Abstract
This study aims to evaluate the clinical value of haptoglobin (Hp) and sCD163 testing for the differential diagnosis of pleural effusion, and investigate the correlation of Hp and sCD163 with the inflammatory response of the body.
Pleural effusion samples were collected from 78 patients (38 tuberculous pleural effusions [TPE] and 40 malignant pleural effusions [MPE]). The concentrations of Hp and sCD163 in the pleural effusion were measured by enzyme-linked immunosorbent assay (ELISA).
The concentrations of Hp and sCD163 were significantly higher in the TPE group than in the MPE group (P < .05). The sensitivity and specificity of the Hp test for the differential diagnosis of TPE and MPE was 82.4% and 86.1%, respectively (P < .01), while the cut off value was 779.05 ug/mL. Furthermore, the sensitivity and specificity of the sCD163 test for the differential diagnosis of TPE and MPE was 76.3% and 85.0%, respectively (P < .01), while the cut off value was 16,401.11 ng/mL. Moreover, the sensitivity and specificity of the combination of Hp and sCD163 tests for diagnosing TPE was 90.0% and 87.5%, respectively. Hp and IL-1β, TNF-α, CRP and ESR were positively correlated in both the TPE group and MPE group (P < .05). Hp and sCD163 were positively correlated in the TPE group (r = 0.3735, P = .0209), but not in the MPE group (r = 0.22, P = .1684). However, there was no correlation between sCD163 and TNF-α, CRP and ESR in either the TPE group, or the MPE group (P > .05). Furthermore, sCD163 and IL-1β were weakly correlated in the TPE group (r = 0.49, P = .0018), but these had no correlation in the MPE group (r = 0.068, P = .6767).
Hp and sCD163 can be used as biological markers for the differential diagnosis of pleural effusion in clinic, and the level of Hp in pleural effusion may reflect the intensity of inflammation in the body to some extent.
Keywords: haptoglobin, malignancy, pleural effusion, soluble CD163, tuberculosis
1. Introduction
A pleural effusion is the excess fluid that accumulates in the pleural cavity, which is caused by various diseases. Tuberculous pleural effusion (TPE), malignant pleural effusion (MPE), and par pneumonic pleural effusion are the most common causes of exudative pleural effusion in clinical practice. According to statistics, tuberculosis and malignant diseases account for 44.1% and 29.6% of the causes of pleural effusion, respectively.[1] Furthermore, both TPE and MPE present with lymphocytic pleural effusions. The early diagnosis of TPE is an important step for treating tuberculous pleural effusion, since it is closely associated with the course and prognosis of the disease. However, conventional diagnostic tests, including microscopic examination of the pleural fluid, biochemical tests, culture of the pleural fluid, sputum, or pleural tissue, and the histopathological examination of pleural tissue, have known limitations, because the sensitivity and specificity could not be taken into account. Therefore, there is a need to develop more sensitive and effective detection methods. In recent years, varieties of differentially expressed proteins in TPEs and MPEs have been identified by proteomics. Those differentially expressed proteins can be used as important biomarkers for the differential diagnosis of TPE and MPE. According to published proteomics studies on TPEs and MPEs, the level of haptoglobin (Hp) and free CD163 (soluble-CD163, sCD163) in TPEs and MPEs significantly differ.[2,3] Therefore, both Hp and sCD163 are potential biomarkers for the differential diagnosis of TPE and MPE. Hp is an acid glycoprotein produced by the liver. Furthermore, Hp is a multiple functional protein that can be detected in serum and other body fluids. The main functions of Hp are as follows:
-
1.
Hp protects hemoglobin (Hb) by combining with free Hb and transporting Hb to macrophages in the form of a Hp-Hb complex, thereby avoiding the oxidative stress damage of excess Hb;
-
2.
as an important acute reaction protein in the process of host anti-infective defense, Hp performs the repair of damaged tissues and maintenance of the stability of the internal environment;
-
3.
