qiao et al[20] reported that anemia is associated with an increased risk for diabetic retinopathy. Ito et al[6] showed a close relationship between anemia and diabetic retinopathy in individuals with type 2 DM. Furthermore, our study showed that anemia was positively associated with diabetic retinopathy after adjusting for sex, age, smoking habits, body mass index, ALT, hypertension, hyperlipidemia, diabetes duration, A1 C, and nephropathy (Model 2 in Table 2). Our data therefore support the preceding findings that anemia is implicated in retinal damage in diabetes.[5,6,20]
With regards to underlying factors linking anemia with organ damage, previous studies have focused on hypoxia. Anemia causes tissue hypoxia, which is a critical etiology of diabetic retinopathy.[5] Retinal hypoxia is proposed to stimulate synthesis of vascular endothelial growth factor (VEGF), a strong stimulant of neovascularization.[5,21] This factor also increases vascular permeability and retinal exudates.[5,21] Individuals with anemia have been reported to have increased systemic levels of VEGF.[22] Therefore, decreased oxygen delivery due to anemia might have detrimental effects on the retina of individuals with diabetes. Additionally, anemia might contribute to increased oxidative stress due to a decrease in the absolute number of red blood cells with antioxidant defense and enhanced free radical production.[23,24]
Furthermore, anemia might be linked to serum bilirubin levels. Bilirubin is generated from the sequential catalytic degradation of the heme in hemoglobin by heme oxygenase and biliverdin reductase.[25] Thus, a reduction in hemoglobin levels might be implicated in a change in physiological serum bilirubin levels. Bilirubin has been recently recognized as a natural antioxidant that scavenges free radicals.[8] Experimental studies have shown that all types of bilirubin, including unconjugated bilirubin, conjugated bilirubin, free bilirubin, and albumin-bound bilirubin, have effective antioxidant capacities.[8,26,27] Bilirubin is suggested to inhibit the formation of advanced glycation end products and protein kinase C[28] and to be involved in inflammatory processes.[29] All of these pathways have been implicated in the pathogenesis of diabetic retinopathy.[30] Further support comes from clinical studies showing that serum bilirubin levels might play a protective role against DM and cardiovascular disease.[31,32] Furthermore, previous clinical studies have reported close associations between serum bilirubin levels and diabetic microangiopathy.[10,11] Yasuda et al[12] reported an inverse relationship between total serum bilirubin levels and diabetic retinopathy. Sekioka et al[13] showed that total serum bilirubin levels were negatively related to the severity of diabetic retinopathy.[13] Thus, we hypothesized that the relationship between anemia and diabetic retinopathy might be linked to a correlated change in serum bilirubin levels. In the current study, a statistically significant association between anemia and diabetic retinopathy was abolished when serum bilirubin level was further entered into Model 2, which adjusted for other confounders in the multiple regression model (Model 2a in Table 2). Our findings, therefore, indicate that the proposed induction of diabetic retinopathy by anemia might be partly mediated by a change in serum bilirubin levels.
In conclusion, our study shows a positive relationship between anemia and diabetic retinopathy in individuals with type 2 diabetes, which might be partly attributed to a correlated decline in total serum bilirubin levels. Further prospective investigations are necessary to explore the causal associations among anemia, bilirubin, and diabetic retinopathy.
Author contributions
Conceptualization: Jin Ook Chung, Seon-Young Park, Min Young Chung.
Data curation: Jin Ook Chung, Seon-Young Park, Dong Jin Chung.
Formal analysis: Jin Ook Chung, Seon-Young Park.
Investigation: Jin Ook Chung, Seon-Young Park, Min Young Chung.
Supervision: Seon-Young Park, Min Young Chung.
Writing – original draft: Jin Ook Chung.
Writing – review & editing: Seon-Young Park, Dong Jin Chung, Min Young Chung.
Footnotes
Abbreviations: ACR = albumin excretion rate; A1C = glycated hemoglobin; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CI = confidence intervals; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; NPDR, non-proliferative diabetic retinopathy; OR = odds ratio; PDR = proliferative diabetic retinopathy; VEGF = vascular endothelial growth factor.
How to cite this article: Chung JO, Park SY, Chung DJ, Chung MY. Relationship between anemia, serum bilirubin concentrations and diabetic retinopathy in individuals with type 2 diabetes. Medicine. 2019;98:43(e17693).
MYC and SYP equally contributed to this work as co-corresponding authors.
This study was financially supported by Chonnam National University (Grant number: 2018-3551) and Chonnam National University Hospital Biomedical Research Institute (CRI18033-1).
The authors have no conflicts of interest to disclose.