Hp is an important player in regulating antioxidation, prostaglandin synthesis, bacteriostasis, angiogenesis, and immune response.
CD163 is a transmembrane glycoprotein with 9 extracellular domains, and belongs to the scavenger receptor cysteine-rich superfamily.[4] The molecular weight of CD163 is 130 kDa, which is mainly expressed on the surface of mature mononuclear macrophage membranes, especially M2 macrophages.[5–7] The anti-inflammatory and antibacterial activities of CD163 have been reported in recent years.[8,9] Studies have found that when macrophages are activated through Toll-like receptors in response to inflammatory and other stimulations, CD163 on the surface of macrophages can be released into the surrounding body fluids through the cleavage of proteins to form free CD163 (sCD163).[10] Therefore, sCD163 is considered as a marker of macrophage activation.[11]
The aim of the present study was to evaluate the utility of both Hp and sCD163 as biomarkers for the differential diagnosis of TPE and MPE, and determine the sensitivity and specificity of Hp and sCD163 in the differential diagnosis of malignant and tuberculous pleurisy. Meanwhile, the correlation of Hp and sCD163 with cytokines, such as Interleukin (IL)-1β and tumor necrosis factor (TNF)-α, as well as clinically applied inflammatory markers, such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), in TPEs and MPEs were also investigated. The present study presents the feasibility of Hp and sCD163 for the differential diagnosis of TPE and MPE.
2. Materials and methods
2.1. Patients
The present study recruited 78 patients with primary pleural effusion in the First Hospital of Jilin University from April 2017 to January 2018. The age of these patients ranged within 18 to 72 years old. Among these patients, 38 patients had TPE, while 40 patients had MPE. Group criteria: The pleural effusion examination for all patients was performed according to Light's criteria for exudative pleural effusion. All patients were diagnosed with tuberculosis or malignant tumor through the pleural effusion cytology specimen obtained by thoracoscopy.
Exclusion criteria:
-
1.
patients who previously had a similar pleural effusion or definite diagnosis of tuberculosis and malignant tumor;
-
2.
patients who previously or recently received treatment for regular anti-tuberculosis, chemotherapy, or regular anti-inflammation, or used immunosuppressive agents;
-
3.
the initial segment of the thorax piercing fluid could not be successfully obtained;
-
4.
patients with serious liver disease, renal diseases, heart diseases, immune system diseases and other related diseases that could affect the expression of Hp and sCD163;
-
5.
patients with severe or incomplete data.
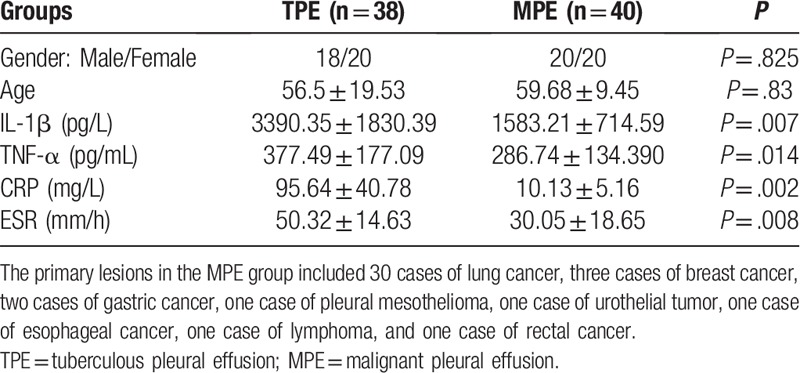
Table 1 presents the samples, which were divided into 2 groups: samples from patients with TPE, and samples from patients with MPE.
Table 1.
Baseline characteristics of patients and levels of inflammatory markers in serum obtained from patients with TPE and MPE.
The protocol and procedure of the present study were approved by the Institutional Ethical Committee, and all patients who agreed to participate in the present study were required to provide a signed consent form.