References
- [1].Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010;376:124–36.. [DOI] [PubMed] [Google Scholar]
- [2].Cheung N, Rogers S, Couper DJ, et al. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke 2007;38:398–401.. [DOI] [PubMed] [Google Scholar]
- [3].Thomas MC, MacIsaac RJ, Tsalamandris C, et al. Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes Care 2003;26:1164–9.. [DOI] [PubMed] [Google Scholar]
- [4].Zoppini G, Targher G, Chonchol M, et al. Anaemia, independent of chronic kidney disease, predicts all-cause and cardiovascular mortality in type 2 diabetic patients. Atherosclerosis 2010;210:575–80.. [DOI] [PubMed] [Google Scholar]
- [5].Thomas MC. Anemia in diabetes: marker or mediator of microvascular disease? Nat Clin Pract Nephrol 2007;3:20–30.. [DOI] [PubMed] [Google Scholar]
- [6].Ito H, Takeuchi Y, Ishida H, et al. Mild anemia is frequent and associated with micro- and macroangiopathies in patients with type 2 diabetes mellitus. J Diabetes Investig 2010;1:273–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Singh DK, Winocour P, Farrington K. Erythropoietic stress and anemia in diabetes mellitus. Nat Rev Endocrinol 2009;5:204–10.. [DOI] [PubMed] [Google Scholar]
- [8].Stocker R, Yamamoto Y, McDonagh AF, et al. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043–6.. [DOI] [PubMed] [Google Scholar]
- [9].Zhu B, Wu X, Bi Y, et al. Effect of bilirubin concentration on the risk of diabetic complications: a meta-analysis of epidemiologic studies. Sci Rep 2017;7:41681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chung JO, Cho DH, Chung DJ, et al. Physiological serum bilirubin concentrations are inversely associated with the prevalence of cardiovascular autonomic neuropathy in patients with Type 2 diabetes. Diabet Med 2014;31:185–91.. [DOI] [PubMed] [Google Scholar]
- [11].Inoguchi T, Sasaki S, Kobayashi K, et al. Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. JAMA 2007;298:1398–400.. [DOI] [PubMed] [Google Scholar]
- [12].Yasuda M, Kiyohara Y, Wang JJ, et al. High serum bilirubin levels and diabetic retinopathy: the Hisayama Study. Ophthalmology 2011;118:1423–8.. [DOI] [PubMed] [Google Scholar]
- [13].Sekioka R, Tanaka M, Nishimura T, et al. Serum total bilirubin concentration is negatively associated with increasing severity of retinopathy in patients with type 2 diabetes mellitus. J Diabetes Complications 2015;29:218–21.. [DOI] [PubMed] [Google Scholar]
- [14].Berk PD, Howe RB, Bloomer JR, et al. Studies of bilirubin kinetics in normal adults. J Clin Invest 1969;48:2176–90.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26Suppl 1:S5–20.. [DOI] [PubMed] [Google Scholar]
- [16].World Health Organization Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: Vitamin and Mineral Nutrition Information System World Health Organization; 2011. [Google Scholar]
- [17].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Craig KJ, Williams JD, Riley SG, et al. Anemia and diabetes in the absence of nephropathy. Diabetes Care 2005;28:1118–23.. [DOI] [PubMed] [Google Scholar]
- [19].Tong PC, Kong AP, So WY, et al. Hematocrit, independent of chronic kidney disease, predicts adverse cardiovascular outcomes in Chinese patients with type 2 diabetes. Diabetes Care 2006;29:2439–44.. [DOI] [PubMed] [Google Scholar]
- [20].Qiao Q, Keinanen-Kiukaanniemi S, Laara E. The relationship between hemoglobin levels and diabetic retinopathy. J Clin Epidemiol 1997;50:153–8.. [DOI] [PubMed] [Google Scholar]
- [21].Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A 1995;92:10457–61.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dunst J, Becker A, Lautenschlager C, et al. Anemia and elevated systemic levels of vascular endothelial growth factor (VEGF). Strahlenther Onkol 2002;178:436–41.. [DOI] [PubMed] [Google Scholar]
- [23].Siems WG, Sommerburg O, Grune T. Erythrocyte free radical and energy metabolism. Clin Nephrol 2000;53(1 Suppl):S9–17.. [PubMed] [Google Scholar]
- [24].Grune T, Sommerburg O, Siems WG. Oxidative stress in anemia. Clin Nephrol 2000;53(1 Suppl):S18–22.. [PubMed] [Google Scholar]
- [25].Fujiwara R, Haag M, Schaeffeler E, et al. Systemic regulation of bilirubin homeostasis: potential benefits of hyperbilirubinemia. Hepatology 2018;67:1609–19.. [DOI] [PubMed] [Google Scholar]
- [26].Wu TW, Fung KP, Wu J, et al. Antioxidation of human low density lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol 1996;51:859–62.. [DOI] [PubMed] [Google Scholar]
- [27].Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem 1994;269:16712–9.. [PubMed] [Google Scholar]
- [28].Kalousova M, Novotny L, Zima T, et al. Decreased levels of advanced glycation end-products in patients with Gilbert syndrome. Cell Mol Biol (Noisy-le-grand) 2005;51:387–92.. [PubMed] [Google Scholar]
- [29].Basiglio CL, Arriaga SM, Pelusa F, et al. Complement activation and disease: protective effects of hyperbilirubinaemia. Clin Sci (Lond) 2009;118:99–113.. [DOI] [PubMed] [Google Scholar]
- [30].Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–25.. [DOI] [PubMed] [Google Scholar]
- [31].Lin JP, O’Donnell CJ, Schwaiger JP, et al. Association between the UGT1A1∗28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation 2006;114:1476–81.. [DOI] [PubMed] [Google Scholar]
- [32].Abbasi A, Deetman PE, Corpeleijn E, et al. Bilirubin as a potential causal factor in type 2 diabetes risk: a Mendelian randomization study. Diabetes 2015;64:1459–69.. [DOI] [PMC free article] [PubMed] [Google Scholar]