2.2. Pleural fluid collection
The initial pleural effusion was collected and stored at a constant temperature of 4°C, centrifuge at 1500 rpm for 5 minutes, and the supernatant was dispensed. Then, the supernatant was centrifuge again at 4000 rpm for 10 minutes. Next, an aliquot of the isolated supernatant was placed in Eppendorf tubes (1 mL/tube) and stored in a freezer at −80°C.
2.3. Measurement of the levels of biomarkers and inflammatory factors
The samples of the pleural effusion were defrosted at 4°C in a room. When precipitates were observed in the samples, the sample was centrifuged again to obtain a clear supernatant for testing. The measurement of the concentration of Hp and CD163 in the pleural effusion samples was performed using an ab108856 human haptoglobin kit (Abcam) and RD Systems human CD163 kit (RD Systems). Then, the level of IL-1β and TNF-α in these pleural effusion samples was determined by ELISA using a microplate reader (BioTek, ELx800).
The examination of ESR and CRP was performed by the laboratory of our hospital. Both ESR and CRP are routine laboratory tests for all patients on the day of admission.
2.4. Statistical analysis
The data analysis was performed using SPSS 18 software. Comparisons for normally distributed measurement datasets were performed using t test, while comparisons for non-normally distributed measurement datasets were performed using non-parametric rank sum test. A P value of <.05 was considered statistically significant. A receiver operating characteristic (ROC) curve was used to define the best cut-off point for the diagnostic biomarker, and calculate the sensitivity and specificity of Hp and sCD163 testing. Pearson linear correlation was used to determine the correlations between various parameters.
3. Results
3.1. Hp and sCD163 levels in pleural effusion samples
In order to evaluate the utility of both Hp and sCD163 as biomarkers for the differential diagnosis of TPE and MPE, the concentration of Hp and sCD163 in specimens collected from patients with TPE and patients with MPE was measured. As shown in Figure 1, the median concentration of Hp was significantly higher in TPE (1,374.936 ± 411.13 ug/mL), than in MPE (505.84 ± 294.65 ug/mL), and the difference in median values between TPE and MPE was statistically significant (P < .05). On the other hand, the median concentration of sCD163 was also significantly higher in the TPE group (2102.58 ± 611.59 ng/mL), when compared with the MPE group (1240.62 ± 428.74 ng/mL), and the difference in median concentration of sCD163 between TPE and MPE was statistically significant (P < .05) (Fig. 2)
Figure 1.
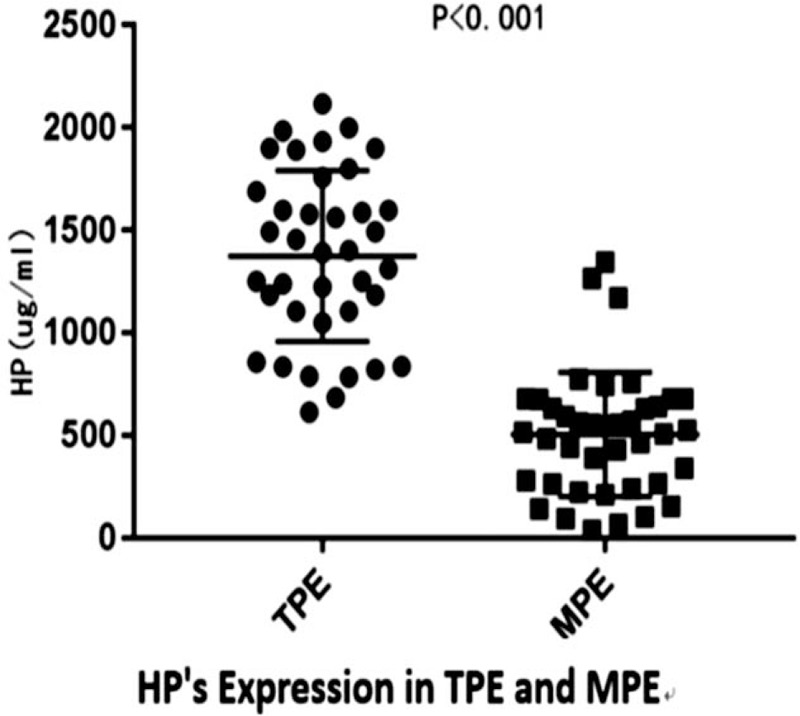
The levels of Hp in pleural effusions obtained from patients with tuberculous and cancer.
Figure 2.
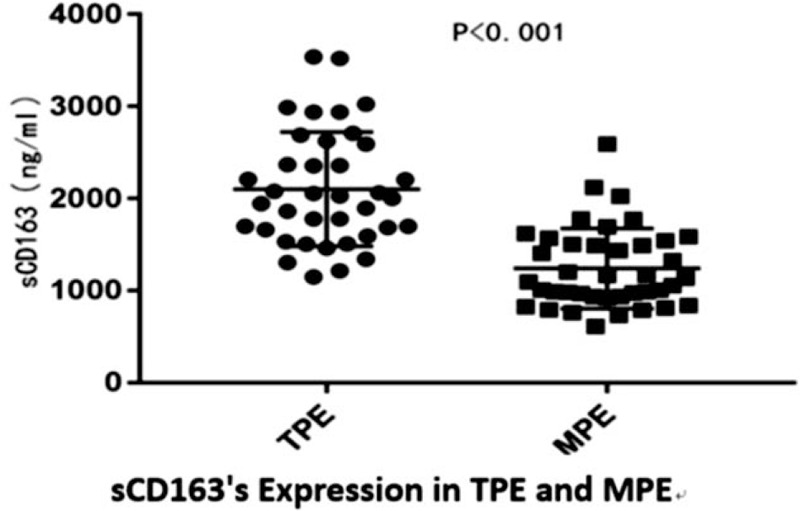
The levels of sCD163 in pleural effusions obtained from patients with tuberculous and cancer.
3.2. Diagnostic value of Hp and sCD163 measurements
In order to determine the sensitivity and specificity of Hp and sCD163 in the differential diagnosis of malignant and tuberculous pleurisy, a ROC curve analysis was performed. The analysis results revealed that Hp offered an area under the curve (AUC) of 0.940 between the TPE and MPE groups. As shown in Figure 3, the diagnostic sensitivity and specificity of the pleural fluid Hp test at an optimal cut-off value of 779.05 ug/mL was 82.4% and 86.1%, respectively (P < .01). Moreover, sCD163 offered an AUC of 0.888 between the TPE and MPE groups. The diagnostic sensitivity and specificity of the pleural fluid sCD163 test at an optimal cut-off value of 16401.11 ng/mL was 76.3%% and 85%, respectively (P < .01) (Fig. 3).
Figure 3.
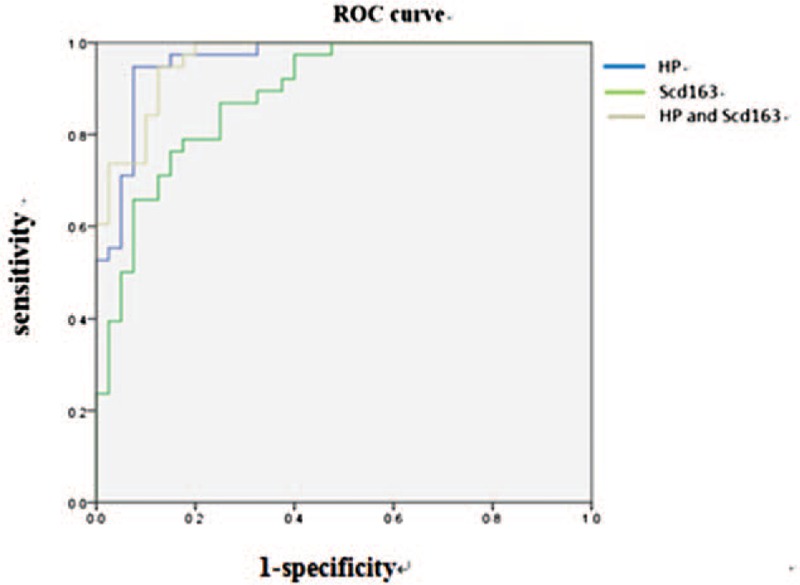
The ROC curve of Hp and sCD163 levels for diagnosing tuberculous pleural effusion.
The strength of the combined measurement of Hp and sCD163 was also estimated. The combined measurement of Hp and sCD163 offered an AUC of 0.963 between the TPE and MPE groups. The diagnostic sensitivity and specificity of the combined measurement of Hp and sCD163 was 90% and 87.5%, respectively (P < .01, Fig. 3), indicating that the combined measurement is better than a single test of Hp or sCD163 for the differential diagnosis of malignant and tuberculous pleurisy.
3.3. Results of Pearson correlation analysis between Hp and sCD163, IL-1β, TNF-α, CRP, ESR
The correlation of Hp and sCD163 with cytokines and inflammatory markers in the TPE and MPE groups was investigated using Pearson linear correlation analysis. The analysis data revealed the following:
-
1.
IL-β was positively correlated with TNF-α in the TPE and MPE groups (P < .0001);
-
2.
Hp was positively correlated with IL-1β, TNF-α, CRP and ESR (P < .05);
-
3.
Hp and sCD163 were positively correlated in the TPE group (r = 0.3735, P = .0209), but not in the MPE group (r = 0.22, P = .1684);
-
4.
there was no correlation between sCD163 and CRP, ESR and TNF-α in the TPE and MPE groups (P > .05);
-
5.
sCD163 was weakly correlated with IL-1β in the TPE group (r = 0.49, P = .0018), but not in the MPE group (r = 0.068, P = .6767).
4. Discussion
The development of novel biomarkers for the differential diagnosis of TPE and MPE has been an area of active investigation in recent years. Numerous studies have found that differentially expressed proteins in pleural effusions have high clinical value as a biomarker for the differential diagnosis of pleural effusion etiology. Among the differential expressed proteins, Hp[12,5] and sCD163[13] have been given more attentions due to the significant difference in their levels in TPE and MPE. Hp is an acid glycoprotein produced mainly by the liver, and detected in serum and other body fluids. It has been reported[14] that the level of Hp was significantly higher in MPEs, than in non-MPEs (P < .001). Another study performed a proteomics analysis of TPE and MPE, and revealed that there were significant differences in Hp level between TPE and MPE.[12] Hp is significantly increased in TPEs.[12] In order to evaluate the utility of Hp as a biomarker for the differential diagnosis of TPE and MPE, the Hp levels between the TPE and MPE groups were compared through measurement by ELISA. The results revealed that Hp was significantly elevated in TPE, when compared to MPE (P < .01). Furthermore, the analysis of the ROC curve revealed that the cut-off value of the Hp test (779.05 ug/mL) has high sensitivity and specificity (82.4% and 86.1%, respectively). However, these present results are contrary to the study conducted by Alexandrakis et al[14] but similar to the study conducted by Lee et al.[12] The insistence of the results of the 2 reports mentioned above may be due to the hypoxic state of macrophages in the tissue granulomas of tuberculosis patients, which can inhibit tuberculosis infection.[15] When erythrocytes are heavily lysed during hypoxia, the Hb in the blood would increase, accordingly. Under that condition, Hp production would increase to remove the excessive Hb. In addition, the body of a patient with tuberculous pleurisy would go to a hypersensitive state, leading to the increase in Hp as an acute reaction protein, which would involve anti-infection, the repair of damaged tissues, and the maintenance of homeostasis. In the study conducted by Alexandrakis et al,[14] Hp was found to be significantly elevated in MPEs, and this was considered to be correlated to the function of secreted Hp in certain tumor cells. The difference in results between these 2 previous reports may be due to the difference in research population.
In the report of Suzuki et al,[13] the level of sCD163 in serum and the pleural effusion supernatant of patients with tuberculosis significantly increased (P < .01). However, the level of sCD163 in the pleural effusion was significantly higher, when compared to that in serum. Similarly, the present study revealed that the level of sCD163 in the TPE group was significantly higher than that in the MPE group (P < .01). This is consistent with the report of Suzuki et al,[13] considering that M2 macrophage activity is significantly increased in active tuberculosis, because M2 macrophages play a leading role in the development of tuberculous granuloma.[16] Furthermore, the ROC curve analysis revealed that the sensitivity and specificity of sCD163 in the diagnosis of TPE was 76.3% and 85.0%, respectively (P < .01). When compared to the best diagnostic value of the Hp test, the sensitivity of the sCD163 test was slightly lower, but the specificity was similar. The analysis also indicated that the combination of the Hp and sCD163 test had a higher sensitivity and specificity (90% and 87.5%, respectively), when compared to a single Hp or sCD163 test for the differential diagnosis of TPE and MPE. Since IL-1β and TNF-α are important inflammatory factors in the body, the correlation of IL-1β and TNF-α with TPE or MPE was also investigated in the present study. The analysis results revealed that IL-β and TNF-α had a positive correlation in TPEs and MPEs. Furthermore, Hp was positively correlated with IL-1β, TNF-α, CRP and ESR in TPE and MPE (P < .05). These results reveal the association of Hp with the inflammatory response of the body, suggesting that Hp may be used as an important indicator of inflammatory response of the body. However, the comparative analysis of sCD163 and related inflammatory markers revealed a weak correlation between sCD163 and IL-β in the TPE group (r = 0.49, P = .0018), while there was no correlation between sCD163 and CRP, ESR and TNF-α in both the TPE and MPE groups (r = 0.3735, P = .0209). Furthermore, the analysis revealed that Hp and sCD163 were positively correlated in the TPE group (r = 0.3735, P = .0209), but not in the MPE group. It remains unclear whether a linear correlation in sCD163 level exists with the number of CD163 on the macrophage membrane surface. Due to the sample size of the present study study, it remains unclear whether Hp and CD163 are correlated.
All specimens tested in the present study were initial pleural effusions collected from patients who did not receive anti-inflammation or anti-tumor treatment. Many factors may affect the level of Hp and sCD163 in TPE and MPE, such as diseases of the liver and kidney, immune system and circulatory system, as well as drug treatment. Therefore, for the accuracy of the tests of these patients, these factors were excluded in the present study. However, the sample size of the present study was small. Hence, there may be some deviations. Future studies on Hp and sCD163 in TPE and MPE would be conducted with an expanded sample size. Furthermore, Suzuki et al[13] found that patients with a serum sCD163 level above 1300 ng/mL had a mortality rate that was five times higher than that of patients with lower sCD163 levels (44.6% vs 6.6%; P < .0001, by log-rank test). Therefore, it is necessary to investigate the effect of Hp and sCD163 in pleural effusions on the prognosis of the disease in future studies.
Author contributions
Data curation: Lei Song.
Funding acquisition: Hui Ding.
Investigation: Jinying Gao.
Methodology: Jinying Gao.
Resources: Liping Peng.
Writing – original draft: Jinying Gao.
Writing – review & editing: Dan Li.
Footnotes
Abbreviations: CRP = C-reactive protein, ELISA = enzyme-linked immunosorbent assay, ESR = erythrocyte sedimentation rate, Hp = haptoglobin, IL-1β = Interleukin-1β, MPE = malignant pleural effusions, sCD163 = soluble CD163, TNF-α = tumor necrosis factor-α, TPE = tuberculous pleural effusions.
How to cite this article: Gao J, Song L, Li D, Peng L, Ding H. Clinical value of haptoglobin and soluble CD163 testing for the differential diagnosis of tuberculous and malignant pleural effusions. Medicine. 2019;98:42(e17416).
This work was supported by the Major National Science and Technology Projects (2017ZX10302301-002 and 2012Z053).
The authors have no conflicts of interest to disclose.
References
- [1].Liam CK, Lim KH, Wong CM. Causes of pleural exudates in a region with a high incidence of tuberculosis. Respirology 2000;5:33–28.. [DOI] [PubMed] [Google Scholar]
- [2].Glaziou P, Floyd K, Raviglione M. Global burden and epidemiology of tuberculosis. Clin chest Med 2009;30:621–36.. vii. [DOI] [PubMed] [Google Scholar]
- [3].Hu S, Loo JA, Wong DT. Human body fluid proteome analysis. Proteomics 2006;6:6326–53.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Onofre G, Kolackova M, Jankovicova K, et al. Scavenger receptor CD163 and its biological functions. Acta Med (Hradec Kralove) 2009;52:57–61.. [PubMed] [Google Scholar]
- [5].Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol 2013;13:621–34.. [DOI] [PubMed] [Google Scholar]
- [6].Etzerodt A, Moestrup SK. CD163 and inflammation: biological, diagnostic,and therapeutic aspects. Antioxid Redox Signal 2013;18:2352–63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Buechler C, Ritter M, Orsó E, et al. Regulation of scavenger receptor CD163 expression in humanmonocytes and macrophages by pro- and anti-inflammatory stimuli. J Leukoc Biol 2000;67:97–103.. [PubMed] [Google Scholar]
- [8].Kneidl J, Löffler B, Erat MC, et al. Soluble CD163 promotes recognition, phagocytosis and killing of Staphylococcus aureus via binding of specific fibronectin peptides. Cell Microbiol 2012;14:914–36.. [DOI] [PubMed] [Google Scholar]
- [9].Fabriek BO, van Bruggen R, Deng DM, et al. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 2009;113:887–92.. [DOI] [PubMed] [Google Scholar]
- [10].Moller HJ, Nielsen MJ, Maniecki MB, et al. Soluble macrophage derived CD163: a homogenous ectodomain protein with a dissociable haptoglobin-hemoglobin binding. Immunobiology 2010;215:406–12.. [DOI] [PubMed] [Google Scholar]
- [11].Fabriek BO, Dijkstra CD, vandenBerg TK. The macrophage scavenger receptor CD163. Immunobiology 2005;210:153–60.. [DOI] [PubMed] [Google Scholar]
- [12].ChangYoul Lee, JiYoung Hong, Myung-Goo Lee, et al. Identification of 10 candidate biomarkers distinguishing tuberculous and malignant pleural fluid by proteomic methods. Yonsei Med J 2017;58:1144–51.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yuzo Suzuki, Masahiro Shirai, Kazuhiro Asada, et al. Utility of macro-phage-activated marker CD163 for diagnosis and prognosis in pulmonary tuberculosis. Orig Res 2017;14:57–64.. [DOI] [PubMed] [Google Scholar]
- [14].alexandrakis M, coulocheri S, kyriakou D, et al. Diagnostic value of ferritin, haptoglobin, (1-antitrypsin, lactate dehydrogenase and complement factors C3 and C4 in pleural effusion differentiation. Respir Med 1997;91:517–23.. [DOI] [PubMed] [Google Scholar]
- [15].Tang Y, Hua SC, Qin GC, et al. Different subsets of macrophages in patients with new onset tubercles pleural effusion. PloS One 2014;9:e88343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang Z, Luo Q, Guo Y, et al. Mycobacterium tuberculosis-induced polarization of humanmacrophage orchestrates the formation and development oftuberculous granulomas in vitro. PLoS One 2015;10:e0129744. [DOI] [PMC free article] [PubMed] [Google Scholar]


